Abstract
Climate change is predicted to increase the probability of soil waterlogging due to severe rainfall, causing significant damage to soybean at the germination stage. Germination under waterlogging is also greatly influenced by temperature. To clarify the variation in germination responses of soybean genotypes to waterlogging at different temperatures, the seeds of 15 soybean genotypes were treated by soaking for 2 days at four temperatures: 21 °C, 23 °C, 25 °C, 27 °C and 29 °C. Differences in the germination rate (GR) and normal seedling rate (NSR) were observed among soybean genotypes after soaking treatments regardless of the temperature. Among the examined genotypes, Iyodaizu was classified as waterlogging tolerant at the germination stage, and Tachinagaha was classified as sensitive. Interestingly, through the analyses of recombinant inbred lines (RILs) developed from a cross between Tachinagaha and Iyodaizu, quantitative trait loci (QTLs) for root development under hypoxia at the seedling stage of soybean were detected on chromosome 12 (Chr.12).We investigated whether the candidate QTL region for root development is involved in seed waterlogging tolerance by using a near-isogenic line (NIL), NIL-9-4-5. Interestingly, under soaking treatment, the GR and NSR of NIL-9-4-5, carrying the candidate QTL region, was nearly the same as that of Iyodaizu and was significantly higher than that of Tachinagaha. These results may indicate that the candidate QTL region for root development under hypoxia at the seedling stage located on Chr.12 contributes to the seed waterlogging tolerance of soybean plants at the germination stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is regarded as a major factor increasing the probability of soil waterlogging due to severe rainfall. When soil is waterlogged, a hypoxic environment is induced due to the low diffusion of gases in water (Jackson and Colmer 2005; Licausi and Giuntoli 2020) and the respiration of organisms (Bailey-Serres and Voesenek 2008). The low-oxygen atmosphere negatively influences plant growth and productivity. To cope with waterlogging-induced hypoxia, three major strategies (adaptation, escape and quiescence) have evolved in plants; for example, plants exhibit certain root traits that prevent the loss of oxygen from the roots, such as adventitious rooting, root aerenchyma formation and the formation of physical barriers.
Great efforts have been made to understand the effects of waterlogging conditions on the growth and development of various upland crops, such as cucumber (Cucumis sativus) (Yeboah et al. 2008) wheat (Triticum aestivum) (Boru et al. 2001; Malik et al. 2001), chickpea (Cicer arietinum) (Cowie et al. 2013), upland cotton (Gossypium hirsutum) (Wang et al. 2017) and maize (Zea mays) (Tian et al. 2019). However, few of these studies have addressed the central question of the hypoxia stress response in soybean (Glycine max), a crop with poor tolerance to waterlogging (Maekawa et al. 2011; Kim et al. 2015; Dhungana et al. 2019).
Soybean is considered the most important legume species to humans and is frequently cultivated from spring to early summer in eastern Asia (Lee et al. 2003; Carpentieri-Pipolo et al. 2012). During this period, an increasing occurrence of heavy rains has been reported, causing significant damage to soybean at the germination and seedling stages (Araki et al. 2012; Kokukun et al. 2013). Genetic variation in germination responses to waterlogging was reported in previous studies (Sung 1995; Sayama et al. 2009; Nanjo et al. 2014), and this response can be greatly affected by temperature (Hou and Thseng 1991; Wuebker et al. 2001). Warmer temperatures are associated with greater losses during seedling emergence and a complete loss of germination was observed in a typical soybean cultivar when seeds were soaked for 4 days at 30 °C (Hou and Thseng 1991). Unfortunately, the variation in seed germination responses to waterlogging at different temperatures remains unclear. However, effect of temperature on the germination capacity of waterlogged seeds has not been tested across soybean varieties.
Although extensive QTLs for waterlogging tolerance of soybean have been detected, most of these QTLs are related to tolerance at the vegetative stage (Van Toai et al. 2001; Reyna et al. 2003; Cornelious et al. 2005; Githiri et al. 2006; Sayama et al. 2009; Nguyen et al. 2012; Van Nguyen et al. 2017). Limited information is available about QTLs for waterlogging tolerance in soybean plant in the germination stage. Only five QTLs, Sft1, Sft2, Sft3, Sft4 (Sayama et al. 2009) and QTN13 (Yu et al. 2019), have been associated with germination and normal seedling rates under seed-soaking stress. Interestingly, one of these QTLs (Sft1) is located in a marker interval containing QTLs on chromosome 12, near a candidate QTL region for root development under hypoxia and waterlogging (Van Nguyen et al. 2017). To obtain a deeper understanding of the effects of candidate QTL regions for root development on waterlogging tolerance of soybean at the germination stage, seeds of a near-isogenic line (NIL) and their parents were used as the study materials in this work. We comprehensively studied the effects of waterlogging at various temperatures on the characteristics of these soybean seeds, including the germination rate and seedling rate. Our findings could provide a valuable reference for alleviating waterlogging conditions in soybean planted around the world.
Materials and methods
Materials
Fifteen soybean genotypes, including seven from Japan (Iyodaizu, Kokubu 7, Komame, Maetsue Zarai 90B, Miyashishirome, Nattou Kotsubu and Tachinagaha), three from India (E C 112,828, M42 and M652), two from Nepal (N 2295 and U1155-4), one from Korea Rep. (Okjo), one from China (Peking) and one from the United States (Williams 82), were used in this study (Table 1). In addition, we used Iyodaizu, Tachinagaha and NIL-9-4-5 to confirm the effects of a major QTL for root development under hypoxia on seed waterlogging tolerance in soybean. Among them, NIL-9-4-5 is reported as a near-isogenic line (NIL) that was selected from the Tachinagaha/Iyodaizu BC6F2 population through marker-assisted selection (Van Nguyen et al. 2017).
Seed waterlogging treatment
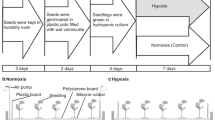
Seeds of each genotype were incubated in Petri dishes (diameter: nine cm) at 23 °C and 80% RH in the dark and the number of germinating seeds was counted for three days. Then, three-day-seeds were treated under waterlogging condition at different temperatures: 21, 23, 25, 27 and 29 °C (Fig. 1). Briefly, control seeds were sown in 0.43 l plastic pots (7.2 cm in top diameter; 11.6 cm in height; and 5.1 cm in bottom diameter) filled with humid vermiculite (Midorisangyou, Fukuoka, Japan) for four days, while waterlogging-treated- seeds (10 per temperature treatment per genotype) were subjected to a water soaking treatment in an Erlenmeyer flask containing 120 ml of deionized distilled water for 48 hours. The pots and Erlenmeyer flasks were then placed on trays in growth chambers (220 µmol m2 s−1 light density, 14 h light/10 h dark) that were set at the target temperatures. Then, the treated seeds were germinated in humid vermiculite for two days as the recovery stage. The experiments were performed under a randomized complete block design with three replications per treatment, and 10 seeds were used per replication.
Measurements
At the end of the seed waterlogging treatment, the water surface in the Erlenmeyer flak was observed for presence or absence of air bubbles. The samples were collected after four days of treatment. Seeds with a radicle longer than 1 cm were recorded as germinating seeds, and seedlings without any damage to the radicle or cotyledon were regarded as normal seedlings. The germination rate (GR) and normal seedling rate (NSR) were calculated with the following formulas:
The relative GR and NSR values at the tested temperatures were calculated as the ratio of the mean value under the control treatment to the mean one under waterlogging.
Statistical analysis
All statistical analyses were performed with Unistat 6.5. The effect of the genotype on the results for each trait was assessed by two-way ANOVA.
Results
Effects of seed waterlogging stress on GR and NSR
No effects of genotype, temperature or their interactions on GR or NSR were detected under control conditions, while these effects were significant for both variables under waterlogging treatment (Table 2). Compared to the control, the mean GR under waterlogging stress was reduced from 4% at 21 °C, to 68% at 29 °C. The mean NSR was reduced from 18% at 21 °C, to 82% at 29 °C (Table 2).
Variation in seed germination responses to waterlogging stress at different temperatures
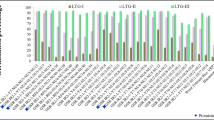
The appearance of air bubbles at the water surface in Erlenmeyer flasks showed an increase with increasing temperatures (Figs. 2 and 3). Significant genetic variations in NR and NSR responses to temperature under seed waterlogging were found (Figs. 4 and 5). At 21 °C, the GR of the soybean genotypes varied from 73% (Okjo and M42) to 100% (10/15 varieties), and the NSR varied from 37% (Williams 82) to 100% (U 1155-4, Iyodaizu and N 2295). At 23 °C, the GR of the soybean genotypes varied from 50% (Tachinagaha) to 100% (U 1155-4 and Iyodaizu), and the NSR varied from 0% (Williams 82) to 100% (U 1155-4). At 25 °C, the GR of the soybean genotypes varied from 33% (Tachinagaha) to 100% (U 1155-4), and the NSR varied from 0% (Williams 82 and Miyagishirome) to 90% (U 1155-4). At 27 °C, the GR of the soybean genotypes varied from 13% (Maetsue Zarai 90B and N 2295) to 90% (U 1155-4), and the NSR varied from 0% (8/15 genotypes including Williams 82, Miyagishirome, Nattou Kotsubu, Komame, Okjo, M42, Kokubu, Maetsue Zarai 90B and N 2295) to 87% (U 1155-4). At 29 °C, the GR and NSR showed clear differences among the genotypes. Only five of the 15 genotypes, including U 1155-4, M 652, E C 112,828, Yodaizu, and Peking showed to have the NSR at 29 °C. Based on the GR, the genotypes were categorized into three groups, including a tolerant (Peking, U 1155-4, Iyodaizu and M 652), a moderately tolerant (E C 112,828, Komame, and Nattou Koshubu) and a sensitive group (others), regardless of the temperature (Figs. 4 and 5).
The seed waterlogging tolerance of soybean is linked to QTLs for root development under hypoxia
Under the control treatment, NSR of Iyodaizu, Tachinagaha and NIL-9-4-5 were all higher than 90% and were not significantly different among genotypes and across all temperature conditions (Fig. 6, Supplementary Fig. S2).Under waterlogging, we found a significant reduction in GR and NSR of the 3 genotypes associated with increasing temperatures from 21 to 29 °C (Figs. 7 and 8, and Supplementary Fig. 3). Among genotypes, Tachinagaha was found to be a waterlogging-sensitive genotype, showing rapid reductions in GR and NSR, while Iyodaizu and NIL-9-4-5 were waterlogging-tolerant compared to controls. GR and NSR of Iyodaizu and NIL-9-4-5 were also significantly greater than those of the recurrent parent Tachinagaha at all temperatures (Figs. 7 and 8, and Supplementary Fig. S3).
Discussion
Recently reports have described the relationship between waterlogging condition and temperature variation. Here, we confirmed a QTL region for root development under hypoxia significantly contributes to the seed waterlogging tolerance of soybean plants at the germination stage. Hypoxia has been proposed as the main problem associated with waterlogging because the available oxygen concentration is rapidly decreased due to the slow diffusion of oxygen in water (Armstrong 1980; Wiengweera et al. 1997; Hossain and Uddin 2011). Reduction of oxygen partial pressures to 2 kPa and 6 kPa, decreased GR to 0% and 50% respectively, compared to the maximum GR under ambient conditions (Al-Ani et al. 1985; Tian and Arihara 1998) conducted the experiments on the effects of O2 supplies on GR of 8 soybean genotypes and reported that GR was decreased by 25–75% at 5% O2 compared to 20% O2. In this study, the GR of soybean under soaking was rapidly decreased by 4% at 21 °C to 68% at 29 °C (Table 2). These results indicated that hypoxia stress causes a decrease in the GR of soybean under waterlogging.
In this study, the interaction between the waterlogging treatment and temperature was consistent with results of other studies obtained under soaking or soil waterlogging conditions. Previous study has indicated that warmer soil temperatures are related to greater losses in seedling emergence under waterlogging compared with lower temperatures (Fausey and McDonald 1985). Under soaking conditions, incubating soybean seeds at 10 or 15 °C for up to 8 days prior to germination caused no loss in germination, but germination decreased as the length of the soaking period at 25 and 30 °C was increased (Hou and Thseng 1991). The data obtained in this study showed that the GR was reduced by only 6% at 21 °C but was decreased by up to 68% at 29 °C (Table 2).
Variation was found among the genotypes included in this study (Figs. 3 and 4). Williams 82 and Tachinagaha showed waterlogging-sensitive genotypes exhibiting rapid reductions in GR and NSR regardless of increasing temperatures. Peking, U 1155-4, Iyodaizu and M 652 were better adapted to waterlogging than the other genotypes. Among these genotypes, Peking has been reported as a seed waterlogging-tolerant genotype exhibiting a delay in germination under hypoxia regardless of temperature (Nakajima et al. 2015). Iyodaizu was selected as a tolerant Japanese variety at 25 °C (Nanjo et al. 2014).The genotypes showing seed waterlogging tolerance might be useful for the genetic improvement of waterlogging tolerance in modern soybean varieties.
In this study, Iyodaizu was classified in the waterlogging-tolerant group at the germination stage, and Tachinagaha was classified in the sensitive group. In line with our observations, Iyodaizu has been previously reported as a genotype that is tolerant to waterlogging at the germination (Nanjo et al. 2014) and seedling stage (Sakazono et al. 2014; Jitsuyama 2015; Suematsu et al. 2017; Van Nguyen et al. 2017), and Tachinagaha has been reported as a moderately sensitive genotype at the germination (Sayama et al. 2009; Nanjo et al. 2014) and seedling stage (Sakazono et al. 2014; Jitsuyama 2015; Suematsu et al. 2017; Van Nguyen et al. 2017). These results provide interesting information for exploring the mechanisms involved in the development of adaptations in response to waterlogging in the germination stage. Through the analyses of inbred lines (RILs) developed from a cross between Tachinagaha and Iyodaizu, Van Nguyen et al. (2017) identified QTLs for root development, including root length development (RLD) and root surface area development (RSAD), on soybean chromosome 12 and developed an NIL-9-4-5 carrying targeted QTLs at BC6F2. The QTLs for RLD and RSAD (Qrld-12, Qrsad-12) on Chr.12 have been shown to be stable across years. The resultant increase in root development in NIL-9-4-5 was most likely inherited from the waterlogging-tolerant parent Iyodaizu. Interestingly, Sayama et al. (2009) also identified a QTL (Sft1) for seed flooding tolerance in soybean, which is located near the candidate QTL region mentioned in this study. Therefore, this study was conducted to confirm the existence of QTL effects on seed waterlogging tolerance related to root development under hypoxia using NIL-9-4-5 line. The obtained results suggested that the normal seedling rate of NIL-9-4-5 presented the same trend as that of the donor parent Iyodaizu and was significantly greater than that of the recurrent parent Tachinagaha, indicating that the marker interval may contain a gene for seed waterlogging tolerance in soybean (Figs. 7 and 8). These results agreed with those of Van Nguyen et al. (2017) showing that QTLs for hypoxia tolerance in soybean at the germination and seedling stages are located in the marker interval on chromosome 12.
The important pathway induced under hypoxia is ethanolic fermentation (Liem et al. 2019). By which under the action of pyruvate decarboxylase and alcohol dehydrogenase, carbohydrates convert to alcohol and CO2 gas (Zabalza et al. 2009). In this study, the formation of gas bubbles indicated that fermentation occurred under seed waterlogging treatment (Figs. 1 and 2). Under waterlogging, the appearance of air bubbles was observed in Erlenmeyer flasks containing the seeds of Iyodaizu and NIL-9-4-5 at 29 °C and those of Tachinagaha at 23 °C (Supplementary Fig. S1). The mechanisms involved in the hypoxia tolerance of soybean are related to the patterns of alanine aminotransferase (AlaAT), aldehyde dehydrogenase (ALDH) as previously described (Liem et al. 2019). More specifically, AlaAT plays an important role in regulating the glycolytic flux by preventing the excessive accumulation of pyruvate (Zabalza et al. 2009) while retaining carbon and nitrogen resources within the cell (Rocha et al. 2010). Unlike the production of lactate and ethanol, alanine accumulation does not have detrimental side effects in cells. Another analysis of expression identified aldehyde dehydrogenase, a fermentative enzyme responsible for the metabolization of aldehyde, which is harmful to cells under hypoxia stress (Nakazono et al. 2000; Fukao et al. 2003; Tsuji et al. 2003). These results suggest that by analysing the expression of genes related to fermentation linked to the seed waterlogging tolerance of soybean evaluated in this study, a more mechanistic understanding of the response to waterlogging stress will be achieved.
Abbreviations
- Chr.:
-
Chromosome
- GR:
-
Germination rate
- NIL:
-
Near isogenic line
- NSR:
-
Normal seedling rate
- QTL:
-
Quantitative trait loci
References
Al-Ani A, Bruzau F, Raymond P, Saint-Ges V, Leblac JM, Pradet A (1985) Germination, respiration, and adenylate energy charge of seeds at various oxygen partial pressures. Plant Physiol 79:885–890
Araki H, Hossain M, Takahashi A (2012) Waterlogging and hypoxia have permanent effects on wheat root growth and respiration. J Agron Crop Sci 198:264–275
Armstrong W (1980) Aeration in higher plants. Adv Bot Res 7:225–332
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339
Boru G, van Ginkel M, Kronstad W, Boersma L (2001) Expression and inheritance of tolerance to waterlogging stress in wheat. Euphytica 117:91–98
Carpentieri-Pipolo V, Pipolo AE, Abdel-Haleem H, Boerma HR, Sinclair TR (2012) Identification of QTLs associated with limited leaf hydraulic conductance in soybean. Euphytica 186:679–686
Cornelious BP, Chen P, ChenN, deLeon N, Shannon JG, Wang D (2005) Identification of QTLs underlying waterlogging tolerance in soybean. Mol Breed 16:103–112
Cowie AL, Jessop RS, MacLeod DA (2013) Effects of waterlogging on chickpeas I. Influence of timing of waterlogging. Plant Soil 183:97–103
Dhungana SK, Kim HS, Kang BK, Seo JH, Kim HT, Shin SO, Park CH, Kwak DY (2019) Evaluation of flooding tolerance of soybean (Glycine max L. Merr.) in greenhouse under upland and paddy soil conditions. J Crop Sci Biotechnol 22:283–290
Fausey N, McDonald MB (1985) Emergence of inbred and hybrid corn following flooding. Agron J 77:51–56
Fukao T, Kennedy RA, Yamasue Y, Rumpho ME (2003) Genetic and biochemical analysis of anaerobically-induced enzymes during seed germination of Echinochloa crusgalli varieties tolerant and intolerant of anoxia. J Exp Bot 54:1421–1429
Githiri SM, Wananabe S, Harada K, Takahashi R (2006) QTL analysis of flooding tolerance in soybean at an early vegetative growth stage. Plant Breed 125:613–618. https://doi.org/10.1111/j.1439-0523.2006.01291.x
Hossain MA, Uddin SN (2011) Mechanism of waterlogging tolerance in wheat: morphological and metabolic adoptions under hypoxia or anoxia. Aust J Crop Sci 5:1094–1101
Hou FF, Thseng FS (1991) Studies on the flooding tolerance of soybean seed: varietal differences. Euphytica 57:169–173
Jackson M, Colmer T (2005) Response and adaptation by plants to flooding stress. Ann Bot 96:501–505
Jitsuyama Y (2015) Morphological root responses of soybean to rhizosphere hypoxia reflect waterlogging tolerance. Can J Plant Sci 95:999–1005
Kim YH, Hwang SJ, Waqas M, Khan A, Lee JH, Lee JD, Nguyen H, Lee IJ (2015) Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front Plant Sci 6:714
Kokubun M (2013) Genetic and cultural improvement of soybean for waterlogged conditions in Asia. Field Crops Res 152:3–7
Lee KH, Park SW, Kwon YW (2003) Enforced early development of adventitious roots increases flooding tolerance in soybean. Jap J Crop Sci 72:82–88
Licausi F, Giuntoli B (2020) Synthetic biology of hypoxia. New Phytol. doi:https://doi.org/10.1111/nph.16441
Liem TB, Giacomo N, Lara L, Cristina I, Jacopo R, Antonietta S, Anna M, Françoise C, Beatrice G, Pierdomenico P, Mirko Z, Francesco L (2019) Conservation of ethanol fermentation and its regulation in land plants. J Exp Bot 70:1815–1827
Maekawa T, Shimamura S, Shimada S (2011) Effects of short-term waterlogging on soybean nodule nitrogen fixation at different soil reductions and temperatures. Plant Prod Sci 14:349–358
Malik AI, Colmer DTD, Lambers H, Schortemeyer M (2001) Changes in physiological and morphological traits of roots and shoots of wheat in response to different depth of waterlogging. Aust J Plant Physiol 28:1121–1131
Nakajima T, Seino A, Nakamura T, Goto Y, Kokubun Y (2015) Does pre-germination flooding-tolerant soybean cultivar germinate better under hypoxia conditions? Plant Prod Sci 18:146–153
Nakazono M, Tsuji H, Li Y, Saisho D, Arimura S, Tsutsumi N, Hirai A (2000) Expression of a gene encoding mitochondrial aldehyde dehydrogenase in rice increases under submerged conditions. Plant Physiol 24:587–598
Nanjo Y, Jang HY, Kim HS, Hiraga S, Woo SW, Komatsu S (2014) Analyses of flooding tolerance of soybean varieties at emergence and varietal differences in their proteomes. Phytochemistry 106:25–36
Nguyen VT, Vuong TD, VanToai T, Lee JD, Wu X, Rouf Mian MA, Dorrance AE, Shannon JG, Nguyen HT (2012) Mapping of quantitative trait loci associated with resistance to Phytophthora sojae and flooding tolerance in soybean. Crop Sci 52:2481–2493
Reyna N, Cornelious B, Shannon JG, Sneller CH (2003) Evaluation ofaQTL forwaterlogging tolerance in southern soybean germplasm. Crop Sci 43:2077–2082
Rocha M, Licausi L, Araújo WL, Nunes-Nesi A, Sodek L, Fernie AR, van Dongen JT (2010) Glycolysis and the TCA-cycle are linked by alanine amino transferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol 152:1501–1513
Sakazono S, Nagata T, Matsuo R, Kajihara S, Watanabe M, Ishimoto M, Shimamura S, Harada K, Takahashi R, Mochizuki T (2014) Variation in root development response to flooding among 92 soybean lines during early growth stages. Plant Prod Sci 17:228–236
Sayama T, Nakazaki T, Ishikawa G, Yagasaki K, Yamada N, Hirota N, Hirat K, Yoshikawa T, Saito H, Teraishi M, Okumoto Y, Tsukiyama T, Tanisaka T (2009) QTL analysis of seed-flooding tolerance in soybean (Glycine max[L.] Merr.). Plant Sci 176:514–521
Suematsu K, Abiko T, Nguyen LV, Mochizuki T (2017) Phenotypic variation in root development of 162 soybean accessions under hypoxia condition at the seedling stage. Plant Prod Sci 20:323–335
Sung FJM (1993) Waterlogging effect on nodule nitrogenase and leaf nitrate reductase activities in soybean. Field Crops Res 35:183–189
Tamang BG, Magliozzi JO, Maroof MAS, Fukao T (2014) Physiological and transcriptomic characterization of submergence and reoxygenation responses in soybean seedlings. Plant Cell Environ 37:2350–2365
Tian L, Li J, Bi W, Zuo S, Li L, Li W, Sun L (2019) Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) under field conditions. Agricul Water Manag 218:250–258
Tian X, Arihara J (1998) Influence of low oxygen concentration stress on germination and growth of crops. Nissaku Kanto Shihou 13:48–49 (in Japanese)
Tsuji H, Tsutsumi N, Sasaki T, Hirai A, Nakazono M (2003) Organ-specific expressions and chromosomal locations of two mitochondrial aldehyde dehydrogenase genes from rice (Oryza sativa L.), ALDH2a and ALDH2b. Genetics 305:195–204
Van Nguyen LV, Takahashi R, Githiri SM, Rodriguez TO, Tsutsumi N, Kajihara S, Sayama T, Ishimoto M, Harada K, Suematsu K, Abiko T, Mochizuki (2017) Mapping quantitative trait loci for root development under hypoxia conditions in soybean (Glycine max L. Merr.). Theor Appl Genet 130:743–755
Van Toai TT, Martin SKSt, Chase K, Boru G, Schnipke V, Schmitthennr AF, Lark KG (2001) Identification of a QTL associated with tolerance of soybean to soil waterlogging. Crop Sci 41:1247–1252
Wang XS, Deng Z, Zhang WZ, Meng ZJ, Chang X, Lv MC (2017) Effect of waterlogging duration at different growth stages on the growth, yield and quality of cotton. PLoS ONE 12:e0169029
Wiengweera A, Greenway H, Thomson CJ (1997) The use of agar nutrient solution to simulate lack of convection in waterlogging soils. Ann Bot 80:115–123
Wuebker EF, Mullen RE, Koehler K (2001) Flooding and temperature effects on soybean germination. Crop Sci 4:1857–1861
Yeboah MA, Xuehao C, Guohua L, Minghong G, Chenwu X (2008) Inheritance of waterlogging tolerance in cucumber (Cucumis sativus L.). Euphytica 162:145–154
Yu Z, Chang F, Lv W, Sharmin RA, Wang Z, Kong J, Bhat JA, Zhao T (2019) Identification of QTN and candidate gene for seed-flooding tolerance in soybean [Glycine max (L.) Merr.] using genome-wide association study (GWAS). Genes 10:957
Zabalza A, van Dongen JT, Froehlich A, Oliver SN, Faix B, Gupta KJ, Schmazlin E, Igal M, Orcaray L, Royuela M, Geigenberger P (2009) Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol 149:1087–1098
Acknowledgements
This work was supported by grants from key project of Vietnam National University of Agriculture (T2019-01-01TĐ) and TIFO postdoctoral Fellowship.
Author information
Authors and Affiliations
Contributions
LVN designed the methodology. TM and TA developed experimental materials. TM, TA and TN oversaw and guided experiments, data analysis, writing and editing. LVN, HDTT, and HDC conducted the experiments and analysed the data. LVN and HDC wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nguyen, V.L., Dang, T.T.H., Chu, H.D. et al. Near-isogenic lines of soybean confirm a QTL for seed waterlogging tolerance at different temperatures. Euphytica 217, 16 (2021). https://doi.org/10.1007/s10681-020-02736-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-02736-1