Abstract
In a sample of individuals with ovarian cancer, we aimed to (a) identify factors associated with the psychosocial impact of genetic counseling and multigene panel testing, (b) identify factors associated with cancer genetics knowledge, and (c) summarize patient-reported recommendations to improve the genetic counseling and multigene panel testing process. Eligible participants in this secondary analysis of quantitative and qualitative survey data were English-speaking adults with ovarian cancer. Psychosocial impact was assessed using the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Knowledge of cancer genetics was assessed using the KnowGene scale. Significant predictors of MICRA and KnowGene scores were identified using multiple regression. Open-ended survey item responses were analyzed using conventional content analysis. Eighty-seven participants met eligibility criteria. A positive genetic test result was associated with greater adverse psychosocial impact (B = 1.13, p = 0.002). Older age (B = − 0.07, p = 0.044) and being a member of a minority racial or ethnic group (B = − 3.075, p = 0.033) were associated with lower knowledge, while a personal history of at least one other type of cancer (B = 1.975, p = 0.015) was associated with higher knowledge. In open-ended item responses, participants wanted clinicians to assist with family communication, improve result disclosure, and enhance patient and family understanding of results. A subset of individuals with ovarian cancer who receive a positive genetic test result may be at risk for adverse psychosocial outcomes. Tailored cancer genetics education is necessary to promote the equitable uptake of targeted ovarian cancer treatment and risk-reducing therapies. Interventions to enhance patient-clinician communication in this setting are a research priority.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is one of the most heritable forms of cancer, with more than 20% of ovarian, fallopian tube, and primary peritoneal carcinomas associated with an inherited pathogenic variant (PV) [1, 2]. Between 30 and 44% of hereditary ovarian cancer cases occur in individuals without a known family history of breast or ovarian cancer[2, 3]; as such, the Society for Gynecologic Oncology and the National Comprehensive Cancer Network have recommended genetic counseling and testing for all individuals with epithelial ovarian carcinoma for over a decade [4, 5]. While PVs in BRCA1 or BRCA2 are implicated in the majority of hereditary ovarian cancers, advances in multigene panel testing have allowed for the identification of at least six additional PVs (BRIP1, MLH1, MSH2/EPCAM, PALB2, RAD51C, and RAD51D) associated with a moderate to high relative or cumulative lifetime risk of epithelial ovarian cancer [6,7,8]. Additional genes under investigation in epithelial ovarian cancer are NBN and MSH6 [6, 9]. Accordingly, the use of multigene panel testing to identify individuals with or at increased risk for hereditary ovarian cancer is rising [10].
Clinicians have been called to adapt to evolving clinical practice guidelines and the advent of multigene panel testing [11]. Genetic test results have the potential to inform ovarian cancer treatment, prognosis, and personal and familial cancer risk [1, 4, 5]. The presence of a PV in BRCA1 or BRCA2 is associated with increased sensitivity to platinum-based chemotherapy, increased sensitivity to poly-ADP ribose polymerase (PARP) inhibitors, and increased survival among individuals with epithelial ovarian cancer [1, 3]. In addition, recent studies have demonstrated that mismatch repair-deficient cancers are sensitive to immune checkpoint blockade, regardless of the cancer’s tissue of origin [12].
The presence of a PV has cancer risk implications for an individual’s relatives. Therefore, both positive and negative genetic test results have psychosocial implications for individuals with ovarian cancer and their families. Little research has evaluated the psychosocial impact of the multigene panel testing process on individuals with ovarian cancer, and knowledge of cancer genetics in this population has not been assessed. Given the developing role of precision oncology in ovarian cancer care, research that informs a patient-centered approach to genetic counseling and multigene panel testing in this setting is warranted.
Prior research suggests the experience of undergoing single gene or syndrome testing is not usually associated with a long-term increase in adverse psychosocial outcomes [13]. However, results from several studies suggest individuals with a prior psychiatric diagnosis may be more vulnerable to adverse psychosocial outcomes following genetic counseling and testing [14, 15]. Multigene panel testing differs from single gene or syndrome testing in that there is an increased likelihood of detecting a clinically non-actionable variant of uncertain significance (VUS). Likewise, risk profiles for PVs of many moderate-penetrance genes are not yet well-defined [16, 17], and the appropriate clinical management of individuals affected with one or more of these variants is often unclear. Given high rates of distress and uncertainty among individuals with ovarian cancer [18], improved understanding of the psychosocial impact of multigene panel testing is necessary to develop implementation strategies that preserve well-being in the face of ambiguous test results.
Multigene panel testing is essential to the identification of individuals and families affected by hereditary cancer syndromes other than Hereditary Breast and Ovarian Cancer (HBOC). Compared to single gene or syndrome testing, multigene panel testing has a higher diagnostic yield of PVs [19]. Regardless of genetic test result, the complex nature of cancer genetics information may lead to communication challenges during genetic counseling and clinical consultations. Individuals who are not confident in their knowledge of cancer genetics may be less likely to share genetic test results with relatives who may benefit from cascade testing [20]. Identifying gaps in cancer genetics knowledge among individuals undergoing multigene panel testing is therefore necessary to promote the uptake of preventive measures by individuals with hereditary ovarian cancer and their at-risk relatives.
The purpose of this study was to (a) assess the psychosocial impact of genetic counseling and multigene panel testing, (b) assess knowledge of cancer genetics, (c) identify factors associated with the psychosocial impact of genetic counseling and multigene panel testing, and (d) identify factors associated with knowledge of cancer genetics in a sample of individuals with ovarian cancer. In addition, this study aimed to summarize recommendations to improve the genetic counseling and multigene panel testing process provided by individuals with ovarian cancer. The results of this study may inform efforts to identify and assist individuals with ovarian cancer who may be at risk for adverse psychosocial or educational outcomes following genetic counseling and multigene panel testing.
Methods
This was a secondary analysis of quantitative and qualitative data collected as part of a larger study of cancer survivors’ experiences undergoing genetic counseling and multigene panel testing [21]. The parent study used a convergent parallel mixed methods design. The Dana-Farber/Harvard Cancer Center Institutional Review Board approved the study procedures.
Participants
Eligible participants for the parent study were English-speaking adults with a personal history of breast or gynecologic cancer who underwent genetic counseling and multigene panel testing in the 18 months prior to study enrollment. Participants were included in the current analysis if they had a primary diagnosis of ovarian cancer.
Setting
Participants were recruited from a single National Cancer Institute-designated cancer center. The genetic counseling and multigene panel testing process at this institution has been described in detail elsewhere [21]. Briefly, genetic counseling and multigene panel testing at this institution are integrated into routine ovarian cancer care. Genetic counselors meet patients in the infusion setting or at an arranged outpatient appointment to collect a family history and provide pre-test genetic counseling prior to genetic testing. Following genetic counseling, patients select from several panels that are appropriate for their personal and family history. Genetic test results are disclosed during a telephone call from the genetic counselor, which is followed by a mailed report. Patients may request a follow-up appointment to review genetic test results, and those with findings indicative of a PV are strongly encouraged to be seen by a cancer genetics physician and genetic counselor for in-depth discussion.
Measures
Demographic characteristics were obtained through self-report and included gender, race, ethnicity, marital status, annual household income, and educational attainment. Clinical characteristics were obtained through medical record review and included age, other cancer diagnoses, genetic test result, PV (if applicable), prior genetic testing, and number of months between genetic counseling and study enrollment. For the purposes of this study, a positive genetic test result was defined as the presence of any PV known to confer increased cancer risk, while a negative genetic test result was defined as the absence of any PV known to confer increased cancer risk. Categories of genetic test result were not mutually exclusive; for example, an individual may have received both a positive and a VUS result.
The psychosocial impact of multigene panel testing was measured with the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire [22]. The MICRA questionnaire is a valid, reliable measure comprised of three subscales: distress, uncertainty, and positive experience [22]. Possible total scores range from 0 to 95, with higher scores representing a more adverse psychosocial impact. Possible scores for the distress, uncertainty, and positive experience subscales range 0–30, 0–45, and 0–20, respectively. The positive experience subscale is reverse scored so that a higher score indicates a less positive experience. In the parent study, the MICRA was found to be reliable with a Cronbach alpha of 0.83 for the total scale, 0.88 for the distress subscale, 0.78 for the uncertainty subscale, and 0.77 for the positive experience subscale [21].
Cancer genetics knowledge related to multigene panel testing was measured using the KnowGene scale [23], which had a Cronbach alpha of 0.78 in the parent study [21]. The KnowGene scale was developed by members of our study team and is comprised of 19 items with three response options: “agree,” “disagree,” and “don’t know” [23]. The total score is calculated by tallying the number of correct responses, with “don’t know” being scored as an incorrect response. Scores can range 0–19, with higher scores representing greater knowledge. Items pertain to genetic test result interpretation, inheritance, screening and risk reduction, and clinical impact of genetic test results.
In addition to the above closed-ended survey measures, four open-ended survey items elicited suggestions to improve the genetic counseling and multigene panel testing process: (1) What parts of the process of genetic counseling and multigene panel testing do you think could be done differently? (2) What suggestions do you have to improve the process of genetic counseling and multigene panel testing? (3) What parts of the process of genetic counseling and multigene panel testing would you like to remain the same? (4) Is there anything else related to genetic counseling and testing that we have not discussed that you would like to share?
Data analysis
Demographic and clinical characteristics, MICRA total and subscale scores, and KnowGene scores were summarized using descriptive statistics. Specific cancer genetics knowledge needs were identified by calculating the proportion of participants who selected the correct response for each item on the KnowGene scale. Based on findings in the parent study, the following factors were assessed for potential associations with MICRA and KnowGene scores: marital status (married/partnered vs. not), educational attainment (college graduate vs. not), annual household income (less than $50,000 vs. $50,000 or more), race/ethnicity (white, non-Hispanic vs. not), pathogenic gene variant (BRCA vs. not), and genetic test result (positive vs. not, negative vs. not, and VUS vs. not) [21]. Consistent with the parent study, a square root transformation was applied to MICRA total and subscale scores to account for positively skewed distributions of these scores.
In univariate analyses, demographic and clinical characteristics associated with MICRA total, MICRA uncertainty, and KnowGene scores were identified using simple linear regression. Associations between MICRA total and subscale scores and KnowGene scores were also assessed using simple linear regression. Categorical demographic and clinical characteristics associated with MICRA distress subscale and MICRA positive experience subscale scores were identified using Wilcoxon’s rank-sum test. Continuous demographic and clinical characteristics associated with MICRA distress subscale and MICRA positive experience subscale scores were identified using Pearson’s correlation coefficient. Purposeful selection was used to identify variables likely to be significant predictors or confounders in multivariable models. Variables that were associated with MICRA total scores, MICRA subscale scores, or KnowGene scores with a p-value < 0.3 were entered into multivariable models. When more than one variable describing genetic test result (i.e., “positive vs. not,” “negative vs. not,” and “VUS vs. not”) met this criterion, the variable with the lowest p-value was entered into the multivariable model. In multivariable analyses, demographic and clinical characteristics significantly associated with MICRA total, MICRA uncertainty, MICRA distress, MICRA positive experience, and KnowGene scores were identified using multiple linear regression. All statistical tests were two-tailed and defined statistical significance as p ≤ 0.05. Given the exploratory nature of the study, no adjustments were made for multiple comparisons. Statistical analyses were conducted using the statistical software environment R (R Core Team 2017).
Open-ended survey item responses were analyzed in NVivo Pro 11 (QSR International 2016) using conventional content analysis [24]. Author RAP conducted the initial analysis, while authors MUB and MMN reviewed and refined the coding framework. Coding discrepancies were resolved through discussion.
Results
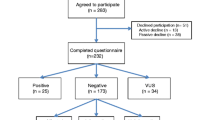
Eighty-seven participants met inclusion criteria. Participant characteristics are provided in Table 1. Participants underwent multigene panel testing through the Ambry Genetics CancerNext, GYNplus, and OvaNext panels; the Invitae Common Hereditary Cancers and Multi-Cancer panels; and the Myriad myRisk Hereditary Cancer panel. Twenty participants (23%) had a positive genetic test result; of these, nine had a BRCA-associated PV and 11 had a non-BRCA-associated PV. Non-BRCA-associated PVs were identified in BRIP1, MSH6, RAD51C, RAD51D, MUTYH, NBN, and RAD50. Forty-three participants (49.4%) had a negative genetic test result. Twenty-seven participants (31%) had a variant of uncertain significance (VUS) on its own or in addition to a positive genetic test result. Twenty-one participants (24.1%) had previously undergone genetic testing for BRCA1 and BRCA2 only, and 25 participants (28.7%) had a personal history of at least one other type of cancer. The mean number of months between pre-test genetic counseling and survey completion was 12.6 (SD = 5.04).
Psychosocial impact
The mean total MICRA score was 20 (SD = 12.4) out of a possible 95, suggesting psychosocial impact varied significantly in this sample. The mean distress, uncertainty, and positive experience subscale scores were 3.93 (SD = 5.36), 10.3 (SD = 7.57), and 5.61 (SD = 5.58), respectively. MICRA score ranges and percentiles, which are provided in Table 2, suggest participants’ ratings of distress, uncertainty, and positive experience were diverse. MICRA responses highlighted three stressors that were endorsed by the majority of participants who responded. Sixty-two of 84 respondents (73.8%) indicated that they “sometimes” or “often” worried about their risk of getting cancer again, 43 of 68 respondents (63.2%) indicated that they “sometimes” or “often” worried about the possibility of their children getting cancer, and 41 of 82 respondents (50%) indicated that they were “sometimes” or “often” uncertain about the impact of their genetic test result on their children’s and/or family’s cancer risk.
In univariate analyses (Tables 3 and 4), having a positive genetic test result was significantly associated with a higher MICRA total score, a higher MICRA distress subscale score, and a higher MICRA positive experience subscale score (indicating a less desirable genetic counseling and multigene panel testing experience). There were no statistically significant associations between any of the selected factors and MICRA uncertainty subscale scores. In multivariable analyses (Table 5), having a positive genetic test result was significantly associated with a higher MICRA total score (B = 1.13, p = 0.002), MICRA distress subscale score (B = 0.998, p = 0.012), and MICRA positive experience subscale score (B = 1.212, p = 0.002). In addition, having an annual household income of at least $50,000 was significantly associated with a lower MICRA positive experience subscale score (B = − 0.689, p = 0.05), indicating a more desirable experience among participants who reported a higher annual household income. None of the predictors entered into the model were significantly associated with MICRA uncertainty subscale scores.
Knowledge of cancer genetics
The mean KnowGene score was 11.9 (SD = 3.5) out of a possible 19. KnowGene percentile scores are provided in Table 2. Fewer than half of participants provided the correct response to five items pertaining to inheritance, clinical impact, and interpretation of genetic test results. Details regarding these items can be found in the first five rows of Table 6.
In univariate analyses, higher knowledge was significantly associated with younger age (Table 4) and an annual household income of $50,000 or more (Table 3). In multivariable analyses (Table 5), older age (B = − 0.07, p = 0.044) and being a member of a minority racial or ethnic group (B = − 3.075, p = 0.033) were significantly associated with lower knowledge, while having a personal history of at least one other type of cancer (B = 1.975, p = 0.015) was significantly associated with higher knowledge.
In univariate analyses, pathogenic variant type (BRCA vs. non-BRCA) met criteria for inclusion in the multivariable model. Given that pathogenic variant type only applies to participants with a positive genetic test result, a second multiple regression model assessed predictors of knowledge among these participants (Table 5). None of the predictors in this model were significantly associated with knowledge.
Responses to open-ended survey items
Four open-ended survey items elicited suggestions to improve the genetic counseling and multigene panel testing process, and participant responses to these items provided context for the quantitative survey results. Of 87 participants, 67 responded to at least one open-ended survey item, resulting in a total of 178 open-ended survey item responses. Of 178 responses, 124 (70%) were expressions of satisfaction with the genetic counseling and multigene panel testing process, while 54 (30%) were recommendations for improvement. Three major categories of recommendations for improvement were identified: (1) family communication and testing, (2) result disclosure and follow-up, and (3) understanding test results.
Family communication and testing
The largest category of recommendations for improvement related to family communication and testing. Several participants wished to have the option to engage family members early in the genetic counseling and multigene panel testing process. Family engagement was perceived to alleviate the participant’s burden of processing and disseminating family genetics knowledge.
“I think it would be helpful [if], along with the individual [being] tested, other family members who want to and should hear the results [are present during results disclosure]. …Then anyone with questions would have them answered right there and then, so it benefits all.” (74 year old, negative genetic test result)
Other open-ended item respondents expressed a desire for written, audio, or visual materials that could be shared with family. As one participant who audio recorded her genetic counseling session explained:
“I knew I was in no state of mind to remember all [the genetic counselor] would say…and it felt so very important to capture it for re-play for myself and for whomever in my family wanted to listen to the discussion.” (57 year old, negative genetic test result)
Several open-ended item respondents expressed a desire for health care providers to facilitate follow-up genetic counseling and testing of at-risk relatives. Some of these participants felt that genetic testing services should be made immediately accessible to relatives following disclosure of a positive genetic test result, while others expressed a need for assistance conveying the need for follow-up testing to members of their families.
“Until my sisters finally went through with the testing, there were many sleepless hours for me.” (70 year old, positive and VUS genetic test result)
“I wish you could send my son a letter telling him to go get his genes tested.” (64 year old, positive genetic test result)
Result disclosure and follow up
Several participants identified needs related to the disclosure of genetic test results. For some, the wait to receive genetic test results was distressing. One 70-year-old participant with a negative result shared that she experienced significant “anxiety while waiting” to receive her genetic test results.
Once results were disclosed, some participants indicated that the timing and method of results disclosure served as a barrier to effective information exchange. Some explained that they would have preferred a follow-up appointment to a telephone call to review and discuss their genetic test results.
“The first results were done via phone call, which caught me off guard. Therefore I didn’t absorb all of the information.” (53 year old, positive genetic test result)
Other participants expressed a desire for improved follow-up after results disclosure, both in terms of addressing psychosocial needs and assisting with the practical aspects of pursuing follow-up care. One 45-year-old participant with a negative result suggested providers “spend more time post-[results] report checking in and seeing how the patient is doing with the information.”
Understanding test results
A number of participants suggested ways for clinicians to enhance patient understanding of genetic test results. These participants emphasized the importance of explaining results in simple, clear language; providing written materials to patients and families; and spending an adequate amount of time explaining genetic test results and their clinical implications. As one 66-year-old participant with a negative result shared, “for those who have no scientific background, [the explanation of results] was very difficult to understand.”
Discussion
The results of this study suggest some individuals with ovarian cancer who receive a positive genetic test result report greater psychosocial impact, greater distress, and a less positive genetic counseling and multigene panel testing experience than those with negative or VUS results. Qualitative responses to open-ended survey items may partially explain the association between genetic test result and psychosocial impact. Participants with a positive genetic test result described the psychological burden of being responsible for processing and disseminating their family’s genetic information. Individuals whose genetic test results are negative may perceive fewer responsibilities related to family communication, which may explain the lower MICRA total and subscale scores in this group. Prior research assessing the psychosocial impact of single gene testing among individuals with ovarian cancer similarly found that carriers of a pathogenic variant reported greater psychosocial impact than non-carriers [25]. The current study corroborates this finding in the setting of multigene panel testing and adds the insight that receipt of a VUS result was not significantly associated with greater psychosocial impact, greater distress, or greater uncertainty.
The results of this study highlight several stressors that were encountered by participants as they underwent genetic counseling and multigene panel testing. These findings have important implications for the ovarian cancer care setting, where the prevalence of psychological distress has been estimated to range from 20 to 30% [26]. Responses to the MICRA questionnaire illustrate that nearly three-quarters of participants “sometimes” or “often” worry about their risk of getting cancer again, underscoring the prevalence of fear of cancer recurrence in this population [27]. In qualitative responses to open-ended items, participants indicated that delays in scheduling and delays in genetic test result disclosure exacerbated their concern. Indeed, in prior studies of women with ovarian cancer undergoing hereditary cancer risk assessment, participants have expressed a preference for genetic testing upon initial diagnosis [28, 29].
In the current study, some participants preferred to discuss genetic test results in person and expressed a desire for follow-up psychosocial care. A recent trial found that telephone disclosure of genetic test results is non-inferior to in-person disclosure for general and state anxiety immediately post-disclosure [30]. However, as the results of the current study indicate, face-to-face results disclosure may be preferable for some individuals. In a survey of 339 individuals who underwent BRCA testing, O’Shea and colleagues [31] found that participants perceived that face-to-face results disclosure facilitated information exchange and provision of emotional support. Likewise, Beri and colleagues [32] evaluated factors associated with preference for in-person result disclosure. They found that individuals who opted for in-person disclosure were more likely to be older and more likely to be undergoing multigene panel testing. Among those undergoing multigene panel testing, those who opted for in-person disclosure had lower baseline knowledge and higher distress.
While knowledge of cancer genetics was moderate in this sample of individuals with ovarian cancer, 50% of participants reported that they were “sometimes” or “often” uncertain about what their genetic test result means for their children’s and/or family’s cancer risk. Confidence in one’s ability to communicate genetic test results is related to the likelihood that an individual will share genetic test results with relatives [20]. In turn, individuals with limited knowledge of cancer genetics may be less likely to recommend cascade testing to relatives who may be at increased risk for ovarian cancer.
In the current sample, older adults had lower KnowGene scores than their younger counterparts. Given that older adults may serve as gatekeepers of family health information [33], intervening to increase cancer genetics knowledge in older adults is critical. Additionally, members of racial and ethnic minority groups had lower KnowGene scores than their white, non-Hispanic counterparts. Given that members of racial and ethnic minority groups comprised only 12.6% of the sample, this finding must be interpreted with caution. Nevertheless, prior research suggests Black and Hispanic individuals with gynecologic cancer face barriers to undergoing hereditary cancer risk assessment [34]. Development and testing of interventions to remediate racial and ethnic disparities in cancer genetics education, referral, and testing are needed to avoid exacerbating existing racial and ethnic disparities in ovarian cancer treatment quality [35].
In open-ended item responses, several participants identified a need for improved explanations of genetic test results and the clinical implications for themselves and their relatives. Indeed, although all participants in the current sample had undergone genetic counseling, KnowGene item scores indicate that many participants had limited knowledge of inheritance and interpretation of genetic test results. In a pilot randomized controlled trial, Vogel and colleagues [36] found that use of a mobile health application significantly improved hereditary cancer knowledge among women with ovarian cancer. Likewise, Tea and colleagues [37] found that the use of a visual tool significantly improved comprehension of cancer genetics information among individuals at high risk for HBOC. Beyond these interventions, development of educational materials that are culturally tailored and appropriate for individuals across the spectrum of literacy and numeracy is warranted. Accessibility of cancer genetics information is a priority consideration for clinicians and researchers engaged in the promotion of equitable uptake of targeted ovarian cancer treatment and risk reduction strategies.
Overall, the psychosocial impact- and knowledge-related needs expressed by participants in this study may reflect a desire for patient-centered communication, which aims to validate the patient’s perspective and understand the patient within his or her own psychological and social context [38]. As Littell and colleagues recently observed [39], this approach to communication has the capacity to promote knowledge retention and alleviate anxiety. Moreover, patient-centered communication may assist patients for whom family communication of genetic test results is burdensome. Communication with health professionals may clarify the relevance of genetic test results to at-risk relatives [40], underscore the importance of genetic testing [41], and stimulate conversations within families about hereditary cancer risk [41]. However, health care providers in the oncology setting have reported challenges communicating about genetics [42]. Additional research that identifies best practices for meeting the communication-related needs of individuals undergoing multigene panel testing in the ovarian cancer care setting is warranted.
The extent to which the findings from this study are generalizable is limited by this study’s cross-sectional design, recruitment from a single institution, and relatively small and homogenous sample. Nevertheless, these findings provide insight into opportunities to improve the genetic counseling and multigene panel testing process within the ovarian cancer care setting.
Conclusion
The psychosocial impact of genetic counseling and multigene panel testing on individuals with ovarian cancer may be highest among those with a positive genetic test result. Moreover, older adults and members of racial or ethnic minority groups may benefit from a personalized approach to cancer genetics education. Research that focuses on the development and testing of interventions that aim to promote patient-centered communication, enhance cancer genetics education, and facilitate family communication is necessary to meet the current and future needs of individuals with ovarian cancer and their families.
References
Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS, Agnew KJ, Pritchard CC, Scroggins S, Garcia RL, King MC, Swisher EM (2014) Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res 20(3):764–775. https://doi.org/10.1158/1078-0432.CCR-13-2287
Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A, Norquist B, Pennington KP, Garcia RL, King MC, Swisher EM (2011) Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci USA 108(44):18032–18037. https://doi.org/10.1073/pnas.1115052108
Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, Dobrovic A, Birrer MJ, Webb PM, Stewart C, Friedlander M, Fox S, Bowtell D, Mitchell G (2012) BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 30(21):2654–2663. https://doi.org/10.1200/JCO.2011.39.8545
Lancaster JM, Powell CB, Chen LM, Richardson DL, Committee SGOCP (2015) Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 136(1):3–7. https://doi.org/10.1016/j.ygyno.2014.09.009
National Comprehensive Cancer Network (2020) Genetic/familial high-risk assessment: breast and ovarian: National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf.
Colas C, Golmard L, de Pauw A, Caputo SM, Stoppa-Lyonnet D (2019) Decoding hereditary breast cancer: benefits and questions from multigene panel testing. Breast 45:29–35. https://doi.org/10.1016/j.breast.2019.01.002
Song H, Dicks EM, Tyrer J, Intermaggio M, Chenevix-Trench G, Bowtell DD, Traficante N, Group A, Brenton J, Goranova T, Hosking K, Piskorz A, van Oudenhove E, Doherty J, Harris HR, Rossing MA, Duerst M, Dork T, Bogdanova NV, Modugno F, Moysich K, Odunsi K, Ness R, Karlan BY, Lester J, Jensen A, Krüger Kjaer S, Høgdall E, Campbell IG, Lázaro C, Pujara MA, Cunningham J, Vierkant R, Winham SJ, Hildebrandt M, Huff C, Li D, Wu X, Yu Y, Permuth JB, Levine DA, Schildkraut JM, Riggan MJ, Berchuck A, Webb PM, Group OS, Cybulski C, Gronwald J, Jakubowska A, Lubinski J, Alsop J, Harrington P, Chan I, Menon U, Pearce CL, Wu AH, de Fazio A, Kennedy CJ, Goode E, Ramus S, Gayther S, Pharoah P (2020) Population-based targeted sequencing of 54 candidate genes identifies PALB2 as a susceptibility gene for high-grade serous ovarian cancer. J Med Genet. https://doi.org/10.1136/jmedgenet-2019-106739
Yang X, Leslie G, Doroszuk A, Schneider S, Allen J, Decker B, Dunning AM, Redman J, Scarth J, Plaskocinska I, Luccarini C, Shah M, Pooley K, Dorling L, Lee A, Adank MA, Adlard J, Aittomäki K, Andrulis IL, Ang P, Barwell J, Bernstein JL, Bobolis K, Borg Å, Blomqvist C, Claes KBM, Concannon P, Cuggia A, Culver JO, Damiola F, de Pauw A, Diez O, Dolinsky JS, Domchek SM, Engel C, Evans DG, Fostira F, Garber J, Golmard L, Goode EL, Gruber SB, Hahnen E, Hake C, Heikkinen T, Hurley JE, Janavicius R, Kleibl Z, Kleiblova P, Konstantopoulou I, Kvist A, Laduca H, Lee ASG, Lesueur F, Maher ER, Mannermaa A, Manoukian S, McFarland R, McKinnon W, Meindl A, Metcalfe K, Mohd Taib NA, Moilanen J, Nathanson KL, Neuhausen S, Ng PS, Nguyen-Dumont T, Nielsen SM, Obermair F, Offit K, Olopade OI, Ottini L, Penkert J, Pylkäs K, Radice P, Ramus SJ, Rudaitis V, Side L, Silva-Smith R, Silvestri V, Skytte A-B, Slavin T, Soukupova J, Tondini C, Trainer AH, Unzeitig G, Usha L, van Overeem HT, Whitworth J, Wood M, Yip CH, Yoon S-Y, Yussuf A, Zogopoulos G, Goldgar D, Hopper JL, Chenevix-Trench G, Pharoah P, George SHL, Balmaña J, Houdayer C, James P, El-Haffaf Z, Ehrencrona H, Janatova M, Peterlongo P, Nevanlinna H, Schmutzler R, Teo S-H, Robson M, Pal T, Couch F, Weitzel JN, Elliott A, Southey M, Winqvist R, Easton DF, Foulkes WD, Antoniou AC, Tischkowitz M (2019) Cancer risks associated with germline PALB2 pathogenic variants: An international study of 524 families. J Clin Oncol 38(7):674–685. https://doi.org/10.1200/JCO.19.01907
Vysotskaia V, Kaseniit KE, Bucheit L, Ready K, Price K, Johansen Taber K (2020) Clinical utility of hereditary cancer panel testing: impact of PALB2, ATM, CHEK2, NBN, BRIP1, RAD51C, and RAD51D results on patient management and adherence to provider recommendations. Cancer 126(3):549–558. https://doi.org/10.1002/cncr.32572
Hall MJ, Patrick-Miller LJ, Egleston BL, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, Brandt A, Chambers R, Clark DF, Forman A, Gaber R, Gulden C, Horte J, Long JM, Lucas T, Madaan S, Mattie K, McKenna D, Montgomery S, Nielsen S, Powers J, Rainey K, Rybak C, Savage M, Seelaus C, Stoll J, Stopfer JE, Yao XS, Bradbury AR (2018) Use and patient-reported outcomes of clinical multigene panel testing for cancer susceptibility in the multicenter communication of genetic test results by telephone study. JCO Precis Oncol. https://doi.org/10.1200/PO.18.00199
Graffeo R, Livraghi L, Pagani O, Goldhirsch A, Partridge AH, Garber JE (2016) Time to incorporate germline multigene panel testing into breast and ovarian cancer patient care. Breast Cancer Res Treat 160(3):393–410. https://doi.org/10.1007/s10549-016-4003-9
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357(6349):409–413. https://doi.org/10.1126/science.aan6733
Hamilton JG, Lobel M, Moyer A (2009) Emotional distress following genetic testing for hereditary breast and ovarian cancer: a meta-analytic review. Health Psychol 28(4):510–518. https://doi.org/10.1037/a0014778
Murakami Y, Okamura H, Sugano K, Yoshida T, Kazuma K, Akechi T, Uchitomi Y (2004) Psychologic distress after disclosure of genetic test results regarding hereditary nonpolyposis colorectal carcinoma: a preliminary report. Cancer 101(2):395–403. https://doi.org/10.1002/cncr.20363
van Oostrom I, Meijers-Heijboer H, Duivenvoorden HJ, Bröcker-Vriends AHJT, van Asperen CJ, Sijmons RH, Seynaeve C, Van Gool AR, Klijn JGM, Tibben A (2007) Prognostic factors for hereditary cancer distress six months after BRCA1/2 or HNPCC genetic susceptibility testing. Eur J Cancer 43(1):71–77. https://doi.org/10.1016/j.ejca.2006.08.023
Liang MI, Wong DH, Walsh CS, Farias-Eisner R, Cohen JG (2018) Cancer genetic counseling and testing: perspectives of epithelial ovarian cancer patients and gynecologic oncology healthcare providers. J Genet Couns 27(1):177–186. https://doi.org/10.1007/s10897-017-0135-2
Lumish HS, Steinfeld H, Koval C, Russo D, Levinson E, Wynn J, Duong J, Chung WK (2017) Impact of panel gene testing for hereditary breast and ovarian cancer on patients. J Genet Couns 26(5):1116–1129. https://doi.org/10.1007/s10897-017-0090-y
Faller H, Brahler E, Harter M, Keller M, Schulz H, Wegscheider K, Weis J, Boehncke A, Reuter K, Richard M, Sehner S, Koch U, Mehnert A (2017) Unmet needs for information and psychosocial support in relation to quality of life and emotional distress: a comparison between gynecological and breast cancer patients. Patient Educ Couns 100(10):1934–1942. https://doi.org/10.1016/j.pec.2017.05.031
Kapoor NS, Curcio LD, Blakemore CA, Bremner AK, McFarland RE, West JG, Banks KC (2015) Multigene panel testing detects equal rates of pathogenic BRCA1/2 mutations and has a higher diagnostic yield compared to limited BRCA1/2 analysis alone in patients at risk for hereditary breast cancer. Ann Surg Oncol 22(10):3282–3288. https://doi.org/10.1245/s10434-015-4754-2
Montgomery SV, Barsevick AM, Egleston BL, Bingler R, Ruth K, Miller SM, Malick J, Cescon TP, Daly MB (2013) Preparing individuals to communicate genetic test results to their relatives: report of a randomized control trial. Fam Cancer 12(3):537–546. https://doi.org/10.1007/s10689-013-9609-z
Underhill-Blazey M, Blonquist T, Chittenden A, Pozzar R, Nayak M, Lansang K, Hong F, Garber J, Stopfer J (2020) Informing models of cancer genetic care in the era of multigene panel testing with patient-led recommendations. J Genet Couns. https://doi.org/10.1002/jgc4.1317
Cella D, Hughes C, Peterman A, Chang C-H, Peshkin BN, Schwartz MD, Wenzel L, Lemke A, Marcus AC, Lerman C (2002) A brief assessment of concerns associated with genetic testing for cancer: the multidimensional impact of cancer risk assessment (MICRA) questionnaire. Health Psychol 21(6):564–572. https://doi.org/10.1037/0278-6133.21.6.564
Underhill-Blazey M, Stopfer J, Chittenden A, Nayak MM, Lansang K, Lederman R, Garber J, Gundersen DA (2019) Development and testing of the KnowGene scale to assess general cancer genetic knowledge related to multigene panel testing. Patient Educ Couns 102(8):1558–1564. https://doi.org/10.1016/j.pec.2019.04.014
Hsieh H-F, Shannon S (2005) Three approaches to qualitative content analysis. Qual Health Res 15(9):1277–1288. https://doi.org/10.1177/1049732305276687
Bjornslett M, Dahl AA, Sorebo O, Dorum A (2015) Psychological distress related to BRCA testing in ovarian cancer patients. Fam Cancer 14(4):495–504. https://doi.org/10.1007/s10689-015-9811-2
Watts S, Prescott P, Mason J, McLeod N, Lewith G (2015) Depression and anxiety in ovarian cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open 5(11):e007618. https://doi.org/10.1136/bmjopen-2015-007618
Ozga M, Aghajanian C, Myers-Virtue S, McDonnell G, Jhanwar S, Hichenberg S, Sulimanoff I (2015) A systematic review of ovarian cancer and fear of recurrence. Palliat Support Care 13(6):1771–1780. https://doi.org/10.1017/S1478951515000127
Fox E, McCuaig J, Demsky R, Shuman C, Chitayat D, Maganti M, Murphy J, Rosen B, Ferguson S, Randall Armel S (2015) The sooner the better: genetic testing following ovarian cancer diagnosis. Gynecol Oncol 137(3):423–429. https://doi.org/10.1016/j.ygyno.2015.03.057
Gleeson M, Meiser B, Barlow-Stewart K, Trainer A, Tucker K, Watts K, Friedlander M, Kasparian N (2013) Communication and information needs of women diagnosed with ovarian cancer regarding treatment-focused genetic testing. Oncol Nurs Forum 40(3):275–283. https://doi.org/10.1188/13.ONF.40-03AP
Bradbury AR, Patrick-Miller LJ, Egleston BL, Hall MJ, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, Brandt A, Chambers R, Clark DF, Forman A, Gaber R, Gulden C, Horte J, Long JM, Lucas T, Madaan S, Mattie K, McKenna D, Montgomery S, Nielsen S, Powers J, Rainey K, Rybak C, Savage M, Seelaus C, Stoll J, Stopfer JE, Yao XS (2018) Randomized noninferiority trial of telephone vs in-person disclosure of germline cancer genetic test results. J Natl Cancer Inst 110(9):985–993. https://doi.org/10.1093/jnci/djy015
O’Shea R, Meany M, Carroll C, Cody N, Healy D, Green A, Lynch SA (2016) Predictive genetic testing and alternatives to face to face results disclosure: a retrospective review of patients preference for alternative modes of BRCA 1 and 2 results disclosure in the Republic of Ireland. J Genet Couns 25(3):422–431. https://doi.org/10.1007/s10897-015-9887-8
Beri N, Patrick-Miller LJ, Egleston BL, Hall MJ, Domchek SM, Daly MB, Ganschow P, Grana G, Olopade OI, Fetzer D, Brandt A, Chambers R, Clark DF, Forman A, Gaber R, Gulden C, Horte J, Long J, Lucas T, Madaan S, Mattie K, McKenna D, Montgomery S, Nielsen S, Powers J, Rainey K, Rybak C, Savage M, Seelaus C, Stoll J, Stopfer JE, Yao XS, Bradbury AR (2019) Preferences for in-person disclosure: patients declining telephone disclosure characteristics and outcomes in the multicenter Communication Of GENetic Test Results by Telephone study. Clin Genet 95(2):293–301. https://doi.org/10.1111/cge.13474
Ashida S, Schafer EJ (2015) Family health information sharing among older adults: reaching more family members. J Commun Genet 6(1):17–27. https://doi.org/10.1007/s12687-014-0197-x
Hinchcliff EM, Bednar EM, Lu KH, Rauh-Hain JA (2019) Disparities in gynecologic cancer genetics evaluation. Gynecol Oncol 153(1):184–191. https://doi.org/10.1016/j.ygyno.2019.01.024
Pozzar RA, Berry DL (2017) Patient-centered research priorities in ovarian cancer: a systematic review of potential determinants of guideline care. Gynecol Oncol 147(3):714–722. https://doi.org/10.1016/j.ygyno.2017.10.004
Vogel RI, Niendorf K, Petzel S, Lee H, Teoh D, Blaes AH, Argenta P, Rivard C, Winterhoff B, Lee HY, Geller MA (2019) A patient-centered mobile health application to motivate use of genetic counseling among women with ovarian cancer: a pilot randomized controlled trial. Gynecol Oncol 153(1):100–107. https://doi.org/10.1016/j.ygyno.2019.01.019
Tea MM, Tan YY, Staudigl C, Eibl B, Renz R, Asseryanis E, Berger A, Pfeiler G, Singer CF (2018) Improving comprehension of genetic counseling for hereditary breast and ovarian cancer clients with a visual tool. PLoS ONE 13(7):e0200559. https://doi.org/10.1371/journal.pone.0200559
Epstein RM, Franks P, Fiscella K, Shields CG, Meldrum SC, Kravitz RL, Duberstein PR (2005) Measuring patient-centered communication in patient-physician consultations: theoretical and practical issues. Soc Sci Med 61(7):1516–1528. https://doi.org/10.1016/j.socscimed.2005.02.001
Littell RD, Kumar A, Einstein MH, Karam A, Bevis K (2019) Advanced communication: a critical component of high quality gynecologic cancer care. A Society of Gynecologic Oncology evidence based review and guide. Gynecol Oncol 155(1):161–169. https://doi.org/10.1016/j.ygyno.2019.07.026
Chivers Seymour K, Addington-Hall J, Lucassen AM, Foster CL (2010) What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research. J Genet Couns 19(4):330–342. https://doi.org/10.1007/s10897-010-9296-y
Mesters I, Ausems M, Eichhorn S, Vasen H (2005) Informing one’s family about genetic testing for hereditary non-polyposis colorectal cancer (HNPCC): a retrospective exploratory study. Fam Cancer 4:163–167
Douma KF, Smets EM, Allain DC (2016) Non-genetic health professionals’ attitude towards, knowledge of and skills in discussing and ordering genetic testing for hereditary cancer. Fam Cancer 15(2):341–350. https://doi.org/10.1007/s10689-015-9852-6
Acknowledgements
The authors wish to acknowledge the Center for Cancer Genetics and Prevention and the Phyllis F. Cantor Center for Research in Nursing and Patient Care Services at Dana-Farber Cancer Institute for providing clinical and research support.
Funding
Rachel A. Pozzar is supported by an American Cancer Society Postdoctoral Fellowship (133063-PF-19–102-01-CPPB) and a Society for Medical Decision Making/Gordon and Betty Moore Foundation Fellowship in Medical Decision Making (GBMF7853). Funding for the parent study was provided by the Friends of Dana-Farber. The funding sources were not involved in the study design; data collection, analysis, and interpretation; manuscript preparation; or decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
RAP: Conceptualization, analysis, writing—original draft. FH: Analysis, writing—review and editing. NX: Analysis, writing—review and editing. JES: Conceptualization, writing—review and editing. MMN: Investigation, analysis, writing—review and editing. MU-B: Conceptualization, investigation, analysis, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
Jill E. Stopfer reports personal fees from Astra Zeneca. Rachel A. Pozzar, Fangxin Hong, Niya Xiong, Manan M. Nayak, and Meghan Underhill-Blazey have no conflict of interest to disclose.
Ethical approval
The Dana-Farber/Harvard Cancer Center Institutional Review Board approved the study protocol.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pozzar, R.A., Hong, F., Xiong, N. et al. Knowledge and psychosocial impact of genetic counseling and multigene panel testing among individuals with ovarian cancer. Familial Cancer 21, 35–47 (2022). https://doi.org/10.1007/s10689-021-00240-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-021-00240-6