Abstract
Oxidative stress is a hypothesis for the association of reactive oxygen species with cerebrovascular and neurodegenerative diseases. Thus, we examined whether oral betaine can act as a preventive agent in ethanol-induced oxidative stress on the cerebellum of rats. Thirty-two adult male Sprague–Dawley rats were divided into four equal groups (control, ethanol, betaine, and betaine plus ethanol) with different dietary regimens and were followed up for 1 month. Total homocysteine (tHcy) of plasma and cerebellum homogenate was determined by an Axis® homocysteine EIA kit, and antioxidant enzyme (glutathione peroxidase (GPx), SOD, and CAT) activities of cerebellum homogenate were measured chemically by a spectrophotometer. Lipid peroxidation of cerebellum was shown by the measurement of thiobarbituric reactive substances (TBARS) via a spectrophotometer. Ethanol-induced hyperhomocysteinemia was manifested by an increase in the concentrations of tHcy in the plasma and cerebellum homogenates of the ethanol group, while ethanol-induced oxidative stress was indicated via an increase in lipid peroxidation marker (TBARS) in cerebellum homogenates of ethanol-treated rats. In contrast, betaine prevented hyperhomocysteinemia and oxidative stress in the betaine plus ethanol group as well as the betaine group. The results of the present investigation indicated that the protective effect of betaine is probably related to its ability to strengthen the cerebellum membrane cells by enhancement of antioxidant enzyme activity principally GPx, while the methyl donor effect of betaine to reduce hyperhomocysteinemia has been explained previously and confirmed in the present study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been increasingly more evidence to support the hypothesis that elevated total homocysteine (tHcy) is an independent risk factor for coronary vascular and neurodegenerative diseases [1, 2, 12, 14, 38, 47]. High homocysteine (Hcy) has been suggested as a mediating factor in alcohol-related brain atrophy [41]. The high prevalence of hyperhomocysteinemia in the population and its easy treatability make Hcy an interesting amino acid for studies in the prevention of degenerative brain disorders [41]. Homocysteine plays a role in a shared biochemical cascade involving overstimulation of N-methyl-d-aspartate receptors, oxidative stress, activation of caspases, DNA damage, and endoplasmic reticulum and mitochondrial dysfunction [13]. The formation of methionine from homocysteine can occur either via betaine or via 5-methyltetrahydrofolate [21]. Animal studies have shown that both pathways are equally important and that betaine is a vital methylating agent [6, 26, 27]. Betaine (trimethylglycine) transfers a methyl group via the enzyme betaine homocysteine methyltransferase (BHMT) to become dimethylglycine. Ethanol feeding can affect several hepatic enzymes in animals, including decreasing methionine synthetase activity [7]. This leads to increased BHMT activity to maintain hepatic S-adenosyl methionine (SAM) at normal concentrations [21] (supplementary file).
During the past decades, several studies have examined the role of oxidative stress on developmental alcohol-mediated neurotoxicity, possibly via the formation of free radicals [18, 31, 48, 52]. Reactive oxygen species (ROS) are generated during oxidative metabolism and can inflict damage on all classes of cellular macromolecules, eventually leading to cell death [11, 48]. Oxidative stress is believed to contribute to neurodegeneration and cognitive and behavioral deficits after ischemia, anoxia, carbon monoxide poisoning, and alcoholism [9, 53, 54]. During the metabolism of alcohol, acetaldehyde is formed as the principal metabolite. When acetaldehyde is oxidized, it produces superoxide free radicals that are able to react with hydrogen peroxide to form other types of free radicals, such as hydroxyl radicals [48]. Chronic and excessive ethanol consumption is associated with various biochemical and physiological changes in the central nervous system (CNS). The most affected brain regions seem to be superior frontal association complex, hypothalamus, and cerebellum [35].
Although the brain has defenses against ROS including dietary free radical scavengers (ascorbate, α-tocopherol), the endogenous tripeptide glutathione, and enzymatic antioxidants superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), there is considerable evidence that oxidative damage directly or indirectly, due to free radical production and ROS, can lead to brain injury [23]. Indeed, brain tissues have unique characteristics that make them especially susceptible to damage due to low levels of antioxidant defenses [10]. Lipid peroxidation is one of the consequences of oxidative stress induced by metabolism of ethanol [35]. In this regard, a previous study demonstrated that chronic gestational exposure to alcohol caused a decrease in the membrane integrity while increasing oxidative stress in cerebellar neurons [48]. Malondialdehyde (MDA) is one of the major aldehyde derivatives of lipid peroxidation, and it is a by-product of the lipid peroxidation process [48]. In this content, oxidative stress and lipid peroxidation due to ethanol consumption were evaluated by MDA concentration in brain tissue via the measurement of thiobarbituric acid reactive substances (TBARS) [35].
It has been shown methyl tetrahydrofolate reductase (MTHFR) deficiency in mice delayed brain development and caused severe cerebellar abnormalities, with effects on granule cell development and neuronal organization [45, 46]. In this regard, previous betaine supplementation showed a beneficial effect on cerebellar development of the pups. It reduced the severity of lamination disruption in the cerebellum and limited the defects to only one or two anterior lobules [19]. Very recently, we proved the beneficial properties of betaine in ethanol-induced hyperhomocysteinemia in a rabbit animal model [1, 2].
Taking the above into consideration, we hypothesized that the oral administration of betaine prior to ethanol can act as an antioxidant agent to increase the activity of GPx, SOD, and CAT in ethanol-induced oxidative stress and decrease TBARS concentration (as a lipid peroxidation marker) in the rat cerebellum. We also investigated how tHcy of plasma and cerebellum varied with betaine therapy in rats.
Materials and methods
Materials
Alcohol (ethanol 95%) and TBARS were purchased from Merck Chemical Company (KGaA, Darmstadt, Germany), and betaine (Betafin® 96%) was obtained from Biochem Company (Brinkstrasse 55, D-49393 Lohne, Germany). GPx and SOD kit were obtained via Randox ® Company (Randox, Germany). The homocysteine kit was prepared by Axis® Homocysteine EIA (Axis-Shield AS, Germany). All chemicals used were of analytical grade.
Animals
Thirty-two adult male Sprague–Dawley rats (weighing 220–250 g, purchased from Shiraz University of Medical Sciences, Animal House Center, Iran) were housed in temperature-controlled conditions under a 12:12-h light/dark photocycle with food and tape water supplied ad libitum. All rats were treated humanely and in compliance with the recommendations of Animal Care Committee for the Shiraz University of Medical Sciences (Shiraz, Iran). All the experimental procedures were carried out between 8.00-11.00 hours, and all treatments were applied orally by gavage.
Experimental design
The rats were divided into four equal groups, and weight gain and food consumption were determined at weekly intervals and treated daily for 1 month in the following order: the control group received 1 ml of normal saline by gavage orally, the ethanol group received ethanol (4 g/kg by gavage), the betaine group received betaine soluble in water (1.5% w/w of the total diet, approximately 180 mg/mouse soluble in water daily by gavage), and the betaine plus ethanol group received betaine (similar to the betaine group) and after 120 min, fed with ethanol solution (4 g/kg by gavage). Doses of ethanol and betaine were determined according to previous studies [1, 33, 49]. One day after the last gavage, the rats were killed using diethyl ether anesthesia (Dagenham, UK) by decapitation. Immediately after rat killing, blood samples were collected via cardiac puncture, whole blood containing EDTA was centrifuged at 3,000×g for 5 min, and plasma was prepared in microtubes. The brain was removed and carefully cleaned of adhering, and then the cerebellum in all groups was separated. Cerebellum and plasma samples were stored at −70°C until analysis.
Tissue preparation for protein measurement, tHcy and TBARS detection, and enzyme assay
Rat cerebellum was thawed and manually homogenized in cold phosphate buffer (pH 7.4) containing 5 mM EDTA, and debris were removed by centrifugation at 2000×g for 5 min (Centrifuge 5415 R; Rotofix 32A, Germany). Supernatants were recovered and used for antioxidant enzyme activities, TBARS and tHcy concentrations, and protein measurement. Protein content of tissue homogenates was determined using a colorimetric method of Lowry with bovine serum albumin as a standard [39].
Measurement of tHcy concentration
Total homocysteine of cerebellum homogenates and plasma, which refers to the sum of protein-bound, free-oxidized, and reduced species of homocysteine, was determined by the Axis® Homocysteine EIA kit [29, 36]. The sample volume used was 25 μl. Absorbance was measured at a wavelength of 450 nm using an ELISA reader (STAT FAX 2100, USA). All estimations were performed in duplicate and the intra assay coefficient of variation was <10%.
Measurement of GPx activity
The activity of GPx was evaluated with Randox GPx detection kit according to the manufacturer’s instructions. GPx catalyze the oxidation of glutathione (GSH) by cumene hydroperoxide. In the presence of glutathione reductase and nicotinamide adenine dinucleotide phosphate (NADPH), the oxidized glutathione (GSSG) is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance was measured spectrophotometrically (S2000 UV model; WPA, Cambridge, UK) against blank at 340 nm. One unit of GPx was defined as l μmol of oxidized NADPH per minute per milligram of tissue protein. The GPx activity was expressed as unit per milligram of tissue protein.
Measurement of SOD activity
The activity of SOD was evaluated with Randox SOD detection kit according to the manufacturer’s instructions. The role of SOD is to accelerate the dismutation of the toxic superoxide produced during oxidative energy processes to hydrogen peroxide and molecular oxygen. This method employs xanthine and xanthine oxidase to generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. The SOD activity is then measured by degree of inhibition of this reaction. One unit of SOD is that which causes 50% inhibition of the rate of reduction of INT under the conditions of the assay. SOD levels were recorded at 505 nm and through a standard curve and expressed as unit per milligram of tissue protein.
Measurement of CAT activity
Tissue catalase activity was assayed using the method described by Claiborne [20]. The reaction mixture (1 ml) consisted of 50 mM potassium phosphate (pH 7.0), 19 mM H2O2, and a 20–50 μl sample. The reaction was initiated by the addition of H2O2, and absorbance changes were measured at 240 nm (25°C) for 30 s. The molar extinction coefficient for H2O2 is 43.6/M cm−1. The CAT activity was expressed as the unit that is defined as 1 μmol of H2O2 consumed per minute. The catalase activity was expressed as unit per milligram of tissue protein.
Measurement of lipid peroxidation
The level of lipid peroxidation was indicated by the content of TBARS in the cerebellum. Tissue TBARS was determined by following the production of thiobarbituric acid reactive substances as described by Subbarao et al. [50]. In short, 40 μl of homogenate was added to 40 μl of 0.9% NaCl and 40 μl of deionized H2O, resulting in a total reaction volume of 120 μl. The reaction was incubated at 37°C for 20 min and stopped by the addition of 600 μl of cold 0.8 M hydrochloride acid, containing 12.5% trichloroacetic acid. Following the addition of 780 μl of 1% TBA, the reaction was boiled for 20 min and then cooled at 4°C for 1 h. In order to measure the amount of TBARS produced by the homogenate, the cooled reaction was spun at 1,500×g in a microcentrifuge for 20 min, and the absorbance of the supernatant was spectrophotometrically read at 532 nm, using an extinction coefficient of 1.56 × 105. The blanks for all of the TBARS assays contained an additional 40 μl of 0.9% NaCl instead of homogenate as just described. TBARS results were expressed as nanomole per milligram of tissue protein.
Statistical analysis
All results are presented as mean ± SEM. Data were compared by one-way analysis of variance (ANOVA) with Tukey's post hoc analysis. A calculated P value of less than 0.05 was considered to be statistically significant. Statistical analysis was performed using the statistical package SPSS version 11.5 (SPSS, Inc., Chicago, IL, USA).
Results
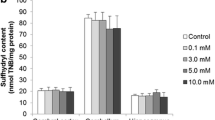
The mean values (±SEM) of the GPx, SOD, and CAT activity of the four groups of rat cerebellum are presented in Fig. 1. GPx activity is significantly higher in the betaine group compared to the control, ethanol, and betaine plus ethanol group (P < 0.05). Interestingly, GPx activity is insignificantly higher in the ethanol group compared to the control group (P > 0.05). SOD activity is significantly higher in the betaine group compared to the control and ethanol group (P < 0.05). However, CAT activity is significantly higher in the betaine group compared to the ethanol group (P < 0.05). Although the activities of SOD and CAT in the betaine plus ethanol group were higher compared to the ethanol group, these enhancements were not statistically significant (P > 0.05). Indeed, when betaine was administered prior to ethanol, it could increase the level of these parameters near to the betaine group.
Comparison of antioxidant enzyme activities: glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) among the control and treatment groups of rats. Values represent mean ± SEM of enzyme activity (unit/mg protein of cerebellum tissue). Means for each enzyme with different superscripts differ statistically (one-way ANOVA followed by Tukey's post hoc test; P < 0.05)
TBARS concentration (mean ± SEM) in the ethanol group (33.60 ± 7.3) was increased significantly compared to the control (11.21 ± 2.15), betaine (13.52 ± 1.85), and betaine plus ethanol group (14.49 ± 1.98; P < 0.05). Although the betaine plus ethanol group showed slightly increased TBARS concentration compared to the control group, this was not significant (P > 0.05; Fig. 2).
.Comparison of thiobarbituric acid reactive substances (TBARS) concentration among the control and treatment groups of rats. Values represent mean ± SEM of TBARS (nanomoles per milligram protein of cerebellum tissue. Means with different superscripts differ statistically (one-way ANOVA followed Tukey's post hoc test; P < 0.05)
Treatment of rats with ethanol significantly increased tHcy in cerebellum of the ethanol group compared to the other groups, while administration of betaine to the betaine and betaine plus ethanol group could prevent increase of tHcy concentration (P < 0.05; Fig. 3). In contrast, the plasma concentration of tHcy in the betaine group was remarkably lower compared to ethanol-ingested rats (P < 0.05). Although plasma homocysteine for ethanol-treated rats was not significant compared to the control group, it tended to approach significance (P = 0.058; Fig. 4).
Comparison of total homocysteine (tHcy) concentration among the control and treatment groups of rats. Values represent mean ± SEM of tHcy (nanomoles per gram protein of cerebellum tissue). Means with different superscripts differ statistically (one-way ANOVA followed by Tukey's post hoc test; P < 0.05)
Discussion
Our data support the hypothesis that betaine can reduce ethanol-induced oxidative stress and suggests it does by promoting the antioxidant enzyme activity including GPx, SOD, and CAT. The observation for antioxidant enzymes, TBARS concentration, and tHcy in the treatment groups supports the idea that betaine is associated with antioxidant and methyl donor properties through its involvement in cell membrane stabilization and homocysteine remethylation [1].
The results of the present investigation indicated that the protective effect of betaine is probably related to its ability to strengthen the cerebellar cell membrane by its membrane-stabilizing action or to a counteraction of free radicals by its antioxidant property [28]. The present data indicate that ethanol consumption increases tHcy and TBARS content in the rat cerebellar tissue [14, 15, 35]. Interestingly, our results indicate that ethanol induces oxidative damage and enhances GPx activity. In this context, a previous report showed that ethanol can enhance glutathione content in the rat pups brain [48].
Chronic alcohol consumption results in brain injury, leading to a number of neuropsychiatric symptoms including alcoholic cerebellum degeneration and dementia [17, 35]. Because brain consumes approximately 29% of oxygen received by organism, ethanol-induced ROS production and consecutive oxidative stress play an important role among the mechanisms of ethanol neurotoxicity [4, 48, 51]. Previous studies showed that betaine exerted cellular and subcellular membrane stabilization in the liver and myocardium cells by restoring both non-enzymatic and enzymatic antioxidants [24, 28]. We have already reported the protective effect of betaine on ethanol-induced hyperhomocysteinemia in our recent studies [1, 2]. However, the protective effect of betaine on cerebellar antioxidant defense system has not yet been previously explored. In the present study, we found significantly elevated antioxidant enzyme activity in cerebellum of rats exposed to betaine, and this is consistent with a previous report [28].
During the past decade, administration of betaine has been shown to exert a significant role within tissue as a methyl donor, which in turn may be used for the synthesis of methionine, carnitine, phosphatidylcholine, creatine, and these substances play a key role in protein and energy metabolism in the cells [21]. Betaine is believed to play a significant role in maintaining the structural and functional integrity of cell membranes. A previous study has been demonstrated that betaine, through its participation in sequential methylation within the cellular membranes, maintains a proper balance between phosphotidyl ethanolamine and phosphotidyl choline, thus sustaining proper membranes [28, 37]. In the present study, betaine, a methyl donor that continuously generates SAM, is shown to lead to long-term lowering of plasma homocysteine during supplementation in the dietary intake range of 1.5% (w/w) of total diet. Furthermore, since humans produce little betaine from choline due to lack of choline oxidase [30], betaine is practical for investigations regarding the treatment of hyperhomocysteinemia in humans.
Although betaine can across the blood–brain barrier, homocysteine remethylation to methionine catalyzed by BHMT occurs mainly in liver [42, 43]. SAM is released from liver and can also across the blood–brain barrier, with partial restoration of the decreased methyl donor pool in brain caused by MTHFR deficiency [18]. This phenomenon may explain why there is still a high level of homocysteine in the brain of betaine plus ethanol group despite the increase in homocysteine remethylation to methionine in liver following betaine administration. Indeed, plasma total homocysteine decreased in the betaine group after betaine treatment, while tHcy in the cerebellum of ethanol-ingested rats due to lack of SAM elevated significantly. In this regard, previously, Broch et al. have shown a much higher concentration of Hcy (about 6 nmol/g tissue) in the cerebellum of the rats, while the concentration in the liver was about 4 nmol/g of tissue in rats [16].
Homocysteine inhibits the expression of antioxidant enzymes which might potentiate the toxic effects of ROS [13, 32]. In addition, autooxidation of homocysteine is known to generate ROS, whereby the prevention of homocysteine-induced toxicity by catalase suggests that hydrogen peroxide acted as a mediator of oxidative injury, leading to oxidative stress [5, 22, 40]. In this regard, only catalase activity was significantly higher in the betaine group compared to the control group. Indeed, catalase prevented H2O2 accumulation and elevated its activity in the betaine plus ethanol group compared to the control group, although it was not significant.
The brain is more vulnerable to oxidative stress than other organs due to its low antioxidant protection system and increased exposure of target molecules to ROS [23]. The nervous tissue has a high content of polyunsaturated fatty acids, which are easy targets to oxidative damage by free radicals due to the unsaturated bonds they contain [48]. In our study, ethanol consumption caused significantly increased TBARS concentration in the ethanol group, and betaine treatment restored this elevated TBARS concentration in the betaine plus ethanol group near to the control group. On the other hand, there were significant differences among the ethanol and other groups for the TBARS concentration, indicating oxidative stress in ethanol-treated rats. These results of ethanol-exposed rats were consistent with previous literature [35].
Animal models have shown that a number of antioxidants prevent oxidative brain injury through a variety of cellular mechanisms which have described oxidative damage on the CNS [10, 23, 53, 54]. Glutathione antioxidant system plays a fundamental role in cellular defense against reactive oxygen species. The cellular tripeptide, GSH (γ-glutamyl cysteinyl glycine), thwarts peroxidative damage by neutralizing the free radicals [28]. In the present study, significant elevation of TBARS concentration in the cerebellum tissue of the ethanol group suggests an enhanced oxidative stress in the experimental animal model.
It is well known that SOD and CAT, which are responsible for the destruction of peroxides, have a specific role in protecting tissues against oxidative damage [3, 28, 34]. In the present study, the unpaired electron present in the hydroxyl free radical might have been trapped and subsequently dismuted by betaine [8, 44]. However, the protective effect of betaine against ethanol-induced oxidative stress observed in this study may also be associated with the restoration of SAM, which contributes to an increase in the supply of substrate needed for the synthesis of glutathione that protects the cell from reactive metabolites and reactive oxygen species [28].
To the best of our knowledge, this is the first in vivo study to show that betaine treatment results in an overall increase in the antioxidant enzyme activities in rat cerebellum. Betaine is a methylating agent like SAM, and it also stabilizes SAM levels via BHMT pathway [1, 2, 14]. Therefore, betaine may have an antioxidant effect against oxidative damage in brain. In addition, betaine may have some advantages than endogenous SAM application because SAM application enhances the levels of homocysteine, which is undesirable due to the toxicity of this amino acid, whereas betaine treatment decreases homocysteine levels by directly inducing the remethylating process, which transforms homocysteine into methionine [38]. With regard to BHMT, which is abundant in primates, the beneficial properties of betaine are promising and reduce the elevated plasma homocysteine concentrations via the BHMT pathway [12, 25, 26]. Although, betaine demonstrated as a potential neuroprotective agent for prevention of ethanol-induced oxidative damage in the cerebellum. However, further studies including extra biochemical parameters and histochemical techniques should be performed to validate this assumption.
References
Alirezaei M, Saeb M, Javidnia K, Nazifi S, Khalighyan N, Saeb S (2010) Betaine reduction of hyperhomocysteinemia and enhancement of 5-hydroxyindoleacetic acid in ethanol-induced hyperhomocysteinemia in rabbits. Afr J Biochem Res 4:246–254
Alirezaei M, Saeb M, Javidnia K, Nazifi S, Saeb S (2011) Hyperhomocysteinemia reduction in ethanol-fed rabbits by oral betaine. Comp Clin Pathol. doi:10.1007/s00580-010-1110-6
Anandan R, Devi KP, Devaki T, Govindaraj P (1998) Preventive effects of Picrochiza kurroa on d-galactosamine-induced hepatitis in rats. J Clin Biochem Nutr 25:87–95
Augustyniak A, Michalak K, Skrzydlewska E (2005) The action of oxidative stress induced by ethanol on the central nervous system (CNS). Postepy Hig Med Dosw 59:464–471
Austin RC, Sood SK, Dorward AM, Singh G, Shaughnessy SG, Pamidi S, Outinen PA, Weitz JI (1998) Homocysteine-dependent alterations in mitochondrial gene expression, function and structure. Homocysteine and H2O2 act synergistically to enhance mitochondrial damage. J Biol Chem 273:30808–30817
Barak AJ, Beckenhauer HC, Badakhsh S, Tuma DJ (1997) The effect of betaine in reversing alcoholic steatosis. Alcohol Clin Exp Res 21:1100–1102
Barak AJ, Beckenhauer HC, Tuma DJ (2002) Methionine synthase a possible prime site of the ethanolic lesion in liver. Alcohol 26:65–67
Barak AJ, Tuma DJ (1983) Betaine, metabolic by-product or vital methylating agent? Life Sci 32:771
Baraona E, Zeballos GA, Shoichet L, Mak KM, Lieber CS (2002) Ethanol consumption increases nitric oxide production in rats, and its peroxynitrite-mediated toxicity is attenuated by polyenylphosphatidylcholine. Alcohol Clin Exp Res 26:883–889
Barichello T, Fortunato JJ, Vitali AM et al (2006) Oxidative variables in the rat brain after sepsis induced by cecal ligation and perforation. Crit Care Med 34:886–889
Bergamini CM, Gambetti S, Dondi A, Cervellati C (2004) Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des 10:1611–1626
Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G (2009) Repeatability and measurement error in the assessment of choline and betaine dietary intake: the Atherosclerosis Risk in Communities (ARIC) Study. Nutr J 8:14–20
Bleich S, Degner D, Sperling W, Bönsch D, Thürauf N, Kornhuber J (2004) Homocysteine as a neurotoxin in chronic alcoholism. Prog Neuropsychopharmacol Biol Psychiat 28:453–464
Bottiglieri T (2005) Homocysteine and folate metabolism in depression. Neuropsychopharmacol Biol Psychiat 29:1103–1112
Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MWP, Reynolds EH (2000) Homocysteine, folate, methylation, and monoamine metabolism in depression. Br Med J 69:228–232
Broch OJ, Ueland PM (1984) Regional distribution of homocysteine in the mammalian brain. J Neurochem 43:1755–1757
Butterworth RF (1999) Pathophysiology of alcoholic brain damage: synergistic effects of ethanol, thiamine deficiency and alcoholic liver disease. Metab Brain Dis 10:1–8
Chen Z, Schwahn BC, Wu Q, He X, Rozen R (2005) Postnatal cerebellar defects in mice deficient in methylenetetrahydrofolate reductase. Inter J Dev Neurosci 23:465–474
Chen SY, Sulik K (1996) Free radicals and ethanol-induced cytotoxicity in neural crest cells. Alcohol Clin Exp Res 20:1071–1076
Claiborne A (1986) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 283–284
Craig SA (2004) Betaine in human nutrition. Am J Clin Nutr 80:539–549
D’Emilia DM, Lipton SA (1999) Ratio of S-nitrosohomocyst(e)ine to homocyst(e)ine or other thiols determines neurotoxicity in rat cerebrocortical cultures. Neurosci Lett 265:103–106
Dal-Pizzol F, Ritter C, Cassol-Jr OJ, Rezin GT, Petronilho F, Zugno AI, Quevedo J, Streck EL (2010) Oxidative mechanisms of brain dysfunction during sepsis. Neurochir Res 35:1–12
Erman F, Balkan J, Cevikbas U, Kocak-Toker N, Uysal M (2004) Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids 27:199–205
Finkelstein JD (2007) Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med 45:1694–1699
Finkelstein JD, Martin JJ (1986) Methionine metabolism in mammals. Adaptation to methionine excess. J Biol Chem 261:1582–1587
Finkelstein JD, Martin JJ, Harris BJ, Kyle WE (1983) Regulation of hepatic betaine-homocysteine methyltransferase by dietary betaine. J Nutr 113:519
Ganesan B, Buddhan S, Anandan R, Sivakumar R, Anbinezhilan R (2010) Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Mole Biol Rep 37(3):1319–1327
Golbahar J, Aminzadeh MA, Hamidi SA, Omrani GR (2005) Association of red blood cell 5-methyltetrahydrofolate folate with bone mineral density in postmenopausal Iranian women. Osteop Inter 16:1894–1898
Haubrich DR, Gerber NH (1981) Choline dehydrogenase. Assay, properties and inhibitors. Biochem Pharmacol 30:2993
Heaton MB, Mitchell JJ, Paiva M (2000) Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcohol Clin Exp Res 24:512–518
Huang RF, Huang SM, Lin BS, Wei JS, Liu TZ (2001) Homocysteine thiolactone induces apoptotic DNA damage mediated by increased intracellular hydrogen peroxide and caspase 3 activation in HL-60 cells. Life Sci 68:2799–2811
Ji C, Kaplowitz N (2003) Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 124:1488–1499
Kalra J, Lautner K, Massey L, Prasad K (1988) Oxygen free radicals induced release of lysosomal enzymes in vitro. Mol Cell Biochem 84:233–238
Kanbak G, Arslan OC, Dokumacioglu A, Kartkaya K, Inal ME (2008) Effects of chronic ethanol consumption on brain synaptosomes and protective role of betaine. Neurochem Res 33:539–544
Karthikeyan G, Thachil A, Sharma S, Kalaivani M, Ramakrishnan L (2007) Elevated high sensitivity CRP levels in patients with mitral stenosis and left atrial thrombus. Inter J Cardiol 122:252–254
Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ (2007) Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol 46:314–321
Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS (2008) Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiat 192:268–274
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Outinen PA, Sood SK, Liaw PC, Sarge KD, Maeda N, Hirsh J, Ribau J, Podor TJ, Weitz JI, Austin RC (1998) Characterization of the stress-inducing effects of homocysteine. Biochem J 332(Pt. 1):213–221
Sachdev PS (2005) Homocysteine and brain atrophy. Prog Neuropsychopharmacol Biol Psychiatry 29:1152–1161
Sachdev P, Parslow R, Salonikas C, Lux O, Wen W, Kumar R, Naidoo D, Christensen H, Jorm A (2004) Homocysteine and the brain in midadult life. Arch Neurol 61:1369–1376
Sachdev PS, Valenzuela M, Brodaty H, Wang XL, Looi J, Lorentz L, Howard L, Jones M, Zagami AS, Gillies D, Wilcken DEL (2003) Homocysteine as a risk factor for cognitive impairment in stroke patients. Dement Geriatr Cogn Disord 15:155–162
Saravanan G, Prakash J (2004) Effect of garlic (Allium sativum) on lipid peroxidation in experimental myocardial infarction in rats. J Ethnopharmacol 94:155–158
Schwahn BC, Chen Z, Laryea MD, Wendel U, Lussier-Cacan S, Genest J Jr, Mar M-H, Zeisel SH, Castro C, Garrow T, Rozen R (2003) Homocysteine–betaine interactions in a murine model of 5,10- methylenetetrahydrofolate reductase deficiency. FASEB J 17:512–514
Schwahn BC, Laryea MD, Chen Z, Melnyk S, Pogribny I, Garrow T, James SJ, Rozen R (2004) Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency. Biochem J 382:831–840
Sher L, Oquendo MA, Grunebaum MF, Burke AK, Huang Y, Mann JJ (2007) CSF monoamine metabolites and lethality of suicide attempts in depressed patients with alcohol dependence. Eur Neuropsychopharmacol 17:12–15
Smith AM, Zeve DR, Grisel JJ, Chen WJA (2005) Neonatal alcohol exposure increases malondialdehyde (MDA) and glutathione (GSH) levels in the developing cerebellum. Dev Brain Res 160:231–238
Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, McClain C (2003) S-adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice. J Nutr Biochem 14:591–597
Subbarao KV, Richardson JS, Ang LC (1990) Autopsy samples of Alzheimer's cortex show increase peroxidation in vitro. J Neurochem 55:342–345
Sun AY, Ingelman-Sundberg M, Neve E et al (2001) Ethanol and oxidative stress. Alcohol Clin Exp Res 25:237–243
Tran TD, Jackson HD, Horn KH, Goodlett CR (2005) Vitamin E does not protect against neonatal ethanol-induced cerebellar damage or deficits in eyeblink classical conditioning in rats. Alcohol Clin Exp Res 29:117–129
Vajragupta O, Boonyarat C, Murakami Y et al (2006) A novel neuroprotective agent with antioxidant and nitric oxide synthase inhibitory action. Free Radic Res 40:685–695
Wang ZJ, Liang CL, Li GM et al (2006) Neuroprotective effects of arachidonic acid against oxidative stress on rat hippocampal slices. Chem Biol Interact 163:207–217
Acknowledgment
The authors wish to thank Dr. A. Tamaddon (A member of Stem Cell and Transgenic Technology Research Center Shiraz University of Medical Sciences Shiraz, Iran) for his scientific comments and expert revision on the final manuscript of this study. We also like to thank Saeedeh Ahmadi for the kind technical assistance. We are most grateful to M. Shoaei and R. Shirazi (the member and manager of Aryadalman Company, Tehran, Iran) for providing betaine (Betafine®).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alirezaei, M., Jelodar, G., Niknam, P. et al. Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat cerebellum. J Physiol Biochem 67, 605–612 (2011). https://doi.org/10.1007/s13105-011-0107-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-011-0107-1