Abstract
The present study was to evaluate the effects of six antioxidants on frozen-thawed sperm motility, viability, membrane integrity and mitochondrial function in red seabream (Pagrus major) by computer-assisted sperm analysis system and flow cytometry, respectively. All the parameters tested in this study were determined using one-way ANOVA and identified using the SNK test (P < 0.05). The results demonstrated that on the first day, the highest motility and longevity occurred in 100 mM trehalose (78.34 ± 3.41 %, 29 ± 4.00 days) and 50 mM taurine (77.46 ± 1.54 %, 29.33 ± 4.04 days), followed by 25 mM vitamin C (79.03 ± 5.37 %, 17 ± 1.00 days), 25 mM vitamin E (69.64 ± 1.64 %, 27.67 ± 1.53 days) and 25 mM vitamin A (78.89 ± 2.81 %, 9.33 ± 1.53 days), which were all higher than frozen-thawed sperm without antioxidant (control) (66.80 ± 5.55, 5.67 ± 1.15 days). Especially, the percentages of class A sperm with the addition of 100 mM trehalose (40.39 ± 5.20 %) and 50 mM taurine (37.78 ± 3.22 %) were significantly improved compared to the control (19.63 ± 5.44 %). The viability of all groups on the third and sixth day showed a similar trend. Moreover, during the 4 °C storage process, the decrease of frozen-thawed sperm motility was closely associated with the decrease in membrane integrity and mitochondrial function. In conclusion, the present study indicated that antioxidant (100 mM trehalose and 50 mM taurine) provided the most pronounced protective effect in improving frozen-thawed quality of red seabream sperm. The addition of antioxidant may be capable of scavenging the ROS generated during the cryopreservation process and 4 °C storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryopreservation of sperm is a useful technique for the guarantee of a constant supply of sperm, the conservation of genetic diversity and genetic improvement of resources (Cabrita et al. 2010). Damage usually occurs during freezing and thawing due to the ice crystal formation, osmotic stress, cryoprotectant toxicity and so on (Cosson et al. 2008). Cellular damage may greatly decrease motility, impair velocity and reduce fertilizing capacity. Computer-assisted sperm analysis (CASA) as well as flow cytometry has been used to characterize different subpopulations of sperm in fish to evaluate the sperm quality (Beirão et al. 2011; De Baulny et al. 1997), which can provide rapid, precise information regarding the viability of thousands of individual sperm cell. However, the exact mechanism of cryodamage has not yet been completely understood.
With the rapid development of sperm cryopreservation, some researches have been conducted on antioxidant supplementation in vitro to improve techniques for sperm storage and cryopreservation (Bucak et al. 2007; Cabrita et al. 2011; Gadea et al. 2013; Hagedorn et al. 2012; Pena et al. 2003). Oxidative stress is a phenomenon associated with increased rate of oxidation of cellular components and excessive production of ROS (Aitken et al. 1996; Alvarez and Storey 1982). In mammalians, several negative effects of ROS on sperm quality have been reported (Peris et al. 2007; Saleh and Hcld 2002; Sikka et al. 1995), such as lipid peroxidation of sperm cell membrane, damage of membrane structure, influence of the mitochondrial function and loss of fertilization capacity (Shiva et al. 2011). Even some researchers suggested that oxidative damage to mitochondrial DNA and membrane structure might be a factor of major importance to explain the impaired fertility and motility of cryopreserved sperm (Fernãndez-Santos et al. 2008; Sharma and Agarwal 1996). In marine fishes, little is known about the protective effects of antioxidant systems on sperm cryopreservation, while in case of fresh water teleost sperm, oxidant and antioxidant status of seminal plasma and sperm have been detected (Lahnsteiner and Mansour 2010; Shaliutina-Kolešová et al. 2013). Studies have demonstrated the important role of the antioxidants for maintaining the motility and the genetic integrity of sperm (Cabrita et al. 2011). The physiological functions of seminal plasma proteins in prolongation and stabilization of sperm viability have been investigated in rainbow trout (Oncorhynchus mykiss) (Lahnsteiner et al. 2004). However, dilution in the extender before cryopreservation decreased the concentration of those components in seminal plasma, diminishing the antioxidant protection of sperm (Martínez-Páramo et al. 2009). For brown trout sperm incubation in vitro (Lahnsteiner et al. 2010a, b), uric acid and catalase increased the sperm motility, sperm membrane integrity and decreased the sperm lipid peroxidation in comparison with the control. The exact mechanism of antioxidant molecules could improve sperm quality after thawing is not yet completely understood. The addition of antioxidant may be capable of neutralizing ROS and maintaining the balance of the production and scavenging of ROS generated during the cryopreservation process.
A great variety of antioxidant substances, including vitamins, enzymes, trehalose, taurine and other free radical scavengers, have been used in sperm cryopreservation (Bucak et al. 2007; Hagedorn et al. 2012; Gadea et al. 2013; Pena et al. 2003). However, the effect of each antioxidant was species-specific (Cabrita et al. 2011), which depended on the type of molecule and concentration used for each species. The objectives of this study were to investigate the effect of the addition of six antioxidants on frozen-thawed sperm motility, membrane integrity and mitochondrial function, and to find the suitable antioxidants to improve red seabream sperm cryopreservation techniques.
Materials and methods
Sperm collection
Naturally mature red seabream (Pagrus major) males were maintained from Qingdao hatchery during the spawning season (from the end of March to the middle of May). About 20 males (weighing 3–4 kg individually, 10 years old) were cultivated in a 20-m3 concrete rearing pond with flow-through seawater. Prior to handling, fish were first anesthetized in a 0.003 % eugenol bath. Sperm was collected into 10-ml centrifuge tube by gently hand-stripping the abdomen of three males. Extreme care was taken to avoid contamination of sperm with seawater, blood, urine and feces. Sperm was kept on crushed ice and transported to the laboratory for further use. The male was chosen randomly, and all the fish were healthy enough. To avoid the quality differences among the sperm of different batches, only the sperm with motility >90 % was used in the experiment, and the motility of sperm was checked using Nikon-YS-100 light microscope (Nikon Corporation, Tokyo, Japan) at 200× magnification.
General procedure for sperm freezing and thawing
The general procedure for sperm freezing and thawing was performed using the method of Liu et al. (2007). The collected sperm (400 μL) were diluted 1:3 (sperm: extender + cryoprotectant) (v/v) with 15 % DMSO containing trehalose (25, 50, 100 mM), taurine (25, 50, 100 mM), DL-cysteine (25, 50, 100 mM), vitamin A (25, 50, 100 mM), vitamin E (25, 50, 100 mM) and vitamin C (25, 50, 100 mM). The sperm concentration was about 1010 cells/mL. The samples (1.6 mL) were mixed thoroughly and placed in 2 mL cryovials. And the cryovials were transferred to a Kryo-360-1.7 programmable freezer (Planer Plc., Middlesex, UK) for freezing. Sperm samples were equilibrated at 0 °C for 5 min in the chamber of the programmer freezer, frozen from 0 to −150 °C at a cooling rate of 20 °C min−1 and then transferred immediately into liquid nitrogen. The cryopreserved sperm were thawed in a 37 °C water bath for 90–110 s and stored at 4 °C till used. The control was defined as the frozen-thawed sperm without antioxidant. Biological replicates were three in each study and three males per cryopreservation treatment. Each treatment had three replicates.
Analysis of sperm parameters
Analysis of frozen-thawed sperm motion parameters was determined using a computer-assisted sperm analysis (CASA) system. The CASA-derived motility characteristics were motile sperm (%), average path velocity (VAP; μm/s), curvilinear velocity (VCL; μm/s), straight line velocity (VSL; μm/s), linearity of the curvilinear trajectory (Lin ratio of VSL/VCL, %) (Kime et al. 2001) and longevity (d) (The longevity was defined as the time that the inactivated frozen-thawed sperm maintained viability in 4 °C).
Briefly, the frozen-thawed sperm was diluted and activated in natural seawater at a ratio of 1:250, and fresh sperm was activated at a ratio of 1:1,000. An aliquot of activated sperm (20 μL) was placed into a special microscopic sample chamber under a 20× negative phase-contrast objective (Nikon E200, Tokyo, Japan); 10 s after activation, sperm motility was analyzed with a computer-assisted sperm motion analysis system (CASASQH-Z, Tsinghua Tongfang Inc., Beijing, China) at room temperature (18–20 °C). According the average path velocity (VAP), sperm were divided into four classes, with a >100 μm/s for A class, 50–100 μm/s for B class, 20–50 μm/s for C class and <20 μm/s for D class. Six antioxidants were investigated and trehalose (100 mM), and taurine (50 mM) were used in the following study.
Fluorescent staining and flow cytometrical analysis
The staining method used was slightly modified from that described for trout sperm (De Baulny et al. 1997). An aliquot of frozen-thawed sperm was incubated for 20 min (in the dark, temperature 4 °C) with 5 μg/ml of Rhodamine 123 (Rh123, Sigma Chemical Co., St. Louis, MO, USA). Thereafter, sperm were incubated for 45 min. Samples were diluted (final concentration, 106 cells/mL) and counterstained with 5 μg/ml of propidium iodide (PI, Sigma Chemical Co.). After 10 min, sperm samples were analyzed with flow cytometry (FAC Svantage SE flow cytometer; Becton–Dickinson, Mountain View, CA, USA). Sperm populations were identified according to their relative red and green fluorescence (staining with PI and Rh123, respectively). Sperm with red (stained with PI) DNA were interpreted as having a damaged plasma membrane, whereas those that were green (stained with Rh123) were interpreted as having intact mitochondrial function. Sperm that were only red (damaged plasma membrane and lacking mitochondrial function), were localized in Region 1, whereas those that were only green (intact membrane and functional mitochondria), were localized in Region 3. Sperm with both red and green fluorescence (damaged plasma membrane and functional mitochondria) were localized in Region 4, and those with no staining (intact plasma membrane, but no mitochondrial activity) were localized in Region 2.
Biochemical assays
Biochemical assays were performed immediately after thawing. The sperm from each sample were centrifuged at 600g for 10 min at 4 °C, the pellet was washed by ice-cold Hanks three times. After centrifugation, the pellet was incubated with 1 mL 0.2 % Triton X-100 for 20 min at 4 °C. The supernatant was collected by centrifugation at 2,000g for 10 min and kept in 4 °C until enzyme determination. Glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) activities and malondialdehyde (MDA) level were analyzed with a spectrophotometric method using GSH kit, SOD kit, CAT kit and MDA kit (Nanking Jiancheng Biological Engineering Research Institute, China).
Statistical analysis
Statistical analysis was carried out using the software package SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA), and data were expressed as mean ± SD (P < 0.05). Significant differences between treatments for the motility, viability and longevity of the frozen-thawed sperm were determined using one-way ANOVA and identified using the SNK test (P < 0.05).
Results
Physiological characteristics
The motility (87.58 ± 2.88 %) of fresh sperm was higher than those of all frozen-thawed groups (from 3.92 ± 2.32 to 79.03 ± 5.37). On the first day, frozen-thawed motility in the 100 mM trehalose (78.34 ± 3.41 %) and 50 mM taurine (77.46 ± 1.54 %) was higher (P < 0.05) than the control (66.80 ± 5.55 %), while the vitamin A and vitamin E groups (50, 100 mM) were lower (Fig. 1). In the sperm viability classification analysis, the percentage of class A sperm decreased significantly before and after cryopreservation. The percentage of class A sperm with the addition of 100 mM trehalose (40.39 ± 5.20) or 50 mM taurine (37.78 ± 3.22) improved significantly compared to the control (19.63 ± 5.44) (P < 0.05), which were still lower than that of fresh sperm (53.75 ± 7.21). The CASA-derived viability characteristics of sperm classified according to the VAP is presented in Table 1. There were no significant differences (P < 0.05) among fresh sperm, frozen-thawed sperm (trehalose 100 mM and taurine 50 mM) and control, except for class A of fresh group. On the third day, frozen-thawed motility in the 100 mM trehalose group (73.17 ± 2.7 %) and 50 mM taurine (74.57 ± 0.61 %) was higher (P < 0.05) than those of the fresh sperm (66.31 ± 8.39 %) and control (40.19 ± 6.75 %) (Fig. 2). A similar trend was also observed on the sixth day (P < 0.05) (Fig. 3). Moreover, the longevity of fresh and frozen-thawed sperm with 100 mM trehalose (29 ± 4 days) and 50 mM taurine (29.33 ± 4.04 days) was higher (P < 0.05) than that of the control (5.67 ± 1.15 days) (Fig. 4). According to the motility and longevity, 100 mM trehalose and 50 mM taurine were selected and used for the following experiments.
Motility of fresh and frozen-thawed sperm on the first day (day 1) in red seabream. Six additives (25, 50, 100 mM trehalose, taurine, DL-cysteine, vitamin A, vitamin E, vitamin C) were used to analyze their protective effects on frozen-thawed sperm. The control was defined as the frozen-thawed sperm without antioxidant. The parameters tested were determined using one-way ANOVA and identified using the SNK test (P < 0.05). Columns with the same letters are not significantly different (P < 0.05). The values were given as mean ± SD, N = 3. Each treatment had three replicates
Motility of fresh and frozen-thawed sperm on the third day (day 3) in red seabream. The parameters tested were determined using one-way ANOVA and identified using the SNK test (P < 0.05). The fresh and frozen-thawed sperm were stored at 4 °C till the third day for motility analysis with CASA. Columns with the same letters are not significantly different (P < 0.05). The values were given as mean ± SD, N = 3. Each treatment had three replicates
Motility of fresh and frozen-thawed sperm on the sixth day (day 6) in red seabream. The parameters tested were determined using one-way ANOVA and identified using the SNK test (P < 0.05).The fresh and frozen-thawed sperm were stored at 4 °C till the third day for motility analysis with CASA. Columns with the same letters are not significantly different (P < 0.05). The values were given as mean ± SD, N = 3. Each treatment had three replicates
Longevity of fresh and frozen-thawed sperm in red seabream. The longevity was defined as the time that the frozen-thawed sperm maintained viability (motility ≥ 95 %) in 4 °C. The parameters tested were determined using one-way ANOVA and identified using the SNK test (P < 0.05). Columns with the same letters are not significantly different (P < 0.05). The values were given as mean ± SD, N = 3. Each treatment had three replicates
Analysis of frozen-thawed sperm quality by flow cytometry
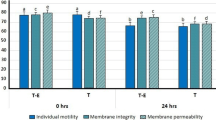
Sperm populations were divided into four distinct regions according to their relative green and red fluorescence after staining with PI and Rh123 as shown in Fig. 5. At the first day, 81.63 % of the frozen-thawed sperm had an intact membrane and functional mitochondria (Region 3); whereas for frozen-thawed sperm with the addition of 100 mM trehalose and 50 mM taurine to the cryoprotectant, the percentage of sperm had an intact membrane and functional mitochondria was 86.18 and 84.18 %, respectively. As time goes on, the percentages were 58.80, 76.16, 80.07 and 8.36, 76.16, 78.42 %, respectively at the third and sixth day. The percentage of the 4 regions of the control and frozen-thawed sperm with the addition of 100 mM trehalose and 50 mM taurine at 1, 3 and 6 days after thawing are presented in Table 2.
Flow-cytometric dot plots (Rh123 and PI fluorescence) of the frozen/thawed control (a) and frozen-thawed sperm with the addition of 100 mM trehalose (b) and 50 mM taurine (c) to the cryoprotectant at day 6. Region 1, sperm with a damaged plasma membrane and abnormal mitochondrial function. Region 2, sperm with an intact plasma membrane but lacking mitochondrial function. Region 3, sperm with an intact plasma membrane and functional mitochondria. Region 4, sperm with a damaged plasma membrane and functional mitochondria
Assays of antioxidant system
The effects of the six antioxidants on antioxidant activities and lipid peroxidation in thawed red seabream sperm are shown in Table 3. The addition of antioxidants did not cause significant differences in levels of SOD, CAT, GSH and MDA compared to the control (P > 0.05).
Discussion
Many considerable improvements have been achieved in sperm cryopreservation during the past decades of research, however, cryodamages still occurred and influenced the sperm quality during the process of sperm freezing and thawing. In recent years, some researchers suggested that excessive ROS production may be a significant contributing factor of cryodamage (Ozkavukcu et al. 2008; Zribi et al. 2010). Addition of antioxidants, such as trehalose, taurine, vitamin A, vitamin C, vitamin E and cysteine, effectively reduced the cryodamage as reported in ram (Aisen et al. 2002, Bucak et al. 2007), bull (Chen et al. 1993) and mouse sperm (Storey et al. 1998).
In the present study, the motility of frozen-thawed sperm of red seabream in 100 mM trehalose and 50 mM taurine groups was significantly improved (P < 0.05) than that in the control. Similarly, the supplementation of taurine or trehalose could significantly improve frozen-thawed sperm quality in Karan fries (Chhillar et al. 2012) and murine spermatogonial stem cells (Lee et al. 2013). Moreover, we found sperm motility was probably antioxidant dosage dependent, which has been demonstrated in zebrafish (Danio rerio) (Hagedorn et al. 2012), gilthead seabream and European seabass (Cabrita et al. 2011). Meanwhile, the longevity of frozen-thawed red seabream sperm in the 100 mM trehalose (29 days) and 50 mM taurine groups (29 days) was significantly prolonged (P < 0.05) compared to the control (5 days). In addition, the vitamin A and vitamin E groups (50 mM, 100 mM) did not significantly increase the sperm motility. Consistent with our study, Cabrita et al. (2011) observed that vitamins had no effect on motility in gilthead seabream and European seabass. However, vitamin E was effective in improving frozen-thawed motility of human (Askari et al. 1994) and boar sperm (Pena et al. 2003). Our results, together with data reported by other authors, suggested that vitamin E presented species-specific characteristics, and more effective in mammals compared to fish. This may be due to the different structural features and internal antioxidant system.
It is known that ROS were probably responsible for the decline in sperm quality in some species during cryopreservation. In this study, the results from flow cytometrical analysis presented that frozen-thawed sperm membrane integrity and mitochondrial function were improved by the addition of 100 mM trehalose or 50 mM taurine. It was well consistent with the results of physiological characteristics from CASA. Similar results were reported that the detrimental effects of ROS were reduced by addition of antioxidants to the extender in alpaca, boar and canine, respectively (Acosta et al. 2013; Pena et al. 2003; Michael et al. 2007).
The imbalance between ROS production and degradation caused oxidative injury to sperm membrane and a consequent impairment of related functional properties (Sharma and Agarwal 1996). ROS was believed to start the cascade of lipid peroxidation in the sperm membrane, while membrane lipid peroxidation was correlated with decreased sperm motility and membrane damage (Storey 1997). Probably, lipid peroxidation promoted sperm membrane alterations, which could be avoided by using antioxidant (Peris et al. 2007).
It was well demonstrated that antioxidant system of sperm in some species could play an important role in preventing oxidative damage (Bell et al. 1993). Some authors reported that SOD, GSH and CAT played an important role in preventing sperm lipid peroxidation of plasma membrane (Lahnsteiner et al. 2010a, b; Shiva et al. 2011; Chitra et al. 2001; Griveau et al. 1995). However, in the present work, SOD, CAT, GSH and MDA activity of the frozen-thawed sperm with addition of 100 mM trehalose or 50 mM taurine were not significantly different from the control. Similarly, Bucak et al. (2007) demonstrated that SOD, CAT and GSH content in ram sperm did not change significantly between frozen-thawed sperm with the addition of antioxidant and the control. These results indicated that the antioxidants provided protective effect to the frozen-thawed sperm not by influencing the antioxidant enzymatic system, but by directly neutralizing the excessive ROS.
In conclusion, the present study was to evaluate the effects of antioxidants, including trehalose, taurine, vitamin A, vitamin C, vitamin E and cysteine on the quality of frozen-thawed sperm in red seabream. The results indicated that some antioxidants provided pronounced protective effect in improving frozen-thawed sperm motility, viability, longevity, membrane integrity and mitochondrial function during the process of sperm cryopreservation and 4 °C storage. The exact nature of protective effect of the antioxidants in fish sperm is not been fully understood yet. Further studies are required to obtain more information on the mechanism of the antioxidant scavenging ROS and keeping sperm normal structure and function.
References
Acosta AS, Vargas SE, Cuya MV, Gonzalez JR, Gutierrez RS (2013) Effect of the addition of two superoxide dismutase analogues (Tempo and Tempol) to alpaca semen extender for cryopreservation. Theriogenology 79(5):842–846
Aisen EG, Medina VH, Venturino A (2002) Cryopreservation and frozen-thaweded fertility of ram semen frozen in different trehalose concentrations. Theriogenology 57(7):1801–1808
Aitken RJ, Buckingham DW, Carreras A, Irvine DS (1996) Superoxide dismutase in human sperm suspensions: relationship with cellular composition, oxidative stress, and sperm function. Free Radical Biol Med 21(4):495–504
Alvarez JG, Storey BT (1982) Spontaneous lipid peroxidation in rabbit epididymal sperm: its effect on sperm motility. Biol Reprod 27(5):1102–1108
Askari HA, Check JH, Peymer N, Bollendorf A (1994) Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process. Syst Biol Reprod Med 33(1):11–15
Beirão J, Cabrita E, Pérez-Cerezales S, Martínez-Páramo S, Herráez MP (2011) Effect of cryopreservation on fish sperm subpopulations. Cryobiology 62(1):22–31
Bell M, Wang R, Hellstrom WJ, Sikka SC (1993) Effect of cryoprotective additives and cryopreservation protocol on sperm membrane lipid peroxidation and recovery of motile human sperm. J Androl 14(6):472–478
Bucak MN, Ateşşahin AH, Varışlı Ö, Yüce A, Tekin N, Akçay A (2007) The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen: microscopic and oxidative stress parameters after freeze–thawing process. Theriogenology 67(5):1060–1067
Cabrita E, Sarasquete C, Martinez-Paramo S, Robles V, Beirao J, Perez-Cerezales S, Herraez MP (2010) Cryopreservation of fish sperm: applications and perspectives. J Appl Ichthyol 26(5):623–635
Cabrita E, Ma S, Diogo P, Martínez-Páramo S, Sarasquete S, Dinis MT (2011) The influence of certain aminoacids and vitamins on frozen-thawed fish sperm motility, viability and DNA fragmentation. Anim Reprod Sci 125(1):189–195
Chen Y, Foote RH, Brockett CC (1993) Effect of sucrose, trehalose, hypotaurine, taurine, and blood serum on survival of frozen bull sperm. Cryobiology 30(4):423–431
Chhillar S, Singh VK, Kumar R, Atreja SK (2012) Effects of Taurine or Trehalose supplementation on functional competence of cryopreserved Karan Fries semen. Anim Reprod Sci 135(1–4):1–7
Chitra KC, Sujatha R, Latchoumycandane C, Mathur PP (2001) Effect of lindane on antioxidant enzymes in epididymis and epididymal sperm of adult rats. Asian J Androl 3(3):205–208
Cosson J, Groison AL, Suquet M, Fauvel C, Dreanno C, Billard R (2008) Studying sperm motility in marine fish: an overview on the state of the art. J Appl Ichthyol 24(4):460–486
De Baulny BO, Le Vern Y, Kerboeuf D, Maisse G (1997) Flow cytometric evaluation of mitochondrial activity and membrane integrity in fresh and cryopreserved rainbow trout (Oncorhynchus mykiss) sperm. Cryobiology 34(2):141–149
Fernández-Santos MR, Dománguez-Rebolledo AE, Esteso MC, Garde JJ, Martı´nez-Pastor F (2008) Catalase supplementation on thawed bull sperm abolishes the detrimental effect of oxidative stress on motility and DNA integrity. Int J Androl 32:353–359
Gadea J, Molla M, Selles E, Marco MA, Garcia-Vazquez FA, Gardon JC (2011) Reduced glutathione content in human sperm is decreased after cryopreservation: effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology 62(1):40–46
Gadea J, Gumbao D, Gómez-Giménez B, Gardón JC (2013) Supplementation of the thawing medium with reduced glutathione improves function of frozen-thawed goat sperm. Reprod Biol 13(1):24–33
Griveau JF, Dumont E, Renard P, Callegari JP, Lannou DL (1995) Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human sperm. J Reprod Fertil 103(1):17–26
Hagedorn M, McCarthy M, Carter VL, Meyers SA (2012) Oxidative Stress in Zebrafish (Danio rerio) Sperm. PLoS One 7(6):e39397
Kime DE, Van Look KJW, McAllister BG, Huyskens G, Rurangwa E, Ollevier F (2001) Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp Biochem Physiol C: Toxicol Pharmacol 130(4):425–433
Lahnsteiner F, Mansour N (2010) A comparative study on antioxidant systems in semen of species of the Percidae, Salmonidae, Cyprinidae, and Lotidae for improving semen storage techniques. Aquaculture 307(1–2):130–140
Lahnsteiner F, Mansour N, Berger B (2004) Seminal plasma proteins prolong the viability of rainbow trout (Oncorynchus mykiss) sperm. Theriogenology 62(5):801–808
Lahnsteiner F, Mansour N, Plaetzer K (2010a) Antioxidant systems of brown trout (Salmo trutta f. fario) semen. Anim Reprod Sci 119(3–4):314–321
Lahnsteiner F, Mansour N, Plaetzer N (2010b) Antioxidant systems of brown trout (Salmo trutta f. fario) semen. Anim Reprod Sci 119(3):314–321
Lee YA, Kim YH, Kim BJ, Kim BG, Kim KJ, Auh JH, Schmidt JA, Ryu BY (2013) Cryopreservation in trehalose preserves functional capacity of murine spermatogonial stem cells. PLoS One 8(1):1–9
Liu QH, Li J, Xiao ZZ, Ding FH, Yu DD, Xu XZ (2007) Use of computer-assisted sperm analysis (CASA) to evaluate the quality of cryopreserved sperm in red seabream (Pagrus major). Aquaculture 263:20–25
Martínez-Páramo S, Martínez-Pastor F, Martínez-Rodríguez G, Herraez P and Cabrita E (2009) Antioxidant status in fresh and cryopreserved sperm from gilthead sea bream (Sparus aurata). In: Second International Workshop on Biology of Fish Gametes, 76–77
Michael A, Alexopoulos C, Pontiki E, Hadjipavlou-Litina D, Saratsis P, Boscos C (2007) Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine sperm. Theriogenology 68(2):204–212
Ozkavukcu S, Erdemli E, Isik A, Oztuna D, Karahuseyinoglu S (2008) Effects of cryopreservation on sperm parameters and ultrastructural morphology of human spermatozoa. J Assist Reprod Genet 25:403–411
Pena FJ, Johannisson A, Wallgren M, Rodriguez Martinez H (2003) Antioxidant supplementation in vitro improves boar sperm motility and mitochondrial membrane potential after cryopreservation of different fractions of the ejaculate. Anim Reprod Sci 78(1):85–98
Peris SI, Bilodeau JF, Dufour M, Bailey JL (2007) Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm. Mol Reprod Dev 74(7):878–892
Saleh RA, Hcld AA (2002) Oxidative stress and male infertility: from research bench to clinical practice. J Androl 23(6):737–752
Shaliutina-Kolešová A, Gazo I, Cosson J, Linhart O (2013) Comparison of oxidant and antioxidant status of seminal plasma and sperm of several fish species. Czech J Anim Sci 58(7):313–320
Sharma RK, Agarwal A (1996) Role of reactive oxygen species in male infertility. Urology 48(6):835–850
Shiva MS, Gautam AK, Verma Y, Shivgotra V, Doshi H, Kumar S (2011) Association between sperm quality, oxidative stress, and seminal antioxidant activity. Clin Biochem 44(4):319–324
Sikka SC, Rajasekaran M, Hellstrom WJ (1995) Role of oxidative stress and antioxidants in male infertility. J Androl 16(6):464–468
Storey BT (1997) Biochemistry of the induction and prevention of lipoperoxidative damage in human sperm. Mol Hum Reprod 3(3):203–213
Storey BT, Noiles EE, Thompson KA (1998) Comparison of glycerol, other polyols, trehalose, and raffinose to provide a defined cryoprotectant medium for mouse sperm cryopreservation. Cryobiology 37(1):46–58
Zribi N, Chakroun FN, El Euch H, Gargouri J, Bahloul A, Ammar Keskes L (2010) Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil Steril 93:159–166
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos. 31072212 and 41076100), Modern Agro-industry Technology Research System (nycytx-50), National High Technology R&D Project of China (863 Program; 2012AA10A402).
Author information
Authors and Affiliations
Corresponding author
Additional information
Qinghua Liu and Xueying Wang have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Liu, Q., Wang, X., Wang, W. et al. Effect of the addition of six antioxidants on sperm motility, membrane integrity and mitochondrial function in red seabream (Pagrus major) sperm cryopreservation. Fish Physiol Biochem 41, 413–422 (2015). https://doi.org/10.1007/s10695-014-9993-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-9993-9