Abstract
This study maps genomic regions associated with terminal heat- and drought-stress tolerance in barley (Hordeum vulgare L.). One hundred lines were randomly sampled from a ND24260 × Flagship doubled haploid population and evaluated for stay-green (SG) expression. SG expression including that of parental controls and commercial check varieties was evaluated in two controlled environments; one simulating terminal heat-stress, the other terminal water-stress. During grain-fill the greenness of the spikes (S), flag leaf (FL) and the first leaf under the flag leaf (FL-1) were phenotyped; visually (using a 0–9 scale) and via single-photon avalanche diode measurements. From the visual assessments, the green leaf area of the plant was determined, by using the difference in green area of the S and FL. Composite interval mapping detected 10 quantitative trait loci (QTL) for SG, positioned on chromosomes 3H, 4H, 5H, 6H and 7H; six of which were associated with terminal heat-stress and four with terminal water-stress. None were co-located with previously reported barley stress-response QTL and thus represent novel barley QTL. Although novel, some SG QTL mapped near chromosomal regions previously reported; such as the two heat-stress QTL mapped to bPb-5529 on 5H, adjacent to QTL reported for root length and root-shoot ratio. Detection of SG QTL in barley grown under simulated heat- and water-stressed conditions offers the potential of high through put screening for these traits. If confirmed in field trials, these genomic regions will be candidates for barley breeding programs targeting improved abiotic stress tolerance via marker-assisted selection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Barley (Hordeum vulgare L.) is extensively used for feed, food, and malt products. The potential end use of barley grain is determined in part by grain quality, which can be influenced by numerous genetic and environmental factors (Fox et al. 2003). Barley is one of the oldest cultivated grains, and is considered one of the most drought-stress tolerant. This is due to a diverse array of morphological traits and adaptations, which has ensured its survival and global wide distribution (Vaezi et al. 2010; Li et al. 2001). However, increases in the frequency and duration of droughts associated with climate change have prompted breeding programs to target the development of more robust cultivars, by incorporating traits for improved adaptation to drought stress tolerance (Ashraf 2010; Rollins et al. 2013).
Stay-green (SG) is a drought-adaptation trait that enables the plant to retain green leaf area post anthesis during the grain-filling period (Thomas and Howarth 2000). In other cereal crops, such as sorghum (Sorghum bicolor L. Moench), wheat (Triticum aestivum L.), rice (Oryza sativa) and maize (Zea mays) (Rong et al. 2013; Arriola et al. 2012; Harris et al. 2007; Jordan et al. 2012), SG phenotypes have given rise to higher yield under water-limited conditions. These yield increases have been associated with traits such as delayed leaf senescence, photosynthetic competence, decreased leaf area, rate and duration of grain filling, the efficient assimilation of carbohydrates and remobilisation of nitrogen from the leaves during drought-stress (Cattivelli et al. 2008; Spano et al. 2003; Ashraf 2010).
Because SG has a genetic basis, the trait has been successfully incorporated into elite breeding germplasm of other cereal crops (Rong et al. 2013; Arriola et al. 2012). In barley, selection for yield per se, even in water-limited environments, may have led to some degree of passive selection for SG. Yield under water-limitation is a complex trait, often confounded by many factors that have low heritability. Targeting heritable traits that likely underpin drought-adaptation, such as SG, could offer higher rates of genetic gain for drought tolerance in breeding programs. However, weather conditions can complicate phenotyping such traits in the field, reducing the efficiency and reliability of screening (Cattivelli et al. 2008; Tao et al. 1999). Identification of molecular markers associated with SG and adoption of marker-assisted selection (MAS) is considered a viable alternative for the development of SG sorghum, wheat and barley cultivars (Chen et al. 2010; Joshi et al. 2007; Spano et al. 2003; Tao et al. 1999). With MAS, the level of expression of the trait of interest is not quantified, but rather a marker linked to the trait of interest is selected. This can make MAS an effective method to screen for complex traits in the early generations of a breeding program (Collard and Mackill 2008).
To date, numerous studies have identified QTL associated abiotic stress tolerance traits, which are predominantly found on Chromosomes 2H, 3H, 4H, 5H and 7H. Most notable of these studies identified QTL associated with osmotic potential (OPQ), water-soluble carbohydrate (WSCQ), and the accumulation of water-soluble carbohydrate at 100 % relative water content (DWSC100Q) on Chromosomes 2H, 3H and 7H (Diab et al. 2004). Chen et al. (2010) identified QTL for relative water content (RWCQ) and root length (RLQ) on Chromosomes 1H, 2H and 5H; while several putative QTL associated with root to shoot ratio (RSRQ), root length (RLQ) and root dry weight (RDWQ) for samples under drought conditions, were identified by Arifuzzaman et al. (2014) across Chromosomes 1H, 2H, 3H, 4H, 5H and 7H. These studies emphasized the importance of root structure and efficient water usage by the plant for the maintenance of osmolality. However, none of these studies identified photosynthetic capacity of the leaf as a factor contributing to drought tolerance. It has not been until recently that the impact of chlorophyll content and fluorescence parameters in the leaves have been evaluated as drought-stress tolerance indicators in barley. Several putative QTL associated with these studies have been reported by Xue et al. (2008) and Guo et al. (2008), with many traits linked to leaf structural parameters. There are reports of QTL for leaf morphology, chlorophyll content and chlorophyll fluorescence in barley under abiotic stress (Xue et al. 2008; Guo et al. 2008). There are also reports of changes in chlorophyll parameters and in leaf senescence as indicators of drought stress in barley (Gregersen et al. 2008; Rong-hua et al. 2006). However, we are not aware of previous reports of QTL for SG in barley.
In this study, we perform QTL mapping of SG in barley using a subset of one hundred lines from the ND24260 × Flagship doubled haploid (DH) population. SG expression was evaluated in two separate experiments performed under controlled conditions; one simulating terminal heat-stress and the other simulating terminal drought-stress. We investigate the alignment of genomic regions harbouring abiotic stress tolerance QTL in barley and discuss implications for further research and potential for MAS in breeding programs targeting development of cultivars incorporating improved heat and drought tolerance.
Materials and methods
Plant materials
A subset of 100 lines was randomly selected from the ND24260 × Flagship doubled-haploid (DH) population, which has been genotyped with DArT markers (Hickey et al. 2011b, 2012), with both parents of the DH population not closely (genetically) related. Flagship (Chieftain/Barque//Manley/VB9104) is a DH Australian malting barley cultivar released in 2005 by the Barley Program at the Waite Campus of the University of Adelaide, Australia (Eglinton 2006). ND24260 (ND19869-1//ND17274/ND19119) (Franckowiak et al. 2007) is an advanced breeding line from North Dakota State University, Fargo, USA, which was selected for its superior grain quality. ND24260 has also been observed to express SG phenotype under drought-stress conditions during field trials (Franckowiak unpublished data). The DH population was developed to combine desirable traits from Australian and North Dakotan germplasm, including disease resistance and abiotic stress tolerance. The population has been recently used to identify QTL for grain dormancy and biotic stress resistance (Hickey et al. 2011b, 2012).
Abiotic stress glasshouse experiments
Grains from selected lines were imbibed in petri dishes and refrigerated at 5 °C for one week to overcome seed dormancy and induce synchronous germination. The petri dishes were transferred to 19 °C for optimal sprouting; five germinating grains per line were transplanted into a 4L pot (ANOVApot, 200 mm diameter, 190 mm height, http://www.anovapot.com/) in the glasshouse. Temperature within the glasshouse located at the University of Queensland, Australia (27°29′43.9″S, 153°00′36.6″E) was maintained at a constant 22 ± 2 °C with a natural diurnal photoperiod of 11 h daylight. A “Twinpot” system (Gous et al. 2013) was used to provide an effective bottom watering system. Once established, seedlings were thinned to leave the strongest plant in each pot, and two partially duplicated (Coombes 2002) trials were set up. A slow release fertilizer with micronutrients was applied at early booting (Z40; Zadoks decimal growth stage scale (Zadoks et al. 1974) to maintain optimal nutrient levels, while the glasshouse was maintained to be disease and pest free. At anthesis (Z61), the two sample populations, each consisting of 100 genotypes, were separated into heat- and water-stress treatments. A set of parental lines (3 pots per parent) were also grown under optimal stress-free conditions (i.e. 22 ± 2 °C), with unrestricted access to water.
In the drought-stress trial, severe water-stress was applied by maintaining pots at 20 % field capacity (FC), relative to the 24 h water consumption of the parental controls, while glasshouse temperature was maintained at an optimal 22 ± 2 °C (Gous et al. 2013). The degree of greenness including that of the parental lines, were visually assessed and measured, in the morning of every second day until the plants were completely senesced. Three methods for monitoring SG expression were used, two visual assessment methods and measurements with a single-photon avalanche diode (SPAD).
The first visual assessment method was taken by scoring of the greenness of the spike (GS), flag leaf (GFL) and the first leaf under the flag leaf (GFL-1) using a 0–9 scale [0 = dead and 9 = dark green; (Joshi et al. 2007)].
The second visual assessment approach used plant area under green, which was determined using the difference in spike and flag leaf (Yi) greenness (Joshi et al. 2007). This difference in green area was referred to as leaf area under green (LAUG). Using the 0–9 score the difference in the green leaf area between the spike and flag leaf at a given time (t i ) was used as Y i , while the time between two consecutive readings (measured in days) was used as t (i+1) − t i (Joshi et al. 2007). The higher the LAUG score the greater the lines capacity to express SG during drought-stress. Leaf area under green is thus calculated as follows:
A third, more objective, technique was used to validate the data obtained by the visual assessments of the FL and the FL-1, using a Minolta SPAD 502 (Konica Minolta, Hong Kong). SPAD is an instantaneous, non-destructive technique based on the quantification of light intensity absorbed by the tissue samples at 650 and 940 nm, which is proportional to the amount of chlorophyll in the sample (Netto et al. 2004; Ling et al. 2011; Hamblin et al. 2014). The readings were taken from the same FL and FL-1, which were marked on their dorsal and ventral surfaces. SPAD measurements taken from these marked positions were annotated as SPFL and SPFL-1, respectively as previously described (Christopher et al. 2008; Harris et al. 2007).
In the heat-stress trial, the DH population had unrestricted access to water using the “Twin-pot” bottom watering system, but severe heat-stress was induced by adopting average day time temperatures of 35 ± 2 °C and night time temperatures of 28 ± 2 °C, under natural diurnal photoperiod of 11 h daylight. The degree of greenness was evaluated using the same techniques and methods as described above in the water-stress trial, until plants had completely senesced.
Once the plants reached maturity, the number of tillers and spikes producing grain was recorded prior to harvest. Samples were threshed and dried for 48 h at 50 °C and the total grain and 100 kernel weights were recorded before being stored in sealed containers.
Statistical analysis
The relationship between SG expression and phenotypic responses in the drought- and heat-stress experiments were explored by conducting principal component analysis (PCA) (Gabriel 1971; Demey et al. 2008) using genotype trait means for GS, GFL, GFL-1, SPFL, SPFL-1and LAUG. PCA and bi-plot construction was performed using GenStat v.16 (VSN International, United Kingdom). Correlation between SG measurements and phenotypic responses in the DH population, was determined using Minitab17 (Minitab Inc., State College, PA, USA), adopting a 95 % CI.
Linkage map and QTL analysis
The linkage map for the ND24260 × Flagship DH population reported by Hickey et al. (2011a) was used for initial marker order and mapping in this study. The map comprises 605 polymorphic (DArT) markers and 10 linkage groups for the seven chromosomes, with two linkage groups each for chromosomes 1H, 5H and 6H.
Composite interval mapping (CIM) was used to detect QTL for GS, GFL, GFL-1, SPFL, SPFL-1and LAUG; and estimate the magnitude of their effects, using the stepwise regression analysis in WinQTLCartographer 2.0 (Wang et al. 2002; Collard et al. 2005; Phuong et al. 2014; Crasta et al. 1999). A series of 100,000 permutations were performed at 1 cM intervals, to determine the experiment-wise significant level at P = 0.05 of the logarithm of odds (LOD) for the trait (Gous et al. 2012). The logarithm of odds scores is a statistical measure of linkage and is defined as the ratio of the probabilities that the observed effect occurs due to linkage, compared to the effect occurring by chance (Collard et al. 2005). QTL were considered significant at a LOD threshold of 2.5 or higher following the genome wide permutations tests. Epistatic interactions between the QTL were identified following analysis in QTLNetwork with a threshold of P = 0.05 (Yang et al. 2007, 2008). With QTL effects estimated using the Monte Carlo Markov chain method. Permutation tests were carried out using 1,000 iterations at 1 cM intervals, using a test and filtration window size of 10 cM (Hickey et al. 2011b, 2012).
Collation of published QTL studies
QTL previously reported as underpinning drought tolerance in other studies in barley were collated (Arifuzzaman et al. 2014; Chen et al. 2010; Diab et al. 2004; Siahsar and Narouei 2010; Teulat et al. 2001). The information collected from these studies included pedigree, population type (i.e. DH, RIL), population size, traits observed, QTL positioning, marker platform used and the phenotypic variance explained (R2) by each of the QTL. From these studies, 10 different traits relating to drought tolerance were analysed with a total of 59 QTLs reported. Using the projection strategy detailed in Mace et al. (2009), the location of QTL identified in this study and those previously reported were projected onto the DArT consensus map (Wenzl et al. 2006). For display purposes, a 4 centimorgans (cM) confidence interval, 2 cM above and below the peak marker location, was implemented. DArT consensus marker data, SG QTL and the aligned QTL reported in previous studies were visually displayed using MapChart 2.2 (https://www.wageningenur.nl (Voorrips 2002). The QTL were annotated using the nomenclature style described on GrainGenes (http://wheat.pw.usda.gov/GG2).
Results
Segregation for stay-green and other phenotypic traits
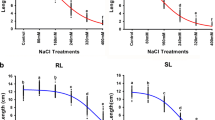
The ND24260 × Flagship DH population segregates for SG under both heat- and water-stress treatments. Examples of variation for SG evaluated using SPAD or by visual assessment of FL-1 are illustrated in Fig. 1. Under water-stress using either evaluation method, ND24260 exhibited a more stay-green phenotype than Flagship. ND24260 was later than Flagship to commence rapid senescence and later to reach full senescence at SPAD or visual scores near zero (Fig. 1c, d). This agrees with previous field observations suggesting that ND24260 has greater stay-green phenotypic expression when compared to Flagship under water-limited condition. In contrast, under heat-stress conditions using either evaluation method, Flagship was later to commence rapid senescence and to reach full senescence (Fig. 1a, b).
Stay-green (SG) response for parental lines (ND24260 and Flagship) and progeny displaying transgressive segregation in the ND24260 × Flagship DH population. a and c display SG response measured in the FL-1, using a Minolta SPAD 502 under terminal heat-stress and water-stress, respectively. b and d display the SG response based on visual assessment of the FL-1 under terminal heat-stress and water-stress, respectively
Transgressive segregation for SG expression of the FL-1 was observed in ND24260 × Flagship DH population for SG traits (Fig. 1). Under water-stress, transgressive segregation in both directions for SG traits is illustrated in Fig. 1, with DH183 exhibiting a more SG phenotype than either parent contrasting with DH310 which was more senescent (Fig. 1c, d). For heat-stress, transgressive segregation for greater SG was observed for genotype(s) such as DH119 (Fig. 1a, d).
As might be anticipated, a number of the SG traits examined were closely correlated (Table 1). A significant correlation was evident between the SPAD measurements for the FL and FL-1 within the heat-stress experiment (R2 = 0.94; Table 1) and this is confirmed in the PCA as vectors for these two traits were of similar direction and length (Fig. 2a). The visually assessed greenness ratings for both FL and FL-1 were also significantly correlated with one another (R2 = 0.95). LAUG scores did not significantly correlate with any of the measured effects of individual leaves (both FL and FL-1) within the heat-stress data set (Table 1; Fig. 2a). In the water-stress experiment, the SPAD measurements of the FL and FL-1 were significantly correlated (R2 = 0.77, P < 0.05) to each other (Table 1; Fig. 2b). The greenness of the FL and FL-1 were also significantly correlated (R2 = 0.91) to each other. However, as was seen in the heat-stress treatment, LAUG in the water-stress treatment was not significantly correlated to any of the measured effects within the water-stress trial (Table 1).
Bi-plots from principal component analysis displaying the correlation between season averages of visually assessed and SPAD measured SG expression of the spike (GS), flag leaf (GFL and SPFL), first leaf under the flag leaf (GFL-1 and SPFL1-1), leaf area under green (LAUG) and key morphological traits (Biomass, 100 kernel weight (Kernel), tiller number (Tiller) and spike number (Spike)). Bi-plot (a) presents the trait relationships under terminal heat-stress, explaining 73.0 % of the variance observed, while bi-plot (b) displays the trait relationships under terminal water-stress, explaining 50.1 % of the variance observed
The relationship between SG traits and morphological traits varied. Figure 2 represents the relationship between SG expression and morphological trait responses in the DH population, averaged for each trait over the duration of the trial. In the heat treatment, the SG traits examined appeared related to each other but showed little relationship with any of the morphological traits (Fig. 2a). Vectors for SPAD and visual assessments as well as LAUG were aligned with each other and the horizontal axis in Fig. 2a but are approximately perpendicular to those for biomass and kernel weight as well as tiller and spike numbers. For the water-stress treatment, a more complex pattern was observed. Vectors for the SPAD measured traits, SFL and SFL-1 as well as for LAUG appeared related as they roughly aligned with biomass as well as tiller and spike numbers in the vertical direction of Fig. 2b. However, these were not closely related to the visual assessment scores GS, GF and GF-1 that aligned more closely with kernel weight in the horizontal direction.
Significant (P < 0.05) correlations were observed between phenotypic responses associated with the SG traits. However, no significant (P > 0.05) correlation was evident between these phenotypic responses within each abiotic stress treatment and number of spikes/tillers and biomass.
Novel SG QTL were identified under both heat- and water-stress
Composite interval mapping identified ten QTL associated with SG expression, corrected for flowering date, under abiotic stress in the ND24260 × Flagship DH population. Six QTL were identified under heat-stress conditions, on chromosomes 3H, 4H, 5H and 6H. Individually these QTL explained between 0.7 and 17.4 % of the phenotypic variance observed in the sample population, but explained a potential total of 52.5 % of the phenotypic variance observed (Table 2; Fig. 3). The most significant QTL detected in the heat-stress experiment was HSPFLQ1, positioned at bPb-5529 on chromosome 5H bin 12 associated with SPFL (LOD = 5.9) and SPFL-1 (HSPFL1Q, LOD = 4.3), explaining 17.4 and 14.6 % of the phenotypic variance respectively (Table 2; Fig. 3). An additional locus for SPFL (HSPFLQ2) was identified and mapped to bPb-3269 (LOD = 3.6) on chromosome 5H bin 13 (Table 2; Fig. 3), explaining 13.7 % of the phenotypic variance. The allele associated with a SG phenotype at HSPFLQ1 was donated by ND24260, while the allele at bPb-3269, HSPFLQ2, was donated by Flagship. CIM using data for spike greenness (GS) detected a minor QTL on chromosome 6H bin 05 (DArT marker bPb-8613), explaining 0.7 % of the observed phenotypic variance, with ND24260 contributing the desirable allele for SG (Table 2; Fig. 3). Two QTL associated with LAUG under heat-stress were mapped to bPb-9672 on chromosome 4H bin 08 (LOD = 2.5, HLAUGQ1) and bPb-8770 on 6H bin 08 (LOD = 2.7, HLAUGQ2) explaining 2.8 and 3.3 % of the phenotypic variance respectively (Table 2; Fig. 3). Flagship contributed the allele for high LAUG at both of these QTL.
QTL for stay-green and drought tolerance traits in barley projected onto the DArT consensus map (Wenzl et al. 2006). A total of 59 QTL were sourced from five discovery papers (Arifuzzaman et al. 2014; Chen et al. 2010; Diab et al. 2004; Siahsar and Narouei 2010; Teulat et al. 2001) and combined with the 10 QTL identified in this study. Twelve traits for drought tolerance are displayed on the map [root length (RLQ), root dry weight (RDWQ), root to shoot ratio (RSRQ), root angle (RAQ), root number (RNQ), relative water content (RWCQ), accumulation water-soluble carbohydrate at 100 % RWC (DWSC100Q), osmotic potential (OPQ), osmotic potential full turgor (OP100Q), water-soluble carbohydrate (WSCQ), WSC full turgor (WSC100Q), osmotic adjustment (OAQ)]. The SG QTL from the current study are indicated in black and detected in the area associated with the greenness of the spike (GS), flag leaf (GFL), first leaf under the flag leaf (GFL-1) and leaf area under green (LAUG) or SPAD measurements of the flag leaf (SPFL) and the first leaf under the flag leaf (SPFL-1) under either heat- or water-stress (annotated as H or W respectively). Identifiers for QTL proposed in the current study are as listed in Table 1. Confidence intervals adjusted to 4 cM for display purposes
Four QTL associated with SG expression during terminal water-stress were detected using phenotype data based on both SPAD and visuals assessments. Altogether, these four QTL explained up to 20.4 % of the total phenotypic variance observed. Two QTL for GFL-1 (WGFL1Q) were identified and mapped to bPb-7425 (LOD = 3.0, WGFL1Q1) on chromosome 3H bin 16 (Table 2; Fig. 3) and bPb-3703 (LOD = 2.6, WGFL1Q2) on chromosome 7H bin 07 (Table 2; Fig. 3). These QTL explained a low portion of the phenotypic variance observed—only 0.8 and 1.1 % respectively. WGFL1Q1, on chromosome 3H was linked to ND24260, while Flagship was associated with the QTL on 7H. The visual assessment data helped identify a QTL near bPb-3703 (LOD = 3.1) in bin 07 of chromosome 7H, explaining 1.7 % of the phenotypic variance observed for spike greenness (GS). Unlike the heat-stress QTL, Flagship was the contributing source for trait expression. Furthermore a WLAUGQ associated with high LAUG was positioned at bPb-6023 on chromosome 6H bin 06 (LOD = 3.0) and explained 16.8 % of the phenotypic variance (Table 2; Fig. 3). The allele for high LAUG at bPb-6023 was donated by Flagship. Analysis of QTL regions using QTLNetwork failed to detect any significant epistatic interactions for QTL reported in this study.
The QTL identified for SG expression under heat-stress did not co-locate with those identified under water-stress (Fig. 3). Despite occurring in regions where QTL for stress-related response have been previously identified in barley (Arifuzzaman et al. 2014; Chen et al. 2010; Diab et al. 2004; Siahsar and Narouei 2010; Teulat et al. 2001), none of the QTL reported in the current study are co-located with the previously reported QTL (Fig. 3). The lack of correlation between SG expression and anthesis date suggest SG expression is independent to maturity. This is supported by the point that a known maturity QTL linked to Flagships was not detected by the association analysis. Thus, the QTL reported here appear to be novel. Although not co-located with any of the previously identified drought stress tolerance QTL (Fig. 3), a number of QTL on 5H for SG traits under heat-stress are positioned in close proximity to previously mapped QTL for some root traits. Similarly, some QTL on 7H associated with SG expression under water-stress were found in close proximity to previously identified drought stress tolerance QTL. These previously identified QTL on 7H were associated with relative water content (RWCQ) and water-soluble carbohydrate (WSCQ). Although not co-located, the SG QTL mapped in near proximity to each other on 6H, are of interest as they represent terminal heat- and water-stress treatments respectively.
Discussion
This is the first study reporting QTL for SG expression in barley grown under simulated heat- and water-stress conditions. Breeding for abiotic stress tolerance has inadvertently been achieved (to some degree) in elite lines, by increasing yield potential under abiotic stress conditions. Although only putative, the identification of these QTL is an important first step looking at the genetic control of SG in barley. However, the mechanisms and genetic controls associated with heat-stress and drought-tolerance in barley are neither clearly defined nor well understood due to their complexity and dependence on environmental effects. Thus the putative QTL identified in this study need to be validated under field conditions, by applying genomic selection models to the whole population, using the 100 sub-sampled DH lines as a ‘training population’. Therefore, lines with extreme predicted phenotypes, representing the tails of the population, could be selected for validation in the field.
Phenotypic responses and SG expression
The aim of this study was to identify QTL associated with SG, using a double haploid ND24260 × Flagship barley population under simulated heat- and water-stressed conditions. Under heat-stress, there was a lack of correlation between SG expression and morphological traits such as number of spikes/tillers and biomass (Fig. 2a). However, these morphological traits are also key components associated with agronomic yield and often driven by water-use or the timing thereof. Therefore, the SG mechanism could potentially be incorporated with other desirable agronomic traits underpinning yield in different environments. Under water-stress, there was more suggestion that SG traits, particularly those measured by SPAD as well as the LAUG calculated from the visual scores, were more related to morphological traits such as biomass, tiller and spike numbers. This suggests that SG traits selection may be more useful for performance under water-limitation than for performance under heat-stress. These trends also indicate that field trials are warranted to validate these observations from the controlled environments.
Transgressive segregation for the SG traits examined, indicates that they are likely controlled by multiple genes. This was supported by evidence that genes contributing to certain traits are derived from both parents (Table 2). From data collected in a pilot study by Gous et al. (2013), it was suggested that ND24260 was the superior parent contributing to abiotic stress tolerance. It is clear from the QTL regions detected in this population, contributed by both parents, that SG is a complex trait which may be underpinned by a number of different mechanisms, each of which may be under its own complex genetic control (Borrell et al. 2010). QTL with small to moderate effects are expected for such complex abiotic tolerance mechanisms in cereals. SPAD measurements were used to quantify the leaf greenness and to validate data collected through visual assessment. In the heat-stressed trial the significant correlation between the SPAD measurements of the FL and FL-1 and the similarly correlated visually assessed greenness of the FL and FL-1, indicated that rating of the leaves greenness was performed consistently within and between each replicate. However, the SPAD readings and greenness scores of the FL and FL-1 were only moderately correlated (R2 = 0.72 and R2 = 0.77 respectively) when compared to each other (Table 1). This is due to variation in the data collected by these different techniques. However, the phenotypic data generated by these different techniques was sufficient to perform QTL analysis with a 95 % CI (P = 0.05).
Although SPAD measurements may provide more precise data in the glasshouse, its application in field trials evaluating large populations may prove to be too time consuming and impractical, when compared to a visual rating system. However, different QTL were identified using these different techniques for monitoring SG expression, indicating the need to validate data collected from these techniques under field trial conditions, to determine which may be more relevant for breeders.
Novel QTL for SG expression
The identification of SG QTL is of considerable interest to industry, as genes controlling SG expression have low heritability. This makes SG genes difficult to accumulate by means of traditional breeding practices without the use of techniques such as MAS. The trait is also expressed late during development making screening in early generations more difficult. Several QTL associated with SG were identified by genome wide association analyses of the ND24260 × Flagship DH population under terminal heat and drought-stress. These SG QTL mapped to chromosomes 3H, 4H, 5H, 6H and 7H, with ND24260 the greatest contributor to the greenness characteristics, thus confirming field trial observations and pilot study data determining greenness expression of the parental lines (Gous et al. 2013). We believe that this is the first report of SG QTL in barley.
Alignment of SG QTL with genomic regions associated with abiotic stress tolerance
None of the novel QTL described here co-located with any previously identified stress-related QTL. However, some of these SG QTL mapped near chromosome regions previously identified in other studies corresponding to QTL for abiotic stress tolerance traits in barley. For example, the LAUG QTL identified under heat-stress conditions linked to DArT marker bPb-9672 on 4H (HLAUGQ1), was in close proximity to QTL reported for two abiotic stress tolerance traits. The first of which was associated with osmotic adjustment (OAQ) found on gene marker CDO541 (Teulat et al. 2001) and the second found on EBmac635 was associated with root dry weight (RDWQ) (Arifuzzaman et al. 2014). Furthermore QTL associated with SPFL and SPFL-1 under terminal heat-stress, mapped to the same DArT marker bPb-5529 on 5H bin 12 (Table 2). This marker is associated with expression of the long basal rachis internode gene lbi1 in Flagship. However, these QTL were found in the proximity of several QTL associated with abiotic stress tolerance expression. The most significant of these was a root length QTL (RLQ) found on VrnH1 and a root to shoot ratio QTL (RSRQ) found on bPb-0071 (Arifuzzaman et al. 2014). However, numerous QTL mapped to 3H, 5H and 7H have been reported to be linked to disease resistance and malt quality (Cakir et al. 2003; Teulat et al. 2001); as is evident in that BMag222 (bPb-3269) from Flagship is associated with cereal cyst nematode (Rha4) resistance (Table 2).
Similarly, QTL analysis using SG data from the water-stressed treatment identified four QTL on chromosomes 3H, 6H and 7H. The region on 6H is also associated with the expression of the Karl low protein gene (Dailey et al. 1988) from ND24260. Two QTL, WLAUGQ and HGSQ, on 6H are in close proximity to each other, and are of interest and worth noting, because they align across both heat- and water-stress treatments.
Two QTL for SG under water-stress mapped to the same DArT marker bPb-3703 on 7H bin 07 (Table 2) are of interest. This region on 7H has been association with other QTL, such as those linked to relative water content (RWCQ) and water-soluble carbohydrate (WSCQ), both found on Acl3. The expression of these QTL could impact on grain quality particularly under abiotic stress and needs to be determined.
The aim of this study was to identify QTL associated with SG in barley. The identification of these SG QTL suggests that trait accumulation through targeted selection using MAS is a possibility. However, the lack of information regarding SG expression in barley is a significant hindrance; although progress is slowly being made in defining this trait and its incorporation into future breeding lines.
Conclusions
The identification and incorporation of SG in other cereal crops, which has shown to improve yield under drought-stress conditions, has led to attempts to identify and incorporate similar traits in barley. Here we report novel SG QTLs associated with stress-response in barley. Molecular markers defining these QTL may be used as an effective tool for SG selection, following validation of the results in the field. With lines expressing high and low levels of SG, identified in this study, selected as population tails and used to more efficiently validate the effect of SG under field conditions. Alternatively lines with specific QTL combinations could be selected and their effect tested, across the whole population, based on marker haplotypes associated with SG that have been identified in this study. SG has shown highly variable expression and is strongly influenced by environmental conditions making the manipulation and selection of these traits, with low heritability or expression late in development, very difficult without the use of modern techniques such as MAS. Thus the identification of these putative SG QTLs in barley is likely to be of considerable benefit for the development of cultivars with stable grain yield of outstanding quality notwithstanding heat- and drought-stress.
Abbreviations
- SG:
-
Stay-green
- MAS:
-
Marker-assisted selection
- GS:
-
Green spike
- GFL:
-
Green flag leaf
- GFL-1:
-
Green first leaf under the flag leaf
- LAUG:
-
Leaf area under green
- SP:
-
SPAD (Single-photon avalanche diode)
- DH:
-
Double haploid
- CIM:
-
Composite interval mapping
- DArT:
-
Diversity Array Technology
- LOD:
-
Logarithm of odds
References
Arifuzzaman M, Sayed MA, Muzammil S, Pillen K (2014) Detection and validation of novel QTL for shoot and root traits in barley (Hordeum vulgare L.). Mol Breed 34(3):1373–1387. doi:10.1007/s11032-014-0122-3
Arriola KG, Kim SC, Huisden CM, Adesogan AT (2012) Stay-green ranking and maturity of corn hybrids: 1. Effects on dry matter yield, nutritional value, fermentation characteristics, and aerobic stability of silage hybrids in Florida. J Dairy Sci 95(2):964–974. doi:10.3168/jds.2011-4524
Ashraf M (2010) Inducing drought tolerance in plants: recent advances. Biotechnol Adv 28(1):169–183. doi:10.1016/j.biotechadv.2009.11.005
Borrell AK, Mullet JE, George-Jaeggli B, Oosterom EJ, Hammer GL, Klein PE, Jordan DR (2010) Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. J Exp Bot 28:1–13. doi:10.1093/jxb/eru232
Cakir M, Poulsen D, Galwey NW, Ablett GA, Chalmers KJ, Platz GJ, Park RF, Lance RCM, Panozzo JF, Read BJ, Moddy DB, Barr AR, Johnston P, Li CD, Boyd WJR, Grime CR, Appels R, Jones MGK, Langridge P (2003) Mapping and QTL analysis of the barley population Tallon × Kaputar. Aust J Agric Res 54(12):1155–1162. doi:10.1071/AR02238
Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, Marè C (2008) Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crop Res 105(1–2):1–14. doi:10.1016/j.fcr.2007.07.004
Chen GX, Krugman T, Fahima T, Chen KG, Hu Y, Röder M, Nevo E, Korol A (2010) Chromosomal regions controlling seedling drought resistance in Israeli wild barley, Hordeum spontaneum C Koch. Genet Resourc Crop Evol 57(1):85–99. doi:10.1007/s10722-009-9453-z
Christopher JT, Manschadi AM, Hammer GL, Borrell AK (2008) Developmental and physiological traits associated with high yield and stay-green phenotype in wheat. Aust J Agric Res 59(4):354–364. doi:10.1071/AR07193
Collard BCY, Jahufer MZZ, Brouwer JB, Pang ECK (2005) An introduction to markers, quantitative trait loci (QTL) mapping and marker-assisted selection for crop improvement: the basic concepts. Euphytica 142(1–2):169–196. doi:10.1007/s10681-005-1681-5
Collard BCY, Mackill DJ (2008) Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Phil Trans R Soc B 363(1491):557–572. doi:10.1098/rstb.2007.2170
Coombes NE (2002) The reactive tabu search for efficient correlated experimental designs. Liverpool John Moores University, Liverpool
Crasta OR, Xu WW, Rosenow DT, Mullet J, Nguyen HT (1999) Mapping of post-flowering drought resistance traits in grain sorghum: association between QTLs influencing premature senescence and maturity. Mol Gen Genet 262(3):579–588. doi:10.1007/s004380051120
Dailey JE, Peterson DE, Osborn TC (1988) Hordein gene expression in a low protein barley cultivar. Plant Physiol 88(2):450–453. doi:10.1104/pp.88.2.450
Demey JR, Vicente-Villardón JL, Galindo-Villardón MP, Zambrano AY (2008) Identifying molecular markers associated with classification of genotypes by External Logistic Biplots. Bioinformatics 24(24):2832–2838. doi:10.1093/bioinformatics/btn552
Diab AA, Teulat-Merah B, This D, Ozturk NZ, Benscher D, Sorrells ME (2004) Identification of drought-inducible genes and differentially expressed sequence tags in barley. Theor Appl Genet 109(7):1417–1425. doi:10.1007/s00122-004-1755-0
Eglinton J (2006) Flagship barley (Hordeum vulgare). Plant Breeders Rights. http://pericles.ipaustralia.gov.au/pbr_db/plant_detail.cfm?AID=10505989
Fox GP, Panozzo JF, Li CD, Lance RCM, Inkerman PA, Henry RJ (2003) Molecular basis of barley quality. Austr J Agric Res 54(12):1081–1101. doi:10.1071/AR02237
Franckowiak JD, Horsley RD, Neate SM, Schwarz PB (2007) Registration of ‘Rawson’ barley. J Plant Regist 1:37–38. doi:10.3198/jpr2006.10.0664crc
Gabriel KR (1971) The biplot graphical display of matrices with application to principal components analysis. Biometrika 58(3):453–467. doi:10.1093/biomet/58.3.453
Gous PW, Hasjim J, Franckowiak J, Fox GP, Gilbert RG (2013) Barley genotype expressing “stay-green”-like characteristics maintains starch quality of the grain during water stress condition. J Cereal Sci 1(6):414–419. doi:10.1016/j.jcs.2013.08.002
Gous PW, Lawson W, Kelly A, Martin A, Fox GP, Sutherland M (2012) QTL associated with barley (Hordeum vulgare) feed quality traits measured through in situ digestion. Euphytica 185(1):37–45. doi:10.1007/s10681-011-0608-6
Gregersen PL, Gregersen PL, Holm PB, Krupinska K (2008) Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol (Stuttg) 10:37. doi:10.1111/j.1438-8677.2008.00114.x
Guo P, Baum M, Varshney RK, GraneR A, Grando S, Ceccarelli S (2008) QTLs for chlorophyll and chlorophyll fluorescence parameters in barley under post-flowering drought. Euphytica 163(2):203–214. doi:10.1007/s10681-007-9629-6
Hamblin J, Stefanova K, Angessa TT (2014) Variation in Chlorophyll Content per Unit Leaf Area in Spring Wheat and Implications for Selection in Segregating Material. PLoS ONE 9(3):e92529. doi:10.1371/journal.pone.0092529
Harris K, Subudhi PK, Borrell A, Jordan D, Rosenow D, Nguyen H, Klein P, Klein R, Mullet J (2007) Sorghum stay-green QTL individually reduce post-flowering drought-induced leaf senescence. J Exp Bot 58(2):327–338. doi:10.1093/jxb/erl225
Hickey LT, Lawson W, Platz GJ, Dieters M, Arief VN, German S, Fletcher S, Park RF, Singh D, Pereyra S, Franckowiak J (2011a) Mapping Rph20: a gene conferring adult plant resistance to Puccinia hordei in barley. Theor Appl Genet 123(1):55–68. doi:10.1007/s00122-011-1566-z
Hickey LT, Lawson W, Platz GJ, Dieters M, Arief VN, Germán S, Fletcher S, Park RF, Singh D, Pereyra S, Franckowiak J (2011b) Mapping Rph20: a gene conferring adult plant resistance to Puccinia hordei in barley. Theor Appl Genet 123(1):55–68. doi:10.1007/s00122-011-1566-z
Hickey LT, Lawson W, Arief VN, Fox G, Franckowiak J, Dieters MJ (2012) Grain dormancy QTL identified in a doubled haploid barley population derived from two non-dormant parents. Euphytica 188:113–122. doi:10.1007/s10681-011-0577-9
Jordan DR, Hunt CH, Cruickshank AW, Borrell AK, Henzell RG (2012) The relationship between the stay-green trait and grain yield in elite sorghum hybrids grown in a range of environments. Crop Sci 52(3):1153–1161. doi:10.2135/cropsci2011.06.0326
Joshi AK, Kumari M, Singh VP, Reddy CM, Kumar S, Rane J, Chand R (2007) Stay green trait: variation, inheritance and its association with spot blotch resistance in spring wheat (Triticum aestivum L.). Euphytica 153(1–2):59–71. doi:10.1007/s10681-006-9235-z
Li JH, Vasanthan T, Rossnagel B, Hoover R (2001) Starch from hull-less barley: I. Granule morphology, composition and amylopectin structure. Food Chem 74(4):395–405
Ling Q, Huang W, Jarvis P (2011) Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth Res 107(2):209–214. doi:10.1007/s11120-010-9606-0
Mace E, Rami J, Bouchet S, Klein P, Klein R, Kilian A, Wenzl P, Xia L, Halloran K, Jordan D (2009) A consensus genetic map of sorghum that integrates multiple component maps and high-throughput Diversity Array Technology (DArT) markers. BMC Plant Biol 9(1):13
Netto AL, Campostrini E, Goncalves de Oliveira J, Bressan-Smith RE (2004) Photosynthetic pigments, nitrogen, chlorophyll α fluorescence and SPAD-502 readings in coffee leaves. Sci Hortic-Amsterdam 104(2005):199–209. doi:10.1016/j.scienta.2004.08.013
Phuong N, Afolayan G, El Soda M, Stützel H, Wenzel W, Uptmoor R (2014) Genetic dissection of pre-flowering growth and development in sorghum bicolor l. moench under well-watered and drought stress conditions. Agric Sci 5(11):923–934. doi:10.4236/as.2014.511100
Rollins JA, Drosse B, Mulki MA, Grando S, Baum M, Singh M, Ceccarelli S, Mv Korff (2013) Variation at the vernalisation genes Vrn H1 and Vrn H2 determines growth and yield stability in barley (Hordeum vulgare) grown under dryland conditions in Syria. Theor Appl Genet 126(11):2803–2824. doi:10.1007/s00122-013-2173-y
Rong-hua L, Pei-guo G, Baum M, Grando S, Ceccarelli S (2006) Evaluation of chlorophyll content and fluorescence parameters as indicators of drought tolerance in Barley. Agric Sci China 5(10):751–757. doi:10.1016/S1671-2927(06)60120-X
Rong H, Tang Y, Zhang H, Wua P, Chen Y, Li M, Wua G, Jiang H (2013) The Stay-Green Rice like (SGRL) gene regulates chlorophyll degradation in rice. J Plant Physiol 170(15):1367–1373. doi:10.1016/j.jplph.2013.05.016
Siahsar BA, Narouei M (2010) Mapping QTLs of physiological traits associated with salt tolerance in ‘Steptoe’x’Morex’ doubled haploid lines of barley at seedling stage. J Food Agric Environ 8:751–759
Spano G, Fonzo ND, Perrotta C, Platani C, Ronga G, Lawlor DW, Napier JA, Shewry PR (2003) Physiological characterization of `stay green’ mutants in durum wheat. J Exp Bot 54(386):1415–1420. doi:10.1093/jxb/erg150
Tao YZ, Henzell RG, Jordan DR, Butler DG, Kelly AM, McIntyre CL (1999) Identification of genomic regions associated with stay green in sorghum by testing RILs in multiple environments. Theor Appl Genet 100(8):1225–1232. doi:10.1007/s001220051428
Teulat B, Borries C, This D (2001) New QTLs identified for plant water status, water-soluble carbohydrate and osmotic adjustment in a barley population grown in a growth-chamber under two water regimes. Theor Appl Genet 103(1):161–170. doi:10.1007/s001220000503
Thomas H, Howarth CJ (2000) Five ways to stay green. J Exp Bot 51(suppl 1):329–337. doi:10.1093/jexbot/51.suppl_1.329
Vaezi B, Bavei V, Shiran B (2010) Screening of barley genotypes for drought tolerance by agro-physiological traits in field condition. Afr J Agric Res 5(9):881–892. doi:10.5897/AJAR09.294
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93(1):77–78. doi:10.1093/jhered/93.1.77
Wang S, Basten CJ, Zeng ZB (2002) Windows QTL Cartographer: WinQtlCart V2.0
Wenzl P, Li H, Carling J, Zhou M, Raman H, Paul E, Hearnden P, Maier C, Xia L, Caig V, Ovesná J, Cakir M, Poulsen D, Wang J, Raman R, Smith KP, Muehlbauer GJ, Chalmers KJ, Kleinhofs A, Huttner E, Kilian A (2006) A high-density consensus map of barley linking DArT markers to SSR, RFLP and STS loci and agricultural traits. BMC Genom 7(206):1–22. doi:10.1186/1471-2164-7-206
Xue D-W, Chen M-C, Zhou M-X, Chen S, Mao Y, Zhang G-P (2008) QTL analysis of flag leaf in barley (Hordeum vulgare L.) for morphological traits and chlorophyll content. J Zhejiang Univ Sci B 9(12):938–943. doi:10.1631/jzus.B0820105
Yang J, Hu C, Hu H, Yu R, Xia Z, Ye X, Zhu J (2008) QTLNetwork: mapping and visualizing genetic architecture of complex traits in experimental populations. Bioinformatics 24(5):721–723. doi:10.1093/bioinformatics/btm494
Yang J, Zhu J, Williams RW (2007) Mapping the genetic architecture of complex traits in experimental populations. Bioinformatics 23(12):1527–1537. doi:10.1093/bioinformatics/btm143
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14(6):415–421. doi:10.1111/j.1365-3180.1974.tb01084.x
Acknowledgments
The authors thank and acknowledge the support of the 1000-Talents Program of the Chinese Government’s Foreign Experts Bureau, and the Grain Research and Development Corporation for a Ph.D. top-up scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gous, P.W., Hickey, L., Christopher, J.T. et al. Discovery of QTL for stay-green and heat-stress in barley (Hordeum vulgare) grown under simulated abiotic stress conditions. Euphytica 207, 305–317 (2016). https://doi.org/10.1007/s10681-015-1542-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1542-9