Abstract
Microcystin-LR (MCLR), one of the most popular microcystins (MCs) found in many field water bodies around the world, poses great health risks to animals and humans. In the present study, healthy common carp (initial weight 24.8 ± 2.3 g) were randomly assigned to five groups. Group I was fed on normal diet as control. Group II was maintained on normal diet and received MCLR intraperitoneal injection (150 μg kg−1 BW). Common carp in groups III, IV, and V were daily pretreated with L-carnitine (LC) at doses of 0.5, 1.0, and 2.0 g kg−1 of the diet for 4 weeks prior to MCLR intraperitoneal injection. The results showed that MCLR alone led to a significant downregulation in immune response, including serum complement C3, lysozyme, and bactericidal activity. However, oxidative stress response: catalase (CAT), superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GPx), and lipid peroxidation (LPO) levels were significantly increased. Similarly, gene expressions of inflammatory IL-1β, TNF-α, IFN I, and heat shock proteins (HSP70 and HSP90) were also upregulated after challenged with MCLR. However, LC pretreated group caused a significant elevation in immune response (C3, lysozyme, and bactericidal activity) and gene expressions of inflammatory IL-1β, TNF-α, IFN I, and heat shock proteins (HSP70 and HSP90) after MCLR stress. Antioxidant activities (CAT, SOD, GSH, GPx, and LPO) were returned to background levels at 96 h after MCLR challenge. Strikingly, LC supplementation at 2.0 g kg−1 has been considered the optimum for common carp since it exhibited enhancement of immune response and antioxidant activity over the level 0.5 and 1.0 g kg−1, and even better than that of control level. It was concluded that LC as a functional feed additive significantly inhibited the progression of MCLR-induced immunotoxicity and oxidative stress in common carp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacterial blooms and the associated cyanotoxins are being increasingly reported worldwide (Palus et al. 2007; Graham et al. 2010; Davis et al. 2012). These toxins can be accumulated in aquatic organisms and transferred to higher trophic levels, representing a health hazard to animals and humans (Chen et al. 2009; Campos and Vasconcelos 2010). Among all the cyanotoxins, microcystins (MCs) are the most frequently studied due to their wide distribution and high toxicity. Microcystin-LR (MCLR) is generally recognized as one of the most toxic microcystin variants, and the concentration in surface waters often exceeds the World Health Organization advisory level of 1 μg l−1 (Organization 2004). MCs are released from the cyanobacterial cells into the water bodies where aquatic organisms especially fish spend their whole life stage, including growth, reproduction, and embryonic development (Zhang et al. 2009).
Although MCs have been demonstrated to cause damages to fish intestine, kidney, gills, heart, and brain (Qiu et al. 2009; Li et al. 2012; Chen et al. 2012; Trinchet et al. 2011; Li et al. 2013), the liver is the most affected organ in fish, with symptoms of hepatocyte dissociation, degeneration, and necrosis (Malbrouck et al. 2003; Li and Xie 2009). A classic toxic mechanism of MCs is their inhibition of protein phosphatase 1 and 2A, leading to increased protein phosphorylation, which is directly related to their cytotoxicity and tumor-promoting activity (Honkanen et al. 1990). Several evidences showed that oxidative stress played an important role in the pathogenesis of MC toxicity in aquatic organisms such as crab (Pinho et al. 2003) and tilapia Oreochromis niloticus (Prieto et al. 2006, 2007).
Because of the rapid, irreversible, and severe damage to the liver caused by MCs, therapy is likely to have little or no value; effective prophylaxis is critical. In spite of the potential human hazards associated with MCs, very little work has been done on the development of effective chemoprotectants or antidotes against these toxins. L-Carnitine (LC) is synthesized from the essential amino acids (lysine and methionine) with the assistance of vitamin C and other secondary compounds produced in the body (Harpaz et al. 2005). The supplementation of LC in fish diets has been advocated in aquaculture for multiple reasons: LC can be used to improved growth performance indices including specific growth rate (SGR), feed conversion ratio (FCR), and survival rate (Saliny et al. 1994), to protect against toxic levels of xenobiotics (Schreiber et al. 1997), to ameliorate stress that is related to water temperature extremes, to facilitate better acclimation to water temperature changes (Harpaz et al. 1999), and to improve immune responses (Safari et al. 2015). Of late, the role of LC as an antioxidant agent has been confirmed on ischemia–reperfusion injury, adriamycin-induced membrane damage, and diphtheria toxins (Ma et al. 2008). However, there is little information about the protective effects of LC on MCLR-induced immunotoxicity and oxidative stress in aquaculture. The aim of the present study is to evaluate the protective effect of LC on the prevention of MCLR stress by evaluating the activities of antioxidant-related enzyme and gene expressions of inflammatory and immune response in common carp.
Materials and methods
Feed and experimental design
The experimental design was completely randomized with four treatment diets, each of which was replicated three times. For each treatment replicate, 30 common carp (24.8 ± 2.3 g) were randomly chosen and placed in 300-l cycling-filtered plastic tanks containing continuously circulating aerated water. The four treatment diets were as follows: group I (control group I) and group II (control group II) were always fed with control (basic) diet throughout the feeding trial; groups III, IV, and V were fed 0.5, 1.0, and 2.0 g kg−1 LC (Sigma, ≥98%), respectively. Fish were fed twice a day (9:00 and 15:00) at a rate of 3% of the bodyweight and kept in glass aquaria at 23 ± 1 °C with laboratory conditions as mentioned above for 4 weeks. Tank bottom debris was removed by siphon daily, and about one third of the water was replaced daily.
MCLR challenge experiments
After 4 weeks of feeding the fish, one set of fish (n = 25) from groups II, III, IV, and V was injected i.p. with MCLR at dose of 150 μg kg−1 BW. Doses of MCLR used in the experiment were based on the result from 48-h LD 50 study of MCLR in our previous experiment, which calculated the LD 50 value (310.5 μg kg−1 BW) with a 95% confidence interval (256.8–364.2 μg kg−1 BW). The control (group I) fish were injected i.p. with equal volume of physiological saline solution (0.85% NaCl). In the experiment, sampling points were set at 0, 12, 24, 48, and 96 h (two and one fish died at 12 and 24 h, and no more dead fish was found at 48 and 96 h. However, no mortality was found in the control.). At each sampling point, five fish from each dose group were anesthetized with 0.02% tricaine methane sulfonate (MS-222) solution. Live of five individuals were excised and immediately frozen in liquid nitrogen and then stored at −80 °C for analysis of gene expression and enzyme activities. Blood of all fish from each group was also analyzed for serum complement C3, lysozyme, and bactericidal activity.
Antioxidant enzyme activity assays
Total superoxide dismutase (SOD) activity was determined following the methods of Beauchamp and Fridovich (Lawrence et al. 1976). Catalase (CAT) activity was determined by measuring the decrease in H2O2 concentration. Glutathione peroxidase (GPx) activity was assayed by following the rate of NADPH oxidation at 340 nm by the coupled reaction with glutathione reductase according to the method by Lawrence and Burk (1976). The contents of GSH were determined using commercial kits (Nanjing, Jiangsu, China) following the manufacturer’s instructions.
Lipid peroxidation level assays
The thiobarbaturic acid (TBA) method of Esterbauer and Cheeseman (1990) was used to determine the lipid peroxidation by determining the amount of TBA reactive substances present in the liver homogenates obtained from common carp.
Serum immune parameter assays
Blood was sampled from the caudal vasculature using a 2.5-ml syringe after the fish were euthanized by overdose of MS-222. Individual fish was sampled only once to avoid the influence on the assays due to multiple bleeding and handling stress on the fish. Lysozyme activity measurement was based on the turbidimetric method described by Ellis (1990). A unit of lysozyme activity was defined as the amount of serum lysozyme that caused a decrease in absorbancy of 0.001 per minute at 530 nm.
The serum bactericidal activity was determined according to the method which previously described (Barnes et al. 2003) with some modification. Staphylococcus aureus isolates were adjusted to 1.0 (OD540; 3.0 × 107 CFU ml−1). Bacterial suspension and serum samples were mixed with 1:1 ratio and incubated for 90 min at 25 °C. Thereafter, 10 ml of serum and bacteria mixtures were transferred to the Shieh broth medium and cultured for 24 h at 25 °C. Phosphate-buffered saline (PBS, Sigma) was used instead of serum as negative control. Viable colonies were counted, and results expressed as percentage of survival in the PBS controls.
The serum complement C3 level was assayed using Complement C3 assay kit (Jiancheng, Nanjing, Jiangsu, China) (Wang et al. 2011). Results are presented as complement C3 milligram per milliliter.
Immune relates gene expression
The gene expressions of inflammatory IL-1β, TNF-α, IFN I, and heat shock proteins (HSP70 and HSP90) were tested according to our previous study (Chen et al. 2015). Total RNA was extracted from blood samples by TRIzol Reagent (SimGEN). Complementary DNA (cDNA) was then synthesized using the Reverse Transcriptase M-MLV Kit (TaKaRa) following the instructions. The real-time quantitative PCR was performed using THUNDERBIRD SYBR qPCR Mix Kit (TOYOBO) and carried out in astratagene MxProSystem (stratagene mx3005p, USA) in 96-well reaction plates. The β-actin gene was used as a housekeeping gene. The reaction mixture included 10 μl of THUNDERBIRD SYBR qPCR Mix, 1 μl of forward and reverse primer (10 mM), and 1 μl of cDNA and was then filled up with ultra-pure water to a final total volume of 20 ml. The cycling conditions were as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s. All PCRs were performed at least three times. Additional dissociation curve analysis was performed and showed a single melting curve in all cases. The PCR efficiency of each primer was between 98.5 and 99.6%. Data were analyzed by the stratagene MxPro software (stratagene mx3005p, USA).
Data analysis
The data in this study were analyzed by Statistical Product and Service Solutions (SPSS 16.0) and expressed as the arithmetic mean ± standard deviation. Data were analyzed by repeated measures analysis of variance (ANOVA) and the LSD post hoc test. The homogeneity of the replicates of the samples was checked by the Mann–Whitney U test. Differences between the three groups were measured and considered statistically different at P < 0.05 or P < 0.01.
Results
Activity of antioxidant enzyme after MCLR stress
The results of antioxidant enzyme activity assays were presented in Tables 1, 2, 3, 4 and 5. As shown in tables, antioxidant activity (CAT, SOD, GSH, GPx, LPO) in group II (MCLR treated but with no LC pretreatment) was significantly increased as compared with the control group, indicating severe oxidative stress in common carp.
Common carp fed the diets containing 0.5, 1.0, and 2.0 g kg−1 LC had significantly higher CAT, SOD, and GPx activities compared to fish fed the control diets (P < 0.05) at 0 h (before MCLR stress) (Tables 1, 2 and 3); however, only SOD activities showed the statistical difference (P < 0.05) (Table 3). After MCLR stress, activities of CAT, SOD, and GPx were increased firstly and then decreased, the highest values were observed at 12 h, and group V had returned to the original values at 48–96 h post-stress. As for GSH, slight increases in GSH content were detected after MCLR stress at 0 h (Table 4), but there was no significant difference among them. High dose of LC pretreated group (group V) showed a significant increase in the GSH levels when compared with MCLR (P < 0.01). Better preventive effects were observed at dose of 2.0 g kg−1 than other LC pretreated groups.
LPO activity
The results of LPO activity assays are presented in Table 5. LPO levels in group II (MCLR treated with no LC pretreatment) were significantly elevated in response to MCLR treatment as compared with fish fed the control (basal) diets (group I, P < 0.05) at 0 h (before MCLR stress); it indicated that MCLR caused obvious oxidative damages on common carp. We found that the increase was dramatically diminished by LC pretreatment at 2.0 g kg−1 dosage.
Immune parameter assays
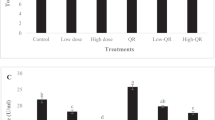
Serum complement C3, bactericidal, and lysozyme activities of common carp among any treatment groups at day 0 had no significant changes. However, all data in group II were significantly decreased as compared to control group (P < 0.05) after MCLR stress. After treated with MCRL, serum complement C3, bactericidal, and lysozyme activities in groups III, IV, and V were increased, and group V had returned to the original values at 96 h post-stress (Figs. 1, 2 and 3).
Bactericidal activity (U ml−1) of common carp fed with LC-containing diet after MCLR stress. Values followed by the different letters are significantly different (P < 0.05). Fish fed with basic diets and challenged with no MCLR (Group I). Fish fed with basic diets and challenged with 150 μg kg−1 BW MCLR (Group II). Fish fed with 0.5 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group III). Fish fed with 1.0 g kg−1 and challenged with 150 μg kg−1 BW MCLR (Group IV). Fish fed with 2.0 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group V)
Lysozyme activity (U ml−1) of common carp fed with LC-containing diet after MCLR stress. Values followed by the different letters are significantly different (P < 0.05). Fish fed with basic diets and challenged with no MCLR (Group I). Fish fed with basic diets and challenged with 150 μg kg−1 BW MCLR (Group II). Fish fed with 0.5 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group III). Fish fed with 1.0 g kg−1 and challenged with 150 μg kg−1 BW MCLR (Group IV). Fish fed with 2.0 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group V)
Complement C3 of common carp fed with LC-containing diet after MCLR stress. Values followed by the different letters are significantly different (P < 0.05). Fish fed with basic diets and challenged with no MCLR (Group I). Fish fed with basic diets and challenged with 150 μg kg−1 BW MCLR (Group II). Fish fed with 0.5 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group III). Fish fed with 1.0 g kg−1 and challenged with 150 μg kg−1 BW MCLR (Group III). Fish fed with 2.0 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group V)
Immune relates gene expression
The transcriptional changes of IL-1β, TNF-α, and IFN I genes in the blood of common carp are shown in (Figs. 4, 5 and 6). All the test gene transcriptions were markedly increased by MCLR stress as compared with group II (P < 0.05). The administration of LC alone (at 0 h) did not affect gene expression of messenger RNA (mRNA) of all the tested genes.
The relative expression of IFN I in the livers of common carp fed with LC-containing diet after MCLR stress. Values followed by the different letters are significantly different (P < 0.05). Fish fed with basic diets and challenged with no MCLR (Group I). Fish fed with basic diets and challenged with 150 μg kg−1 BW MCLR (Group II). Fish fed with 0.5 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group III). Fish fed with 1.0 g kg−1 and challenged with 150 μg kg−1 BW MCLR (Group IV). Fish fed with 2.0 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group V)
The relative expression of IL-1β in the livers of common carp fed with LC-containing diet after MCLR stress. Values followed by the different letters are significantly different (P < 0.05). Fish fed with basic diets and challenged with no MCLR (Group I). Fish fed with basic diets and challenged with 150 μg kg−1 BW MCLR (Group II). Fish fed with 0.5 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group III). Fish fed with 1.0 g kg−1 and challenged with 150 μg kg−1 BW MCLR (Group IV). Fish fed with 2.0 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group V)
The relative expression of TNF-α in the liver of common carp fed with LC-containing diet after MCLR stress. Values followed by the different letters are significantly different (P < 0.05). Fish fed with basic diets and challenged with no MCLR (Group I). Fish fed with basic diets and challenged with 150 μg kg−1 BW MCLR (Group II). Fish fed with 0.5 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group III). Fish fed with 1.0 g kg−1 and challenged with 150 μg kg−1 BW MCLR (Group IV). Fish fed with 2.0 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group V)
Compared with group II, transcriptional level of IL-1β and IFN I in groups III, IV, and V was significantly decreased in most cases after MCLR treatment (P < 0.05). The TNF-α mRNA levels were downregulated in the fish fed the diets containing 0.5, 1.0, and 2.0 g kg−1 LC as compared to the control after MCLR stress; however, only group V showed the statistical difference (P < 0.05); it had recovered to the normal level at 96 h (Table 5).
The effects of LC in the expression of HSP70 and HSP90 genes after MCLR stress were determined. The relative expression of HSP70 gene (Fig. 7) transcript level was increased before 24 h but fell back to the blank levels at 96 h. The expression of HSP90 in groups III, IV, and V was increased firstly and then decreased; the highest values were observed at 12 h and had returned to the original values at 96 h (Fig. 8).
The relative expression of HSP70 in the liver of common carp fed with LC-containing diet after MCLR stress. Values followed by the different letters are significantly different (P < 0.05). Fish fed with basic diets and challenged with no MCLR (Group I). Fish fed with basic diets and challenged with 150 μg kg−1 BW MCLR (Group II). Fish fed with 0.5 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group III). Fish fed with 1.0 g kg−1 and challenged with 150 μg kg−1 BW MCLR (Group IV). Fish fed with 2.0 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group V)
The relative expression of HSP90 in the liver of common carp fed with LC-containing diet after MCLR stress. Values followed by the different letters are significantly different (P < 0.05). Fish fed with basic diets and challenged with no MCLR (Group I). Fish fed with basic diets and challenged with 150 μg kg−1 BW MCLR (Group II). Fish fed with 0.5 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group III). Fish fed with 1.0 g kg−1 and challenged with 150 μg kg−1 BW MCLR (Group IV). Fish fed with 2.0 g kg−1 LC and challenged with 150 μg kg−1 BW MCLR (Group V)
Discussion
The monocyclic heptapeptide MCs are the most common and abundant cyanotoxins and pose a world health threat to humans and animals (de Figuereido et al. 2004). Oxidative stress is considered the major cytotoxic mechanism of MCLR. Various antioxidants and detoxicants have been studied to identify new compounds that can regulate the oxidative stress and toxic effects caused by MCLR (Prieto et al. 2008). Recently, there is an increasing interest in natural feed additives as potent antioxidants (Ma et al. 2008). In the present study, we explored the usefulness of dietary pretreatment with LC (0.5, 1.0, and 2.0 g kg−1 of fish over a 4-week period) as prophylaxis for MCLR-induced oxidative stress in common carp for the first time.
Increasing evidences suggest that oxidative stress as a result of excessive reactive oxygen species (ROS) production may play an important role in the toxic mechanism of MCs (Li et al. 2003; Amado and Monserrat 2010; Chen et al. 2012). Under normal physiological situations, there is a balance between ROS production and the antioxidant defense system. Cellular oxidative stress occurs when the physiological antioxidant protection does not counteract the elevated ROS levels (Prieto et al. 2008; Jiang et al. 2012), and the antioxidant system can counteract the ROS and reduce the oxidative stress with the antioxidant enzymes (SOD, CAT, and GPx). SOD catalyzes the conversion of superoxide to hydrogen peroxide, while CAT or GPx reduces hydrogen peroxide to H2O (Cadenas 1989). The results of our study indicate that MCLR induces oxidative stress in live common carp and altered activity of antioxidant enzymes. Concretely, the activity of SOD, GPx, and GST was increased after MCLR exposure, possibly indicating their scavenging activity against ROS. In line with our study, Li et al. (2003) also reported an enhancement of SOD activity in hepatocytes of common carp (Cyprinus carpio L.) exposed to MCLR. Prieto et al. (2006) showed that i.p.-administered pure MCLR (500 μg kg−1) induced a significant increase in the activity of CAT, SOD, and glutathione reductase (GR) in the liver of tilapia fish. In the case of oral uptake route, a time-dependent increase in the activity of CAT, SOD, and GPx was also observed in the liver of tilapia fed with crush lyophilized cyanobacterial cells (approximately 60.0 μg MCLR fish−1 day−1) for 21 days (Jos et al. 2005). On the contrary, Liu et al. (2014) found that activities of SOD, CAT, and GPx drastically decreased in parental MCLR-treated groups compared with the control. The activities of GPx, GR, SOD, and CAT enzymes decreased after fish were orally exposed to a single dose of cyanobacterial cells containing 120 g per fish MCLR (Prieto et al. 2007). Under balneation conditions, Pavagadhi et al. (2012) found that enzyme activities including GST, GPx, and SOD increased at lower concentrations (≤5.0 μg l−1) and decreased at higher concentrations (≥5.0 μg l−1) in the liver of adult zebrafish after MCLR exposure. The higher MC dose may damage the enzyme proteins, while the lower dose, given for a longer period of time, may induce a defensive response. The antioxidant response to MC exposure varied largely and mainly depended upon the dose of the toxins and the exposure route (Malbrouck and Kestemont 2006).
GSH is one of the major antioxidant proteins, protecting the cell against the effects of reactive oxygen species (Nanda et al. 1996; Shila et al. 2005). It is also an essential protein in maintaining other antioxidant proteins, since it is responsible for preserving the reductive nature of the cell and regulating the binding of xenobiotics with cellular thiol. It is present in higher concentrations in metabolizing organs, such as the liver (Shila et al. 2005). In the present study, GSH level was drastically increased in MCLR-treated groups compared with the control. Lipid peroxidation is particularly important for aquatic animals since they normally contain greater amounts of highly unsaturated fatty acids (HUFAs) than other species (Huang et al. 2003). In the present study, the increase of LPO, as assessed by the formation of MDA, found in the liver of common carp treated with MCLR, suggests oxidative stress during MCLR intoxication. LC pretreated fish showed significantly decreased levels as compared to MCLR. This observation directly demonstrates the antiperoxidative and antioxidant effects of LC. Similarly, Ma et al. (2008) reported that dietary L-carnitine decreased the iron-induced lipid peroxidation in liposomes through formation of free iron complexes. This advised that dietary L-carnitine lowered lipid peroxidation and improved resistance to oxidative stress in crayfish.
Some studies reported that the toxicity of MCLR was related to the stimulation of immune system (Li et al. 2012; Rymuszka 2013; Qiao et al. 2013). Recently, some cytokines have been studied to explore the immunomodulatory effects of MCs. In the present study, after the fish were fed with LC for 4 weeks, serum complement C3, lysozyme, and bactericidal activity were significantly (P < 0.05) increased after treated with MCRL as compared to the control group (group II). It indicated that LC enhances the protective effect against MCRL-induced immune response. TNF-α is considered to be an important component in innate immunity and inflammatory responses in fish (Rosa et al. 2008) and essential for inflammatory response to pathogenic germs or toxicants (Liew 2003). IFN 1 provides an important first line of defense against toxicants (McBeath et al. 2007). Some evidences demonstrated that MCLR affected the transcription of TNF-α and IFN I in fish (Wei et al. 2009; Rymuszka and Adaszek 2012). In the present study, after the fish were fed with LC for 4 weeks, a strong downregulation in IL-1β, TNF-α, and IFN I expression was observed as compared to the control group after treated with MCLR (group II). The downregulated genes might have controlled the inflammatory response of the stimulated pro-inflammatory cytokines, thereby minimizing damage to the host due to an excessive response (Raida et al. 2008)
HSPs are another protection system to protect the organisms from oxidative stress by preventing the irreversible loss of vital proteins and facilitating their subsequent regeneration (Jiang et al. 2012). When organisms are exposed to a variety of stress factors such as cold, heat, CO2, heavy metal, and various chemicals (Hoffmann and Parsons 1991; Ferrando et al. 1995), they synthesize a set of HSPs, which usually act as molecular chaperones, and play diverse roles in transporting, folding, and assembling of degraded or misfolded proteins (Johnston et al. 1998; Sφrensen et al. 2003). Heat shock proteins, particularly HSP70, have been proposed as biochemical markers of environmental stress. The HSP induction may rely on perturbation of the cellular redox status (Rai et al. 2005). In the present study, HSP70 and HSP90 gene expressions in the serum of common carp were significantly higher as compared to control group after treated with MCLR. The dramatically increased transcription of HSP90 and HSP70 may indicate their important roles as molecular chaperones under oxidative stress caused by MCLR, and the expression level recovered to original level at 96 h. In agreement with the results of the present study, HSP70 gene expression in serum was significantly (P < 0.05) downregulated in the treatment group fed with Immunogen® in rainbow trout as compared with control group. Downregulation of HSP70 expression is possibly due to elevated tolerance toward usual stresses during culture-like stresses caused by sampling for monitoring water quality, fish biometry, or other unwanted stresses (Wang et al. 2011).
In conclusion, dietary supplementation with LC induced expression of several antioxidant enzyme-related genes, stimulated PO, CAT, SOD, AST, and ALT activity, and improved the survival rate against ammonia stress. The results obtained indicated that LC has a protective effect against ammonia-induced oxidative stress in common carp.
References
Amado LL, Monserrat JM (2010) Oxidative stress generation by microcystins in aquatic animals: why and how. Environ. Int. 36:226–235
Barnes AC, Young FM, Horne MT, Ellis AE (2003) Streptococcus iniae: serological differences, presence of capsule and resistance to immune serum killing. Dis Aquat Org 53:241–247
Cadenas E (1989) Biochemistry of oxygen toxicity. Annu Rev Biochem 58:79–110
Chen XM, Ru HM, Niu XT, Wang GQ, Zhang DM (2015) Enhancement of secondary metabolites from Bacillus Licheniformis XY-52 on immune response and expression of some immune-related genes in common carp, Cyprinus carpio. Fish Shellfish Immunol 45(1):124–131
Campos A, Vasconcelos V (2010) Molecular mechanisms of microcystin toxicity in animal cells. Int J Mol Sci 11:268–287
Chen J, Zhang D, Xie P, Wang Q, Ma Z (2009) Simultaneous determination of microcystin contaminations in various vertebrates (fish, turtle, duck and water bird) from a large eutrophic Chinese lake, Lake Taihu, with toxic Microcystis blooms. Sci Total Environ 407:3317–3322
Chen Y, Zeng SF, Cao YF (2012) Oxidative stress response in zebrafish (Danio rerio) gill experimentally exposed to subchronic microcystin-LR. Environ Monit Assess 184:6775–6787
Davis TM, Koch F, Marcoval MA, Wilhelm SW, Gobler CJ (2012) Mesozooplankton and microzooplankton grazing during cyanobacterial blooms in the western basin of Lake Erie. Harmful Algae 15:26–35
de Figuereido DR, Azeiteiro UM, Esteves SM, Goncalves FJM, Pereira JM (2004) Microcystin-producing blooms—a serious global public health issue. Ecotox Environ Saf 59(2):151–163
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxnonenal. Methods Enzymol 186:407–421
Ellis AE, Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB (1990) Techniques in fish immunology. SOS Publications, NJ, pp 101–103
Ferrando RE, Schuschereba ST, Quong JA, Bowman PD (1995) Carbon dioxide laser induction of heat shock protein 70 synthesis: comparison with high temperature treatment. Lasers Med Sci 10:207–212
Graham JL, Loftin KA, Meyer MT, Ziegler AC (2010) Cyanotoxin mixtures and taste-and-odor compounds in canobacterial blooms from the Midwestern United States. Comp Biochem Physiol B: Environ Sci Technol 44:7361–7368
Harpaz S (2005) L-Carnitine and its attributed functions in fish culture and nutrition-a review. Aquacult 249:3–21
Harpaz S, Becker K, Blum R (1999) The effect of dietary lcarnitine supplementation on cold tolerance and growth of ornamental cichlid fish (Pelvicachromis pulcher)—preliminary results. J Thermal Biology 24:57–62
Honkanen RE, Zwiller JEMR, Moore RE, Daily SL, Khatra BS, Dukelow M, Boynton AL (1990) Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. J Biol Chem 265:19401–19404
Hoffmann AA, Parsons PA (1991) Evolutionary genetics and environmental stress. Oxford University Press, New York
Huang CH, Chang RJ, Huang SL, Chen W (2003) Dietary vitamin E supplementation affects tissue lipid peroxidation of hybrid tilapia. Oreochromis niloticus × O. aureus. Comp Biochem Physiol B 134:265–270
Jiang JL, Shi Y, Shan ZJ, Yang LY, Wang XR, Shi LL (2012) Bioaccumulation, oxidative stress and HSP70 expression in Cyprinus carpio L. exposed to microcystin-LR under laboratory conditions. Comp Biochem Phys C 155:483–490
Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular response to misfolded proteins. J Cell Biol 143:1883–1898
Jos Á, Pichardo S, Prieto AI, Repetto G, Vázquez CM, Morenoa I (2005) Toxic cyanobacterial cells containing microcystins induce oxidative stress in exposed tilapia fish (Oreochromis sp.) under laboratory conditions. Aquat Toxicol 72:261–271
Lawrence A, Burk RF (1976) Glutathione peroxidase activity in selenium deficient rat liver. Bioch Bioph Res Co 71:952–958
Li G, Yan W, Qiao Q, Chen J, Cai F, He Y, Zhang X (2012) Global effects of subchronic treatment of microcystin-LR on rat splenetic protein levels. J Proteome 77:383–393
Li L, Xie P (2009) Hepatic histopathological characteristics and antioxidant response of phytoplanktivorous silver carp intraperitoneally injected with extracted microcystins. Biomed Environ Sci 22:297–302
Li L, Xie P, Lei H, Zhang X (2013) Renal accumulation and effects of intraperitoneal injection of extracted microcystins in omnivorous crucian carp (Carassius auratus). Toxicon 49:1150–1157
Li X, Liu Y, Song L, Liu J (2003) Responses of antioxidant systems in the hepatocytes of common carp (Cyprinus carpio L.) to the toxicity of microcystin-LR. Toxicon 42:85–89
Liew FY (2003) The role of innate cytokines in inflammatory response. Immunol Lett 85:131–134
Liu WJ, Qiao Q, Chen YY, Wu K, Zhang XZ (2014) Microcystin-LR exposure to adult zebrafish (Danio rerio) leads to growth inhibition and immune dysfunction in F1 offspring, a parental transmission effect of toxicity. Aquatic Toxicol 155:360–367
Ma JJ, Xu ZRQ, Shao JJ, Xu Z, Hung SSO, Hu WL, Zhou LY (2008) Effect of dietary supplemental L-carnitine on growth performance, body composition and antioxidant status in juvenile black sea bream, Sparus macrocephalus. Aquac Nutr 14:464–471
Malbrouck C, Trausch G, Devos P, Kestemont P (2003) Hepatic accumulation and effects of microcytin-LR on juvenile goldfish Carassius auratus L. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 135:39–48
Malbrouck C, Kestemont P (2006) Effects of microcystins on fish. Environ Toxicol Chem 25:72–86
McBeath A, Snow M, Secombes C, Ellis A, Collet B (2007) Expression kinetics of interferon and interferon-induced genes in Atlantic salmon (Salmo salar) following infection with infectious pancreatic necrosis virus and infectious salmon anaemia virus. Fish. Shellfish. Immun. 22:230–241
Nanda D, Tolputt J, Collard KJ (1996) Changes in brain glutathione levels during postnatal development in the rat. Dev Brain Res 94:238–241
Organization, W.H 2004. Guidelines for Drinking Water Quality, vol. 1 Recommendations. World Health Organization, Geneva
Palus J, Dziubałtowska E, Stanczyk M, Lewinska D, Mankiewicz-Boczek J, Izydorczyk K, Bonisławska A, Jurczak T, Zalewski M, Wasowicz W (2007) Biomonitoring of cyanobacterial blooms in polish water reservoir and the cytotoxicity and genotoxicity of selected cyanobacterial extracts. Int J Occup Med Environ 20:48–65
Pavagadhi S, Gong Z, Hande MP, Dionysiou DD, de la Cruz AA, Balasubramanian R (2012) Biochemical response of diverse organs in adult Danio rerio (zebrafish) exposed to sub-lethal concentrations of microcystin-LR and microcystin-RR: a balneation study. Aquat Toxicol 109:1–10
Pinho GLL, Moura da Rosa C, Yunes JS, Luquet CM, Bianchini A, Monserrat JM (2003) Toxic effects of microcystins in the hepatopancreas of the estuarine crab Chasmagnathus granulatus (Decapoda, Grapsidae). Comp Biochem Physiol C: Toxicol Pharmacol 135:459–468
Prieto AI, Jos Á, Pichardo S, Moreno I, Cameán AM (2006) Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat Toxicol 77:314–321
Prieto AI, Pichardo S, Jos Á, Moreno I, Cameán AM (2007) Time-dependent oxidative stress responses after acute exposure to toxic cyanobacterial cells containing microcystins in tilapia fish (Oreochromis niloticus) under laboratory conditions. Aquat Toxicol 84:337–345
Prieto AI, Jos Á, Pichardo S, Moreno I, Cameán AM (2008) Protective role of vitamin E on the microcystin-induced oxidative stress in tilapia fish (Oreochromis niloticus). Environ Toxicol Chem 27:1152–1159
Qiu T, Xie P, Liu Y, Li G, Xiong Q, Hao L, Li H (2009) The profound effects of microcystin on cardiac antioxidant enzymes, mitochondrial function and cardiac toxicity in rat. Toxicology 257:86–94
Qiao Q, Liang H, Zhang X (2013) Effect of cyanobacteria on immune function of crucian carp (Carassius auratus) via chronic exposure in diet. Chemosphere 90:1167–1176
Raida MK, Buchmann K (2008) Development of adaptive immunity in rainbow trout, Oncorhynchus mykiss (Walbaum) surviving an infection with Yersinia ruckeri. Fish Shellfish Immunol 25:533–541
Rai R, Richardson C, Flecknell P, Robertson H, Burt A, Manas DM (2005) Study of apoptosis and heat shock protein (HSP) expression in hepatocytes following radiofrequency ablation (RFA). J Surg Res 129:147–151
Rosa CE, Figueiredo MA, Lanes CFC, Almeida DV, Monserrat JM, Marins L (2008) Metabolic rate and reactive oxygen species production in different genotypes of GH-transgenic zebrafish. Comp Biochem Phys B 149:209–214
Rymuszka A, Adaszek Ł (2012) Pro-and anti-inflammatory cytokine expression in carp blood and head kidney leukocytes exposed to cyanotoxin stress—an in vitro study. Fish Shellfish Immun 33:382–388
Rymuszka A (2013) Microcystin-LR induces cytotoxicity and affects carp immune cells by impairment of their phagocytosis and the organization of the cytoskeleton. J Appl Toxicol 33:1294–1302
Safari O, Atash MMS, Paolucci M (2015) Effects of dietary L-carnitine level on growth performance, immune responses and stress resistance of juvenile narrow clawed crayfish, Astacus leptodactylus leptodactylus Eschscholtz, 1823. Aquaculture 439:20–28
Saliny, G. 1994. Effect of L-carnitine on growth responses of the fresh water prawn, Macrobrachium idella idella (Hilgendorf). University of Kerala, India. PhD thesis (213 pp)
Schreiber S, Becker K, Bresler V, Fishelson L (1997) Dietary l-carnitine protects the gills and skin of guppies (Poecilia reticulata) against anionic xenobiotics. Comp Biochem Physiol 117C:99–102
Sφrensen JG, Kristensen TN, Loeschcke V (2003) The evolutionary and ecological role of heat shock proteins. Ecol Lett 6:1025–1037
Shila S, Subathra M, Devi MA, Panneerselvam C (2005) Arsenic intoxicationinduced reduction of glutathione level and of the activity of related enzymes in rat brain regions: reversal by DL-alpha-lipoic acid. Arch Toxicol 79:140–146
Trinchet I, Djediat C, Huet H, Dao SP, Edery M (2011) Pathological modifications following sub-chronic exposure of medaka fish, Oryzias latipes to microcystin-LR. Reprod Toxicol 32:329–340
Wang GX, Wang Y, Wu ZF, Jiang HF, Dong RQ, Li FY et al (2011) Immunomodulatory effects of secondary metabolites from the rmophilic Anoxybacillus kamchatkensis XA-1 on carp, Cyprinus carpio. Fish Shellfish Immunol 30:1331–1338
Wei L, Sun B, Chang M, Liu Y, Nie P (2009) Effects of cyanobacterial toxin microcystin-LR on the transcription levels of immune-related genes in grass carp Ctenopharyngodon idella. Environ Biol Fish 85:231–238
Zhang DW, Xie P, Liu YQ, Qiu T (2009) Transfer, distribution and bioaccumulation of microcystins in the aquatic food web in Lake Taihu, China, with potential risks to human health. Sci Total Environ 407:2191–2199
Acknowledgments
The research was supported by the National Natural Science Foundation of China (31372540) and Foundatioh projects in Jilin Province (20140303049ZY).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, XM., Guo, GL., Sun, L. et al. Modulatory role of L-carnitine against microcystin-LR-induced immunotoxicity and oxidative stress in common carp. Fish Physiol Biochem 43, 1081–1093 (2017). https://doi.org/10.1007/s10695-017-0354-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0354-3