Abstract
Flubendiamide is a widely used diamide insecticide with many adverse effects on environmental organisms. This study assessed its bioaccumulation and toxicity effects in zebrafish (Danio rerio) using LC-MS/MS. The concentrations of flubendiamide in the whole zebrafish increased in the early stages and achieved steady levels at 14 days. The bioconcentration factors (BCFs) of flubendiamide was 1.125-2.011. Although flubendiamide did not significantly affect the growth phenotypes of zebrafish, it significantly changed the hepatic somatic index (HSI) of zebrafish. Histopathological analysis showed that flubendiamide could cause structural damage to the liver tissue of zebrafish. Further physiological and biochemical analysis showed that flubendiamide significantly changed the activity of catalase (CAT) and the contents of malondialdehyde (MDA) and glutathione (GSH) in liver of zebrafish. Moreover, flubendiamide significantly changed the mRNA expression levels of cell apoptosis-related genes, including p53, puma, caspase-3, caspase-9, apaf-1, and bax in liver of zebrafish. In summary, these results indicate that flubendiamide can cause liver damage by inducing oxidative stress and apoptosis in the liver of zebrafish. This study provides a background for further safety evaluation of flubendiamide to aquatic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diamide insecticides are broad-spectrum and high-efficiency pesticides widely used in agricultural production (Teixeira and Andaloro 2013). As a result, they are ubiquitous in the natural environment (Caboni et al. 2008; Sharma et al. 2014; Song et al. 2019) and pose potential toxicity risks to environmental organisms. Previous studies have shown that diamide insecticides exist in aquatic ecosystems (Song et al. 2019). For instance, Song et al. found that the concentrations of chlorantraniliprole in farmland water and water sediments were 0.01~0.09 μg/L and 0.24~0.56 μg/L, respectively. Therefore, the toxicity risks of diamide insecticides in aquatic organisms have attracted much attention (Barbee et al. 2010). However, available research on the toxicity effects of diamide insecticides on fish is very limited. Flubendiamide (CAS: 272451-65-7) is one of the earliest applied diamide insecticides. Moreover, flubendiamide has adverse effects on environmental organisms (Sarkar et al. 2014). Chronic sub-lethal flubendiamide exposure can induce cell apoptosis in larval imaginal discs of Drosophila melanogaster (Sarkar et al. 2017). Liu et al. (2017) found that flubendiamide causes oxidative stress and DNA damage in earthworms (Eisenia fetida). Meanwhile, long-term flubendiamide exposure induces oxidative stress in water buffalo (Bubalus bubalis) calves (Ranjan et al. 2018). A previous study showed that μg/L of flubendiamide can seriously affect the survival, reproduction, and growth of Daphnia magna (Cui et al. 2017). These studies suggest that flubendiamide has adverse effects on aquatic organisms. Therefore, it is necessary to conduct a toxicological study of flubendiamide on aquatic organisms.
Zebrafish (Danio rerio) is commonly used as a representative aquatic organism because it is easily available, less costly, sensitive to chemicals, and has a short life cycle. Zebrafish also have a high degree of conservation in the structure and function of genes and proteins. As a result, it is widely used in the toxicity assessment of environmental pollutants (Jia et al. 2020, 2021, Liu et al. 2022, Tian et al. 2021). No study has reported the toxicological effects of flubendiamide on zebrafish. Oxidative stress characterizes the damage caused by pesticides to organisms. Antioxidant enzymes remove reactive oxygen species (ROS) in organisms. Pesticides can disturb the balance of oxidizing substances and antioxidant systems, leading to excessive ROS production, thus causing biological damage (Hirooka et al. 2010; Li et al. 2018). Some studies have also shown that pesticides can affect the expression level of apoptosis-related genes in organisms (Jin et al. 2011; Teng et al. 2019). This study aimed to assess the bioaccumulation behaviors of flubendiamide in zebrafish using LC-MS/MS analysis. The toxic effects of flubendiamide on zebrafish were also assessed via physiological and biochemical, histopathological, and gene expression analysis. Therefore, this study can help to further understand the potential risks of flubendiamide to aquatic organisms, thus providing a theoretical basis for a comprehensive assessment of the environmental health risks associated with flubendiamide.
Materials and methods
Reagents
Flubendiamide (analytical standards, purity > 99.0%) was obtained from Sigma-Aldrich (Sigma, USA). All other chemicals were of analytical grade and were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Zebrafish maintenance
Four-month-old male zebrafish (AB wild-type strain) were obtained from Yangzhou aquarium supermarket (Yangzhou, China) and maintained at a regular 14-h photoperiod and 26 ± 2 °C temperature. The fish were fed once a day on a commercial diet. Every 15 zebrafish were randomly put in a 10-L glass tank with 5 L oxygen-enriched water. All zebrafish were acclimated for two weeks before experiments.
Zebrafish exposure and sample collection
The zebrafish were exposed to flubendiamide (0.1, 0.5, and 1.0 mg/L) based on the 96 h LC50 value (> 30 mg/L) of our previous study (Supporting information S1). The control group was exposed to 0.1% DMSO. For flubendiamide bioaccumulation, each treatment had 60 zebrafish (three replicates). The exposure media were changed every 48 h and the experimental conditions were consistent with the adaptation period. All zebrafish were fed on commercial fish feed once a day during the exposure period. Five zebrafish and 5-mL water samples were collected from each treatment group before changing the exposure media on days 1, 3, 5, 7, 10, 14, 17, and 21, then stored at − 20 °C. For toxicological effect analysis, zebrafish were sampled on the 7th and 14th days. For biochemical analysis, two zebrafish were obtained from each sample, then stored at − 20 °C. For histopathological analysis, three zebrafish were collected from each treatment group, anesthetized on ice, and dissected to retrieve the liver tissue and stored in tissue fixative. For the qPCR analysis, at least six zebrafish were obtained from each treatment group, anesthetized on ice, then dissected to obtain liver samples, pooled as one sample and stored at − 80 °C. Each treatment group had three biological replicates.
Determination of growth indexes
Five zebrafish were obtained from each treatment group on the 7th and 14th days, then body weight and length were measured. The zebrafish were anesthetized on ice and dissected to obtain liver tissues. The weight of the liver tissues was then determined. The growth factors (K-factors) and hepatic somatic index (HSI) were calculated using Eqs. (1) and (2), respectively.
Extraction and determination of flubendiamide
Sample extraction and purification
Each whole zebrafish was freeze-dried in a vacuum freeze drier (FD-1B-50, Beijing Boyikang Instruments Co. Ltd) at temperature < − 80 °C and vacuum < 5 Pa for 24 h, then grounded using a tissue grinder. The zebrafish samples (0.5 g) were put into 10-mL centrifuge tubes, 5 mL of acetonitrile was added, then ground for 5 min using a tissue grinder. Flubendiamide was added to the blank zebrafish samples to final concentrations of 0.1, 1.0, and 10 mg/kg. Sodium chloride (0.2 g) and 1 g of anhydrous magnesium sulfate were added into the samples, then agitated in a rotary shaker at 5000 rpm for 5 min. The supernatant (2 mL) was collected into a new glass tube containing 50 mg of C18 and agitated in a rotary shaker at 5000 rpm for 5 min. The supernatant (1 mL) was collected and concentrated to dryness. Finally, the extracts were re-dissolved into 1 mL of acetonitrile, filtered using a 0.22-μm membrane, and subjected to LC-MS/MS chromatographic analysis. Moreover, water samples (5 mL) were collected into 50-mL centrifuge tubes, then flubendiamide was added to the blank water samples to final concentrations of 0.1, 1.0, and 10 mg/L. Subsequently, 5 mL of n-hexane was used for shaking extraction (repeated thrice). The organic phases were combined and collected in a new centrifuge tube and concentrated with nitrogen to dry. Finally, the extracts were re-dissolved into 2 mL of acetonitrile, then filtered using a 0.22-μm membrane for LC-MS/MS chromatographic analysis.
Flubendiamide determination via LC-MS/MS
Flubendiamide concentrations in zebrafish and water were analyzed using liquid chromatography-mass spectrometry (LC-MS/MS) according to the previous research (Chen et al. 2012). The detailed analysis method of flubendiamide is shown in the supporting information S1.
Biochemical indicators and histopathology analysis
Malondialdehyde (MDA) and glutathione (GSH) contents and superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities in liver samples were detected using the MDA (Product ID: A003-1-2), GSH (Product ID: A006-2-1), SOD (Product ID: A001-3-2), CAT (Product ID: A007-1-1), and GPx (Product ID: A005-1-2) assay kits, respectively, from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) following the manufacturer’s instructions. The liver tissues from each treatment group were randomly selected for histopathological analysis. The liver tissues were fixed in 4% formaldehyde solution, paraffin-embedded, sectioned then stained with hematoxylin-eosin (H&E).
RNA extraction and qPCR analysis
TRIzol reagent was used to extract total RNA from 20 mg of zebrafish liver samples. The RNA samples (1.5 μg) were then reverse transcribed into cDNA using Fast Quant RT kit (with gDNase). After two-fold dilution of the cDNA with dd-H2O, SuperReal PreMix Plus (SYBR Green) kit was used to perform real-time fluorescent quantitative PCR analysis via the Bio-Rad CFX 96 PCR system (Bio-Rad, USA). Each sample contained three technical replicates. TRIzol reagent (Product ID: DP405), Fast Quant RT Kit (Product ID: KR116), and SuperReal PreMix Plus kit (Product ID: FP205) were obtained from Tiangen Biochemical Technology (Beijing, China). The above analysis methods were conducted following the manufacturer’s instructions. β-actin was used as an internal reference gene. The cycle threshold (Ct) method was used for homogenization. The PCR primers were obtained from Sangon Biotech (Shanghai, China.). The primer sequences are listed in Table S2.
Statistical analysis
The bioconcentration factor (BCF) of flubendiamide in zebrafish was estimated using the following Eq. (3):
C zebrafish C water is the concentration of flubendiamide in the zebrafish at steady state and in the exposure solution, respectively.
Data are expressed as mean ± standard deviation (SD). One-way ANOVA was performed using SPSS 19.0 (IBM, USA) to compare the statistical differences between different treatment groups. GraphPad Prism version 6.0 (Graph Pad) was used for graphical illustrations. Asterisk (*) indicates a statistically significant difference between the control (CK) and treatment groups (p < 0.05).
Results
Optimal conditions for flubendiamide detection using LC-MS/MS
The highest flubendiamide sensitivity was obtained using methanol and 10 mmol/L ammonium acetate aqueous solution containing 0.1% acetic acid as mobile phases at the MRM negative mode. The m/z 214 and m/z 254 were selected as the qualitative and quantitative ions of flubendiamide, respectively. The collision energies of ion pairs 681/214 and 681/254 were − 45 eV and − 20 eV, respectively. The selected ionization chromatography of flubendiamide is shown in Fig. S1. Furthermore, the fortified recoveries of flubendiamide from the zebrafish and water samples are listed in Table S2. The fortified recoveries of flubendiamide in water and zebrafish samples were 82.58–97.36% (relative standard deviation 2.95–3.29%) and 80.16–87.95% (relative standard deviation 1.55–3.24%), respectively. Therefore, the method can be used to study flubendiamide bioaccumulation in zebrafish.
Flubendiamide bioaccumulation in zebrafish
The bioaccumulation effects of flubendiamide in zebrafish in the 0.1, 0.5, and 1.0 mg/L flubendiamide treatment groups showed a similar trend. Flubendiamide concentrations in zebrafish increased from day 1 to 5, then decreased from day 5 to 7. Subsequently, flubendiamide concentrations increased after day 7, reaching a steady stage at 14 days (Fig. 1A, Table S3). The flubendiamide concentration changes in zebrafish may be due to the balance between bioaccumulation and metabolism. Moreover, flubendiamide concentration in water was stable. The actual flubendiamide concentrations in the 0.1, 0.5, and 1.0 mg/L treatment groups were 0.0958 ± 0.0040, 0.4970 ± 0.0084, and 1.0200 ± 0.0720 mg/L, respectively (Fig. 1B, Table S4). The BCFs of flubendiamide in zebrafish in the 0.1, 0.5, and 1.0 mg/L treatment groups at 14 days were 2.011, 1.125, and 1.444, respectively (Fig. 1C, Table S5).
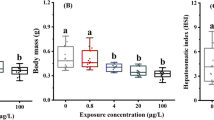
Effects of flubendiamide on the growth phenotypes of zebrafish
Flubendiamide did not significantly alter the body weight and length of zebrafish after 7 and 14 days of exposure compared with the control group (Fig. 2A, B). This study further assessed the effect of flubendiamide on the growth phenotype of zebrafish using growth factors (K-factors). Similarly, flubendiamide did not significantly affect K-factors of zebrafish (Fig. 2C). Particularly, 1.0 mg/L flubendiamide significantly increased hepatic somatic index (HSI) of zebrafish after 14 days compared with the control group (Fig. 2D). In contrast, the HSI did not significantly change in other treatment groups. These results indicate that flubendiamide exposure may adversely affect the liver tissues of zebrafish. Therefore, we conducted a histopathological analysis of liver samples after zebrafish exposure to flubendiamide for 14 days (Fig. 3). The cytoplasm of the liver in the control group was uniform, with a regular round nucleus located in the center of the liver cells. However, the liver tissues were significantly damaged after flubendiamide exposure. Several massive micro- or macro-vesicular intracellular lipid droplets occurred in the flubendiamide treatment groups. Furthermore, the degrees of liver cell pathology increased with increasing flubendiamide concentration. These results further indicate that flubendiamide exposure can cause liver malfunction in zebrafish.
Effects of flubendiamide on liver oxidative stress of zebrafish
This study evaluated the effect of flubendiamide on liver oxidative stress of zebrafish by measuring the enzymatic activities of SOD, CAT, and GPx and the contents of MDA and GSH. Flubendiamide did not significantly change SOD and GPx activities after 7 and 14 days of exposure (Fig. 4). In contrast, 1.0 mg/L flubendiamide significantly increased CAT activity after 14 days of exposure compared with the control group (Fig. 4B, E). The 1.0 mg/L flubendiamide also significantly decreased GSH contents after 14 days of exposure compared with the control group. Flubendiamide (1.0 mg/L) significantly increased MDA content after even days of exposure. Moreover, MDA contents were significantly increased after 14 days of 0.1, 0.5, and 1.0 mg/L flubendiamide exposure compared with the control group (Fig. 4E). These results indicate that flubendiamide can change the content or activity of biomarkers related to oxidative stress in the liver tissues of zebrafish.
Effects of flubendiamide on liver cell apoptosis of zebrafish
This study assessed the effects of flubendiamide on cell apoptosis in zebrafish liver by detecting the mRNA expression levels of apoptosis-related genes, such as apaf-1, p53, puma, caspase-3, caspase-9, bcl-2, and bax (Fig. 5). Flubendiamide (1.0 mg/L) significantly increased the mRNA expression levels of apaf-1 and bax after seven days of exposure (Fig. 5A, G). The 0.5 and 1.0 mg/L flubendiamide exposure significantly increased the mRNA expression levels of caspase-9 (Fig. 5E). Furthermore, 0.1, 0.5, and 1.0 mg/L flubendiamide significantly increased the mRNA expression levels of puma compared with the control group (Fig. 5C). Flubendiamide (0.5 and 1.0 mg/L) significantly increased the mRNA expression levels of apaf-1 and caspase-9 after 14 days of exposure (Fig. 5A, E). Particularly, the mRNA expression levels of p53, puma, caspase-3, and bax were significantly increased in the 0.1, 0.5, and 1.0 mg/L flubendiamide groups compared with the control group (Fig. 5B, C, D, G). However, flubendiamide did not significantly affect the mRNA expression levels of bcl-2 after 7 and 14 days of exposure.
Discussion
The bioaccumulation experiment showed that zebrafish had a low bioaccumulation capacity for flubendiamide based on classification standards of bioaccumulation capacities (Jia et al. 2017). Meanwhile, HIS significantly increased significantly after 14 days of 1.0 mg/L flubendiamide exposure. The liver tissue is the largest gland in vertebrates, and it plays a crucial role in the metabolism of carbohydrates, fats, proteins, vitamins, and hormones (Qiu et al. 2019). Furthermore, histopathological analysis confirmed that flubendiamide damaged the liver structure in zebrafish. Several studies have shown that liver tissue damage is often accompanied by oxidative stress (Meng et al. 2019, 2021; Wang et al. 2015). Herein, CAT activity in zebrafish liver significantly changed after flubendiamide exposure. CAT is an important antioxidant enzyme that can efficiently catalyze H2O2 into water and oxygen. Besides, it can scavenge free radicals and protect against cell damage in organisms (Zhang et al. 2018). These results indicate that flubendiamide can destroy the antioxidant enzyme system of zebrafish. Furthermore, flubendiamide significantly decreased GSH content in zebrafish liver. GSH is an important regulatory metabolite in cells. The decreased GSH content is a potential early activation signal of apoptosis, leading to subsequent generation of oxygen free radicals, thus promoting cell apoptosis (Meng et al. 2019). Importantly, MDA is a product of lipid peroxidation and is usually used to reflect the degree of oxidative damage (Gupta et al. 2009). MDA content significantly increased after flubendiamide exposure, indicating that flubendiamide can induce oxidative stress in zebrafish liver. Increased MDA may also severely damage cell membranes (Teng et al. 2019). Herein, flubendiamide caused structural damage to the liver tissue of zebrafish. Previous studies have also shown that flubendiamide can induce oxidative stress in earthworms and buffalo calves (Liu et al. 2017; Ranjan et al. 2018). Meanwhile, flubendiamide can induce oxidative stress and produce cytogenotoxic effects and histoarchitectural changes in the spleen of rats (Mandil et al. 2020).
Oxidative stress and lipid peroxidation may lead to apoptosis (Teng et al. 2019). Herein, flubendiamide altered the mRNA expression of various cell apoptosis related-genes. Increased mRNA expression of p53 and puma can induce apoptosis (Wang et al. 2004; Yu and Zhang 2008). caspase-3 and caspase-9 are the key genes essential to apoptosis (Soengas et al. 1999). However, apaf-1 can activate the mRNA expression of caspase-3 and caspase-9 (Zimmermann et al. 2001). Herein, flubendiamide significantly increased the mRNA expression levels of p53, puma, caspase-3, caspase-9, and apaf-1 in zebrafish liver. Bax is the target gene of p53 and can promote the release of cytochrome C from mitochondria. Bcl-2 can inhibit cell apoptosis. Both bax and bcl-2 belong to the bcl-2 protein family and are antagonistic proteins (Pena-Blanco and Garcia-Saez 2018). Bax can inhibit the bcl-2 expression, thus alleviating cell apoptosis inhibition. Therefore, the decreased ratio of expression levels of bcl-2/bax can affect the cell apoptosis inhibition effect (Whiteman et al. 2007). Herein, flubendiamide significantly decreased the ratios of expression levels of bcl-2/bax in zebrafish liver. These results suggest that flubendiamide can regulate the expression of apoptosis-related genes in zebrafish liver. In conclusion, flubendiamide can cause liver damage in zebrafish by inducing oxidative stress and cell apoptosis. Similarly, several studies have assessed the toxic effects of pesticides on zebrafish liver. Atrazine can cause oxidative stress, thus leading to liver dysfunction in zebrafish (Jin et al. 2010). Cypermethrin can also cause oxidative stress, DNA damage, and apoptosis in zebrafish liver (Jin et al. 2011). Although this study indicates that flubendiamide can cause oxidative stress and apoptosis in zebrafish liver tissues, the underlying mechanism is unclear. Histopathological analysis showed that flubendiamide induced increased lipid accumulation in liver tissue. However, further studies are needed to assess whether lipid accumulation is related to oxidative stress and apoptosis.
Conclusion
The results of this study indicate that flubendiamide has a low bioaccumulation ability in zebrafish. In addition, flubendiamide can significantly alter CAT activity and the levels of GSH and MDA in zebrafish liver. Also, flubendiamide can significantly change the mRNA expression of apoptosis-related genes in zebrafish liver. Therefore, this study provides better understandings of the toxic effects of flubendiamide on zebrafish through oxidative stress and apoptosis, thus enhancing the evaluation of the biological effects of flubendiamide on aquatic vertebrates.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Barbee GC, McClain WR, Lanka SK, Stout MJ (2010) Acute toxicity of chlorantraniliprole to non-target crayfish (Procambarus clarkii) associated with rice-crayfish cropping systems. Pest Manag Sci 66:996–1001
Caboni P, Sarais G, Angioni A, Vargiu S, Pagnozzi D, Cabras P, Casida JE (2008) Liquid chromatography-tandem mass spectrometric ion-switching determination of chlorantraniliprole and flubendiamide in fruits and vegetables. J Agric Food Chem 56:7696–7699
Chen X, Lu C, Fan S, Lu H, Cui H, Meng Z, Yang Y (2012) Determination of residual flubendiamide in the cabbage by QuEChERS-liquid chromatography-tandem mass spectrometry. Bull Environ Contamination Toxicol 89:1021–1026
Cui F, Chai T, Qian L, Wang C (2017) Effects of three diamides (chlorantraniliprole, cyantraniliprole and flubendiamide) on life history, embryonic development and oxidative stress biomarkers of Daphnia magna. Chemosphere 169:107–116
Gupta A, Bhatt MLB, Misra MK (2009) Lipid peroxidation and antioxidant status in head and neck squamous cell carcinoma patients. Oxidative Med Cellular Longevity 2:68–72
Hirooka Y, Sagara Y, Kishi T, Sunagawa K (2010) Oxidative stress and central cardiovascular regulation - pathogenesis of hypertension and therapeutic aspects. Circulation J 74:827–835
Jia M, Teng M, Tian S, Yan J, Meng Z, Yan S, Li R, Zhou Z, Zhu W (2020) Developmental toxicity and neurotoxicity of penconazole enantiomers exposure on zebrafish (Danio rerio). Environ Pollut 267:115450
Jia M, Teng M, Tian S, Yan J, Meng Z, Yan S, Li R, Zhou Z, Zhu W (2021) Effects of penconazole enantiomers exposure on hormonal disruption in zebrafish Danio rerio (Hamilton, 1822). Environ Sci Pollut Res 28:43476–43482
Jin Y, Zhang X, Shu L, Chen L, Sun L, Qian H, Liu W, Fu Z (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78:846–852
Jin Y, Zheng S, Pu Y, Shu L, Sun L, Liu W, Fu Z (2011) Cypermethrin has the potential to induce hepatic oxidative stress, DNA damage and apoptosis in adult zebrafish (Danio rerio). Chemosphere 82:398–404
Li H, Cao F, Zhao F, Yang Y, Teng M, Wang C, Qiu L (2018) Developmental toxicity, oxidative stress and immunotoxicity induced by three strobilurins (pyraclostrobin, trifloxystrobin and picoxystrobin) in zebrafish embryos. Chemosphere 207:781–790
Liu X, Zhao H, Chen Z, Lin Y, Lin W, Liu T, Wang X, Yang L (2017) Biochemical toxicity and cytotoxicity of flubendiamide on earthworms (Eisenia fetida). Asian J Ecotoxicol 12:293–301
Liu L, Wu Q, Miao X, Fan T, Meng Z, Chen X, Zhu W (2022) Study on toxicity effects of environmental pollutants based on metabolomics: a review. Chemosphere 286:131815
Mandil R, Prakash A, Rahal A, Singh SP, Sharma D, Kumar R, Garg SK (2020) In vitro and in vivo effects of flubendiamide and copper on cyto-genotoxicity, oxidative stress and spleen histology of rats and its modulation by resveratrol, catechin, curcumin and alpha-tocopherol. Bmc Pharmacol Toxicol 21:29
Meng Z, Tian S, Yan J, Jia M, Yan S, Li R, Zhang R, Zhu W, Zhou Z (2019) Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ Pollut 247:935–943
Meng Z, Tian S, Sun W, Liu L, Yan S, Huang S, Zhu W, Zhou Z (2021) Effects of exposure to prothioconazole and its metabolite prothioconazole-desthio on oxidative stress and metabolic profiles of liver and kidney tissues in male mice. Environ Pollut 269:116215
Nareshkumar B, Akbar SM, Sharma HC, Jayalakshmi SK, Sreeramulu K (2017) Evaluation of flubendiamide-induced mitochondrial dysfunction and metabolic changes in Helicoverpa armigera (Hubner). Archives Insect Biochem Physiol 96:e21401
Pena-Blanco A, Garcia-Saez AJ (2018) Bax, Bak and beyond - mitochondrial performance in apoptosis. Febs J 285:416–431
Ranjan A, Dumka VK, Ranjan R (2018) Chronic flubendiamide exposure induces oxidative stress in water buffalo (Bubalus bubalis) calves. Current Sci 114:1610–1612
Sarkar S, Dutta M, Roy S (2014) Potential toxicity of flubendiamide in Drosophila melanogaster and associated structural alterations of its compound eye. Toxicol Environ Chem 96:1075–1087
Sarkar S, Khatun S, Dutta M, Roy S (2017) Trans-generational transmission of altered phenotype resulting from flubendiamide-induced changes in apoptosis in larval imaginal discs of Drosophila melanogaster. Environ Toxicol Pharmacol 56:350–360
Sharma AK, Zimmerman WT, Singles SK, Malekani K, Swain S, Ryan D, McQuorcodale G, Wardrope L (2014) Photolysis of chlorantraniliprole and cyantraniliprole in water and soil: verification of degradation pathways via kinetics modeling. J Agric Food Chem 62:6577–6584
Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW (1999) Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284:156–159
Song C, Zhang J, Hu G, Meng S, Fan L, Zheng Y, Chen J, Zhang X (2019) Risk assessment of chlorantraniliprole pesticide use in rice-crab coculture systems in the basin of the lower reaches of the Yangtze River in China. Chemosphere 230:440–448
Teixeira LA, Andaloro JT (2013) Diamide insecticides: global efforts to address insect resistance stewardship challenges. Pesticide Biochem Physiol 106:76–78
Teng M, Zhou Y, Song M, Dong K, Chen X, Wang C, Bi S, Zhu W (2019) Chronic toxic effects of flutolanil on the liver of zebrafish (Danio rerio). Chemical Res Toxicol 32:995–1001
Tian S, Yan S, Meng Z, Huang S, Sun W, Jia M, Teng M, Zhou Z, Zhu W (2021) New insights into bisphenols induced obesity in zebrafish (Danio rerio): activation of cannabinoid receptor CB1. J Hazardous Mater 418:126100
Wang T, Chen F, Chen Z, Wu Y-F, Xu X-L, Zheng S, Hu X (2004) Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J Gastroenterol 10:2205–2208
Wang Y, Xu L, Li D, Teng M, Zhang R, Zhou Z, Zhu W (2015) Enantioselective bioaccumulation of hexaconazole and its toxic effects in adult zebrafish (Danio rerio). Chemosphere 138:798–805
Whiteman M, Chu SH, Siau JL, Rose P, Sabapathy K, Schantz J-T, Cheung NS, Spencer JPE, Armstrong JS (2007) The pro-inflammatory oxidant hypochlorous acid induces Bax-dependent mitochondrial permeabilisation and cell death through AIF-/EndoG-dependent pathways. Cellular Signalling 19:705–714
Yu J, Zhang L (2008) PUMA, a potent killer with or without p53. Oncogene 27:S71–S83
Zhang Z, Lin L, Gai Y, Hong Y, Li L, Weng L (2018) Subchronic bisphenol S exposure affects liver function in mice involving oxidative damage. Regulatory Toxicol Pharmacol 92:138–144
Zimmermann KC, Bonzon C, Green DR (2001) The machinery of programmed cell death. Pharmacol Therapeutics 92:57–70
Funding
This work was supported by the Key Research and Development Program of Jiangsu Province (BE2019340), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJB610028), the Science and Technology Projects Fund of Yangzhou City (YZ2020178), and Practicality and Innovation Training Project for Undergraduate Students in Jiangsu Province.
Author information
Authors and Affiliations
Contributions
Zhiyuan Meng: conceptualization, methodology, writing-review & editing. Zhichao Wang: methodology, validation, formal analysis. Xiaojun Chen: writing- review & editing, supervision, project administration. Yueyi Song: methodology, data curation, writing-original draft. Miaomiao Teng: methodology, data curation. Tianle Fan: methodology, data curation. Yang Zheng: resources, writing-review & editing. Jiajia Cui: validation, supervision. Wangjin Xu: methodology, data curation.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were in accordance with the current Chinese legislation and were approved by the independent Animal Ethical Committee at Yangzhou University.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 94 kb)
Rights and permissions
About this article
Cite this article
Meng, Z., Wang, Z., Chen, X. et al. Bioaccumulation and toxicity effects of flubendiamide in zebrafish (Danio rerio). Environ Sci Pollut Res 29, 26900–26909 (2022). https://doi.org/10.1007/s11356-021-17868-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17868-7