Abstract
An 8-week feeding experiment was conducted to determine the effect of dietary betaine levels on the growth performance, antioxidant capacity, and lipid metabolism in high-fat diet-fed blunt snout bream (Megalobrama amblycephala) with initial body weight 4.3 ± 0.1 g [mean ± SEM]. Five practical diets were formulated to contain normal-fat diet (NFD), high-fat diet (HFD), and high-fat diet with betaine addition (HFB) at difference levels (0.6, 1.2, 1.8%), respectively. The results showed that the highest final body weight (FBW), weight gain ratio (WGR), specific growth rate (SGR), condition factor (CF), and feed intake (FI) (P < 0.05) were obtained in fish fed 1.2% betaine supplementation, whereas feed conversion ratio (FCR) was significantly lower in the same group compared to others. Hepatosomatic index (HSI) and abdominal fat rate (AFR) were significantly high in fat group compared to the lowest in NDF and 1.2% betaine supplementation, while VSI and survival rate (SR) were not affected by dietary betaine supplementation. Significantly higher (P < 0.05), plasma total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), aspartate transaminase (AST), alanine transaminase (ALT), cortisol, and lower high-density lipoprotein (HDL) content were observed in HFD but were improved when supplemented with 1.2% betaine. In addition, increase in superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) in 1.2% betaine inclusion could reverse the increasing malondialdehyde (MDA) level induced by HFD. Based on the second-order polynomial analysis, the optimum growth of blunt snout bream was observed in fish fed HFD supplemented with 1.2% betaine. HFD upregulated fatty acid synthase messenger RNA (mRNA) expression and downregulated carnitine palmitoyltransferase 1, peroxisome proliferator-activated receptor α, and microsomal triglyceride transfer protein mRNA expression; nevertheless, 1.2% betaine supplementation significantly reversed these HFD-induced effects, implying suppression of fatty acid synthesis, β-oxidation, and lipid transport. This present study indicated that inclusion of betaine (1.2%) can significantly improve growth performance and antioxidant defenses, as well as reduce fatty acid synthesis and enhance mitochondrial β-oxidation and lipid transportation in high-fat diet-fed blunt snout bream, thus effectively alleviating fat accumulation in the liver by changing lipid metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In general, feed is regarded as the main cost of the aquaculture industry. This is due to the dependence on high-cost protein sources such as fish meal which come from marine animals to meet the high dietary protein requirements of fish (Gui et al. 2010). As the demand for fish meal is increasing with the rapid development of the aquaculture industry, global yield of fish meal is failing to meet the production demand in the feeding industry. This situation has given rise to studies on finding alternative sources of high-quality additives to replace the high-cost fish meal. In recent years, several sources of additives such as plant protein, dietary lipid, and probiotic have been experimented on different fish species to either partially or completely replace the more expensive fish meal (Allen Davis and Arnold 2000; Aksnes et al. 2008; Ronnestad et al. 2003).
In fish nutrition, dietary lipids play a major role by supplying energy, essential fatty acids (EFAs), and phospholipids (Sargent et al. 1999; Watanabe 1982). As Li et al. (2012a) reports, increase in dietary lipid content can enhance feed efficiency and growth performance in fish. Thus, fat-rich diets have been extensively used in the intensive fish farming system. However, excess dietary lipid often leads to unwanted fat deposition in the liver (Du et al. 2006; Lu et al. 2013), resulting in high mortality rate, poor growth performance, and immune suppression of the fish (Bolla et al. 2011; Lu et al. 2014a). In order to prevent excessive lipid deposition, various ways have been researched by fish nutritionist. Some additives have been experimented and successfully used in controlling excess fat accumulation in the liver, and they have been shown to regulate the abnormal expression of key genes involved in lipid metabolism, i.e., berberine (Chen et al. 2016), choline (Zhou et al. 2015), zinc (Kang et al. 2009), and in grape seed proanthocyanidins (Quesada et al. 2009).
Betaine is a naturally occurring tertiary amine (trimethylglycine) present in animals, plants, and microorganisms, and it is a methyl derivative of the amino acid glycine with the chemical formula (CH3)3N + CH2COO- and a molecular weight of 117.2 (Patel and Mehta 2015). It is characterized as a methylamine because of its three chemically reactive methyl groups. The rich dietary sources include seafood, beets, broccoli, spinach, as well as grains such as wheat germ and bran. The main physiologic role of betaine is as an osmolyte and methyl donor (transmethylation), which, in turn, may be used for the synthesis of methionine, carnitine, phosphatidyl choline, and creatine, and plays a key role in protein and energy metabolism (Sheard and Zeisel 1989). As an osmolyte, betaine protects cells, proteins, and enzymes from environmental stress (e.g., low water, high salinity, or extreme temperature). Betaine has been found to protect the liver from the damaging effects of CCl4 (Junnila et al. 2000). It has been revealed that betaine regulates erythrocyte (red blood cell) membrane ATPases through conformational changes, which results in cell volume control (Moeckel et al. 2002). In mammals, numerous studies have documented the protective effects of betaine on antioxidant status (Ganesan et al. 2010), mitochondrial function (Ganesan et al. 2007a), protein and glycoprotein metabolism, and lipid metabolism in isoprenaline-induced myocardial infarction in Wistar rats (Ganesan et al. 2007b). Whereas in livestock, betaine supplementation in diet reduces fat deposition in pigs (Huang et al. 2008), meat ducks (Wang et al. 2000, 2004), and laying hens (Zou and Jian-Jun 2002). While in broilers, both abdominal adipose and percent abdominal adipose were decreased in chicken fed with diets containing 0.06% betaine (Esteve-Garcia and Mack 2000; Mcdevitt et al. 2000). Betaine supplementation has also proven to decrease the activities of acetyl-CoA carboxylase, fat acid synthase, malic enzyme, and the messenger RNA (mRNA) levels of fatty acid synthase (FAS) gene in abdominal adipose tissue in finishing pigs (Huang et al. 2008).
In aquaculture, betaine can protect cells against dramatic changes in osmotic pressure, since it is related to osmoregulation and methyl donation. Moreover, studies have shown a positive effect of betaine as a flavor component, acting as a dietary feeding attractant in feed leading to improved growth in some fish species such as Sparus auratus (Kolkovski et al. 1997), Morone saxatilis (Papatryphon and Soares Jr. 2000), Oreochromis niloticus (Kasper et al. 2002), Carassius auratus gibelio (Xue and Cui 2001), red sea bream (Goh and Tamura 1980), and with the European eel (Anguilla anguilla) (Mackie and Mitchell 2006). Moreover, dietary betaine has also shown to have sparing effect on the dietary requirement for choline and methionine in rainbow trout (Rumsey 1991).
Blunt snout bream (Megalobrama amblycephala), commonly known as Wuchang bream, is an herbivorous freshwater fish species native to China. It has also been introduced to North America (north Canada to southern Mexico), Africa, and Eurasia (Habte-Tsion et al. 2013). This fish has been recognized as a main aquaculture species in the Chinese freshwater polyculture system with high economic value species. In 2012, its production level was approximately 0.7 million tons (Ministry of Agriculture of the People’s Republic of China 2013). Due to its fast growth rate, adaptability to local environment conditions, high larval survival rate, compatibility with native species, disease resistance, and tender flesh, blunt snout bream has been regarded as a good candidate species for aquaculture (Zhou et al. 2008). Nevertheless, compared to a number of other commercially produced fishes, its artificial rearing often suffers from liver steatosis, which correlates directly with a high rate of mortality or poor growth (Lu et al. 2013). To better understand the biological processes of excess fat accumulated in liver in this species, and identify a good additive for therapeutic intervention, this study was carried out to determine the effect of dietary betaine supplementation on growth performance, antioxidant capacity, and lipid metabolism in fingerling blunt snout bream fed high-fat diet.
Materials and methods
Fish and the feeding trial
Healthy blunt snout bream fingerlings were obtained from Yangzhou Fish Hatchery (Jiangsu, China). Prior to the experiment, fish were acclimatized for 2 weeks during which they were fed with a commercial diet. After the acclimation, fish of similar size (4.3 ± 0.1 g [mean ± SEM]) were stocked into 15 tanks at a stocking density of 25 fish per tank. The experimental diets were assigned to the tanks in a completely randomized design and each replicated three times. Fish were hand-fed to apparent satiation three times daily (08:30, 12:30, and 16:30 h) for 8 weeks. During the entire experimental period, all the water quality parameters were monitored and kept within the optimum ranges as follows: water temperature ranged from 26 to 28 °C, dissolved oxygen (DO) of ≥6 mg/l, pH of 7.2–7.6, total ammonia nitrogen of 0.02–0.04 mg/l, and photoperiod of 12 h (dark/light). To further maintain good water quality, water was changed three times a week.
Experimental design and diets
All feed ingredients were analyzed for proximate composition, and the data obtained were used as a basis for the feed formulation. Fish meal, soybean meal, cottonseed meal, and rapeseed meal were used as protein sources. In each diet, equal portion of fish oil and soybean oil was used as lipid sources (Table 1). Wheat flour was used as carbohydrate source. Five practical diets were formulated to contain normal-fat diet (NFD), high-fat diet (HFD), and high-fat diet with betaine addition (HFB) at difference levels (0.6, 1.2, 1.8%), respectively. Feed ingredients were ground into fine powder then completely mixed and blended oil and sufficient water to form soft dough. And then, dough was pelleted and air dried. The pellets were stored at −20 °C until used. The ingredients and composition of the diets are given in Table 1.
Sampling and analysis
At the end of the feeding trial, fish were starved for 24 h to evacuate the alimentary tract contents prior to sampling. Thereafter, 15 fish from each replicate were anesthetized in diluted MS-222 (tricaine methanesulfonate, Sigma, USA) at a concentration of 100 mg/l for sampling. Blood was quickly drawn from the caudal vein and then transferred immediately to heparinize capillary tubes and shaken gently in order to avoid hemolysis, and thereafter centrifuged at 2500 rpm at 4 °C for 10 min. The supernatant was then stored at −80 °C for subsequent analysis. It should be mentioned that blood sampling of fish was executed in the morning around 7:00 a.m., and each group was sampled at equally timed interval (about 10 min for each group). Also, individual liver sample was quickly removed and stored at −80 °C for subsequent assays.
Growth performance
At the end of the feeding trial, body weight and length of the fish were recorded and data collected were used in the following equations to calculate growth performance and feed utilization:
where W is weight of body, W 2 is final weight, W 1 is initial weight, t is the period of the trial, and L is total length.
Proximate analysis
Experimental diets and whole fish were analyzed for proximate composition based on the standard AOAC method (AOAC and Chemists 1990). Moisture was determined by oven drying at 105 °C until constant weight. Crude protein (nitrogen × 6.25) was determined by the Kjeldahl method using an Auto Kjeldahl System (FOSS KT260, Switzerland), crude lipid by ether extraction using Soxtec System HT (Soxtec System HT6, Tecator, Sweden), and ash by combustion at 550 °C for 4 h.
Analysis of liver antioxidant status
Liver samples were homogenized on ice in 10 volumes (v/w) in a tissue homogenizer and centrifuged at 3000 rpm at 40 °C for 10 min. The supernatants were separated in aliquots and stored at −70 °C for subsequent analysis. Superoxide dismutase (SOD) activity was measured following the methods described by Wang and Chen (2005). Catalase (CAT) and reduced glutathione (GSH) were determined enzymatically with a commercial kit (Nanjing Jian Cheng Bioengineering Institute, China). The malondialdehyde (MDA) concentration was determined by the thiobarbituric acid test according to the published protocol by Zhang et al. (2008).
Determination of biochemical parameters
Plasma triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), aspartate transminase (AST), and alanine transaminase (ALT) contents were analyzed within 24 h of sampling using commercial assay kits produced by Jian Cheng Bioengineering Institute (Nanjing, China). Plasma cortisol level was measured using a validated and characterized radioimmunoassay.
Total RNA extraction, reverse transcription, and real-time PCR
Total RNA was extracted from the liver tissue using RNAiso Plus (Takara Co. Ltd., Dalian, China). RNA samples were treated by RQ1 RNase-Free DNase prior to RT-PCR (Takara Co. Ltd., Dalian, China) to avoid genomic DNA amplification. Complementary DNA (cDNA) was generated from 500 ng DNase-treated RNA using ExScript™ RT-PCR kit (Takara Co. Ltd., Dalian, China), and the mixture consisted of 500 ng RNA, 2 μl buffer (5×), 0.5 μl dNTP mixture (10 mM each), 0.25 μl RNase inhibitor (40 U/μl), 0.5 μl dT-AP primer (50 mM), 0.25 μl ExScript™ RTase (200 U/μl), and DEPC H2O, with total volume up to 10 μl. The reaction conditions were as follows: 42 °C for 40 min, 90 °C for 2 min, and 4 °C thereafter. Real-time PCR was employed to determine mRNA levels based on the SYBR® Green I fluorescence kit. Specific primers were designed using Primer 5.0 version (Table 7). Primer characteristics used for real-time PCR are listed in the Supplementary Material. Real-time PCR was performed in a Mini Option real-time detector (Bio-Rad, USA). The RT-qPCR reactions were carried out in a final volume of 20 μl, containing 10 μl 1× SYBR Premix Ex Taq™, 0.4 μM of each primer, 0.4 μl ROX, 6.8 μl DEPC water, and 2 μl of cDNA template. The reactions were initially denatured at 95 °C for 10 min and then 40 cycles at 95 °C for 15 s, followed by annealing at 60 °C for 34 s. To assess the specificity of each amplicon, the melt curve analysis of 5 s per step from 65 to 95 °C was performed at the end of each PCR thermal profile. All amplicons were initially separated by agarose gel electrophoresis to ensure that they were of correct size. A dissociation curve was determined during the PCR program to make sure that specific products were obtained in each run. At the end of the reaction, the fluorescent data were converted into C t values. To calculate relative expression levels, blunt snout bream β-actin was used as internal control to normalize the C t value in each sample, and the relative expression levels under different experimental diets were calculated by 2−△△Ct method.
Statistical analysis
Data were compared by one-way analysis of variance (ANOVA) using the SPSS program version 16.0 (SPSS Inc., Michigan Avenue, Chicago, IL, USA) for Windows. If significant differences were found (P < 0.05), Duncan’s multiple range tests was used to rank the means. The results were presented as mean ± SEM of three replicates.
Results
Effect of dietary supplementation on growth performance
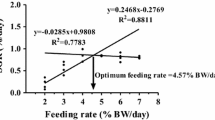
In this present study, betaine supplementation at 1.2% affected final body weight (FBW), weight gain ratio (WGR), specific growth rate (SGR), feed intake (FI), condition factor (CF), feed conversion rate (FCR), hepatosomatic index (HSI), and abdominal fat rate (AFR). Conversely, VSI and SR were not affected by betaine supplementation among the groups (Tables 2 and 3). Significantly higher (P < 0.05) FI, FBW, WGR, and SGR were observed in fish fed dietary betaine supplementation at 1.2%, while FCR showed an opposite trend. The relationship between WG and dietary betaine supplementation levels was best expressed by the second-order polynomial: Y = −29.472x 2 + 65.643x + 178.05, and the optimum dietary betaine level suitable for maximum growth performance of blunt snout bream was estimated to be 1.2% (Fig. 1).
Whole body composition
The results presented in Table 4 demonstrate that dietary betaine supplementation did not affect moisture content among the groups (P < 0.05). Meanwhile, crude protein and ash content were significantly higher (P < 0.05), in 1.2% betaine supplementation groups, whereas crude lipid was significantly lower than those in the HFD group or HFD supplemented with 0.6% betaine groups.
Hepatic antioxidant activities
Hepatic oxidative status parameters affected by betaine supplementation in HFD are shown in Table 5. The results reveal that betaine-supplemented groups increased SOD, CAT, and GSH activities in the liver and reduced the content of hepatic MDA compared to HFD group. The activities of SOD, CAT, and GSH were significantly higher (P < 0.05) in fish fed 1.2% betaine supplementation, while MDA levels showed an opposite trend in the same group compared to others.
Effects of betaine on lipid metabolites
Blood biochemistry of blunt snout bream is shown in Table 6. The activities of TG, TC, LDL, HDL, AST, and ALT and the contents of cortisol were significantly (P < 0.05), affected by diet treatments. Higher TG, TC, AST, and ALT activities and higher cortisol levels in fish given HFD were significantly reduced (P < 0.05), with the inclusion of betaine from 1.2 to 1.8%. Higher LDL and lower HDL content observed in HFD compared to NFD were improved when HFD was supplemented with 1.2% betaine.
Effect of betaine on the expression of lipid metabolism-related genes
FAS mRNA expression was significantly elevated in HFD group compared to NFD group, and this increase was reserved by 1.2% betaine supplementation (Fig. 2a). Whereas carnitine palmitoyltransferase 1 (CPT1), peroxisome proliferator-activated receptor α (PPARα), and microsomal triglyceride transfer protein (MTTP) expression levels were significantly decreased in fish fed HFD in comparison with NFD, but were significantly upregulated with betaine supplementation at 1.2% (Fig. 2b–d). These results indicate that betaine supplementation reversed HFD-induced dysregulation of the mRNA expression levels of lipid metabolism-related genes (Table 7).
Effect of dietary betaine on hepatic lipid metabolism-related gene expression. a Fatty acid synthase (FAS), b carnitine palmitoyltransferase 1 (CPT), c peroxisome proliferator-activated receptor α (PPARα), and d microsomal triglyceride transfer protein (MTTP). The expression level of each gene was normalized to that of the gene encoding β-actin. Data are represented as the mean ± SEM of n = 3 replicates. If significant differences were found (P < 0.05), Duncan’s multiple range tests was used to rank the means
Discussion
The supplementation of betaine with a HFD enhanced feed intake and growth performance in blunt snout bream. In the present study, FBW, WGR, SGR, and FI were significantly improved at 1.2% betaine supplementation compared to other treatments, whereas FCR was significantly reduced. This clearly shows that betaine supplementation has beneficial effects when given at an optimal level, but beyond that level, the supplement may have negative impacts on catabolic activities. The improved growth of fish in 1.2% group could be attributed to improved feed intake and palatability through stimulation of the cephalic reflex induced by smell and taste of attractive substances in the diet (Fange and Grove 1979). Similarly, previous reports found improved growth performance and feed intake and reduced FCR in juvenile gibel carp (Xue and Cui 2001), African catfish (Clarias gariepinus) (Turan and Akyurt 2005), common carp (Akshayamanai and PrakashPatil 2016), and tilapia (And and Davis 2005). However, VSI and SR were not affected by betaine supplementation, but significantly increased the HSI and AFR in high-fat group compared to NFD and 1.2% betaine supplementation. The decrease in HSI and AFR in the present study suggests that betaine is a lipotropic agent which can prevent or reduce accumulation of fat in the liver. Our results concur with the previous findings on pigs (Huang et al. 2008), meat ducks (Wang et al. 2000), and laying hens (Zou and Jian-Jun 2002).
The relationship between WG and dietary betaine supplementation % levels was best expressed by the second-order polynomial: Y = −29.472x 2 + 65.643x + 178.05, and the optimum dietary betaine level suitable for maximum growth performance of blunt snout bream was estimated to be 1.2%. The whole body moisture content was not affected by dietary betaine levels among the groups in this study, but crude protein and ash contents were significantly higher, while crude lipid content was significantly lower in fish fed 1.2% of betaine supplementation than HFD group. Betaine has been reported to play a role in protein metabolism and also has the ability to reduce fat. The increased crude protein and reduced lipid content in the present study could have resulted due to betaine supplementation in the diet. Our results are similar to earlier findings obtained in Oreochromis aureus reared in fresh and seawater (Genc et al. 2006) and in rabbit (Hassan et al. 2011), but conflicting with the report obtained in GIFT tilapia (Luo et al. 2011). The dissimilarity of the results could be related to the change in the nutrient concentration, quality of the diet, ration size, feeding frequency, and other factors (Jobling et al. 2001). However, the effect of dietary betaine supplementation on body composition in high-fat diet has not been previously reported and needs further study.
Oxidative stress occurs when reactive forms of oxygen are produced faster than they can be safely neutralized by antioxidant mechanisms. This may lead to a damage in biological macromolecules, disruption of normal metabolism and physiology of the affected fish (Trevisan et al. 2001; Sies 1992), eventually leading to the inception of health disorders (Miller et al. 1993). SOD is the first superoxide enzyme which has been shown to catalyze the dismutation of superoxide radical O2 − into O2 and hydrogen peroxide (H2O2) (Kohen and Nyska 2002). CAT plays a relatively minor role in the catabolism of H2O2 at low rates of H2O2 generation (Jones et al. 1981). It becomes indispensable when the rate of H2O2 production is enhanced during oxidative stress (Cañavate et al. 2007), and it has been observed that CAT and SOD are responsible for the elimination of peroxides, hence protecting tissues against oxidative damage (Ganesan et al. 2007b; Alirezaei et al. 2011). GSH plays an important role in detoxifying reactive oxygen species (ROS), and MDA is an indicator commonly used to evaluate lipid peroxidation (Parvez and Raisuddin 2005). In the present study, an improved level of SOD, CAT, and GSH activities was observed in 1.2 and 1.8% betaine supplementation. MDA content was significantly low in 1.2% supplementation compared to the HFD group. In this experiment, it was observed that an increase in SOD, CAT, and GSH activities could have decreased lipid peroxidation in blunt snout bream and subsequently improved fish health, an indication that supplementation of betaine in HFD might decrease oxidative damage. It can be suggested that betaine might have antioxidant and nutrient effects against oxidative damage in the cells of blunt snout bream fed HFD. Furthermore, the protective effect of betaine against oxidative stress observed in this study may also support the idea that betaine is associated with antioxidant and methyl donor properties through its involvement in cell membrane stabilization and homocysteine remethylation (Alirezaei et al. 2011).
Betaine is regarded as a lipotropic agent and has been tested in many animal models (Song et al. 2007; Wang et al. 2010). Long-term feeding of HFD can induce liver dysfunction, which might lead to stress and eventually cause fish deaths. In this study, high plasma TG, TC, LDL, AST, ALT, cortisol, and low HDL contents were observed in fish given HFD. The increase in plasma TC, TG, and LDL in this study may show metabolic disorders of lipids and lipoproteins as well as liver damage (Mensinger et al. 2005; Takeuchi-Yorimoto et al. 2013), leading to high cortisol level resulting into poor health condition of the fish in HFD group. Our results indicated that inappropriate increase of HFD may cause stress response of fingerling blunt snout bream, which is in agreement with the findings documented by Li et al. (2012b). This suggests that high dietary lipid might lead to increased lipid peroxidation and cause oxidative stress of fish.
On the other hand, an improved plasma TC, TG, LDL, HDL, AST, ALT, and cortisol concentration were observed in 1.2 and 1.8% supplementation compared to the HFD and 0.6% supplementations of betaine. This improved plasma component that might be related to its lipolytic effects on adipose tissue which could positively influence body composition by reducing TG and TC synthesis, thereby improving LDL and HDL content and equally enhancing liver functioning enzyme activities such as AST and ALT of fish. The lipolytic effect of the betaine in this study concurs with the findings reported by Wang et al. (2014b), whereby betaine supplementation reduced the visceral fat accumulation and plasma TG level in the HFD-fed mice and in broiler when subjected to chronic heat stress (He et al. 2015).
The expressions of genes involved in lipid metabolism were detected. FAS is the key enzyme in de novo lipogenesis which is sensitive to both nutritional and hormonal modulation (Moustaïd et al. 1996). Fatty acid (FA) oxidation is essential in liver lipid metabolism, especially in animals fed HFD (Du et al. 2006). When dietary lipid intake exceeds the capacity of the hepatic cells to oxidize FAS, large amount of triglyceride is synthesized and deposited in vacuoles, leading to steatosis (Lu et al. 2014b). In the present study, FAS expression was significantly decreased in the 1.2% betaine-supplemented diet compared to others, an indication that betaine supplementation partly inhibits the HFD-induced excessive synthesis of fatty acids.
As a specific property, CPT1 is a key rate-limiting enzyme of β-oxidation (Korman et al. 2005), and it is also mentioned as a target of PPARα (Ferré 2004). The β-oxidation of fatty acids plays a key role in the production of energy and mostly occurs in the mitochondria. CPT1 located in outer membrane of the mitochondria mediates the uptake of long-chain fatty acids into the mitochondria. PPARα as a nuclear receptor is activated by fatty acids and regulates the transcription of numerous gene encoding enzymes in fatty acid oxidation, such as CPT1 in mitochondria and CYP2E1 in extra mitochondria (Yang et al. 2012). In this study, CPT1 was significantly downregulated in fish fed a HFD and significantly upregulated when fed HFD supplemented with 1.2 and 1.8% betaine, respectively. The results are in accordance with the previous report, which found that betaine supplementation increased CPT1 expression level and reduced the expression of FAS in mammals (Wang et al. 2012). In this present study, downregulations of PPARα and its target (CPT1) were obtained in the group treated with high-fat diet, which is similar with the previous study by Xu et al. (2015) who reported that high-fat diet decreased mRNA levels for PPARα as well as its downstream target CPT1. However, in this study, betaine supplementation in the high-fat diet of blunt snout bream increased the gene expression of PPARα and CPT1. Similar results were also found in a study by Wang et al. (2014a), which showed that betaine supplementation increased both PPARα and CPT1 expressions of apoE2/2 mice and reversed the inhibition of CPT1 induced by HFD. MTTP plays an essential role in lipoprotein (Wetterau et al. 1992) and assists in the assembly and secretion of apolipoprotein B (apoB)-containing lipoprotein, chylomicrons, and very low-density lipoprotein. In the present study, a downregulation of MTTP expression was observed in the HFD group compared to NFD group, and 1.2% betaine supplementation reversed HFD-induced inhibition of MTTP mRNA expression. According to the current results, it can be highlighted that hepatic MTTP is very crucial in regulating the assembly and secretion of triglyceride-rich lipoproteins, which further confirms that dietary betaine decreases fat accumulation in the liver.
In conclusion, the study confirms that supplementation of betaine at 1.2% can improve growth performance and antioxidant capacity, as well as reduce fatty acid synthesis and enhance mitochondrial β-oxidation and lipid transportation in high-fat diet-fed blunt snout bream, thus effectively alleviating fat accumulation in the liver by changing lipid metabolism.
References
Akshayamanai SMH, Prakash P (2016) Effect of betaine hydrochloride as feed attractant on growth, survival and feed utilization of common carp (Cyprinus carpio). J Aquaculture Marine Biol 4(3):00083. doi:10.15406/jamb.2016.04.00083
Aksnes A, Mundheim H, Toppe J, Albrektsen S (2008) The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets. Aquaculture 275(1–4):242–249
Alirezaei M, Jelodar G, Niknam P, Ghayemi Z, Nazifi S (2011) Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat, cerebellum. J Physiol Biochem 67(4):605–612
Allen Davis D, Arnold CR (2000) Replacement of fish meal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture 185(3–4):291–298
And, G.W., Davis, D.A. 2005. Interrelationship among methionine, choline, and betaine in channel catfish Ictalurus punctutus. J World Aquacult Soc, 36(3), 337–345
AOAC (1990) AOAC Official Methods of Analysis. 15th edition, Association of Official Analytical Chemists, Arlington
Bolla S, Nicolaisen O, Amin A (2011) Liver alterations induced by long term feeding on commercial diets in Atlantic halibut (Hippoglossus hippoglossus L.) females. Histological and biochemical aspects. Aquaculture 312(1–4):117–125
Cañavate JP, Prieto A, Zerolo R, Sole M, Sarasquete C, Fernández-Díaz C (2007) Effects of light intensity and addition of carotene rich Dunaliella salina live cells on growth and antioxidant activity of Solea senegalensis Kaup (1858) larval and metamorphic stages. J Fish Biol 71(3):781–794
Chen Q-Q, Liu W-B, Zhou M, Dai Y-J, Xu C, Tian H-Y, Xu W-N (2016) Effects of berberine on the growth and immune performance in response to ammonia stress and high-fat dietary in blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol 55:165–172
Du ZY, Clouet P, Zheng WH, Degrace P, Tian LX, Liu YJ (2006) Biochemical hepatic alterations and body lipid composition in the herbivorous grass carp (Ctenopharyngodon idella) fed high-fat diets. Br J Nutr 95(5):905–915
Esteve-Garcia E, Mack S (2000) The effect of dl-methionine and betaine on growth performance and carcass characteristics in broilers. Anim Feed Sci Technol 87(1–2):85–93
Fange R, Grove DS (1979) Digestion in Fish Physiology, vol VIII. Acad. Press, NY. pp 162–260
Ferré P (2004) The biology of peroxisome proliferator-activated receptors. Diabetes 53(1):43–50
Ganesan B, Buddhan S, Anandan R, Sivakumar R, Anbinezhilan R (2010) Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Mol Biol Rep 37(3):1319
Ganesan B, Rajesh R, Anandan R, Dhandapani N (2007a) Biochemical studies on the protective effect of betaine on mitochondrial function in experimentally induced myocardial infarction in rats. J Health Sci 53(6):671–681
Ganesan B, Rajesh R, Anandan R, Dhandapani N (2007b) Protective effect of betaine on changes in the levels of protein, glycoproteins and amino acids in isoprenaline-induced myocardial infarction in rats. Afr J Biochem Res 1:117–123
Genc MA, Tekelioglu N, Yilmaz E, Hunt AO, Yanar Y (2006) Effect of dietary betaine on growth performance and body composition of Oreochromis aureus reared in fresh and sea watera comparative study. J Animal Veterinary Advances 5(12)
Goh Y, Tamura T (1980) Olfactory and gustatory responses to amino acids in two marine teleosts—red sea bream and mullet. Comparative Biochemistry & Physiology Part C Comparative Pharmacology 66(2):217–224
Gui D, Liu W, Shao X, Xu W (2010) Effects of different dietary levels of cottonseed meal protein hydrolysate on growth, digestibility, body composition and serum biochemical indices in crucian carp (Carassius auratus gibelio). Anim Feed Sci Technol 156(3–4):112–120
Habte-Tsion HM, Liu B, Ge XP, Xie J, Xu P, Mingchun R (2013) Effects of dietary protein levels on the growth performance, muscle composition, blood composition and digestive enzymes activities of Wuchang bream, Megalobrama amblycephala fry.Nanjing, Nanjing Agricultural University,M.Sc.degree Thesis, p. 83
Hassan RA, Ebeid TA, El-Lateif AIA, Ismail NB (2011) Effect of dietary betaine supplementation on growth, carcass and immunity of New Zealand white rabbits under high ambient temperature. Livest Sci 135(2):103–109
He S, Zhao S, Dai S, Liu D, Bokhari SG (2015) Effects of dietary betaine on growth performance, fat deposition and serum lipids in broilers subjected to chronic heat stress. Anim Sci J 86(10):897–903
Huang QC, Xu ZR, Han XY, Li WF (2008) Effect of dietary betaine supplementation on lipogenic enzyme activities and fatty acid synthase mRNA expression in finishing pigs. Anim Feed Sci Technol 140(3):365–375
Jobling M (2001) Nutrient partitioning and the influence of feed composition on body composition. In: Houlihan D, Boujard T, Joblin M (eds) Food intake in fish. 2001:354–376
Jones DP, Eklöw L, Thor H, Orrenius S (1981) Metabolism of hydrogen peroxide in isolated hepatocytes: relative contributions of catalase and glutathione peroxidase in decomposition of endogenously generated H2O2. Arch Biochem Biophys 210(2):505–516
Junnila M, Rahko T, Sukura A, Lindberg LA (2000) Reduction of carbon tetrachloride-induced hepatotoxic effects by oral administration of betaine in male Han-Wistar rats: a morphometric histological study. Vet Pathol 37(3):231–238
Kang X, Zhong W, Liu J, Song Z, Mcclain CJ, Kang YJ, Z.Z. (2009) Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4α and peroxisome proliferator-activated receptor-α. Hepatology 50(4):1241
Kasper CS, White MR, Brown PB (2002) Betaine can replace choline in diets for juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 205(1–2):119–126
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30(6):620
Kolkovski S, Arieli A, Tandler A (1997) Visual and chemical cues stimulate microdiet ingestion in sea bream larvae. Aquac Int 5(6):527–536
Korman SH, Waterham HR, Gutman A, Jakobs C, Wanders RJA (2005) Novel metabolic and molecular findings in hepatic carnitine palmitoyltransferase I deficiency. Mol Genet Metab 86(3):337–343
Li X, Jiang Y, Liu W, Ge X (2012a) Protein-sparing effect of dietary lipid in practical diets for blunt snout bream (Megalobrama amblycephala) fingerlings: effects on digestive and metabolic responses. Fish Physiol Biochem 38(2):529–541
Li XF, Liu WB, Lu KL, Xu WN, Wang Y (2012b) Dietary carbohydrate/lipid ratios affect stress, oxidative status and non-specific immune responses of fingerling blunt snout bream, Megalobrama amblycephala. Fish Shellfish Immunol 33(2):316–323
Lu KL, Xu WN, Li JY, Li XF, Huang GQ, Liu WB (2013) Alterations of liver histology and blood biochemistry in blunt snout bream, (Megalobrama amblycephala) fed high-fat diets. Fish Sci 79(4):661–671
Lu KL, Xu WN, Liu WB, Wang LN, Zhang CN, Li XF (2014a) Association of mitochondrial dysfunction with oxidative stress and immune suppression in blunt snout bream (Megalobrama amblycephala) fed a high-fat diet. J Aquat Anim Health 26(2):100–112
Lu KL, Xu WN, Wang LN, Zhang DD, Zhang CN, Liu WB (2014b) Hepatic β-oxidation and regulation of carnitine palmitoyltransferase (CPT) I in blunt snout bream (Megalobrama amblycephala) fed a high fat diet. PLoS One 9(3):e93135
Luo Z, Tan XY, Liu XJ, Wen H (2011) Effect of dietary betaine levels on growth performance and hepatic intermediary metabolism of GIFT strain of Nile tilapia (Oreochromis niloticus) reared in freshwater. Aquac Nutr 17(4):361–367
Mackie AM, Mitchell AI (2006) Studies on the chemical nature of feeding stimulants for the juvenile European eel, Anguilla anguilla (L.) J Fish Biol 22(4):425–430
Mcdevitt RM, Mack S, Wallis IR (2000) Can betaine partially replace or enhance the effect of methionine by improving broiler growth and carcase characteristics? Br Poult Sci 41(4):473–480
Mensinger AF, Walsh PJ, Hanlon RT (2005) Blood biochemistry of the oyster toadfish. J Aquat Anim Health 17(17):170–176
Miller JK, Brzezinska-Slebodzinska E, Madsen FC (1993) Oxidative stress, antioxidants, and animal function. J Dairy Sci 76(9):2812–2823
Ministry of Agriculture of the People’s Republic of China (2013) Chinese fishery statistical yearbook. Chinese Agricultural Press, Beijing (in Chinese)
Moeckel GW, Shadman R, Fogel JM, Sadrzadeh SM (2002) Organic osmolytes betaine, sorbitol and inositol are potent inhibitors of erythrocyte membrane ATPases. Life Sci 71(20):2413
Moustaïd N, Jones BH, Taylor JW (1996) Insulin increases lipogenic enzyme activity in human adipocytes in primary culture. J Nutr 126(4):865–870
Papatryphon E, Soares JH Jr (2000) Identification of feeding stimulants for striped bass, Morone saxatilis. Aquaculture 185(3–4):339–352
Parvez S, Raisuddin S (2005) Protein carbonyls: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch). Environ Toxicol Pharmacol 20(1):112–117
Patel VB, Mehta K (2015) Betaine in context. Food & Nutritional Components in Focus 2015(7):3–8
Quesada H, Bas JMD, Pajuelo D, Díaz S, Fernandezlarrea J, Pinent M, Arola L, Salvadó MJ, Bladé C (2009) Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int J Obes 33(9):1007–1012
Ronnestad I, Tonheim SK, Fyhn HJ, Rojasgarcia CR, Kamisaka Y, Koven W, Finn RN, Terjesen BF, Barr Y, Lec C (2003) The supply of amino acids during early feeding stages of marine fish larvae: a review of recent findings. Aquaculture 227(1–4):147–164
Rumsey GL (1991) Choline-betaine requirements of rainbow trout (Oncorhynchus mykiss). Aquaculture 95(1–2):107–116
Sargent J, Bell G, McEvoy L, Tocher D, Estevez A (1999) Recent developments in the essential fatty acid nutrition of fish. Aquaculture 177(1–4):191–199
Sheard NF, Zeisel SH (1989) Choline: an essential dietary nutrient? Nutrition 5(1):1–5
Sies H (1992) Oxidative stress: oxidants and antioxidants. Cardiovasc Res 82(8):291
Song Z, Deaciuc I, Zhou Z, Song M, Chen T, Hill D, Mcclain CJ (2007) Involvement of AMP-activated protein kinase in beneficial effects of betaine on high-sucrose diet-induced hepatic steatosis. Am J Physiol Gastrointest Liver Physiol 293(4):G894–G902
Takeuchi-Yorimoto A, Noto T, Yamada A, Miyamae Y, Oishi Y, Matsumoto M (2013) Persistent fibrosis in the liver of choline-deficient and iron-supplemented l-amino acid-defined diet-induced nonalcoholic steatohepatitis rat due to continuing oxidative stress after choline supplementation. Toxicol Applied Pharmacol 268(3):264–277
Trevisan M, Browne R, Ram M, Muti P, Freudenheim J, Carosella AM, Armstrong D (2001) Correlates of markers of oxidative status in the general population. Am J Epidemiol 154(4):348–356
Turan F, Akyurt I (2005) Effects of androstenedione, a phytoandrogen, on growth and body composition in the African catfish (Clarias gariepinus). Isr J Aquacult Bamidgeh 57(1):62–66
Wang L, Zhang H, Zhou J, Liu Y, Yang Y (2014a) Betaine attenuates hepatic steatosis by reducing methylation of the MTTP promoter and elevating genomic methylation in mice fed a high-fat diet. J Nutr Biochem 25(3):329-36
Wang L, Chen L, Tan Y, Wei J, Chang Y, Jin T, Zhu H (2012) Betaine supplement alleviates hepatic triglyceride accumulation of apolipoprotein E deficient mice via reducing methylation of peroxisomal proliferator-activated receptor alpha promoter. Lipids Health Dis 12(1):34
Wang LJ, Zhang HW, Zhou JY, Liu Y, Yang Y, Chen XL, Zhu CH, Zheng RD, Ling WH, Zhu HL (2014b) Betaine attenuates hepatic steatosis by reducing methylation of the MTTP promoter and elevating genomic methylation in mice fed a high-fat diet. J Nutr Biochem 25(3):329
Wang YZ, Xu ZR, Chen ML (2000) Effect of betaine on carcass fat metabolism of meat duck. Chinese J Veterinaryence 20:409–413
Wang YZ, Xu ZR, Feng J (2004) The effect of betaine and dl-methionine on growth performance and carcass characteristics in meat ducks. Anim Feed Sci Technol 116(1–2):151–159
Wang SH, Chen JC (2005) The protective effect of chitin and chitosan against vibrio alginolyticus, in white shrimp Litopenaeus vannamei[J]. Fish Shellfish Immunol 19(19):191–204
Wang Z, Yao T, Pini M, Zhou Z, Fantuzzi G, Song Z (2010) Betaine improved adipose tissue function in mice fed a high-fat diet: a mechanism for hepatoprotective effect of betaine in nonalcoholic fatty liver disease. Ajp Gastrointestinal & Liver Physiology 298(5):G634
Watanabe T (1982) Lipid nutrition in fish. Comparative Biochemistry & Physiology B Comparative Biochemistry 73(1):3–15
Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE (1992) Absence of microsomal triglyceride transfer protein in individuals with Abetalipoproteinemia. Science 258(258):999–1001
Xu L, Huang D, Hu Q, Wu J, Wang Y, Feng J (2015) Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br J Nutr 113(6):1835–1843
Xue M, Cui Y (2001) Effect of several feeding stimulants on diet preference by juvenile gibel carp (Carassius auratus gibelio), fed diets with or without partial replacement of fish meal by meat and bone meal. Aquaculture 198(3–4):281–292
Yang H, Li F, Xiong X, Kong X, Zhang B, Yuan X, Fan J, Duan Y, Geng M, Li L (2012) Soy isoflavones modulate adipokines and myokines to regulate lipid metabolism in adipose tissue, skeletal muscle and liver of male Huanjiang mini-pigs. Mol Cell Endocrinol 365(1):44–51
Zhang XD, Zhu YF, Cai LS, TX W (2008) Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 280:136–139
Zhou J, Li C, Wang L, Ji H, Zhu T (2015) Hepatoprotective effects of a Chinese herbal formulation, Yingchen decoction, on olaquindox-induced hepatopancreas injury in Jian carp (Cyprinus carpio var. Jian). Fish Physiol Biochem 41(1):153–163
Zhou Z, Ren Z, Zeng H, Yao B (2008) Apparent digestibility of various feedstuffs for bluntnose black bream Megalobrama amblycephala Yih. Aquac Nutr 14(2):153–165
Zou XT, Jian-Jun LU (2002) Effect of betaine on the regulation of the lipid metabolism in laying hen. J Integr Agric 1(9):1043–1049
Acknowledgements
This work was supported by the China Agriculture Research System (grant number CARS-46-20).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adjoumani, JJ.Y., Wang, K., Zhou, M. et al. Effect of dietary betaine on growth performance, antioxidant capacity and lipid metabolism in blunt snout bream fed a high-fat diet. Fish Physiol Biochem 43, 1733–1745 (2017). https://doi.org/10.1007/s10695-017-0405-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0405-9