Abstract
Tris (2-chloroethyl) phosphate (TCEP), a typical organophosphate flame retardant, is of increasingly great concern considering their ubiquitous presence in aquatic environments and potential ecotoxicity. The present work was aimed to investigate the potential growth inhibition and hepatic stress induced by whole life-cycle exposure to TCEP (0.8, 4, 20 and 100 μg/L) in zebrafish. The results revealed that the body length, body mass and hepatic-somatic index (HSI) of zebrafish were significantly declined after exposure to TCEP for 120 days. GPx activity and GSH content were increased in the liver of zebrafish treated with low concentrations (0.8 and 4 μg/L) of TCEP, while exposure to high concentrations (20 and 100 μg/L) of TCEP reduced antioxidative capacity and elevated lipid peroxidation (LPO) levels. Gene transcription analysis demonstrated that the mRNA levels of nrf2 were altered in a similar manner to the transcription of the downstream genes nqo1 and hmox1, suggesting that Nrf2-Keap1 pathway mediated TCEP-induced oxidative stress in zebrafish liver. In addition, TCEP exposure might alleviate inflammatory response through down-regulating transcription of inflammatory cytokines (il-1β, il-6 and inos), and induce apoptosis via activating the p53-Bax pathway. Moreover, whole life-cycle exposure to TCEP caused a series of histopathological anomalies in zebrafish liver. Overall, our results revealed that lifetime exposure to environmentally relevant concentrations of TCEP could result in growth retardation and induce significant hepatotoxicity in zebrafish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the primary substitutes for brominated flame retardants (BFRs), organophosphate flame retardants (OPFRs) are widely used as additives in various products, such as plastics, electronic equipment and textiles (Zhou et al. 2020). Tris (2-chloroethyl) phosphate (TCEP) is one of the typical OPFRs, which is of increasing concern due to its widely application and ubiquitous presence in environmental media (Abdallah and Covaci 2014). Given that TCEP is hardly bound to polymers chemically, it can be transferred from synthetic products to the environment under physical effects such as volatilization, abrasion and dissolution (Bollmann et al. 2012). Additionally, TCEP has a relatively high water solubility (7820 µg/L at 20 ◦C) and cannot be eliminated effectively via the current sewage treatment technology. Thus, it is not surprising that TCEP has been frequently detected in aquatic environments such as rain, wastewater, drinking water and surface water (Marklund et al. 2005). For example, TCEP was detected in drinking water at the concentration of up to 120 ng/L in US (Benotti et al. 2009). In the Songhua River, China, the measured concentrations of TCEP ranged from 38 to 3700 ng/L (Wang et al. 2011). The highest TCEP level (87.4 μg/L) was ever reported in the raw water from a Japanese sea-based solid waste disposal site (Kawagoshi et al. 1999). The extensive existence of TCEP in environments is posing great threats to wild animals and also human beings.
A growing number of studies demonstrated that exposure to TCEP exhibited a variety of adverse effects, such as neurotoxicity, developmental toxicity, reproductive toxicity, endocrine disrupting effects, and even carcinogenicity (Sun et al. 2016; Li et al. 2019; Wang et al. 2020; Sutha et al. 2022). For instance, after exposure to TCEP, the genes and proteins associated with central nervous system (CNS) development were changed, inducing neurotoxicity during the early stages of zebrafish (Li et al. 2019). Treatment with 1250 or 6250 μg/L TCEP produced a significant inhibition on the growth of Japanese medaka (Oryzias latipes) (Sun et al. 2016). A recent work elucidated that TCEP exposure resulted in reproductive toxicity in zebrafish, causing variations in sexual plasma sex hormones, and gonadal damage (Sutha et al. 2022). Furthermore, TCEP exhibited carcinogenicity in mice, evidenced by the regulation of tumor-associated factors (Wang et al. 2020). Nevertheless, the exposure concentrations adopted in most previous studies were much higher than environmentally realistic levels. Besides, considering that aquatic organisms are normally exposed to environmental pollutants constantly in natural waters, life-cycle toxicity assessment may be of more realistic meaning.
Liver is the main target organ for toxic substances, performing multiple functions such as detoxification, metabolism and immunity of vertebrate body (Van den Eede et al. 2013). Several studies have so far been focused on the adverse impacts of OPFRs on fish liver (Fernandes et al. 2008; Moser et al. 2015; Chen et al. 2018; Ramesh et al. 2018). For example, exposure to TCEP significantly elevated the hepatic mRNA levels of antioxidant genes (gst and gpx) in juvenile salmon (Arukwe et al. 2016). Tris (1,3-dichloro-2-propyl) phosphate (TDCIPP), another typical OPFR, triggered inflammation in adult zebrafish liver, evidenced by the upregulation of inflammation biomarker genes and histological alterations (Liu et al. 2016). Besides, histological structure alterations such as necrosis and vacuolation were observed in the liver of Cirrhinus mrigala after a 21-day exposure to TCEP (Sutha et al. 2020). A recent study reported that TCEP might exert hepatotoxic effects on zebrafish by disrupting the HPT and gut-liver axes and thereafter inducing hepatic inflammation and oxidative stress (Tian et al. 2023). However, a systematic study on the hepatotoxicity resulted from whole lifetime exposure to TCEP is still required.
Due to small body size (adults reaching only 3–4 cm), high fecundity and high sensitivity to environmental stressors, zebrafish has become an important model for toxicological studies (Vliegenthart et al. 2014). The objective of this study was to investigate the antioxidant defense, inflammatory response, apoptosis and histological changes in the liver of zebrafish after lifetime exposure to environmentally relevant concentrations of TCEP. These results will broaden our understanding of the hepatotoxicity resulted from long-term exposure to TCEP in fish, and highlight the environmental hazards posed by TCEP in aquatic ecosystems.
Materials and methods
Chemicals and reagents
TCEP (CAS: 115–96–8; purity ≥ 97%), TCEP-d12 (purity ≥ 97%) and ethyl 3-aminobenzoate methanesulfonate (MS-222, CAS: 886–86–2; purity ≥ 98%) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, USA). TCEP were dissolved in dimethyl sulfoxide (DMSO; CAS: 67–68–5; purity ≥ 99.7%; Sigma-Aldrich, USA) as a stock solution. All other reagents used in this work were of analytical or HPLC grade.
Fish husbandry and TCEP exposure
5-month-old zebrafish (wild-type, AB strain) were selected and maintained in aquariums (40 L water and 50 individuals per tank) with water temperature 27 ± 1℃, pH 7.0 ± 0.5 and a 14-h light/10-h dark cycle. The fish were fed twice daily with newly hatched brine shrimp larvae (Artemia salina). After one-week acclimation, 25 males and 50 females were randomly selected and allowed to spawn. Embryos were collected and transferred to plastic culture dishes with lids (60 embryos per dish) and exposed to 0, 0.8, 4, 20 and 100 μg/L TCEP, with three replicates for each treatment. Every petri dish contained 40 mL (maximum volume 70 mL) of exposure solution. The larvae were transferred to breeding aquariums after two weeks and each aquarium contained 6 L (maximum volume was 10 L) of exposed solution and 30 individuals. Fish were fed a commercial diet (Hai Feng Feeds Co. Ltd.) 3 times daily till 120 dpf. Half of exposure medium were renewed with freshly prepared solutions daily. The final concentrations of DMSO were 0.0001% (v/v) in both solvent control and TCEP-treated groups.
Exposure solutions were sampled before and after water renewal at 119 dpf and stored at -80℃ until the quantification of TCEP. At 120 dpf, 10 fishes were randomly selected from each tank, euthanized with 0.03% MS-222. After the record of body length and body weight, liver tissues were collected, immediately frozen in liquid nitrogen and stored at -80 °C till further analysis. Another 3 individuals from each replicate were dissected to obtain liver tissues for histological analysis. The body weight and the liver weight were used for the calculation for hepatic-somatic index (HSI).
TCEP quantification
TCEP was quantified in collected water samples as previously described (Wang et al. 2022). Firstly, the internal standard TCEP-d12 was spiked into the water samples. After then, water samples were cleaned up using solid phase extraction (SPE) method and eluted with acetonitrile. The eluents were reduced to dryness under a gentle stream of nitrogen, and dissolved in 1 mL methanol. The quantification of TCEP was performed on a Waters ACQUITY UPLC® H-Plus Class system (UHPLC) coupled to a Waters® Xevos™ TQ-XS mass spectrometer (TQ-XS/MS) (Milford, MA, USA). Detailed protocols for the extraction, clean up and analysis was provided in Text S1 (Supporting Information).
Histological examination
Freshly dissected liver tissues were fixed in 4% paraformaldehyde (PFA) for 24 h. Then the tissues were dehydrated in ethanol, decontaminated in xylene, embedded in paraffin, and sectioned into 5 µm thick slices. Afterwards, these sections were stained with hematoxylin–eosin (H&E) staining and examined under a light microscope.
Biochemical analysis
Liver tissues were homogenized (1:9, w/v) in 0.9% physiological saline with a high-throughput tissue homogenizer (Scientz, Ningbo, China). The homogenates then were kept in ice-cold condition and finally centrifuged (3000 × g) for 10 min at 4℃ to obtain supernatants for biochemical measurements. Superoxide dismutase (SOD), hydrogenase (CAT), and glutathione peroxidase (GPX) activities, as well as glutathione (GSH) and malondialdehyde (MDA) content were determined using commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Gene transcription analysis
Total RNA isolation was conducted with FastPure® Cell/Tissue Total RNA Isolation Kit V2 (Vazyme Biotech Co. Ltd., Nanjing, China). The cDNA was synthesized using PrimeScript® RT reagent Kit (Takara, China) according to the manufacturer’s instructions. The qPCR was carried out on Light-Cycler® 480II (Roche, Switzerland). The primer sequences used for qPCR analysis were designed using the online Primer-BLAST tool on NCBI website and are given in Table S1 (Supporting Information). β-actin was chosen as an internal reference gene because the transcription level of β-actin did not vary significantly under different TCEP exposure concentrations. The relative expression levels of target genes were calculated by the 2−ΔΔCt method (Livak and Schmittgen 2001).
Statistical analysis
Results were expressed as mean ± standard deviation (S.D.). Prior to statistical analysis, all data were checked for normality and homogeneity of variance using Kolmogorov–Smirnov test and Levene’s test. The differences between the solvent control and treatment groups were evaluated using the one-way analysis of variance (ANOVA) and Tukey’s HSD test with SPSS Statistics 19.0 (SPSS, Chicago, IL). p < 0.05 was considered statistically significant.
Results
TCEP concentrations in exposure media
The actual concentrations of TCEP in 0.8, 4, 20, and 100 μg/L exposure solutions were 0.85 ± 0.12, 3.79 ± 0.43, 19.24 ± 0.31 and 102.07 ± 3.66 μg/L after renewal and 0.72 ± 0.14, 3.72 ± 0.31, 18.68 ± 1.55 and 93.77 ± 6.29 μg/L before next renewal (Fig. S1, Supporting information). No TCEP was detected in the solvent control group.
Body length, body mass and HSI
At 120 dpf, the body length and body mass of zebrafish did not show significant changes in 0.8 μg/L TCEP-treated group, but were markedly declined in 4, 20 and 100 μg/L groups compared with the solvent control group (Fig. 1A, 1B). The HSI values were significantly lower in all exposure groups in comparison to the solvent control (Fig. 1C).
Histopathological changes
Normal hepatocyte structure without signs of degeneration or necrosis was observed in the control fish (Fig. 2A). In comparison to the solvent control group, exposure to 0.8 μg/L TCEP caused a mild granular degeneration and slight vacuolization (Fig. 2B). In addition, increased vacuoles, parenchyma disorganization and pyknotic nucleus occurred in 4, 20 and 100 μg/L TCEP-treated groups, appearing to be more severe with the increase of exposure concentrations (Fig. 2C, 2D, 2E). Especially, lifetime exposure to 100 μg/L TCEP resulted in extensive areas of vacuolar degeneration in the liver of zebrafish (Fig. 2E).
Effects of lifetime exposure to TCEP on liver histology of zebrafish at 120 dpf. A Liver from the solvent control, showing normal hepatocytes structure; B Liver from 0.8 μg/L TCEP group, exhibiting parenchyma disorganization (ellipse), pyknotic nucleus (yellow arrows); C Liver from 4 μg/L TCEP group, pyknotic nucleus (yellow arrows), nuclear deformation (red arrows), vacuolation (black triangle); D Liver from 20 μg/L TCEP group, pyknotic nucleus (yellow arrows), nuclear deformation (red arrows) and showing more severe vacuolation (black triangle); E Liver from 100 μg/L TCEP group, pyknotic nucleus (yellow arrows), nuclear deformation (red arrows) and showing more severe vacuolation (black triangle)
Antioxidant enzyme activities, GSH content and MDA level
Lifetime exposure to 20 and 100 μg/L TCEP significantly reduced the activities of SOD and GPx, while CAT activity was declined in all TCEP exposure groups (Fig. 3). The noticeable increase of GSH content was observed in 0.8, 4 and 20 μg/L TCEP exposure groups (Fig. 3). MDA contents were significantly elevated in 4, 20 and 100 μg/L TCEP-treated groups in comparison to the solvent control (Fig. 3).
mRNA levels of antioxidant genes
Exposure to 0.8 and 100 μg/L TCEP significantly increased the mRNA level of keap1 in zebrafish liver (Fig. 4). Significant up-regulated mRNA expression of nrf2 was only observed in 0.8 μg/L TCEP-treated group (Fig. 4). The mRNA levels of nqo1 were significantly increased in 0.8 and 4 μg/L TCEP-treated groups (Fig. 4). The mRNA expression of homx-1 was up-regulated in 0.8 μg/L group, while sharply down-regulated in 4, 20 and 100 μg/L TCEP-treated groups. Besides, the transcription of gst were significantly suppressed in all TCEP treatments in a dose-dependent manner (Fig. 4).
mRNA levels of inflammatory genes
The mRNA level of il-β was significantly raised after exposure to 0.8 μg/L TCEP, while declined in 20 and 100 μg/L groups (Fig. 5). Significant down-regulation of il-6 and inos expressions were observed in all TCEP treatments (Fig. 5). The transcriptional level of il-10 was only decreased markedly in 100 μg/L TCEP-treated group (Fig. 5).
mRNA levels of apoptosis-related genes
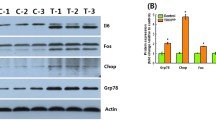
Life-time exposure to TCEP significantly induced the up-regulation of the expression of p53, while down-regulated the expression of bcl-2 (Fig. 6). The mRNA level of bax was only significantly increased in 0.8 μg/L TCEP-treated group (Fig. 6). The transcriptional levels of ced-4 were remarkedly elevated in 4 and 100 μg/L exposure groups (Fig. 6). Exposure to 0.8 and 4 μg/L TCEP augmented the transcription of cyp1a and decreased the transcription of cas3 compared with the solvent control (Fig. 6). The mRNA expression of cas8 was significantly up-regulated in the 0.8 μg/L group, but down-regulated in the 4, 20 and 100 μg/L TCEP-treated groups (Fig. 6). The mRNA level of cas9 was apparently declined in 0.8, 20 and 100 μg/L exposure groups, and was increased in 4 μg/L TCEP-treated group (Fig. 6).
Discussion
Various OPFRs such as triphenyl phosphate (TPP) and TDCIPP exhibited growth-inhibiting effects on Daphnia magna and zebrafish (Li et al. 2017; Yu et al. 2017). Our previous findings also demonstrated that exposure to 20 and 200 μg/L TCEP significantly decreased the body length of 5-dpf larval zebrafish (Hu et al. 2021). In this study, reduced body mass and body length were observed in zebrafish after 120-d exposure to 4, 20 and 100 μg/L TCEP, suggesting that lifetime exposure to environmentally relevant concentrations of TCEP can cause significant growth retardation in fish.
Liver is a target organ for the toxicity of numerous organic substances (Hinton et al. 2017). HSI is an indicator of the growth status of the liver in fish, which is sensitive to various environmental stressors (Larsson et al. 1984; Deng et al. 2010). Our results showed that TCEP exposure significantly reduced the HSI of zebrafish. Consistent results were found in previous studies on zebrafish exposed to TPP, TDCIPP and tris (2-butoxyethyl) phosphate (TBOEP) (Liu et al. 2013; Xu et al. 2017). This might be attributed to the hepatic TCEP accumulation after long-term exposure, which might interfere the synthesis of storage products such as glycogen and fat in liver, causing a decrease in liver weight and a reduction in HSI (Kopecka and Pempkowiak 2008). Thereby, the overall decline of HSI indicated abnormal liver development and function.
In accordance with that reported in freshwater fishes Cirrhinus mrigala and zebrafish sub-chronic exposed to TCEP (Sutha et al. 2020; Tian et al. 2023), occurrence of severe liver injuries including vacuoles, parenchyma disorganization and pyknotic nucleus were clearly observed after whole life-cycle exposure to TCEP in this study, which were more serious with the increase of concentrations. Cavitation of the liver is one of the main signs of liver damage, while parenchyma disorganization and pyknotic nucleus might be indications of apoptosis and necrosis in hepatocytes (Erkmen et al. 2017; Chen et al. 2017). Therefore, these hepatic histopathological alterations provide strong evidence for TCEP-induced hepatotoxicity in zebrafish. Similarly, histological changes such as vacuolization and pyknotic nuclei were presented in the liver of juvenile yellow catfish (Pelteobagrus fulvidraco) (Hu et al. 2022). Moreover, previous study also pointed out that structural damage of the liver might affect the secretion of IGF, inhibiting the normal growth and development in zebrafish (Wang et al. 2019a, b). Hence, TCEP-induced growth inhibition might be attributed to these severe hepatic histological anomalies.
Oxidative damage in fish is due to excessive intracellular production of reactive oxygen species (ROS) under exposure to environmental pollutants (Jin et al. 2010). High concentrations of ROS can be countered by the action of ROS-scavenging enzymes (Arukwe et al. 2016). SOD, CAT and GPx are essential antioxidant enzymes that play crucial role in scavenging excessive ROS to maintain cellular environment dynamic balance (Lackner 1998). Glutathione (GSH) can eliminate excess ROS directly or through the ascorbate–glutathione cycle, protecting cells against oxidative damage (Polekhina et al. 1999). In the current study, the enhanced GPx activity and GSH content were observed in the liver of zebrafish treated with low doses of TCEP groups (0.8 and 4 μg/L), suggesting a defensive response or physiological adaptation to TCEP-duced oxidative stress (Moalem et al. 1999; Zhang et al. 2004). Similar results have also been reported in C. mrigala following exposure to TCEP (Sutha et al. 2020). Conversely, 20 and 100 μg/L TCEP remarkedly reduced the activities of SOD, CAT and GPx, indicating that exposure to high concentrations of TCEP would impair the antioxidant defense in the liver of zebrafish. Significant declines in SOD activity were also observed in the liver of zebrafish after a 28-day exposure to 0.5 and 5 μg/L TCEP (Tian et al. 2023). Malondialdehyde (MDA) is the end product of lipid peroxidation in living organisms, and it is usually employed as an indicator of the extent of oxidative damage in cells (Ali et al. 2012). Our results showed a significant increase of MDA content in 4, 20 and 100 μg/L TCEP treatments, demonstrating that elevated formation of ROS induced by TCEP exceeded the antioxidant capacity, and exacerbated hepatocyte oxidative damage.
To further uncover the molecular mechanisms of oxidative stress, we detected the transcriptional regulation of genes involved in the Nrf2-Keap1 pathway. Nrf2 is a crucial nuclear transcription factor and a signal pathway activator highly expressed in the liver, regulating the expressions of downstream antioxidant genes (Shaw et al. 2019). When the balance between ROS production and clearance is disrupted, Keap1 will be inactivated, which blocked the clearance of Nrf2, and ultimately lead to the excessive accumulation and the activation of Nrf2 (Ray et al. 2012). Nrf2 enters the nucleus to combine with antioxidant response elements (ARE) and transcribe a series of antioxidant response element genes, such as gst, homx-1 and nqo1 in response to oxidative stress (Sule et al. 2022). In the present work, exposure to 0.8 μg/L TCEP significantly elevated the mRNA levels of nrf2 and its downstream genes (nqo1 and homx-1) in liver, indicating that low concentration of TCEP could induce the antioxidative defense through activating the Nrf2-Keap1 pathway. However, with the increase of TCEP exposure concentrations, mRNA levels of keap1 were significantly up-regulated, whereas the levels of downstream genes homx-1 and gst were markedly down-regulated in 4, 20 and 100 μg/L TCEP groups. These results implied that high concentration of TCEP suppressed the transcriptional activation of the Nrf2-Keap1 pathway on downstream genes through up-regulating keap1 expression, ultimately reducing the defense capacity of zebrafish.
Inflammation is a response of the immune system to tissue damage and infection, and hepatic inflammatory disorder can reflect hepatotoxicity induced by environmental pollutants (Wang et al. 2021). Cytokines are critical regulators of inflammation as well as major mediators of immune function (Hermann and Kim 2005). Among them, il-1β and il-6 are two important pro-inflammatory cytokines modulating inflammatory processes (Engelsma et al. 2002; Zanotti et al. 2002). In a recent work, after 28-day TCEP exposure, higher levels of IL-6, IL-1β, and TNF-α were observed in zebrafish livers (Tian et al. 2023). Conversely, in the present study, mRNA levels of both il-1β and il-6 were declined in 4, 20 and 100 μg/L TCEP-treated groups, reflecting the suppressive effect of TCEP on the immune system of zebrafish liver. It was reported that the activation of Nrf2 could negatively regulate pro-inflammatory mediators (Kim et al. 2010; Getachew et al. 2016), thus the down-regulation of inflammatory cytokines after TCEP exposure was possibly ascribed to the activation of Nrf2 in zebrafish liver. The transcription of inos can be promoted by interleukins, producing large amounts of toxic NO and regulating the process of inflammatory response (Saha and Pahan 2006). In this work, the mRNA expression of inos was significantly down-regulated, possibly contributing to alleviate inflammatory responses. il-10 is an anti-inflammatory factor playing roles in down-regulating inflammatory response and antagonizing inflammatory mediators (Karan et al. 2016). Our study revealed that the mRNA expression of il-10 was down-regulated only in the highest concentration exposure group, which might suppress the function of liver immune cells, resulting in an aggravated inflammatory response and liver damage.
Apoptosis is a genetically controlled cell death of self-ordered and can be regulated by multiple genes (Zhao et al. 2009). p53 is a tumor suppressor gene responsible for mediating the apoptosis process (Calaf et al. 2009). bcl-2 and bax are two members of Bcl-2 family that play critical roles in the regulation of apoptosis. bcl-2, an anti-apoptotic gene, prevents the release of cytochrome c from mitochondria (Bernardi et al. 2001). bax is a p53 response gene, inducing the release of cytochrome to promote apoptosis (Cory and Adams 2002). In this study, TCEP exposure elevated the mRNA levels of p53 and bax, while down-regulated the transcription of bcl-2, suggesting that TCEP might trigger apoptosis via the p53-Bax pathway in zebrafish liver. Similar to TCEP, triazophos, an organic phosphate ester, promoted apoptosis by transcriptional activation of p53 and bax in zebrafish (Wang et al. 2019a, b). Caspases family is closely related with apoptosis and can be activated by external and internal pathways (McIlwain et al. 2013). Previous studies demonstrated that caspase-8 (cas8) was involved in the extrinsic pathway, while caspase-9 (cas9) participated in the intrinsic pathway of apoptosis, both of which induced apoptosis by activating the downstream target gene caspase-3 (cas3) (D’Arcy 2019; Wang et al. 2023). CED-4 binds with cytochrome c to activate caspase cascade, and finally leads to programmed cell death (Kumar 2007). Though elevated transcriptional levels of ced-4 were observed in the present work, the transcription of cas3, cas8 and cas9 mainly exhibited downward tendency, indicating that the caspase-dependent apoptotic pathway might be negatively involved in the TCEP-induced apoptosis in zebrafish. CYP1A is a member of the cytochrome P450 superfamily, and the induction of CYP1A by environmental pollutants might cause apoptosis (Tsuchiya et al. 2005; Özdemir et al. 2018). Previous studies have revealed a positive correlation between the induction of cyp1a and cell apoptosis in zebrafish and medaka (Cantrell et al. 1996; Xu et al. 2015). In our study, the transcription of cyp1a was only significantly increased in 0.8 and 4 μg/L TCEP exposure groups, suggesting that TCEP at low concentrations might induce apoptosis through the activation of cyp1a expression.
Inflammation and apoptosis may lead to irreversible damage and structural changes in liver tissue. In this study, alterations including vacuoles, parenchyma disorganization and pyknotic nucleus were clearly observed in the liver of zebrafish after whole life-cycle exposure to TCEP, which were more severe with the increasing concentrations. Cavitation of the liver is one of the main signs of liver damage, while parenchyma disorganization and pyknotic nucleus might be indications of apoptosis and necrosis in hepatocytes (Erkmen et al. 2017; Chen et al. 2017). Therefore, these hepatic histopathological alterations provide strong evidence for TCEP-induced hepatotoxicity in zebrafish. Similarly, histological changes such as vacuolization and pyknotic nuclei were presented in the liver of juvenile yellow catfish (Pelteobagrus fulvidraco) (Hu et al. 2022). Moreover, previous study also pointed out that structural damage of the liver might affect the secretion of IGF, inhibiting the normal growth and development in zebrafish (Wang et al. 2019a, b). Hence, TCEP-induced growth inhibition might be attributed to these severe hepatic histological anomalies.
Conclusion
In summary, our findings suggested that life-cycle exposure to TCEP at environmental relevant concentrations could lead to growth inhibition in zebrafish and exerted significant hepatotoxicity via inducing oxidative stress, inflammatory disorder, apoptosis and histological alterations. These data provide insight into the toxicological effects of TCEP in target organ of fish and highlight the environmental hazard of TCEP in aquatic environments.
Data availability
Data and materials will be made available on request.
References
Abdallah MAE, Covaci A (2014) Organophosphate flame retardants in indoor dust from egypt: implications for human exposure. Environ Sci Technol 48:4782–4789
Ali D, Alarifi S, Kumar S, Ahamed M, Siddiqui MA (2012) Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aquat Toxicol 124–125:83–90
Arukwe A, Carteny CC, Eggen T (2016) Lipid peroxidation and oxidative stress responses in juvenile salmon exposed to waterborne levels of the organophosphate compounds tris (2-butoxyethyl)- and tris (2-chloroethyl) phosphates. J Toxicol Environ Health A 79:515–525
Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA (2009) Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ Sci Technol 43:597–603
Bernardi P, Petronilli V, Di Lisa F, Forte M (2001) A mitochondrial perspective on cell death. Trends Biochem Sci 26:112–117
Bollmann UE, Möller A, Xie Z, Ebinghaus R, Einax JW (2012) Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters. Water Res 46:531–538
Calaf GM, Echiburu-Chau C, Roy D (2009) Organophosphorous pesticides and estrogen induce transformation of breast cells affecting p53 and c-Ha-ras genes. Int J Oncol 35(5):1061–1068
Cantrell SM, LuTz LH, Tillitt DE, Hannlnk M (1996) Embryotoxicity of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD): the embryonic vasculature is a physiological target for TCDD-induced DNA damage and apoptotic cell death in Medaka (Orizias iatipes). Toxicol Appl Pharmacol 141(1):23–34
Chen Q, Sun Y, Liu Z, Li Y (2017) Sex-dependent effects of subacute mercuric chloride exposure on histology, antioxidant status and immune-related gene expression in the liver of adult zebrafish (Danio rerio). Chemosphere 188:1–9
Chen H, Wang P, Du Z, Wang G, Gao S (2018) Oxidative stress, cell cycle arrest, DNA damage and apoptosis in adult zebrafish (Danio rerio) induced by tris (1,3-dichloro-2-propyl) phosphate. Aquat Toxicol 194:37–45
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43:582–592
Deng S, Tian L, Liu F, Jin S, Liang G, Yang H, Du Z, Liu Y (2010) Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus×O. aureus) during long-term dietary exposure. Aquaculture 307:233–240
Engelsma MY, Huising MO, Van Muiswinkel WB, Flik G, Kwang J, Savelkoul HF, Verburg-van Kemenade BL (2002) Neuroendocrine–immune interactions in fish: a role for interleukin-1. Vet Immunol Immunopathol 87:467–479
Erkmen B, Karasu Benli AÇ, Ağuş HH, Yıldırım Z, Mert R, Erkoç F (2017) Impact of sublethal di-n-butyl phthalate on the aquaculture fish species Nile tilapia (Oreochromis niloticus): histopathology and oxidative stress assessment. Aquac Res 48:675–685
Fernandes C, Fontaínhas-Fernandes A, Rocha E, Salgado MA (2008) Monitoring pollution in Esmoriz-Paramos lagoon, Portugal: liver histological and biochemical effects in Liza saliens. Environ Monit Assess 145:315–322
Getachew Y, Cusimano FA, Gopal P, Reisman SA, Shay JW (2016) The synthetic triterpenoid RTA 405 (CDDO-EA) halts progression of liver fibrosis and reduces hepatocellular carcinoma size resulting in increased survival in an experimental model of chronic liver injury. Toxicol Sci 149:111–120
Hermann AC, Kim CH (2005) Effects of arsenic on zebrafish innate immune system. Mar Biotechnol 7:494–505
Hinton DE, Segner H, Braunbeck T (2017) Toxic responses of the liver. CRC Press
Hu F, Zhao Y, Yuan Y, Yin L, Dong F, Zhang W, Chen X (2021) Effects of environmentally relevant concentrations of tris (2-chloroethyl) phosphate (TCEP) on early life stages of zebrafish (Danio rerio). Environ Toxicol Pharmacol 83:1–7
Hu F, Zhao Y, Dong F, Wang H, Zheng M, Zhang W, Chen X (2022) Insights into the mechanisms of tris (2-chloroethyl) phosphate-induced growth inhibition in juvenile yellow catfish Pelteobagrus fulvidraco. Aquat Toxicol 247:106170
Jin Y, Zhang X, Shu L, Chen L, Sun L, Qian H, Liu W, Fu Z (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78:846–852
Karan S, Dash P, Kaushik H, Sahoo PK, Garg LC, Dixit A (2016) Structural and functional characterization of recombinant interleukin-10 from Indian major carp Labeo rohita. J Immunol Res 2016:1–11
Kawagoshi Y, Fukanaga I, Itoh H (1999) Distribution of organophosphoric acid triesters between water and sediment at a sea-based solid waste disposal site. J Mater Cycles Waste Manage 1:53–61
Kim J, Cha Y, Surh Y (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res-Fundam Mol Mech Mutagen 690:12–23
Kopecka J, Pempkowiak J (2008) Temporal and spatial variations of selected biomarker activities in flounder (Platichthys flesus) collected in the Baltic proper. Ecotoxicol Ecotoxicol Environ Safety 70:379–391
Kumar S (2007) Caspase function in programmed cell death. Cell Death Diff Integral Equ 14:32–43
Lackner R (1998) “Oxidative stress” in fish by environmental pollutants. Fish Ecotoxicol 203–224
Larsson Å, Haux C, Sjöbeck M-L, Lithner G (1984) Physiological effects of an additional stressor on fish exposed to a simulated heavy-metal-containing effluent from a sulfide ore smeltery. Ecotoxicol Environ Saf 8:118–128
Li H, Yuan S, Su G, Li M, Wang Q, Zhu G, Liu C (2017) Whole-life-stage characterization in the basic biology of Daphnia magna and effects of TDCIPP on growth, reproduction, survival, and transcription of genes. Environ Sci Technol 51:13967–13975
Li R, Wang H, Mi C, Feng C, Zhang L, Yang L, Zhou B (2019) The adverse effect of TCIPP and TCEP on neurodevelopment of zebrafish embryos/larvae. Chemosphere 220:811–817
Liu X, Ji K, Jo A, Moon HB, Choi K (2013) Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat Toxicol 134–135:104–111
Liu C, Su G, Giesy JP, Letcher RJ, Li G, Agrawal I, Li J, Yu L, Wang J, Gong Z (2016) Acute exposure to tris (1,3-dichloro-2-propyl) phosphate (TDCIPP) causes hepatic inflammation and leads to hepatotoxicity in zebrafish. Sci Rep 6(1):19045
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Marklund A, Andersson B, Haglund P (2005) Traffic as a source of organophosphorus flame retardants and plasticizers in snow. Environ Sci Technol 39:3555–3562
McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5(4):a008656
Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M (1999) Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med 5:49–55
Moser VC, Phillips PM, Hedge JM, McDaniel KL (2015) Neurotoxicological and thyroid evaluations of rats developmentally exposed to tris (1,3-dichloro-2-propyl) phosphate (TDCIPP) and tris (2-chloro-2-ethyl) phosphate (TCEP). Neurotoxicol Teratol 52:236–247
Özdemir S, Altun S, Arslan H (2018) Imidacloprid exposure cause the histopathological changes, activation of TNF-α, iNOS, 8-OHdG biomarkers, and alteration of caspase 3, iNOS, CYP1A, MT1 gene expression levels in common carp (Cyprinus carpio L.). Toxicol Rep 5:125–133
Polekhina G, Board PG, Gali RR, Rossjohn J, Parker MW (1999) Molecular basis of glutathione synthetase deficiency and a rare gene permutation event. EMBO J 18:3204–3213
Ramesh M, Anitha S, Poopal RK, Shobana C (2018) Evaluation of acute and sublethal effects of chloroquine (C18H26CIN3) on certain enzymological and histopathological biomarker responses of a freshwater fish Cyprinus carpio. Toxicol Rep 5:18–27
Ray P, Huang B, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990
Saha RN, Pahan K (2006) Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal 8:929–947
Shaw P, Mondal P, Bandyopadhyay A, Chattopadhyay A (2019) Environmentally relevant concentration of chromium activates Nrf2 and alters transcription of related XME genes in liver of zebrafish. Chemosphere 214:35–46
Sule RO, Condon L, Gomes AV (2022) A common feature of pesticides: oxidative stress-the role of oxidative stress in pesticide-induced toxicity. Oxidative Med Cell Longev 2022
Sun L, Tan H, Peng T, Wang S, Xu W, Qian H, Jin Y, Fu Z (2016) Developmental neurotoxicity of organophosphate flame retardants in early life stages of Japanese medaka (Oryzias latipes). Environ Toxicol Chem 35:2931–2940
Sutha J, Anila PA, Umamaheswari S, Ramesh M, Narayanasamy A, Poopal RK, Ren Z (2020) Biochemical responses of a freshwater fish Cirrhinus mrigala exposed to tris (2-chloroethyl) phosphate (TCEP). Environ Sci Pollut Res 27:34369–34387
Sutha J, Anila PA, Gayathri M, Ramesh M (2022) Long term exposure to tris (2-chloroethyl) phosphate (TCEP) causes alterations in reproductive hormones, vitellogenin, antioxidant enzymes, and histology of gonads in zebrafish (Danio rerio): in vivo and computational analysis. Comp Biochem Physiol C-Toxicol Pharmacol 254:109263
Tian D, Yu Y, Lu L, Tong D, Zhang X, Shi W, Liu G (2023) Tris(2-chloroethyl) Phosphate Exerts Hepatotoxic Impacts on Zebrafish by Disrupting Hypothalamic–Pituitary–Thyroid and Gut-Liver Axes. Environ Sci Technol 57(24):9043–9054
Tsuchiya Y, Nakajima M, Yokoi T (2005) Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett 227:115–124
Van den Eede N, Maho W, Erratico C, Neels H, Covaci A (2013) First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett 223:9–15
Vliegenthart ADB, Tucker CS, Del Pozo J, Dear JW (2014) Zebrafish as model organisms for studying drug-induced liver injury. Br J Clin Pharmacol 78:1217–1227
Wang X, Liu J, Yin Y (2011) Development of an ultra-high-performance liquid chromatography–tandem mass spectrometry method for high throughput determination of organophosphorus flame retardants in environmental water. J Chromatogr A 1218:6705–6711
Wang G, Shao J, Wu M, Meng Y, Gul Y, Yang H, Xiong D (2019a) Effect of acute exposure of triazophos on histological structure and apoptosis of the brain and liver of zebrafish (Danio rerio). Ecotoxicol Environ Saf 180:646–655
Wang Y, Zhang Y, Li W, Yang L, Guo B (2019b) Distribution, metabolism and hepatotoxicity of neonicotinoids in small farmland lizard and their effects on GH/IGF axis. Sci Total Environ 662:834–841
Wang C, Chen Z, Lu Y, Wang L, Zhang Y, Zhu X, Song J (2020) Neurotoxicity and related mechanisms of flame retardant TCEP exposure in mice. Toxicol Mech Methods 30:490–496
Wang Y, Tian J, Shi F, Li X, Hu Z, Chu J (2021) Protective effect of surfactin on copper sulfate-induced inflammation, oxidative stress, and hepatic injury in zebrafish. Microbiol Immunol 65:410–421
Wang H, Jing C, Peng H, Liu S, Zhao H, Zhang W, Chen X, Hu F (2022) Parental whole life-cycle exposure to tris (2-chloroethyl) phosphate (TCEP) disrupts embryonic development and thyroid system in zebrafish offspring. Ecotoxicol Environ Saf 248:114313
Wang X, Zhang J, Lu C, Liu Y, Yang X, Hou K, Du Z, Li B, Juhasz A, Zhu L (2023) Development toxicity and cytotoxicity of pyroxsulam on embryos and adults of zebrafish (Danio rerio). Environ Pollut 319:121040
Xu T, Wang Q, Shi Q, Fang Q, Guo Y, Zhou B (2015) Bioconcentration, metabolism and alterations of thyroid hormones of Tris (1,3-dichloro-2-propyl) phosphate (TDCPP) in Zebrafish. Environ Toxicol Pharmacol 40:581–586
Xu Q, Wu D, Dang Y, Yu L, Liu C, Wang J (2017) Reproduction impairment and endocrine disruption in adult zebrafish (Danio rerio) after waterborne exposure to TBOEP. Aquat Toxicol 182:163–171
Yu L, Jia Y, Su G, Sun Y, Letcher RJ, Giesy JP, Yu H, Han Z, Liu C (2017) Parental transfer of tris (1,3-dichloro-2-propyl) phosphate and transgenerational inhibition of growth of zebrafish exposed to environmentally relevant concentrations. Environ Pollut 220:196–203
Zanotti S, Kumar A, Kumar A (2002) Cytokine modulation in sepsis and septic shock. Expert Opin Investig Drugs 11:1061–1075
Zhang J, Shen H, Wang X, Wu J, Xue Y (2004) Effects of chronic exposure of 2,4-dichlorophenol on the antioxidant system in liver of freshwater fish Carassius auratus. Chemosphere 55:167–174
Zhao M, Zhang Y, Wang C, Fu Z, Liu W, Gan J (2009) Induction of macrophage apoptosis by an organochlorine insecticide acetofenate. Chem Res Toxicol 22:504–510
Zhou X, Liang Y, Ren G, Zheng K, Wu Y, Zeng X, Zhong Y, Yu Z, Peng P (2020) Biotransformation of tris (2-chloroethyl) phosphate (TCEP) in sediment microcosms and the adaptation of microbial communities to TCEP. Environ Sci Technol 54:5489–5497
Funding
This work was supported by National College Students Innovation and Entrepreneurship Training Program (China, 202310389025).
Author information
Authors and Affiliations
Contributions
FH: Writing -Writing - Review & Editing, Investigation, Supervision, Project administration. WL: Conceptualization, Methodology, Validation, Investigation, Writing - original draft & Review, Funding acquisition. HW: Conceptualization, Methodology, Formal analysis, Investigation. HP: Validation, Visualization. JH: Investigation. JD: Validation. WZ: Validation. All the authors revised and approved the ms.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Our experimental protocols were approved by Laboratory Animals Ethics and Welfare Committee of College of Animal Science, Fujian Agriculture and Forestry University (PZCASFAFU22039).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
Whole life-cycle exposure to environmental relevant concentrations of TCEP could inhibit the growth of zebrafish.
Exposure to TCEP induced oxidative stress and led to lipid peroxidation in zebrafish liver.
Inflammatory response might be alleviated through the down-regulation of inflammatory cytokines mRNA expression.
Whole life-cycle exposure to TCEP might induce apoptosis through the activation of p53-Bax pathway.
Whole life-cycle exposure to TCEP resulted in a series of histopathological anomalies in zebrafish liver.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, F., Li, W., Wang, H. et al. Environmentally relevant concentrations of tris (2-chloroethyl) phosphate (TCEP) induce hepatotoxicity in zebrafish (Danio rerio): a whole life-cycle assessment. Fish Physiol Biochem 49, 1421–1433 (2023). https://doi.org/10.1007/s10695-023-01265-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01265-7