Abstract
This study was conducted to evaluate the effects of different dipeptides (lysine-leucine, lysine-glycine, and leucine-glycine) and free amino acids (lysine and leucine) on the growth, gene expression of intestinal peptide and amino acid transporters, and serum free amino acid concentrations in turbot. Fish (11.98 ± 0.03 g) were fed four experimental diets supplementing with crystalline amino acids (CAA), lysine-leucine (Lys-Leu), lysine-glycine (Lys-Gly), and leucine-glycine (Gly-Leu). Fish protein hydrolysate (FPH) containing a mixture of free amino acids and small peptides was designed as a positive control diet. There was no significant difference in the growth and feed utilization among three dipeptide diets (Lys-Leu, Lys-Gly, and Gly-Leu). Compared with the CAA group, feed efficiency ratio was significantly higher in the Lys-Leu and Lys-Gly groups, and protein efficiency ratio was significantly higher in the Lys-Leu group. For peptide transporter, oligopeptide transporter 1 (PepT1) mRNA level was not affected by dietary treatments. For amino acid transporters, lower expression of B0 neutral amino acid transporter 1 (B0AT1) and proton-coupled amino acid transporter 1 (PAT1) were observed in fish fed the dipeptide and FPH diets compared with the CAA diet. In conclusion, juvenile turbot fed Lys-Leu, Gly-Leu, and Lys-Gly had a similar growth performance, whereas lysine and leucine in the Lys-Leu form can be utilized more efficiently for feed utilization than those in free amino acid from. In addition, compared to free amino acids, dipeptides and fish protein hydrolysate in diets may down-regulate the expression of amino acid transporters but did not affect the expression of PepT1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish from aquaculture are important sources of animal protein for millions of people. Global aquaculture has remained at an annual growth rate of 5.8% during the period 2000–2016, which has become one of the fastest-growing major food production sectors in the world. Fish meal in aquafeeds is the major protein source for carnivorous fish. However, fish meal production has followed a fluctuating but overall declining trend since 1994 (FAO 2018). If aquaculture maintains a steady growth over the next few years, fish meal production will not be able to keep up with the demand of feeds for aquaculture. Thus, aquafeeds have a strong need for sustainable alternative ingredients from plant proteins (Perez-Velazquez et al. 2018; Gatlin III et al. 2007).
Since plant proteins are always limited in essential amino acids, such as lysine, methionine, arginine, and leucine, concentrations of essential amino acids are particularly important in evaluating the possibility of fish meal substitution (Gatlin III et al. 2007; Kaushik and Seiliez 2010). Supplementation of essential amino acids in diets as per the requirement of fish is necessary in formulating plant protein-based diets (Furuya et al. 2004; El-Husseiny et al. 2017). Generally, essential amino acids in feed are mainly derived from protein-bound amino acids, free amino acids, or peptides (Zhang et al. 2006). Researchers proposed that exogenous essential amino acids in both synthetic peptides and crystalline forms could be supplemented to high plant protein-based diets for optimum growth of fish (Dabrowski et al. 2003; Ostaszewska et al. 2013). These studies focused on comparing the utilization efficiency of free amino acid and dipeptide in some fish species, such as common carp (Cyprinus carpio) (Kamaszewski et al. 2014; Ostaszewska et al. 2010a), olive flounder (Paralichthys olivaceus) (Kim and Lee 2013; Rahimnejad and Lee 2014), yellow perch (Perca flavescens) (Kwasek et al. 2012; Ostaszewska et al. 2013). However, the effect of dietary dipeptides on growth in different fish species was controversial compared with crystalline amino acids. In addition, to our knowledge, few studies on turbot were conducted to compare the efficiency of free essential amino acids and their small peptides in term of growth performance and amino acid transport.
Generally, the transport of free amino acids and di/tripeptide hydrolyzed from dietary protein was modulated by amino acid and peptide transporters in the intestine (Poncet and Taylor 2013; Verri et al. 2017). Previous studies found that feed containing dipeptides or free amino acids up-regulated the oligopeptide transporter 1 (PepT1) gene expression in the intestine of rainbow trout (Oncorhynchus mykiss) (Ostaszewska et al. 2010b) and yellow perch (Kwasek et al. 2012). However, it was not clear whether a similar response of PepT 1 was also appeared in turbot due to species-specific uptake of di/tripeptides via teleost fish PepT 1 (Margheritis et al. 2013). In addition, the other issue is how to be affected amino acid transporters by dietary free amino acids and dipeptides, because free amino acids from intestinal lumen were transported by amino acid transporters (Bröer 2008).
Among the ten essential amino acids, lysine was an amino acid commonly deficient in plant protein-based diets, and its imbalance and bioavailability could affect fish growth and feed efficiency (Abimorad et al. 2014). Leucine, a member of branched-chain amino acids family, had an important anabolic function, which stimulated protein synthesis in fish via the target of rapamycin signaling pathway (Lansard et al. 2010). Therefore, the two essential amino acids were chosen as representative essential amino acids in the present study. The objectives of this study were to evaluate the effect of different dipeptides (lysine-leucine, lysine-glycine, and leucine-glycine) and free amino acids (lysine and leucine) on growth, muscle amino acid composition, expression of intestinal peptide and amino acid transporters, and postprandial serum free amino acids in turbot fed high plant protein diets.
Materials and methods
Diets and feeding management
Five isoproteic (480 g kg−1 crude protein) and isolipidic (110 g kg−1 crude lipid) diets were formulated to contain fish meal and peanut meal as the protein source as well as fish oil and soy oil as the lipid source. A mixture of crystalline amino acids without lysine, leucine, and glycine was prepared according to Peres and Oliva-Teles (2005). Fish protein hydrolysate containing 822 g kg−1 crude protein and 3.6 g kg−1 crude lipid was produced from by-products of Pollock (Theragra chalcogramma) by enzymatic treatment (Wei et al. 2016). Four experimental diets (the CAA, Lys-Leu, Lys-Gly, and Gly-Leu diets) were prepared to contain the equivalent of lysine, leucine, and glycine: (1) the CAA diet supplemented with crystalline lysine, leucine, and glycine; (2) the Lys-Leu diet supplemented with synthetic lysine-leucine and crystalline glycine; (3) the Lys-Gly diet supplemented with synthetic lysine-glycine and crystalline leucine; (4) the Gly-Leu diet supplemented with synthetic leucine-glycine and crystalline lysine. Fish protein hydrolysate containing a mixture of free amino acids and small peptides was supplemented to the FPH diet as a positive control diet. The formulation and amino acid composition of experimental diets are respectively shown in Tables 1 and 2.
The growth trial was conducted at Yantai Tianyuan Aquatic Product Co., Ltd. (Shandong, China). Turbot were kept in cylindrical fiberglass tanks for 2 weeks to acclimate to the experimental conditions. Then, the fish were fasted for 24 h and weighed. Juvenile turbot (initial weight 11.98 ± 0.03 g) were randomly stocked into 15 tanks (water volume 120 L) at a density of 25 fish per tank (five treatments with three replicates). Sea water was supplied to the tanks at a rate of 5 L min−1. During the whole experimental period, water quality parameters measured were water temperature (15 ± 1 °C), pH (7.7 ± 0.2), salinity (32 ppt), and dissolved oxygen (7 mg L−1). Fish were subjected to natural photoperiod (from August to October 2017) during the trial. During the 8 weeks of feeding period, fish were hand-fed two times a day (06:30 and 16:30).
Sample collection
At the end of feeding trail, fish were fasted for 24 h and then anesthetized with eugenol (1:10000) before sampling. Fish were counted and weighed by each tank. Five fish were randomly sampled from each tank and frozen for the determination of whole-body chemical composition. Another five fish per tank were euthanized with eugenol overdose (3:10000) for dissection. Muscle and proximal intestine were immediately dissected, frozen in liquid nitrogen, and stored at − 80 °C until the determination of concentrations of amino acids and gene expression, respectively. Then, the remaining fish from each tank continued to be fed their allocated diets until a visible satiation. Three fish from each tank were collected at 8 h after refeeding. Blood samples were collected from the caudal vein using heparinized syringes. Then serum was separated with centrifugation at 3000×g for 10 min and stored at − 80 °C.
Chemical analysis of fish and diets
The chemical composition of the diets and fish samples was analyzed using standard methods (AOAC 2005). Dry matter content was determined by drying the samples to constant weight at 105 °C. Crude protein content was determined by the Kjeldahl method (UDK142 automatic distillation unit, VELP, Usmate, MB, Italy). Crude lipid content was determined using Soxhlet method (Foss Tecator, Hoganas, Sweden). Ash content was determined by incinerating in a muffle furnace at 550 °C for 16 h.
Determination of amino acid in muscle and diet
Muscle and experimental diets were hydrolyzed in 6 N HCl for 22 h at 110 °C for amino acid analyses. Serum and experimental diets were de-proteinized by thoroughly mixing samples with trichloroacetic acid (6%), followed by centrifugation at 10000g for 10 min and the supernatant used for free amino acid analyses. The contents of amino acids in hydrolyzed muscle and feed samples as well as free amino acids in de-proteinized serum and feed samples were analyzed using an amino acid analyzer (Hitachi L-8900 automatic amino acid analyzer; Hitachi High-Technologies Corporation, Tokyo, Japan).
Gene expression analysis
Total RNA was extracted from proximal intestine using RNAiso plus kit (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China) and reversely transcribed with Prime Script™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China). Relatively quantitative mRNA expression between different treatments was analyzed using quantitative real time-polymerase chain reaction (qRT-PCR). β-Actin was used as the housekeeping gene. Specific primer sequences of target genes for the qRT-PCR are listed in Table 3. The gene expression analyses were focused on oligopeptide transporter 1 (PepT1, SLC15A1), B0 neutral amino acid transporter 1 (B0AT1, SLC6A19), cationic amino acid transporter 1 (CAT1, SLC7A1), proton-coupled amino acid transporter 1 (PAT1, SLC36A1), and y+ L-type amino acid transporter 2 (y+LAT2, SLC7A6). The qRT-PCR was performed on SYBR Green Real-time PCR Master Mix (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China). Target gene expression was normalized relative to β-actin expression. The relative expression levels of target gene were analyzed using the 2-ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis
The results are presented as mean ± standard error (SE) of three replicate tanks. Normality of distributions and homogeneity of variances were confirmed by Shapiro-Wilk test and Levene’s test, respectively. All data were subjected to one-way ANOVA followed by a Tukey’s multiple comparison test. The differences at P ≤ 0.05 were considered to be statistically significant. The software SPSS 16.0 (SPSS Company, Chicago, IL, USA) was used to compute statistical analyses.
Results
Growth performance and feed utilization
Growth results are shown in Table 4. The survival of experimental fish in all groups was more than 98%. Final body weight and specific growth rate in fish fed the FPH diet were significantly higher than those in fish fed the CAA diet (P < 0.05). Similarly, an increasing trend of specific growth rate had been observed in the Lys-Leu group (P = 0.107), the Gly-Leu group (P = 0.107), and the Lys-Gly group (P = 0.140) compared with fish fed the CAA diet. Feed intake in the Lys-Leu treatment was significantly lower than that of fish in the CAA, Gly-Leu, and FPH treatments (P < 0.05). Fish fed the Lys-Leu and Lys-Gly diets showed significantly higher feed efficiency ratios compared to fish receiving the CAA diet (P < 0.05). Compared to the CAA treatment, protein efficiency ratio significantly increased in the Lys-Leu treatment (P < 0.05) and the trend of the increase was also observed in the Lys-Gly treatment (P = 0.117). There were no significant differences for protein productive value between dietary treatments (P > 0.05).
Whole fish body composition and amino acid composition of muscle
The whole-body proximate composition for the fish fed the different diets is presented in Table 5. Data for moisture, crude protein, crude lipid, and ash on whole fish analysis showed no significant differences between dietary treatments (P > 0.05). As seen in Table 6, except for phenylalanine and taurine, the concentrations of essential and non-essential amino acids were the lowest in the fish fed the CAA diet. The lowest concentration of phenylalanine and taurine was observed in the fish fed the FPH diet.
Proximal intestinal mRNA expressions of peptide and AA transporters
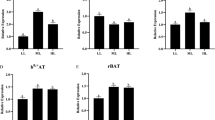
The expression of PepT1 is presented in Fig. 1. PepT1 mRNA level was not affected by dietary treatments (P > 0.05). As shown in Fig. 2, mRNA levels of B0AT1 and PAT1 with fish fed the CAA diet were significantly higher than all other diets (P < 0.05). The expression level of y+LAT2 in fish fed the CAA diet was significantly higher than that of fish fed the Lys-Gly and FPH diets (P < 0.05), while no significant difference was observed among the Lys-Leu, Lys-Gly, and Gly-Leu groups (P > 0.05). Fish fed the CAA diet had significantly higher mRNA level of CAT1 than that of fish fed the FPH diet (P < 0.05), while there was no significant difference among the FPH, Lys-Leu, Lys-Gly, and Gly-Leu groups (P > 0.05).
Relative mRNA expression of amino acid transporters (proton-coupled amino acid transporter 1, PAT1; y+ L-type amino acid transporter 2, y+LAT2; B0 neutral amino acid transporter 1, B0AT1; and cationic amino acid transporter 1, CAT1) in proximal intestine. Data are presented as mean ± SE of three replicate tanks (n = 3). Bars of with same letters are not significantly different by Tukey’s test (P > 0.05)
Serum amino acid profiles
Serum free amino acid concentrations at 8 h post-feeding are shown in Table 7. Regarding essential amino acids, concentrations of valine, methionine, isoleucine, leucine, and lysine had been affected by dietary treatments (P < 0.05), which the lowest amounts for those essential amino acids were observed in fish fed the FPH diet. To non-essential amino acids, taurine, glycine, and cystine in serum showed the lowest concentration in fish fed the FPH diet compared with fish fed other diets. On the contrary, the levels of tyrosine and proline in the FPH group were the highest among all the groups.
Discussion
Fish protein hydrolysates contained short-chain peptides and free amino acids with high nutritional properties, which had been demonstrated to have a beneficial biological effect on fish growth, feed intake, protein and amino acid digestibility, and non-specific immune response (Hevrøy et al. 2005; Olsen and Toppe 2017; Swanepoel and Goosen 2018; Wei et al. 2016). In the present study, higher growth performance in fish fed diets containing fish protein hydrolysate was observed compared with fish fed diets containing crystalline amino acid mixtures at the equivalent protein content, which was consistent with our previous study of turbot (Wei et al. 2016). It indicated that low molecular weight peptides from fish protein hydrolysate may be utilized more efficiently than crystalline amino acids in turbot fed high plant protein diets. This result was supported by the study of Dabrowski et al. (2003) and Dabrowski et al. (2005), which showed that a mixture of synthetic dipeptides improved growth performance of rainbow trout compared to a mixture of crystalline amino acids. For a single dipeptide, an interesting result in the current study was that growth performance was relatively close among three dipeptide groups (Lys-Leu, Gly-Leu, and Lys-Gly), it indicated that turbot had a similar utilization efficiency for these three dipeptides. In addition, compared to fish fed the CAA diets, specific growth rate showed an obviously increasing trend in fish fed the Lys-Leu diet (P = 0.107), the Gly-Leu diet (P = 0.107) and the Lys-Gly diet (P = 0.140), and the trend of the increase in feed efficiency ratio and protein efficiency ratio were also observed in the Lys-Leu group (P < 0.05) and the Lys-Gly group (feed efficiency ratio, P < 0.05; protein efficiency ratio, P = 0.117). Although significant statistical differences were not observed in growth and feed utilization, those change trends still indicated that dietary amino acids in the form of dipeptide, especially Lys-Leu, seemed to improve growth performance of turbot than those in the form of free amino acids. This may be supported by the fact that the levels of most amino acids in muscle were higher in fish fed the dipeptides diets (the Lys-Leu, Gly-Leu, and Lys-Gly diets) than those of fish fed the CAA diets. Because the increase of muscle amino acid concentrations in the dipeptides groups indicated that those amino acids in dipeptide form may more effectively increase amino acid deposition of muscle compared with free amino acid from when concentrations of amino acids in feed were similar among all the groups. Meanwhile, these results of growth were consistent with previous results of olive flounder, which showed that lysine and leucine availability could be better in fish growth when the fish were fed dipeptide (leucine-glycine or lysine-glycine) than free forms (Kim and Lee 2013; Rahimnejad and Lee 2014). However, for common carp and yellow perch, no significant differences were found in the growth when the diets were supplemented with free or dipeptide forms of lysine and glycine (Kwasek et al. 2012; Ostaszewska et al. 2010a, 2013). The possible reason for this contradiction was that substantial species-specific differences may appear in PepT1 of teleost fish in response to various dipeptides transport (Romano et al. 2014).
In teleost fish, PepT1, known as a member of Solute Carrier 15 (SLC15) family, is chiefly responsible for the absorption of di/tripeptides from dietary protein digestion in intestinal lumen (Verri et al. 2017). Previous study reported that the dipeptide uptake occurred in the proximal of the intestine in Mozambique tilapia (Oreochromis mossambicus) (Thamotharan et al. 1996a, 1996b), which was demonstrated by assessing the regional distribution of PepT1 in the whole intestine (Orozco et al. 2017). Similarly, turbot PepT1 was found to express primarily in proximal intestine (Xu et al. 2016), which indicated that dietary dipeptide absorption may be mainly mediated by PepT1 in proximal intestine of turbot (Wei et al. 2020a, b). However, the present experimental diets supplemented with synthetic dipeptide or fish protein hydrolysate did not cause any different changes in PepT1 expression compared to the CAA diet in proximal intestine. For the regulation of PepT1 by dietary dipeptide alone, Ostaszewska et al. (2013) reported that a wheat gluten-based diet supplemented with dipeptide (lysine-glycine) induced a stronger PepT1 immuno-positive reaction in proximal intestine of yellow perch. For the regulation of PepT1 by dietary crystalline amino acids or fish protein hydrolysate, Bakke et al. (2010) also reported that fish meal substituted by a mixture of crystalline amino acids or fish protein hydrolysate could up-regulate PepT1 expression in proximal intestine of Atlantic cod. Taken together, we speculated that PepT1 expression in turbot proximal intestine may be simultaneously up-regulated by dipeptide, fish protein hydrolysate, and crystalline amino acids, which caused no significant differences observed in PepT1 expression between dietary treatments.
Amino acid transporters play an important role in the uptake of amino acids in intestinal lumen, nevertheless, they are different from peptide transporters (PepT1) due to exhibiting distinct substrate specificities for transporting amino acids into the enterocyte (Chen et al. 2018). Based on amino acid profile and functional studies, five amino acid transport systems had been proposed, the “neutral system,” the “basic system,” the “acidic system,” the “iminoglycine system,” and the “β-amino acid system,” respectively, which are transporting groups of amino acids rather than individual amino acid (Bröer 2008). Some studies revealed that the form (free vs. protein-bound) of dietary amino acids may affect the absorption of amino acids from the lumen of the small intestine into enterocytes by regulating intestinal expression of amino acid transporters (Osmanyan et al. 2018; Morales et al. 2017; Zhang et al. 2013). For this reason, amino acid transporters related to the absorption of leucine, lysine, and glycine in the intestine were measured in the present experiment. PAT1 was identified as a proton/amino acid transporter responsible for the absorption of protons and neutral amino acids such as glycine (Boll et al. 2004; Thwaites and Anderson 2011). B0AT1 was a Na+-dependent neutral AA transporter and transported leucine efficiently (Margheritis et al. 2016; Rimoldi et al. 2015). CAT1 was the classical cationic amino acid transporter system, in which its expression can be affected by dietary lysine (Fotiadis et al. 2013; He et al. 2013). y+LAT2 mediated the influx of several neutral and cationic amino acids with similar efficiency (Verrey et al. 2004). Our data found that fish fed the dipeptides and FPH diets down-regulated the expression of those amino acid transporters in proximal intestine relative to fish fed the CAA diets. Similarly, Morales et al. (2017) reported that the expression of amino acid transporters in duodenum of pig was down-regulated fed the high-protein diet (protein-bound) compared with those fed the low-protein diet supplemented with free amino acids. It indicated that dietary amino acids from dipeptide or fish protein hydrolysate (a mixture of small peptides) except for protein-bound amino acids may also down-regulate amino acid transporter expression in the intestine relative to dietary free amino acids. Additionally, the expression of test amino acid transporters and PepT1 had no significant difference among the three dipeptide treatments. It may further prove that turbot has the similar utilization efficiency for Lys-Leu, Gly-Leu, and Lys-Gly in diets.
Free amino acid concentrations in serum can be influenced by a variety of factors including the amino acid forms in diets, the levels of dietary amino acids, and the types of protein sources (Ambardekar et al. 2009; Larsen et al. 2012; Yun et al. 2016). In the present study, leucine and lysine concentrations from serum at 8 h post-feeding were obviously higher in fish fed the CAA and dipeptides diets compared with fish fed the FPH diets, which was in agreement with Yun et al. (2016) study that postprandial serum lysine concentrations tended to increase as the increase of dietary lysine concentration. It suggested that concentrations of leucine and lysine in serum fairly reflected concentrations of amino acids in diets consumed by turbot. In addition, Bogé et al. (1981) reported that glycine from glycine-glycine in rainbow trout was absorbed more rapidly than that from the equivalent free amino acid. However, there were no significant differences in serum concentrations of leucine, lysine, and glycine in the CAA, Lys-Leu, Lys-Gly, and Gly-Leu groups at 8-h post-feeding. The different results may be explained that serum was sampled at 8 h after meal, which missed the optimal absorption time of amino acids, resulting in no significant changes of leucine, lysine, and glycine contents in serum between the CAA diet and the dipeptides diets (Wei et al. 2020a, b). Thus, 8-h postprandial serum free amino acids may not be affected by their forms (free vs. dipeptides) in diets. Further studies could examine concentrations of serum free amino acids at different sampling times.
In conclusion, the growth and feed utilization were similar among the Lys-Leu, Gly-Leu, and Lys-Gly groups. However, lysine and leucine in Lys-Leu forms were utilized more efficiently for feed utilization compared with those in free amino acid form. Dietary amino acids from dipeptide or fish protein hydrolysate may down-regulate amino acid transporter expression but did not affect the expression of PepT1 in the proximal intestine compared with those from free amino acid form. Meanwhile, the present study also found that concentrations of serum free amino acids at 8-h post-feeding may not be affected by amino acid forms (free vs. dipeptides) in diets.
References
Abimorad EG, Ducatti C, Castellani D, Jomori RK, Portella MC, Carneiro DJ (2014) The use of stable isotopes to investigate the effects of supplemental lysine and methionine on protein turnover and amino acid utilization in pacu, Piaractus mesopotamicus, juveniles. Aquaculture 433:119–124. https://doi.org/10.1016/j.aquaculture.2014.06.006
Ambardekar AA, Reigh RC, Williams MB (2009) Absorption of amino acids from intact dietary proteins and purified amino acid supplements follows different time-courses in channel catfish (Ictalurus punctatus). Aquaculture 291:179–187. https://doi.org/10.1016/j.aquaculture.2009.02.044
Association of Official Analytical Chemists (AOAC) (2005) Official Methods of Analysis of Official Analytical Chemists International, 18th edn. Association of Official Analytical Chemists, Gaithersburg, MD
Bakke S, Jordal AEO, Gómez-Requeni P, Verri T, Kousoulaki K, Aksnes A, Rønnestad I (2010) Dietary protein hydrolysates and free amino acids affect the spatial expression of peptide transporter PepT1 in the digestive tract of Atlantic cod (Gadus morhua). Comp Biochem Physiol B-Biochem Mol Biol 156(1):48–55. https://doi.org/10.1016/j.cbpb.2010.02.002
Bogé G, Rigal A, Peres G (1981) Rates of in vivo intestinal absorption of glycine and glycylglycine by rainbow trout (Salmo gairdneri R.). Comp Biochem Physiol A-Physiol 69(3):455–459. https://doi.org/10.1016/0300-9629(81)93004-8
Boll M, Daniel H, Gasnier B (2004) The slc36 family: proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflügers Arch-Eur J Physiol 447(5):776–779. https://doi.org/10.1007/s00424-003-1073-4
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88(1):249–286. https://doi.org/10.1152/physrev.00018.2006
Chen C, Yin Y, Tu Q, Yang H (2018) Glucose and amino acid in enterocyte: absorption, metabolism and maturation. Front Biosci 23:1721–1739. https://doi.org/10.2741/4669
Dabrowski K, Lee KJ, Rinchard J (2003) The smallest vertebrate, teleost fish, can utilize synthetic dipeptide-based diets. J Nutr 133(12):4225–4229. https://doi.org/10.1093/jn/133.12.4225
Dabrowski K, Terjesen BF, Zhang Y, Phang J, Lee KJ (2005) A concept of dietary dipeptides: a step to resolve the problem of amino acid availability in the early life of vertebrates. J Exp Biol 208(15):2885–2894
El-Husseiny O, El-Haroun ER, Goda A, Hassan I, Woodward B, Suloma A (2017) Effects of lysine and tryptophan supplementations in plant protein-based diets on the performance of Nile tilapia (Oreochromis niloticus). J Appl Aquac 29(3-4):266–276. https://doi.org/10.1080/10454438.2017.1364684
FAO (2018) The State of World Fisheries and Aquaculture. Rome, Italy, pp 5–7
Fotiadis D, Kanai Y, Palacín M (2013) The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34(2-3):139–158. https://doi.org/10.1016/j.mam.2012.10.007
Furuya WM, Pezzato LE, Barros MM, Pezzato AC, Furuya VR, Miranda EC (2004) Use of ideal protein concept for precision formulation of amino acid levels in fish-meal-free diets for juvenile Nile tilapia (Oreochromis niloticus L.). Aquac Res 35(12):1110–1116. https://doi.org/10.1111/j.1365-2109.2004.01133.x
Gatlin DM III, Barrows F, Bellis D, Brown P, Campen J, Dabrowski K, Gaylord TG, Hardy RW, Herman EM, Hu G, Krogdahl A, Nelson R, Overturf KE, Rust M, Sealey W, Skonberg D, Souza EJ, Stone D, Wilson RF (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38(6):551–579. https://doi.org/10.1111/j.1365-2109.2007.01704.x
He L, Yang H, Hou Y, Li T, Fang J, Zhou X, Yin Y, Wu L, Nyachoti M, Wu G (2013) Effects of dietary L-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids 45(2):383–391. https://doi.org/10.1007/s00726-013-1514-0
Hevrøy EM, Espe M, Waagbø R, Sandnes K, Ruud M, Hemre GI (2005) Nutrient utilization in Atlantic salmon (Salmo salar L.) fed increased levels of fish protein hydrolysate during a period of fast growth. Aquacult Nutr 11(4):301–313. https://doi.org/10.1111/j.1365-2095.2005.00357.x
Kamaszewski M, Prasek M, Ostaszewska T, Dabrowski K (2014) The influence of feeding diets containing wheat gluten supplemented with dipeptides or free amino acids on structure and development of the skeletal muscle of carp (Cyprinus carpio). Aquacult Int 22(1):259–271. https://doi.org/10.1007/s10499-013-9683-0
Kaushik SJ, Seiliez I (2010) Protein and amino acid nutrition and metabolism in fish: current knowledge and future needs. Aquac Res 41(3):322–332. https://doi.org/10.1111/j.1365-2109.2009.02174.x
Kim SS, Lee KJ (2013) Comparison of leucine requirement in olive flounder (Paralichthys olivaceus) by free or synthetic dipeptide forms of leucine. Anim Feed Sci Technol 183(3-4):195–201. https://doi.org/10.1016/j.anifeedsci.2013.05.008
Kwasek K, Terova G, Wojno M, Dabrowski K, Wick M (2012) The effect of dietary dipeptide lysine-glycine on growth, muscle proteins, and intestine PepT1 gene expression in juvenile yellow perch. Rev Fish Biol Fisheries 22(3):797–812. https://doi.org/10.1007/s11160-012-9266-6
Lansard M, Panserat S, Plagnes-Juan E, Dias K, Seiliez I, Skiba-Cassy S (2010) L-Leucine, L-methionine, and L-lysine are involved in the regulation of intermediary metabolism-related gene expression in rainbow trout hepatocytes. J Nutr 141(1):75–80. https://doi.org/10.3945/jn.110.124511
Larsen BK, Dalsgaard J, Pedersen PB (2012) Effects of plant proteins on postprandial, free plasma amino acid concentrations in rainbow trout (Oncorhynchus mykiss). Aquaculture 326:90–98. https://doi.org/10.1016/j.aquaculture.2011.11.028
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Margheritis E, Terova G, Oyadeyi AS, Renna MD, Cinquetti R, Peres A, Bossi E (2013) Characterization of the transport of lysine-containing dipeptides by PepT1 orthologs expressed in Xenopus laevis oocytes. Comp Biochem Physiol A-Mol Integr Physiol 164(3):520–528. https://doi.org/10.1016/j.cbpa.2012.12.016
Margheritis E, Imperiali FG, Cinquetti R, Vollero A, Terova G, Rimoldi S, Girardello R, Bossi E (2016) Amino acid transporter B0AT1 (slc6a19) and ancillary protein: impact on function. Pflügers Arch-Eur J Physiol 468(8):1363–1374. https://doi.org/10.1007/s00424-016-1842-5
Morales A, Buenabad L, Castillo G, Vázquez L, Espinoza S, Htoo JK, Cervantes M (2017) Dietary levels of protein and free amino acids affect pancreatic proteases activities, amino acids transporters expression and serum amino acid concentrations in starter pigs. J Anim Physiol Anim Nutr 101(4):723–732. https://doi.org/10.1111/jpn.12515
Olsen RL, Toppe J (2017) Fish silage hydrolysates: Not only a feed nutrient, but also a useful feed additive. Trends Food Sci Technol 66:93–97. https://doi.org/10.1016/j.tifs.2017.06.003
Orozco ZGA, Soma S, Kaneko T, Watanabe S (2017) Effects of fasting and refeeding on gene expression of slc15a1a, a gene encoding an oligopeptide transporter (PepT1), in the intestine of Mozambique tilapia. Comp Biochem Physiol B-Biochem Mol Biol 203:76–83. https://doi.org/10.1016/j.cbpb.2016.09.006
Osmanyan AK, Harsini SG, Mahdavi R, Fisinin VI, Arkhipova AL, Glazko TT, Kovalchuk SN, Kosovsky GY (2018) Intestinal amino acid and peptide transporters in broiler are modulated by dietary amino acids and protein. Amino acids 50(2):353–357. https://doi.org/10.1007/s00726-017-2510-6
Ostaszewska T, Dabrowski K, Kamaszewski M, Grochowski P, Verri T, Rzepkowska M, Wolnicki J (2010a) The effect of plant protein-based diet supplemented with dipeptide or free amino acids on digestive tract morphology and PepT1 and PepT2 expressions in common carp (Cyprinus carpio L.). Comp Biochem Physiol A-Mol Integr Physiol 157(2):158–169. https://doi.org/10.1016/j.cbpa.2010.06.162
Ostaszewska T, Kamaszewski M, Grochowski P, Dabrowski K, Verri T, Aksakal E, Szatkowska I, Nowak Z, Dobosz S (2010b) The effect of peptide absorption on PepT1 gene expression and digestive system hormones in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A-Mol Integr Physiol 155(1):107–114. https://doi.org/10.1016/j.cbpa.2009.10.017
Ostaszewska T, Dabrowski K, Kamaszewski M, Kwasek K, Grodzik M, Bierla J (2013) The effect of dipeptide, Lys-Gly, supplemented diets on digestive tract histology in juvenile yellow perch (Perca flavescens). Aquacult Nutr 19(1):100–109. https://doi.org/10.1111/j.1365-2095.2012.00948.x
Peres H, Oliva-Teles A (2005) The effect of dietary protein replacement by crystalline amino acid on growth and nitrogen utilization of turbot Scophthalmus maximus juveniles. Aquaculture 250:755–764. https://doi.org/10.1016/j.aquaculture.2005.04.046
Perez-Velazquez M, Gatlin DM III, González-Félix ML, García-Ortega A (2018) Partial replacement of fish meal and fish oil by algal meals in diets of red drum Sciaenops ocellatus. Aquaculture 487:41–50. https://doi.org/10.1016/j.aquaculture.2018.01.001
Poncet N, Taylor PM (2013) The role of amino acid transporters in nutrition. Curr Opin Clin Nutr Metab Care 16(1):57–65. https://doi.org/10.1097/MCO.0b013e32835a885c
Rahimnejad S, Lee KJ (2014) Comparison of free and dipeptide lysine utilization in diets for juvenile olive flounder Paralichthys olivaceus. Fish Aquat Sci 17(4):433–439. https://doi.org/10.5657/FAS.2014.0433
Rimoldi S, Bossi E, Harpaz S, Cattaneo AG, Bernardini G, Saroglia M, Terova G (2015) Intestinal B0AT1 (SLC6A19) and PEPT1 (SLC15A1) mRNA levels in European sea bass (Dicentrarchus labrax) reared in fresh water and fed fish and plant protein sources. J Nutr Sci 4:1–13. https://doi.org/10.1017/jns.2015.9
Romano A, Barca A, Storelli C, Verri T (2014) Teleost fish models in membrane transport research: the PEPT1 (SLC15A1) H+-oligopeptide transporter as a case study. J Physiol 592(5):881–897. https://doi.org/10.1113/jphysiol.2013.259622
Swanepoel JC, Goosen NJ (2018) Evaluation of fish protein hydrolysates in juvenile African catfish (Clarias gariepinus) diets. Aquaculture 496:262–269. https://doi.org/10.1016/j.aquaculture.2018.06.084
Thamotharan M, Gomme J, Zonno V, Maffia M, Storelli C, Ahearn GA (1996a) Electrogenic, proton-coupled, intestinal dipeptide transport in herbivorous and carnivorous teleosts. Am J Physiol-Regulatory Integrative Comp Physiol 270(39):R939–R947. https://doi.org/10.1152/ajpregu.1996.270.5.R939
Thamotharan M, Zonno V, Storelli C, Ahearn GA (1996b) Basolateral dipeptide transport by the intestine of the teleost Oreochromis mossambicus. Am J Physiol-Regulatory Integrative Comp Physiol 270(5):R948–R954. https://doi.org/10.1152/ajpregu.1996.270.5.R948
Thwaites DT, Anderson CM (2011) The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br J Pharmacol 164(7):1802–1816. https://doi.org/10.1111/j.1476-5381.2011.01438.x
Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflügers Arch-Eur J Physiol 447(5):532–542. https://doi.org/10.1007/s00424-003-1086-z
Verri T, Barca A, Pisani P, Piccinni B, Storelli C, Romano A (2017) Di-and tripeptide transport in vertebrates: the contribution of teleost fish models. J Comp Physiol B 187(3):395–462. https://doi.org/10.1007/s00360-016-1044-7
Wei Y, Liang M, Mu Y, Zheng K, Xu H (2016) The effect of ultrafiltered fish protein hydrolysate level on growth performance, protein digestibility and mRNA expression of PepT1 in juvenile turbot (Scophthalmus maximus L.). Aquacult Nutr 22(5):1006–1017. https://doi.org/10.1111/anu.12319
Wei Y, Liang M, Xu H (2020a) Fish protein hydrolysate affected amino acid absorption and related gene expressions of IGF-1/AKT pathways in turbot (Scophthalmus maximus). Aquacult Nutr 26(1):145–155. https://doi.org/10.1111/anu.12976
Wei Y, Xu H, Liang M (2020b) Amino acid absorption and protein synthesis responses of turbot Scophthalmus maximus to lysine and leucine in free, dipeptide and tripeptide forms. Aquacult Nutr 26(2):358–367. https://doi.org/10.1111/anu.12998
Xu D, He G, Mai K, Zhou H, Xu W, Song F (2016) Postprandial nutrient-sensing and metabolic responses after partial dietary fish meal replacement by soyabean meal in turbot (Scophthalmus maximus L.). Br J Nutr 115(3):379–388. https://doi.org/10.1017/S0007114515004535
Yun H, Park G, Ok I, Katya K, Hung S, Bai SC (2016) Determination of the dietary lysine requirement by measuring plasma free lysine concentrations in rainbow trout Oncorhynchus mykiss after dorsal aorta cannulation. Fish Aquat Sci 19(1):1–7. https://doi.org/10.1186/s41240-016-0004-1
Zhang Y, Dabrowski K, Hliwa P, Gomulka P (2006) Indispensable amino acid concentrations decrease in tissues of stomachless fish, common carp in response to free amino acid-or peptide-based diets. Amino Acids 31(2):165–172. https://doi.org/10.1007/s00726-006-0345-7
Zhang S, Qiao S, Ren M, Zeng X, Ma X, Wu Z, Thacker P, Wu G (2013) Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 45(5):1191–1205. https://doi.org/10.1007/s00726-013-1577-y
Funding
This research was supported by National Natural Science Foundation of China (31672663, 31902387, 31972803), China Agriculture Research System (CARS-47-G15, Government of China), and Central Public-interest Scientific Institution Basal Research Fund, CAFS (2020TD48).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental methods and procedures used in this study were approved by Institutional Animal Care and Use Committee of the Yellow Sea Fisheries Research Institute.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, Y., Li, B., Xu, H. et al. Effects of lysine and leucine in free and different dipeptide forms on the growth, amino acid profile and transcription of intestinal peptide, and amino acid transporters in turbot (Scophthalmus maximus). Fish Physiol Biochem 46, 1795–1807 (2020). https://doi.org/10.1007/s10695-020-00828-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00828-2