Abstract
Persian sturgeon (Acipenser persicus) is an endangered species and genetic resource banking such as gametes and embryo preservation could be one of the most pursued conservation approaches. In this study, deleterious effects of the traditional cryopreservation technique and the effect of different doses of 2-hydroxypropyl-beta-cyclodextrin (HβCD) on thawed spermatozoa quality (motility duration and percentage) of Persian sturgeon (Acipenser persicus) were investigated from metabolic aspects of view. For cryopreserving, semen was diluted with Tris–HCl (100 mM) extenders containing 0, 5, 10, and 15 mM of HβCD in a ratio of 1:1 (semen/extenders). Semen-extenders were filled into 0.5-mL straws and were frozen with the vapor of liquid nitrogen, and then immersed into liquid nitrogen. Cryopreserved spermatozoa were thawed in water baths in 15 s. Two treatments with the highest and the lowest motility percentages (0 and 10 mM of HβCD) were chosen to reveal the extremes of the metabolites change range and were objected to 1H NMR spectroscopy. Univariate (ANOVA) and multivariate (PCA) analysis of the obtained metabolic profiles showed significant changes (P < 0.05) in metabolites. The use of 10 mM of HβCD was completely successful in the preservation of creatinine, glucose, guanidoacetate, O-phosphocholine, and N, N-dimethylglycine and probably their corresponding biochemical pathways, but it failed to preserve lactate, carnitine, betain, β-alanin, and trimethylamine N-oxide. It was also partially successful in preserving acetate, creatine, creatine phosphate, and glycine, all suggesting how HβCD can be effective as a cryoprotectant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persian sturgeon (Acipenser persicus) of the Caspian Sea is a commercially valuable and critically endangered species that was listed on CITES Appendix II in 1998 (Abed-Elmdoust et al. 2017). Sturgeons are producers of caviar which is considered a luxurious source of protein for human consumption (Niksirat et al. 2017). They also play important roles in the maintenance of ecological balance in the aquatic ecosystems (Pikitch et al. 2005).
Spawning populations of the wild Persian sturgeon are dramatically decreasing because of many disruptions in their ecological cycle caused by human activities. Due to the limited success in the artificial reproduction and habitat protection approaches, genetic resource banking such as gametes and embryo cryopreservation can be considered as the final choice for conservation (Sarvi et al. 2006).
Few reports are available aiming to improve Persian sturgeon semen cryopreservation (Aramli and Nazari 2014). Cryoprotectants including dimethyl sulfoxide (DMSO), methanol (MeOH), glycerol, ethylene glycol, propane-diol, and dimethyl acetamide are used in different studies and according to the reports, DMSO and MeOH are the most efficient ones for fish semen cryopreservation (Glogowski et al. 2002). On the other hand, DMSO is toxic to the cell and Aramli and Nazarib (2014) demonstrated that the increase in the concentration of DMSO from 8 to 15% reduces the percentage of motile cells and motility duration. Therefore, applying new approaches and efficient cryoprotectants are necessary to obtain more motile spermatozoa after thawing.
Freeze-thawing process induces damages that dramatically reduce cryopreserved spermatozoa viability and fertility (Jun et al. 2006). During freezing periods, phase separation and ice crystallization occur, and these phenomena cause an increase in the concentration of the solutes (Bhatnagar et al. 2007). Moreover, it is well known that chilling and freezing process change spermatozoa membrane fluidity (Canvin and Buhr 1989).
Hydroxypropyl-beta-cyclodextrin (HβCD) is a member of cyclodextrins (CDs) which are cyclic oligosaccharides, and it is the most common substance used in protein freeze-drying process in protein engineering (Serno et al. 2011), and there are some promising reports about its cryoprotection abilities in cryopreservation approaches. Zeng and Terada (2001) reported the protective effects of HβCD on the boar cryopreserved spermatozoa. In another study, HβCD in combination with cholesterol inhibits tyrosine phosphorylation increase in spermatozoa pigs that were under thermal shock (Galantino-Homer et al. 2006).
Investigation of changes in spermatozoa at molecular level such as comparative profiling of the quantified metabolites can reveal some of the cell functions and dysfunctions during cryopreservation (Nicholson et al. 1999; Lin et al. 2009). Many metabolites can be measured simultaneously using NMR (nuclear magnetic resonance) spectroscopy by either quantitative, or chemometrics approaches. In a study, 31P 1H NMR spectroscopy revealed how changes in high-energy phosphate compounds are effective in turbot (Psetta maxima) spermatozoa motility (Dreanno et al. 1999).
Metabolites can be quantified by comparing the NMR spectra of each metabolite to its pure compound spectra reference database in targeted profiling and in this way, spectral data is reduced to single concentrations for each compound while chemometrics approach is directly based on extensive spectral data. Compared to traditional spectral binning (chemometric analysis), targeted metabolite profiling is more stable in PCA-based pattern recognition and is more insensitive to water suppression, different relaxation times, and scaling factors (Smith et al. 1997; Wang et al. 1997; Sieczyński et al. 2015).
Although monitoring the changes in metabolite profile of the semen during cryopreservation can be very useful in designing a conservative strategy, there have been few reports on the literature. There has been a report of reduction in semen quality and increase in sperm DNA damage in relation to urinary metabolites of pyrethroid insecticides (Meeker et al. 2008). Profiling of the semen of Persian sturgeon also shows that fish antifreeze protein can be a good cryoprotectant in sperm cryopreservation (Abed-Elmdoust et al. 2017). The present study was conducted to profile the compounds related to energetics and some other compounds using 1H NMR spectroscopy in Persian sturgeon cryopreserved semen, and HβCD was used as a cryoprotectant to enhance the cryopreservation efficiency.
Materials and methods
Semen collection
Semen collection was done in Dr. Shahid Beheshti Artificial Sturgeon Propagation and Rearing Center (BASPRC), Rasht, Iran, during spawning season. Broodstock (100–155 cm total length and 15–22 kg weight) were caught from the Sefidrood River (Southern Caspian Sea) and transported to concrete tanks of at BASPRC where they spent a 28-day period for maturation before propagation. Water temperature was12–16 °C and dissolved oxygen was 8.4 ppm during this period. Sturgeon pituitary homogenized extract (SPE) was injected intramuscularly into the fish at doses of 50–70 mg per fish and 24 h after the injection the semen was collected. Restocking programs on wild Persian sturgeon is monopole, done by the governmental BASPRC institution and all ARRIVE, and National Institutes of Health Guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978) guidelines were followed in our experiments.
High-quality semen (motility percentage higher than 70) was selected from seven males and stored on ice. Five microliters of the semen was diluted with 195 μL of activation solution (3.5 mM NaCl, 12 mM Tris–HCl and pH = 8.5), and spermatozoa quality assessment (spermatozoa motility percentage and motility duration) was performed visually using light microscopy under × 400 magnification in room temperature condition (16–21 °C) in replicate of three.
Cryopreservation approach
In advance of the cryopreservation, the semen from seven males was individually diluted with extender containing Tris–HCl (100 mM), pH = 8 (Billard et al. 2004) at 1:1 ratio and split into three aliquots. Each aliquot further divided into five samples. Four samples received cryopreservation treatment containing 0, 5, 10, and 15 mM of HβCD respectively and one sample did not receive any cryopreservation treatment and cryoprotectant as a control group (called fresh semen). HβCD concentrations were chosen according to Zeng and Terada (2001). For freezing, semen was filled into 0.5 mL straws, and then the straws were frozen with the vapor of liquid nitrogen at 4 cm above the surface of liquid nitrogen. After 3 min, straws were immersed into the liquid nitrogen, and stored for 2 and 8 weeks. After storage, 40 °C water baths for 15 s was used as a thawing protocol (Glogowski et al. 2002).
Metabolite extraction
After thawing and semen quality evaluations, frozen cell suspensions were quickly crushed in a porcelain mortar with 3 M perchloric acid (HClO4) and centrifuged at 10,000×g for 15 min at 4 °C. The supernatant was adjusted to pH = 7.6 by the addition of 3 M KOH and centrifuged at 10,000×g for 15 min at 4 °C (Scott et al. 1988). Labconco lyophilizer set at a pressure of 10 mTorr, shelf temperature − 35 °C, condenser temperature − 110 °C, and drying time of 48 h lyophilized the resulting supernatant. Then, the samples were stored at − 80 °C until NMR measurements. The same procedures were done on extender-fresh semen aliquot.
Sample preparation and NMR measurements
Prior to NMR spectroscopy, 50 mg of the lyophilized supernatant (from each replicate) was suspended in 500 μl D2O (deuterium oxide) containing 1.5 mM of 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) (Sigma-Aldrich Co) as an internal reference and transferred to a 5-mm NMR tube for subsequent high-resolution NMR analysis (Weljie et al. 2006). One-dimensional (1-D) 1H NMR spectra were obtained using a 400 MHz NMR spectrometer (BrukerBiospin, Inc., Billerica, MA) equipped with a 5-mm probe and 11.0 μs (90) pulse, 8002.710 Hz spectral width and 1.5 s relaxation delay with pre-saturation of the residual water resonance. One hundred forty transients collected into 30 k data points require a 2-min acquisition time. All 1H NMR spectra were manually phased, baseline-corrected, and calibrated (DSS at 0.0 ppm) using Chenomx processor, and profiler tools from Chenomx NMR Suite software (version 7.6; ChenomxInc., Edmonton, Canada) were used for identification and quantification of metabolites (Wang et al. 1997).
Statistical analysis
Statistical analysis of spermatozoa motility parameters
For statistical analysis of motility percentage and motility duration of spermatozoa, IBM SPSS 21 software (IBM, Armonk, NY, USA) was used. Kolmogorov–Smirnov test was used to assess the normality of distributions. All percentage data were subjected to arcsine transformation before statistical analysis. Parameters were compared using one-way analysis of variance (ANOVA) and Tukey’s multiple-range test.
Statistical analysis of metabolite profiling
Before statistical analysis, obtained metabolite concentrations from cryopreserved treatments with the best and the worst spermatozoa motility results and fresh semen were normalized by median (for row-wise normalization) and range scaling (mean-centered and divided by the range of each variable for column-wise normalization) (Craig et al. 2006). Data normalization and univariate (ANOVA) and unsupervised multivariate pattern recognition (PCA) statistical analyses were performed using MetaboAnalyst, which is a web-based tool for processing, analyzing, and interpreting metabolomic data (Wyss and Kaddurah-Daouk 2000).
Results
Results from semen quality assessments
Motility duration and the percentage of the spermatozoa have been considered as quality indexes. On day 14, all treatments had significant differences with the control group (fresh spermatozoa) except 10 mM treatment (P < 0.05). The results also showed that motility of spermatozoa in all treatments was significantly lower than the control group (P < 0.05), but spermatozoa motility percentage at 10 mM of HβCD was significantly higher compared with other cryopreserved treatments (P < 0.05).
The results for the spermatozoa motility indices after storage for 14 and 56 days have been shown in Tables 1 and 2. Motility duration of thawed spermatozoa in day 56 was significantly lower than the fresh spermatozoa (P < 0.05) in all treatments, but there was not any significant difference in cryopreserved treatments (P > 0.05). Besides, spermatozoa motility percentage of all cryopreserved treatments was significantly lower than fresh spermatozoa (P < 0.05).
In general, the best results for the motility percentage and duration of the spermatozoa were in treatment with 10 mM and the worst results were in the treatment with 0 mM of HβCD; therefore, these two treatments were chosen and objected to 1H NMR spectroscopy to evaluate the maximum metabolite changing range during the cryopreservation process.
Results from NMR spectroscopy and metabolite profiling
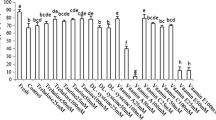
Identified and quantified metabolites obtained from one-dimensional 1H NMR spectroscopy from Persian sturgeon cryopreserved and the fresh semen (Fig. 1). Metabolites are divided into three categories (compounds related to spermatozoa energetics, lipid metabolism, and other detected metabolites), and significant differences among groups (p < 0.05) from ANOVA test are shown by different superscripts above the means ± SD column for each metabolite (Table 3).
1-D 400 MHz spectrum of Persian sturgeon semen. Cryopreserved semen with 0 mM of HbCD (a), 10 mM of HβCD (b), and fresh semen (c). Keys: (1) DSS, (2) acetate, (3) creatine, (4) creatine phosphate, (5) creatinine, (6) glucose, (7) guanidoacetate, (8) lactate, (9) O-phosphocholine, (10) carnitine, (11) betaine, (12) N, N-dimethylglycine, (13) Glycine, (14) b-alanine, and (15) trimethylamine N-oxide
Figure 2a shows the score plot of the first two principal components (PCs) with well-separated clear clusters corresponding to two different cryopreservation treatments and fresh semen showing the great impact of the cryopreservation process and HβCD as the cryoprotectant on metabolite concentrations. The loading plot in Fig. 2b shows the responsible metabolites in cluster derivation for three groups. Although two cryopreserved treatments with different HβCD concentrations are completely separated from each other, and the fresh sperm cluster, the treatment with 10 mM HβCD and the fresh semen cluster are much closer to each other (in PC1 axis), showing the importance of the role of HβCD in preservation of semen metabolome from alteration during cryopreservation and thawing process.
Principal component analysis (PCA) of the metabolite profile. a The PCA score plot distinguishes the metabolic profiles of droplet vitrified (DV), straw cryopreserved (SC), and fresh semen (FS) from seven males. Semen from each individual in treatments was subjected to NMR spectroscopy with replicate of three. b Loadings plot, important metabolites in the separation of the three groups
Results obtained from ANOVA tests revealed almost all identified and quantified metabolites had significant (P < 0.05) changes during cryopreservation process. The use of 10 mM of HβCD was completely successful in the preservation of creatinine, glucose, guanidoacetate, O-phosphocholine, and N, N-dimethylglycine and probably their corresponding biochemical pathways, but it failed to preserve lactate, carnitine, betain, β-alanin, and trimethylamine N-oxide. It can also be concluded that the use of 10 mM of HβCD was partially successful in preserving acetate, creatine, creatine phosphate, and glycine. Based on the results obtained from PCA loading plot, carnitine, guanidoacetate, glycine, O-phosphocholine, and N, N-dimethylglycine were the responsible metabolites in cluster derivation of the treatment with 0 mM HβCD and glucose, creatine, creatine phosphate, and creatinine were the responsible metabolites in 10 mM HβCD cluster derivation. The responsible metabolites for fresh sperm cluster derivation were acetate, lactate, betaine, β-alanin, and trimethylamine N-oxide.
Discussion
There have been many unsuccessful efforts in cryopreservation of the reproductive materials of the sturgeon species (Chao and Chiu Liao 2001; Williot et al. 2009). Investigations on possible metabolome alterations during cryopreservation and thawing process in cryopreserved gametes may give a good prospect of the pitfalls in the present techniques. Here, NMR spectroscopy was used to evaluate the effects of the cryopreservation and HβCD on metabolite profile of the endangered wild Persian sturgeon.
Based on quality assessments of the spermatozoa, motility duration of thawed spermatozoa using 10 mM HβCD at days 14 and 56 were longer than other cryopreserved groups, but it significantly reduced from day 14 to day 56. Motility percentage in all cryopreserved treatments were significantly lower than the fresh spermatozoa but the treatment with 10 mM HβCD had significantly higher motility percentage than the other cryopreserved treatments, and it remained unchanged from days 14 to 56 (Tables 1 and 2). It is believed that damages to the cell happen during the freezing and thawing processes, and storage duration is not effective in spermatozoa quality. Due to the reduction of the motility duration in 10 mM HβCD treatment from day 14 to day 56, this idea can be rejected but it is worth to note that the motility percentage was not affected by the storage period in this treatment. This suggests that the quality changes during the storage period in this treatment were not structural, and the compounds related to spermatozoa energetics faced partial disruption. Metabolite investigations that will be referred below support our idea and suggest the use of 10 mM of HβCD can be partially effective in metabolite cryopreservation.
In the present study, univariate results and patterns obtained from PCA analysis correspond to each other. In PCA score plot, a clear separation in different treatments shows severe effects of cryopreservation process on the metabolite profile of the semen. The treatments with 10 mM HβCD and the fresh spermatozoa are geometrically closer to each other (however, they are still completely apart) comparing to treatment without HβCD suggesting that the use of HβCD was effective in the prevention of the metabolite changes of the semen. All identified and quantified metabolites are divided into three categories (compounds related to spermatozoa energetics, lipid metabolism, and other detected metabolites) and discussed below.
Compounds related to spermatozoa energetics
Motility is the most significant ATP demanding process in the fish spermatozoa (Cosson et al. 2008). Creatine kinase action is one of the major ATPs providing reactions in the cell. Creatine kinase catalyzes the high-energy creatine phosphate to compensate the reduction in intracellular ATP concentration and/or to restore the ATP levels close to the physiological values (Saudrais et al. 1998). It is reported that with spermatozoa activation, creatine phosphate sharply decreases suggesting the importance of creatine phosphate as an energy resource for the spermatozoa (Dreanno et al. 1999). In the present study, significant decreases of creatine and creatine phosphate in both cryopreserved treatments show the destructive effects of the cryopreservation process on these two compounds. On the other hand, significantly higher concentrations of these two compounds in the treatment with 10 mM comparing to 0 mM of HβCD indicate that HβCD can successfully preserve these two important metabolites and probably creatine kinase structure and activity during the cryopreservation-thawing process.
Creatinine is formed by non-enzymatic dehydration of creatine, and this process is reversible in vitro depending on both pH and temperature. However, some studies using 15 N isotope labeling have disapproved the reversibility of this process in the body (Wyss and Kaddurah-Daouk 2000). High concentration of creatinine in cryopreserved treatment without HβCD suggests that the temperature changes during cryopreservation-thawing process enhance creatine dehydration and supports our findings for significant reduction of creatine in this treatment that is required in creatine phosphate synthesis (Huszar et al. 1988). The significantly higher concentration of creatinine in cryopreserved treatment with 10 mM HβCD suggests HβCD can be efficient in inhibiting this event.
Guanidinoacetate is a transitional compound in creatine synthesis. Amidinotransferase (AGAT) catalyzes the transfer of an amidino group from arginine to glycine and produces guanidinoacetate. In the next step, guanidinoacetate N-methyltransferase (GAMT) uses S-adenosylmethionine as a methyl group donator to guanidinoacetate to produce creatine (Wyss and Kaddurah-Daouk 2000; Persky and Brazeau 2001). Lower amounts of guanidinoacetate along with higher amounts of creatine in the fresh semen indicate that AGAT and GAMT are active while a higher concentration of guanidoacetate in the cryopreserved treatment without HβCD suggests cryopreservation–thawing process might disturb the action of these two enzymes. Lower amounts of guanidinoacetate in cryopreserved treatment with 10 mM HβCD without any significant difference compared to fresh semen suggest this cryoprotectant can completely prevent this interruption probably by the preservation of the structures and actions of AGAT and GAMT enzymes.
Monosaccharides, lactate, and pyruvate are used as the energy source for motility in spermatozoa of some species (Rodríguez-gil 2013). Glucose plays a role in spermatozoa motility of some teleost species, and its levels decrease after spermatozoa activation, but there is not any report for Acipenseriformes. In non-respiratory situations of the storage, the levels of lactate increase in equine spermatozoa because most of the glucose converts to lactate (Ponthier et al. 2014). This lactate later can form pyruvate by the action of lactate dehydrogenase (LDH). Positive effects of pyruvate on spermatozoa motility are reported when it was added to spermatozoa incubation medium. Therefore, lactate also can have a positive effect on spermatozoa motility elevation through pyruvate production (Lahnsteiner et al. 1993). In the present study, fresh semen had higher concentrations of lactate compared to both cryopreserved treatments suggesting its conversion in cryopreservation storage condition. No difference in lactate concentrations in both cryopreserved treatments suggests the failure of the HβCD to preserve this compound and related pathways. On the other hand, glucose levels in fresh spermatozoa and cryopreserved treatment with 10 mM HβCD had no significant differences while they were both significantly higher compared to cryopreserved without HβCD which shows this cryoprotectant was effective in glucose decomposition.
Acetate is a precursor in acetyl-CoA synthesis and is used in the tricarboxylic acid (TCA) cycle in aerobic respiration to produce energy and electron carriers (Schwer et al. 2006). It is reported ram and bull spermatozoa oxidize acetate in preference to glucose (Balmain et al. 1954); however; the ability of Persian sturgeon spermatozoa to metabolize acetate is under question. Concentrations of acetate in both cryopreserved treatments were lower compared to fresh semen suggesting that TCA cycle may suffer from some deficiencies in cryopreserved spermatozoa, and the significant difference between two cryopreserved treatments shows positive effects of HβCD in cryopreservation.
Compounds related to lipid metabolism
O-phosphocholine is the precursor of choline in glycine, serine, and threonine metabolism pathways (KEGG map 00260). It also plays a role as a choline providing compound to form cytidine-diphosphatidylcholine in glycerophospholipid metabolism pathway (KEGG map 000564). Glycerophospholipid is one of the most important of biochemical constituents of seminal plasma involved in metabolic activities of spermatozoa by its choline derivatives. Phosphocholine synthesis is by means of choline-CDP pathway and the action of choline kinase enzyme. This molecule is also a part of phosphatidylcholine, the only membrane phospholipid that does not contain glycerol in its structure and is involved in membrane signaling of the cell (Simons and Ikonen 1997; Jackowski et al. 2000). Here, the significant phosphocholine increases in treatment without HβCD may be due to alteration of the early stages of choline-CDP or the disruption of phosphatidylcholine in which 10 mM HβCD can completely prevent them.
Carnitine is synthesized by lysine and methionine metabolism, and it is responsible for fatty acid transfer from cytosol to mitochondria in lipid metabolism to provide energy to the cell. By transferring acyl groups of long-chain fatty acids to the mitochondria and breaking to acetyl-coA by means of beta-oxidation, this molecule provides energy in TCA cycle. It is also considered as an antioxidant that inhibits phospholipid peroxidation in the membrane and reduces oxidative stress and apoptosis (Rauchová et al. 2002). In humans, carnitine leads to higher spermatozoa concentrations and fertility (Cavallini et al. 2004). It also supports phospholipids and increases membrane sustainability (Cavallini et al. 2012). In the present study, the significantly higher levels of carnitine in both cryopreserved treatments comparing to the fresh semen is detected. The mechanism of such an increase due to freeze–thawing process is unclear, and further investigations are needed.
Other compounds
It has been reported that betaine has hypoxia cytoprotective and redox balancing properties. In plants, it has been demonstrated that betaine is also involved in reducing lipid peroxidation (Cushman 2001). In testicular tissues of the rat, betaine acts as an antioxidant and neutralizes hydroxyl radicals and lipid peroxidation in oxidative stress by increasing methylation reactions. Since cryopreservation process causes severe oxidative stress (Wang et al. 1997), lower betaine concentrations in treated semen may be due to its consumption in reactive oxidative species (ROS) caused by severe temperature changes in the cryopreservation-thawing process. Moreover, glycine is used in betaine structure (Frackman et al. 1998), and at this study, changes in glycine levels were vice versa compared to betaine levels. Significant higher levels of glycine in cryopreserved treatments also show decomposition of betaine during ROS process. It is worth to note that glycine can function as an organic intracellular osmolyte (Dawson and Baltz 1997; Summers and Biggers 2003), and it is also used as a cryoprotectant to cryopreserve spermatozoa in many species (He and Woods III 2004), so the increase of the glycine concentration can enhance the potential of cryoresistance of semen during cryopreservation.
β-alanine is used in coenzyme A precursors, and it is operative in redox balancing and hypoxia-like betaine. Trimethylamine N-oxide is a derivative of choline. These osmolytes can inhibit disruptions of protein molecules during physiological stresses (Yancey and Siebenaller 1999; Richards et al. 2010). A sharp decrease in β-alanine and trimethylamine N-oxide concentrations in cryopreserved treatments compared to fresh semen without any significant changes between them shows disruption of these metabolites during the cryopreservation–thawing process that may lead to some loss of osmolyte balancing properties of the cell; it also indicates that HβCD fails to protect them in cold temperatures.
N, N-dimethylglycine is an intermediate compound of converting choline to glycine. This compound also acts as an antioxidant in cells and prevents cell oxidation (Dagmar et al. 2011). There was a significant decrease in N, N-dimethylglycine concentrations in cryopreservation treatment without HβCD, but there was not any significant difference between cryopreserved treatment with 10 mM of HβCD and fresh spermatozoa. This implies the effect of HβCD in preserving the antioxidant effect of the N, N-dimethylglycine in cryopreserved spermatozoa.
Conclusion
In general, we report that HβCD as a new cryoprotectant is effective in cryopreservation of Persian persicus spermatozoa in a dose-dependent manner and the best dosage for its usage is estimated to be at 10 mM. Contrary to the general belief that the greatest changes occur during freezing and thawing processes, here, we also showed that the storage time is effective in frozen sperm quality. HβCD was completely successful in preserving some compounds such as glucose, guanidinoacetate, N, N-dimethylglycine, and O-phosphocholine; for some others such as creatine, creatine phosphate, creatinine, and acetate, it was partially effective. All these results may suggest how HβCD can be effective as a cryoprotectant, and the relevant biochemical pathways interrupted in cryopreservation process were discussed in here. We believe that the study of physical damages using electronic microscope can help us for further understanding of the deleterious effects of cryopreservation techniques alongside the results we obtained in the present study.
References
Abed-Elmdoust A, Farahmand H, Mojazi-Amiri B, Rafiee G, Rahimi R (2017) Metabolic changes in droplet vitrified semen of wild endangered Persian sturgeon Acipenser persicus (Borodin, 1997). Cryobiology 76:111–118. https://doi.org/10.1016/j.cryobiol.2017.03.008
Aramli MS, Nazari RM (2014) Motility and fertility of cryopreserved semen in Persian sturgeon, Acipenser persicus, stored for 30–60 min after thawing. Cryobiology 69:500–502. https://doi.org/10.1016/J.CRYOBIOL.2014.10.006
Balmain JH, Folley SJ, Glascock RF (1954) Relative utilization of glucose and acetate carbon for lipogenesis by mammary gland slices, studies with tritium, 13C and 14C. Biochem J 56:234–239
Bhatnagar BS, Bogner RH, Pikal MJ (2007) Protein stability during freezing: separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol 12:505–523. https://doi.org/10.1080/10837450701481157
Billard R, Cosson J, Noveiri S, Pourkazemi M (2004) Cryopreservation and short-term storage of sturgeon sperm, a review. Aquaculture 236:1–9. https://doi.org/10.1016/J.AQUACULTURE.2003.10.029
Canvin AT, Buhr MM (1989) Effect of temperature on the fluidity of boar sperm membranes. J Reprod Fertil 85:533–540. https://doi.org/10.1530/jrf.0.0850533
Cavallini G, Caracciolo S, Vitali G, Modenini F, Biagiotti G (2004) Carnitine versus androgen administration in the treatment of sexual dysfunction, depressed mood, and fatigue associated with male aging. Urology 63:641–646. https://doi.org/10.1016/J.UROLOGY.2003.11.009
Cavallini G, Biagiotti G, Lo Giudice C (2012) Association between Peyronie disease and low serum testosterone levels: detection and therapeutic considerations. J Androl 33:381–388. https://doi.org/10.2164/jandrol.111.012948
Chao N-H, Chiu Liao I (2001) Cryopreservation of finfish and shellfish gametes and embryos. Reprod Biotechnol Finfish Aquac:161–189. https://doi.org/10.1016/B978-0-444-50913-0.50011-4
Cosson J, Groison A-L, Suquet M, Fauvel C, Dreanno C, Billard R (2008) Marine fish spermatozoa: racing ephemeral swimmers. Reproduction 136:277–294. https://doi.org/10.1530/REP-07-0522
Craig A, Cloarec O, Holmes E, Nicholson JK, Lindon JC (2006) Scaling and normalization effects in NMR spectroscopic metabonomic data sets. Anal Chem 78:2262–2267. https://doi.org/10.1021/ac0519312
Cushman JC (2001) Osmoregulation in plants: implications for agriculture. Am Zool 41:758–769. https://doi.org/10.1093/icb/41.4.758
Dagmar S, Veronika H, Katrin S, Hans N, Erich E (2011) Studies on the chemical identity and biological functions of pangamic acid. Arzneimittelforschung 49:335–343. https://doi.org/10.1055/s-0031-1300424
Dawson KM, Baltz JM (1997) Organic osmolytes and embryos: substrates of the Gly and β transport systems protect mouse zygotes against the effects of raised osmolarity. Biol Reprod 56:1550–1558. https://doi.org/10.1095/biolreprod56.6.1550
Dreanno C, Cosson J, Suquet M, Seguin F, Dorange G, Billard R (1999) Nucleotide content, oxydative phosphorylation, morphology, and fertilizing capacity of turbot (Psetta maxima) spermatozoa during the motility period. Mol Reprod Dev 53:230–243. https://doi.org/10.1002/(SICI)1098-2795(199906)53:2<230::AID-MRD12>3.0.CO;2-H
Frackman BS, Kobs G, Simpson D et al (1998) Betaine and DMSO : enhancing agents for PCR. Promega Notes 65:9–12
Galantino-Homer HL, Zeng W, Megee SO, Dallmeyer M, Voelkl D, Dobrinski I (2006) Effects of 2-hydroxypropyl-β-cyclodextrin and cholesterol on porcine sperm viability and capacitation status following cold shock or incubation. Mol Reprod Dev 73:638–650. https://doi.org/10.1002/mrd.20437
Glogowski J, Kolman R, Szczepkowski M, Horváth Á, Urbányi B, Sieczyński P, Rzemieniecki A, Domagała J, Demianowicz W, Kowalski R, Ciereszko A (2002) Fertilization rate of Siberian sturgeon (Acipenser baeri, Brandt) milt cryopreserved with methanol. Aquaculture 211:367–373. https://doi.org/10.1016/S0044-8486(02)00003-0
He S, Woods LC III (2004) Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology 48:254–262. https://doi.org/10.1016/J.CRYOBIOL.2004.01.009
Huszar G, Vigue L, Corrales M (1988) Sperm Creatine phosphokinase activity as a measure of sperm quality in normospermic, variablespermic, and oligospermic men. Biol Reprod 38:1061–1066. https://doi.org/10.1095/biolreprod38.5.1061
Jackowski S, Wang J, Baburina I (2000) Activity of the phosphatidylcholine biosynthetic pathway modulates the distribution of fatty acids into glycerolipids in proliferating cells. Biochim Biophys Acta - Mol Cell Biol Lipids 1483:301–315. https://doi.org/10.1016/S1388-1981(99)00203-6
Jun L, Qinghua L, Shicui Z (2006) Evaluation of the damage in fish spermatozoa cryopreservation. Chin J Oceanol Limnol 24:370–377. https://doi.org/10.1007/BF02842852
Lahnsteiner F, Patzner RA, Weismann T (1993) Original article energy resources of spermatozoa of the rainbow trout Oncorhynchus mykiss (Pisces, Teleostei). Reprod Nutr Dev 33:349–360
Lin C-Y, Hung P, VandeVoort CA, Miller MG (2009) 1H NMR to investigate metabolism and energy supply in rhesus macaque sperm. Reprod Toxicol 28:75–80. https://doi.org/10.1016/J.REPROTOX.2009.03.005
Meeker JD, Barr DB, Hauser R (2008) Human semen quality and sperm DNA damage in relation to urinary metabolites of pyrethroid insecticides. Hum Reprod 23:1932–1940. https://doi.org/10.1093/humrep/den242
Nicholson JK, Lindon JC, Holmes E (1999) “Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29:1181–1189. https://doi.org/10.1080/004982599238047
Niksirat H, Andersson L, Golpour A, Chupani L, James P (2017) Quantification of egg proteome changes during fertilization in sterlet Acipenser ruthenus. Biochem Biophys Res Commun 490:189–193. https://doi.org/10.1016/J.BBRC.2017.06.019
Persky AM, Brazeau GA (2001) Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev 53:161–176. https://doi.org/10.1124/pharmrev1
Pikitch EK, Doukakis P, Lauck L, Chakrabarty P, Erickson DL (2005) Status, trends and management of sturgeon and paddlefish fisheries. Fish Fish 6:233–265. https://doi.org/10.1111/j.1467-2979.2005.00190.x
Ponthier J, de Tullio P, Blommaert D, Parrilla-Hernandez S, Deleuze S (2014) Sperm motility and lactate production at different sperm concentrations. J Equine Vet Sci 34:75–76. https://doi.org/10.1016/j.jevs.2013.10.049
Rauchová H, Koudelová J, Drahota Z, Mourek J (2002) Hypoxia-induced lipid peroxidation in rat brain and protective effect of carnitine and phosphocreatine. Neurochem Res 27:899–904. https://doi.org/10.1023/A:1020339530924
Richards T, Wang F, Liu L, Baltz JM (2010) Rescue of postcompaction-stage mouse embryo development from hypertonicity by amino acid transporter substrates that may function as organic osmolytes1. Biol Reprod 82:769–777. https://doi.org/10.1095/biolreprod.109.081646
Rodríguez-gil JE (2013) Energy management of mature mammalian spermatozoa. Success in Artificial Insemination - Quality of Semen and Diagnostics Employed. IntechOpen. https://doi.org/10.5772/51711
Sarvi K, Niksirat H, Mojazi Amiri B, Mirtorabi SM, Rafiee GR, Bakhtiyari M (2006) Cryopreservation of semen from the endangered Caspian brown trout (Salmo trutta caspius). Aquaculture 256:564–569. https://doi.org/10.1016/J.AQUACULTURE.2006.02.012
Saudrais C, Fierville F, Loir M, le Rumeur E, Cibert C, Cosson J (1998) The use of phosphocreatine plus ADP as energy source for motility of membrane-deprived trout spermatozoa. Cell Motil Cytoskeleton 41:91–106. https://doi.org/10.1002/(SICI)1097-0169(1998)41:2<91::AID-CM1>3.0.CO;2-I
Schwer B, Bunkenborg J, Verdin RO, Andersen JS, Verdin E (2006) Reversible lysine acetylation controls the activity of the mitochondrial enzyme acetyl-CoA synthetase 2. Proc Natl Acad Sci U S A 103:10224–10229. https://doi.org/10.1073/pnas.0603968103
Scott GK, Hayes PH, Fletcher GL, Davies PL (1988) Wolffish antifreeze protein genes are primarily organized as tandem repeats that each contain two genes in inverted orientation. Mol Cell Biol 8:3670–3675. https://doi.org/10.1128/MCB.8.9.3670
Serno T, Geidobler R, Winter G (2011) Protein stabilization by cyclodextrins in the liquid and dried state. Adv Drug Deliv Rev 63:1086–1106. https://doi.org/10.1016/J.ADDR.2011.08.003
Sieczyński P, Cejko BI, Grygoruk C, Glogowski J (2015) Cryopreservation of Siberian sturgeon (Acipenser baerii, Brandt, 1869) and sterlet (Acipenser ruthenus, Linnaeus, 1758) semen and its influence on sperm motility parameters assessed using a computer-assisted sperm analysis (CASA) system. J Appl Ichthyol 31:99–103. https://doi.org/10.1111/jai.12719
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572. https://doi.org/10.1038/42408
Smith AD, Anthony D, Bender DA, Smith GH (1997) Oxford dictionary of biochemistry and molecular biology. Oxford University Press, Oxford
Summers MC, Biggers JD (2003) Chemically defined media and the culture of mammalian preimplantation embryos: historical perspective and current issues. Hum Reprod Update 9:557–582. https://doi.org/10.1093/humupd/dmg039
Wang AW, Zhang H, Ikemoto I, Anderson DJ, Loughlin KR (1997) Reactive oxygen species generation by seminal cells during cryopreservation. Urology 49:921–925. https://doi.org/10.1016/S0090-4295(97)00070-8
Weljie AM, Newton J, Mercier P et al (2006) Targeted profiling: quantitative analysis of 1H NMR metabolomics data. https://doi.org/10.1021/AC060209G
Williot P, Rochard E, Rouault T, Kirschbaum F (2009) Acipenser sturio recovery research actions in France. In: Biology. Conservation and Sustainable Development of Sturgeons. Springer Netherlands, Dordrecht, pp 247–263
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213. https://doi.org/10.1152/physrev.2000.80.3.1107
Yancey PH, Siebenaller JF (1999) Trimethylamine oxide stabilizes teleost and mammalian lactate dehydrogenases against inactivation by hydrostatic pressure and trypsinolysis. J Exp Biol 202:3597–3603
Zeng WX, Terada T (2001) Protection of boar spermatozoa from cold shock damage by 2-hydroxypropyl-beta-cyclodextrin. Theriogenology 55:615–627. https://doi.org/10.1016/S0093-691X(01)00430-7
Acknowledgments
We would like to thank Mr. Abbas Alizadeh who provided us laboratory facilities at Dr. Beheshti Artificial Sturgeon Propagation and Rearing Center (BASPRC) and Dr. Ahmad Imani from University of Urmia for their valuable advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahimi, R., Farahmand, H., Mirvaghefi, A. et al. 1H NMR metabolic profiling of the cryopreserved spermatozoa of the wild endangered Persian sturgeon (Acipenser persicus) with the use of beta-cyclodextrin as an external cryoprotectant. Fish Physiol Biochem 45, 1029–1040 (2019). https://doi.org/10.1007/s10695-019-00615-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00615-8