Abstract
The presence of a gassy ground condition is an important problem in tunneling. In this study, the effects of groundwater H2S and CH4 emissions are investigated and characterized together with the factors that created these conditions in Nosoud tunnel in Iran. Through the geological investigations, the presence of these gasses was not detected prior to the construction of the tunnel. Groundwater sampling indicated that about 1 L of H2S is released per 100 L of the water inflow into the Nosoud tunnel under normal conditions. However, the volume of the released gas was varying with the changes in the groundwater discharge rate. Thus, estimation of groundwater inflow into the tunnel is necessary for predicting the volume of gas emission. Based on the experience of the Nosoud tunnel excavations, there are several geological and hydrogeological factors that must be considered as the indicators of gas emissions during tunneling. Considering the importance of ground water gas emission into the tunnels located in gassy conditions, the present work was conducted to predict the H2S seepage before the excavation using geological and hydrogeological indicators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prediction of the groundwater inflows in excavations in fractured bedrock is an important issue not only for controlling the rate of the excavation but also because of potential environmental and occupational health impacts associated with groundwater management. Identifying the presence of gassy ground conditions is particularly important in tunneling, especially in cases with toxic and corrosive gasses, which are rarely considered during the geological investigations prior to tunnel construction.
H2S emission into tunnels and other underground structures is a significant health hazard that can cause fatalities during infrastructure construction. The H2S emission from groundwater into tunnels is reported in many studies (Szilas 1985; Gritchfield 1985; Hendry et al. 1985; Novakowski and Lapcevic 1988; O’Brian and Gere 1993; Doyle 2001; Plummer 2002; Schafer et al. 2007; William and Hansmire 2008; Mirmehrabi et al. 2008; Morsali et al. 2008; Hansmire and Jafri 2008; Wightman and Mackay 2008; Wenner and Wannenmacher 2009; Shahriar et al. 2009; Mirmehrabi et al. 2012; Jalilian Khave 2013; Tali et al. 2014).
Gasses together with soils, rocks, and water are among the important components of the underground environments. Hydrogen sulfide, methane, and carbon dioxide are usually considered as hazardous gasses generated by the natural biologic and geologic processes. Underground H2S is generated from different sources including the atmosphere, the mantle, the bacterial decomposition of organic matter, thermal decomposition of organic and inorganic compounds, and inorganic geochemical reactions. Although H2S is widely dispersed at low-level concentrations in the environment, abnormally high concentrations of this gas may occur under some geologic conditions. H2S is a hazardous gas in underground constructions such as tunnels, shafts, and underground chambers.
In natural waters, hydrogen sulfide concentrations of 100–400 mg/L are considered to be high (Hem 1985). There are substantial natural emissions of sulfur compounds into the troposphere (Blunden and Aneja 2007; Blunden et al. 2007). Hydrogen sulfide is a colorless gas with a strong odor similar to “rotten eggs” and can be readily identified even in a small volume [0.001–0.1 parts per million (ppm)] in the air. However, the odor will be lost in 2–15 min of exposure and can no longer be detected. Higher exposure levels (above 50 ppm) can cause headaches, dizziness, nausea, burning eyes, a sore throat, and respiratory problems. Hydrogen sulfide causes a loss of consciousness and possibly death in 30 min to 1 h when present in the air at concentrations in excess of about 500 ppm. Hydrogen sulfide can also be explosive, but only in much higher concentrations. H2S also causes corrosion to metals, in particular to the electrical installations on tunnel boring machines (TBMs).
Hydrogen sulfide gas (H2S) emissions that during the tunnel construction may pose a number of challenges during the process. In this regard, test results show that exposures above 10 ppm in a tunnel can cause significant occupational health and safety problems. Gas emissions were previously reported in several constructions in Iran by Shahriar et al. (2009) and Jalilian Khave (2013) who reported and studied gas emission into the Zagros tunnel. Similarly, this problem is present in Nosoud tunnel where toxic and potentially explosive gasses including hydrogen sulfide and methane were present simultaneously.
High rates of gas seepage into these tunnels have caused serious problems with tunnel workers and TBM components. In this regard, the recorded H2S caused a four-month pause in tunneling operations in extreme conditions in Nosoud tunnel. Gas emissions were found to increase in direct proportion to the water ingress rate. Therefore, rapid dewatering systems were established to reduce the rate of groundwater ingress. According to Doyle (2001), a safe excavation in gassy ground can be conducted through controlling the atmosphere by: (1) preventing hazardous concentrations of gas from accumulating; (2) eliminating potential ignition sources in the presence of combustible gas; and (3) by sounding the alarm to evacuate in the event that gas concentrations approach hazardous levels.
Hydrogen sulfide concentration in groundwater can be high enough to generate corrosion and pose environmental problems for underground construction (Doyle 2001).
As a result of methane and hydrogen sulfide gas emission into Nosoud tunnel, tunneling operations were slowed down and high costs were imposed on the TBM (Tali et al. 2014).
This study is conducted to investigate the impacts of groundwater and gassy ground conditions on the construction of the Nosoud tunnel (H2S and methane) and present the information on the geological and hydrogeological conditions leading to gas emissions.
Physical setting
Geology and geographical setting
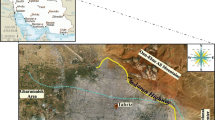
Nosoud tunnel, as a structure designed to transfer water from Sirvan River to Zahab plain, is located in the Zagros Mountains in the west of Iran. The tunnel has a diameter of 6.73 m and is 26 km long (Fig. 1). The study area is underlain by sedimentary rocks that are extensively folded and faulted and contain abundant fractured zones and joints. The area is known as the folded Zagros Zone.
Geologically, this region includes simple structures of reverse faulting and symmetrical anticlines and synclines. The main structure in the project area is the Aspar anticline that extends in a northwesterly to southeasterly direction. The oldest geologic unit along the tunnel axis is a brownish-gray limestone of the Illam formation (with a chainage—distance from start of tunnel boring excavation—of 3710–4927 m) that is located in the core of the Aspar anticline (Fig. 2). Overlying this unit is the Gurpi formation that consists of an alternating sequence of calcareous shales and argillaceous limestones. Some layers of the Illam Formation transitional with Gurpi formation are rich in pyrite. The youngest unit in the study area is the Pabdeh Formation (chainage 0–2300 m) which is comprised of an alternating sequence of dark gray calcareous shales and limestones.
Along the sub-crop of the Illam Formation in the tunnel, several discontinuities are recognized. The main joint sets and random joints were surveyed along this formation. The system of joints which is oriented sub-parallel to the anticline axis is mostly filled by calcite. The face of joint set walls is usually rough. The occasional slickenside surfaces and joint filling are mainly calcite veins. The most significant geological future in this area is the karst aquifer developed in the Illam Formation within the Aspar Anticline. The primary porosity of this aquifer consists of open joints, locally enlarged by solution process. A system of frequent vertical joints sub-parallel to the anticline axis is the dominant system in this formation. In limestone, the joints and fractures play a key role in controlling the orientation and extent of karst features (Milanovic 2000).
Hydrogeological conditions
There are three main formations encountered in the Nosoud tunnel route. Essentially, the Pabdeh and Gurpi Formations are aquiclude layers and, thus, contain a very limited amount of water. On the other hand, the section of the Illam Formation within the anticlinal structure is a karstic aquifer. Accordingly, the major water inflows occur in limestone of Illam Formation. This observation is not reported for the Pabdeh and Gurpi Formations, except locally in association with some major discontinuities. A large aquifer has been formed in the Aspar Anticline. Also, the anticline has deep valleys (Jalekouse, Aspar, Abdalan) including important and permanent springs.
The presence of a recharge area (the surface of the anticline) and a discharge area (deep valleys within the anticline) has caused significant groundwater flow from many joints and fractures in this formation. There are not enough piezometers or observation wells in this hard rock aquifer to adequately characterize the groundwater flow regime. However, according to topography and discharge fluctuations of springs located in Aspar anticline, the main direction of groundwater flow is along anticline axes and the nose of the structure. Under natural conditions, the groundwater table is 100 m above the Nosoud tunnel route crossing Aspar anticline.
There are two sulfur springs around Jalekouse village and five unsulfurated springs, consisting of Aspar l and 2, Jalekouse l and 2, Abdalan springs, one borehole (named BH-27), and Aspar well in the Illam formation. The discharge rate of this spring is about 1–30 L per min. The altitude of the springs is almost 100 m above the tunnel level (Morsali 2007).
Causes and consequences of gas emissions
Gas emission has not been considered in geological investigations undertaken prior to the commence of tunneling project. However, during the constructions of the tunnel, H2S and methane were detected in problematic amounts. These gasses were initially attributed to the anaerobic decomposition of natural petroleum hydrocarbons in the area. Further investigations were then undertaken to determine the characteristics, effects, sources, and geological indicators of H2S. Gasses tend to seep into excavations by geostatic, hydrostatic, and barometric pressure reductions associated with the excavation.
Sampling
A sampling of seepage gas into the tunnel from air and water inflow was carried out in two steps. Sampling from the inside of the tunnel and sampling from the dissolved gas were performed in cooperation with an oil company and a water and wastewater utility, respectively.
Seepage of hydrogen sulfide (H2S)
Hydrogen sulfide was only detected in sections of the tunnel with groundwater inflows. As H2S is soluble in water, there is a direct relationship between dissolved gas and pressure. Thus, H2S gas is released to air when groundwater leaks to the tunnel.
Three H2S air samples were taken from different locations around the TBM (Fig. 3). Table 1 shows the result of this analysis and consulting of the expert Oil Company. The results, however, could not prove the hydrocarbons origin for inner gas of the tunnel as there was no obvious association between H2S and the presence of hydrocarbons in the area.
Additional sampling was performed to determine the relationship between H2S levels in groundwater and in the air within the tunnel. A sampling of air and groundwater was done at specific distances along the tunnel. Analysis of H2S levels was carried out in the field and laboratory. The water sampling and the H2S volume measurement (35–50 ppm) in the tunnel were done simultaneously.
The dissolved H2S content in water was sampled at five locations in the tunnel (Fig. 3). The detection threshold for H2S by this type of sampling of H2S is 5 mg/L. Table 2 shows the results of the sampling process.
The samples were transferred to the laboratory from the cutter head location in the middle of the tunnel (Chainage 2000 m) and from the entrance of the tunnel. Then, they were transferred to the laboratory of the Water & Wastewater Utility within a 24-h period under the standard condition. The results of laboratory sampling shown in Table 3 were found to be similar to those from field sampling.
The sampling indicated that H2S is dissolved in water at the entrance to the tunnel. Along the tunnel, a noticeable amount of H2S from the water by the end of the tunnel has decreased significantly. It is likely that H2S in this region originates from hydrocarbon materials. Some geological indicators can be applied to predict of probable H2S seepage before beginning of the excavation. Among these indicators are geological indicators (formations with high potential for being the sources or reservoirs for hydrocarbons); lithological indicators (depth of faults and particular smells) together with the excavation of boreholes and fresh surface fractures and oil or similar indicators on the surfaces of fractures); and hydrogeological indicators (springs with particular smell (sulfur odor) and springs with sulfate chemical composition and high porosity).
Each of above indicators can implicate the existence of H2S in the underground environment. Most of above indicators were investigated in Aspar anticline. When the tunnel is excavated in a saturated zone, the volume of the entrance gas to the tunnel depends on hydrogeological conditions, which are discussed later.
Methane (CH4)
During the excavation of underground spaces, methane is the most commonly detected gas and causes more deaths and injuries than the other gasses (Hanifi et al. 2012). Two major risks of methane presence are that it is highly flammable and explosive. For this reasons, scientist have tried for years to diminish the hazards in underground spaces (Rodriguez et al. 2012).
Methane (CH4) is only a trace constituent of the atmosphere, but an important greenhouse gas. Although groundwater is unlikely to be a major source of atmospheric CH4, its contribution to the CH4 budget has been poorly characterized (Gooddy and Darling 2004). Methane is an odorless gas and only explosive when mixed with air between 5% (the Lower Explosive Limit) and 15% (the Upper Explosive Limit). Hence, an adequate ventilation is the main safeguard in gassy tunnels. This gas was recorded in the all excavated sections of the Nosoud tunnel. Special monitoring and testing program were implemented to control the methane gas intrusions. Automatic alarm monitors were installed at the three fixed gas detector stations in the machine. The results showed that, in a few days, gas concentrations exceed the specified levels and caused automatic shutoff of the TBM. All workers were educated about the dangers of gas and fire prevention and additional firefighting equipment were installed.
Results and discussion
Estimation of the emission rate of H2S
The emission rate of H2S to air in the tunnel was estimated using Henry’s Law. This law describes the concentration of a gas in water as a function of the partial pressure of the gas in the atmosphere under equilibrium conditions as below:
The concentration of H2S in the entrance water is about 15 mg/L, which is equivalent to 1.5 g H2S per 100 L discharge of entrance water (Morsali et al. 2008).
Based on Henry’s law, the Henry constant of H2S under standard conditions is 0.1 M/L (Lide and Frederikse 1995; Dean 1992; Carroll and Mather 1989). Therefore, the majority of gas is released into the atmosphere.
To design a ventilation system, it can be conservatively assumed that almost all gas dissolved in water is released into the air. Under standard conditions (a temperature of 25 °C and a pressure of 1 atmosphere), each mole of H2S is 22.4 L in volume. The volume of H2S release in the tunnel atmosphere for discharge of 100 L/s is calculated as:
According to Eq. 1, under standard conditions, for 100 L of water entering the tunnel, about 1 L of H2S is released in the tunnel atmosphere. As the volume of gas released to the tunnel varies with the rate of discharge of water, in order to estimate the rate of gas emissions into the tunnel, the rate of groundwater inflow into the tunnel needs to be determined. There are analytical (El Tani 2003; Yoo 2004; Park et al. 2008; Yang et al. 2009; Lachassagne et al. 2015) and experimental (Heuer 2001) methods for estimation groundwater inflow into the tunnel. In this regard, there are also some methods previously applied at tunnels in Iran (Katibeh and Aalianvari 2009; Morsali et al. 2010; Hosseini et al. 2011). Lo Russo et al. (2016) focused on quantifying the amount of water infiltrating into the mine drifts, using a water balance model in the geographic information system (GIS).
Generally, the discharge rate of the water inflow into tunnel depends on several factors such as hydrostatic pressure (water height around the tunnel) and rock mass permeability. The rock permeability is controlled by joints properties such as continuity of the joints, sizes of the joint spaces and openings, and the relationship between joints and discontinuities or lithology. Discontinues, joints, and faults are the most important parameters affecting the permeability of fractured bedrock. The permeability of bedrock is highest at the intersection of faults and joints. The permeability was measured directly using Lugeon tests in boreholes. Also, more than 15 piezometers were bored in the tunnel walls for direct calculation of the water head during the tunneling.
These data were used to estimate the rate of water inflow into the tunnel. In the Nosoud tunnel, a significant correlation was derived between the water inflow into the tunnel and the gas leaking in the tunnel (Fig. 4). In the Nosoud tunnel, there are correlation between the water inflow into the tunnel and the gas leaking in the tunnel (Figs. 5, 6).
Based on the daily observation (measured) data through the boring operation carried out in the saturated zone, it is seen that sulfide hydrogen increases by a rise in groundwater inflow into the tunnel.
Origin of gas and fluctuation prediction
H2S has various sources in gas and oil resources including the bacterial reduction of sulfate, thermochemical sulfate reduction (TSR), pyrite dissolution, inorganic reduction, oxidation reactions, and H2S penetration from the external sources. Although it is not possible to completely exclude any of these possible sources at the Nosoud tunnel site, TSR is more likely than other possible sources. Ford and Williams (2007) used the following reaction as the main source of H2S:
Hydrogen sulfide (H2S) in a shallow subsurface environment usually originates from bacterial decomposition of organic matter under anaerobic conditions. It can also occur in geothermal systems, where it originates from magma degassing and thermal metamorphism (Doyle 2001). Hydrogen sulfide in the air has a half-life of about 12 h (Jaeschke et al. 1978). Ongoing sulfate reduction is manifested in most boreholes by the presence of H2S. Groundwater in the area is characterized by a foul smell caused by sulfate-reducing bacteria, which convert sulfate into H2S (Adamsa et al. 2001).
Geological evidence of gas emissions
According to the Nosoud tunnel experience, the following geological and hydrogeological factors should be considered to assess the probability of gas emissions taking place during the tunneling:
The occurrence of cap and source rocks for hydrocarbons
In an oil field, the presence of hydrocarbons is accompanied with both cap and source rocks. The presence of a low permeability formation overlaying a high permeability formation increases the potential of the gas trapping. In the Aspar anticline, the Gurpi and Pabddeh Formations act as cap rocks while Illam Formation acts as the source rock. From the perspective of tunnel construction, oil or gas are not considered as valuable substances as even relatively low levels of hydrocarbons can lead to significant H2S emissions.
The presence of sulfur-rich lithologies
The presence of rocks containing sulfur-bearing minerals such as pyrite, gypsum, and anhydrite along the tunnel excavation path must be seriously taken into account. For example, the presence of pyrite often shows that the oxidation of hydrogen sulfide has taken place. Hence, this phenomenon can be a sign of the potential presence of H2S in groundwater. Pyrite can be seen in the Illam Formation at the Aspar Anticline (Fig. 7). Some lithologies like carbonaceous shale and anhydrite also are problematic in some cases. During an excavation in Pabdeh and Gurpi formations, the evidence of hydrocarbon materials and weak seepage H2S were observed in some rocks of the inner face of the tunnel segment excavation. The Illam Formation contains organic-rich shale interbedded with limestone (Fig. 7). These indicators must be checked during hydrogeological field investigations.
The detection of odors on fresh rock surfaces during the drilling of boreholes
Any smell of gas observed during drilling and other on-site investigations should be recorded in geotechnical logs of boreholes. The use of gas sensors can be helpful in this regard. Although gas seepage in some boreholes drilled in the Aspar anticline was reported, due to the temporal nature of theses emissions it was not recorded in the boreholes.
When detecting CH4 or H2S during probe drilling in joints far ahead of the cutter head, these joints were cement grouted for reducing gas conductivity in the rock mass. In addition, about 6-m-long gas drainage holes were drilled in the close vicinity of the cutter head to drain the gas (Wenner and Wannenmacher 2009).
Hydrogen sulfide has been previously encountered in test borings and in shaft and tunnel excavations on a number of projects (O’Brian and Gere 1993; Doyle 2001). The ongoing gas bubbling observed in groundwater in the verification drill hole can gas detection. Gas monitoring equipment in a borehole can be used to measure gas concentrations (Wightman and Mackay 2008). At the Nosoud tunnel site, hydrogen sulfide gas was observed in most of the site investigation borings in concentrations exceeding 100 ppm (0.01%) at the borehole collar (Hansmire and Jafri 2008).
Oil leakage from fractures
Oil leakage from joint and fractures of rock and tunnel segments is one of the most important indicators of gas seepage in a tunnel. However, oil leakage from the joints in the Nosoud tunnel was reported without any gas seepage in the most cases.
Deep fault occurrence
Deep faults can provide a hydraulic connection between oil-containing formations at depth to the near-surface environment. In the case of the Nosoud site, H2S migrates upward from the Illam Formation (original or host formation) via faults, large joints, and their intersections.
Hydrogeological evidence of gas emission
The presence of springs with strong odors
Springs that have water with a strong sulfurous odor provide important evidence about the gas presence in the groundwater. These springs show the chemical quality of groundwater in the research area and, consequently, in the tunnel.
There are two low-discharge sulfur springs and many unsulfurated and high-discharge springs in the Aspar Anticline. Gas emissions in the Nosoud tunnel indicate that despite the high-quality spring and the low discharge rates of sulfurated springs, the existence of even one sulfurated spring or seepage face in the study area is an important alarm. In the Aspar Anticline, sulfurated springs discharge water from a deep and old groundwater source, whereas high-quality springs are recharged from shallow and meteoric groundwater. These factors should be assessed during the excavation of tunnels.
The distance of springs from the tunnel path was detected as an important factor in the Nosoud tunnel. The Aspar sulfurated springs located about 50 and 600 m from tunnel path. In Zagros tunnel (with the similar lithological condition), there are two sulfureted springs at two parts of tunnel axis. These springs are located about 3 km away from the tunnel path and there is no H2S emission in the tunnel. Demattis et al. (2001) cited many factors for evaluating spring drawdown due to tunneling. Based on this method, it was found that a tunnel-spring distance exceeding 800 m decreases the mutual effect of springs and tunnel on each other. Dermatitis’s method was used in the Karaj tunnel and verified in Iran (Morsali et al. 2010).
Sulfate type springs
Anomalously high sulfate concentrations in groundwater are a good indicator of gaseous zones. This will only be the case when there is sufficient organic carbon present to drive sulfate-reducing conditions. While there is no evidence of sulfuric springs in the research area, the sulfate content of springs around the sulfuric spring increases noticeably in some area. In limestone and karstic areas, it would be generally expected that the anionic composition of groundwater would be dominated by bicarbonate. The increased sulfate concentrations in such an environment would indicate a significant risk of H2S presence.
Other factors
Other hydrogeological factors can be used to predict gas emission into tunnels. For example, the presence of high porosity rock in a fractured-rock environment can provide a pathway for gas transfer. In the case of the Nosoud tunnel, there are major and minor open joints and calcite filling (Fig. 8) in the Aspar limestone which influence groundwater inflow into the tunnel. There are not thermal springs in Aspar area, but H2S is reported at high concentration in thermal springs (Daldal et al. 2010). Dye or isotope tracers and the geophysics study result can be used to trace groundwater flow paths in fractured-rock environments.
Conclusions
Gaseous emissions, particularly highly toxic and corrosive gasses like H2S which occur in groundwater inflows, can be a significant hazard encountered during tunnel construction. Groundwater inflows into Nosoud tunnel were induced by drawdowns in groundwater levels and decreases in spring discharges associated with construction pose serious health hazards and operational problems.
In Nosoud tunnel, the highest concentrations of H2S gas were detected in limestone because of hydrogen sulfide gas (H2S) dissolution in groundwater and its release when the groundwater entering the excavated tunnel. H2S gas seepage into the tunnel causes many problems, for instance, corrosion in the electronics of the tunneling equipment and unfavorable conditions for working.
It is likely that H2S in this region has originated from the anaerobic decay of hydrocarbon materials. Some indicators to predict the probable H2S seepage prior to tunnel excavation are geological indicators (formations with high potential for being hydrocarbon sources and trap); lithological indicators [depth of faults, odors (e.g., sulfurous smells) together with drilling of boreholes, and fresh surface fracture sand oil similar indicators in surface of fractures]; and hydrogeological indicators (springs with particular smell -sulfur spring-, thermal springs, springs with sulfate chemical composition, thermal springs and high porosity).
Each of above indicators can implicate the existence of H2S in underground lonely. Most of above indicators were investigated in the Aspar Anticline. As the bottom line of this study, we state that the volume of gas seepage into tunnel depends on hydrogeological condition.
References
Adamsa S, Titusa R, Pietersenb K, Tredouxc G, Harrisd C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241(2001):91–103
Blunden J, Aneja VP (2007) Characterizing ammonia and hydrogen sulfide emissions from a swine waste treatment lagoon in North Carolina. Atmos Environ. doi:10.1016/j.atmosenv.2007.02.026
Blunden J, Aneja VP, Westerman WP (2007) Measurement, analysis, and modeling of hydrogen sulfide emission from a swine facility in North Carolina. Atmos Environ. doi:10.1016/j.atmosenv.2007.06.040
Carroll JJ, Mather AE (1989) The solubility of hydrogen sulphide in water from 0 to 90° C and pressures to 1 MPa. Geochim Cosmochim Acta 53:1163–1170
Daldal H, Beder B, Serin S, Sungurtekin H (2010) Hydrogen sulfide toxicity in a thermal spring. Clin Toxicol 48:755–756
Dean JA (1992) Lange’s Handbook of Chemistry. McGraw-Hill Inc., New York
Demattis A, Kalamaras G, Eusebio A (2001) A system approach for evaluating spring drawdown due to tunneling.In: Progress in tunneling, Milan, Italy
Doyle BR (2001) Hazardous gases underground. Marcel Dekker Inc., New York
El Tani M (2003) Circular tunnel in a semi-infinite aquifer. Tunn Undergr Space Technol 18:49–55
Ford DC, Williams PW (2007) Karst geomorphology & hydrology. Chapman & Hall, London
Gooddy DC, Darling WG (2004) The potential for methane emissions from groundwater of the UK, British Geological Survey, Crowmarsh Gifford, Maclean Building, Wallingford, Oxfordshire, OX10 8BB, UK
Gritchfield JW (1985) Tunneling in gassy ground. In: Proceedings Rapid Excavation and Tunneling Conference (RETC), pp 441–461
Hanifi C, Muammer C, Gunduz O, Nuh B (2012) A case study on the methane explosion in the excavation chamber of an EPB-TBM and lessons learnt including some recent accidents. Tunn Undergr Space Technol 27(1):159–167
Hansmire H, Jafri P (2008) Slurry-face rock TBM outfall tunnel in Detroit, Michigan, USA. World Tunnel Congress––Underground Facilities for Better Environment and Safety––India
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. Third printing 1989. U.S. Geological Survey Water-Supply Paper 2254. U.S. Government Printing Office, Washington
Hendry RD, Kimball FE, Shtenko VW (1985) The Tumbler Ridge branch line tunnel, northeastern British Columbia, Canada. In Tunneling’85. Fourth International Symposium; 1985 March 10–15; Institution of Mining and Metallurgy, Brighton
Heuer RE (2001) Estimating Rock tunnel water inflow. Geotechnical consulting McHenry
Hosseini P, Naseri H, Hassanpour J, Morsali M (2011) Prediction of the of groundwater inflow into tunnel in Karaj––Tehran water conveyance tunnel. In: 27th Association of Geology Congress, Tehran, Iran (in Persian)
Jaeschke W, Georgii H, Malewski H (1978) Contributions of H2S to the atmospheric sulfur cycle. Pure Appl Geophys 116:465–475
Jalilian Khave GH (2013) TBM Tunnelling in Hydrogen Sulfide Gas Bearing Ground and its solutions. Geotech Geol Eng 31(5):1621–1638
Katibeh H, Aalianvari A (2009) Development of a new method for tunnel site rating from groundwater hazard point of view. J Appl Sci 9(8):1496–1502
Lachassagne P, Marechal JC, Bienfait P, Lacquement F, Lamotte C (2015) Computing the Water Inflows Discharge and Assessing the Impacts of Tunnels Drilled in Hard Rocks: The A89 (France) Motorway Case Study, Engineering Geology for Society and Territory, vol 3
Lide DR, Frederikse HPR (1995) Handbook of Chemistry and Physics, 76th edn. CRC Press Inc, Boca Raton
Milanovic P (2000) Geological engineering in karst. Zebra Publishing Ltd.
Mirmehrabi H, Hassanpour J, Morsali M, Tarighazali S (2008) Experiences gained from gas and water inflow toward the tunnel, case study: Aspar anticline, Kermanshah, Iran. In: 5th Asian rock mechanics symposium, Tehran, Iran
Mirmehrabi H, Ghafoori M, Lashkaripour S, Tarighazali S, Hassanpour J (2012) Hazard of mechanized tunnel excavation in H2S bearing ground in Aspar tunnel. Environ Earth Sci 66(2):529–535
Morsali M (2007) Hydrogeology studies of Nousoud tunnel route, Iran Water and Power Resources Development, SHE Co., pp 147 (in Persian)
Morsali M, Hassanpour J, Shamsi G (2008) Water inflow and gas seepage to tunnel in Aspar area. In: 26th Geosciences Congress, Mining Explorations and Geology Organization, Tehran, Iran. (in Persian)
Morsali M, Naseri H, Hassanpour J (2010) Determination of the effect of tunnel boring activities on spring drawdown in Karaj–Tehran water conveyance tunnel. In: 14th Association of Geology Congress, Tehran, Iran, (in Persian)
Novakowski KS, Lapcevic PA (1988) Regional hydrogeology of the Silurian and Ordovician sedimentary rock underlying Niagara Falls, Ontario, Canada. J Hydrol 104:211–236
O’Brian and Gere Engineers (1993) Geotechnical design summary report, Raw Water Intake Tunnel, New Drinking Water Treatment Plant, and City of Niagara Falls, New York. O’Brian & Gere Engineers, Syracuse
Park KH, Lee JG, Owatsiriwong A (2008) Seepage force in a drained circular tunnel: an analytical approach. Can Geotech J 45(3):432–436
Plummer PG (2002) Impacts for hydrogen sulfide at reservoirs, Dams and Tail waters. U. S. Army Corps of Engineers, Huntington, p 25705
Rodriguez R, Diaz-Aguado MB, Lombardia C (2012) Complementation of CH4 emission during tunneling works in Asturias, A proposal with benefits both for local councils and for the affected population. J Environ Manag 104:175–185
Russo SL, Gnavi L, Peila D, Suozzi E (2016) Rough evaluation of the water-inflow discharge in abandoned mining tunnels using a simplified water balance model: the case of the Cogne iron mine (Aosta Valley, NW Italy). Environ Earth Sci. doi:10.1007/s12665-013-2335-x
Schafer M, Pintabona R, Lukajic B, Kritzer M, Janosko S, Switalski R (2007) Gas mitigation in the Mill Creek tunnel, Proc. Rapid Excavation and Tunneling Conference (RETC), pp. 168–175
Shahriar K, Rostami J, Khademi J (2009) TBM Tunneling and analysis of high gas emission accident in Zagros long tunnel. In: Proceedings of the ITA-AITES World Tunnel Congress, Budapest, Hungary
Szilas AP (1985) Production and transport of oil and gas: Part A-Flow mechanics and production. Elsevier, Amsterdam
Tali N, Lashgaripour GR, Ghafoori M, Hafezimoghadas N (2014) Problem of mechanized tunneling in gassy ground condition a case study: nousoud water transmission tunnel. Int J Geogr Geol 3(8):101–113
Wenner D, Wannenmacher H (2009) Alborz Service tunnel in Iran: TBM tunnelling in difficult ground conditions and its solutions, Amberg Engineering Ltd. In: 1st Regional and 8th Iranian Tunneling Conference, Tehran, Iran
Wightman R, Mackay A (2008) Gas ground investigation for tunneling works at Hung Hom freight depot, Hong Kong, World Tunnel Congress––Underground Facilities for Better Environment and Safety––India
William H, Hansmire PJ (2008) Slurry-face rock TBM outfall tunnel in Detroit, Michigan, USA. World Tunnel Congress 2008––Underground Facilities for Better Environment and Safety––India
Yang F, Lee C, Kung W, Yeh H (2009) The impact of tunneling construction on the hydrogeological environment of Tseng-Wen Reservoir Trans basin Diversion Project” in Taiwan. Eng Geol 101:39–58
Yoo C (2004) Interaction between tunneling and groundwater. Tunn Undergr Space Technol 19(2004):523–524
Acknowledgements
The authors would like to thank professor Milanovic, Dr. Sahabi and Dr. Aghasi for their guides that greatly improved this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morsali, M., Rezaei, M. Assessment of H2S emission hazards into tunnels: the Nosoud tunnel case study from Iran. Environ Earth Sci 76, 227 (2017). https://doi.org/10.1007/s12665-017-6493-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6493-0