Abstract
Salinity is an adverse environmental stress that limits the yield and quality of maize. As one of the most important osmolytes present in higher plants, glycinebetaine helps stabilize metabolism in plant cells and protects the constituents of cells from damage. In this study, a gene from Atriplex micrantha that encodes betaine aldehyde dehydrogenase was introduced by Agrobacterium-mediated transformation into maize inbred lines Zheng58 and Qi319 under the control of the maize ubiquitin promoter. Putative transgenic plants were confirmed by PCR and Southern blotting analysis. The transgenic maize plants expressed higher amounts of betaine aldehyde dehydrogenase activity and also grew better than the WT plants under NaCl stress. Compared with the wild type, the transgenic plants had increased fresh weight, lower malondialdehyde content, lower relative electrical conductivity, higher chlorophyll content, taller plant height, and higher grain yield under salt stress, which indicated that the expression of BADH gene in maize seedlings enhanced the salt tolerance of these plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is one of the important crops in the world, and serves as food for humans, feed for livestock and poultry, and as a raw material for agriculture-based industries. Meeting human demand will require cereal production to increase to over 400 million tons by 2050, which represents an increase in yield of 37 % from current values (Tester and Langridge 2010). Salinity is an adverse environmental stress that limits the yield and quality of maize. Estimates indicate that about 6 % of the world’s total land area, including 20 % of the world’s irrigated area, is affected by salinity (UNESCO Water Portal 2007). Because demand for maize production is increasing and arable land becomes more limited every year, it is critical to improve the salt tolerance of maize germplasm via efficient breeding technologies. With the application of the transgenic technology, researchers can accelerate the breeding process by introducing genes that encode proteins involved in abiotic stress-protective mechanisms. One common mechanism plants use for self-protection is the accumulation of osmoprotectants that stabilize metabolism and protect cellular components from damage due to abiotic stress (Wood et al. 1996).
Glycinebetaine (GB), a common osmoprotectant in many organisms and in higher plants, raises osmolarity within cells in response to different stresses and stabilizes biological macromolecules (Rhodes and Hanson 1993; Holmstrom et al. 2000). GB is synthesized from choline in eukaryotes by a two-step process with betaine aldehyde as an intermediate compound. The choline is oxidised to betaine aldehyde by choline monooxygenase in the first step, followed by another oxidation reaction catalysed by betaine aldehyde dehydrogenase (BADH) that generates glycinebetaine. GB significantly increases the cellular osmotic levels of many salt-tolerant plants (Rhodes and Hanson 1993). Many studies have shown that overexpression of the BADH gene in transgenic plants could enhance tolerance to a wide range of abiotic stresses including salt, heat, drought, and chilling (Park et al. 2004; Quan et al. 2004; Su et al. 2006; Fan et al. 2012). In previous studies, heterologous overexpression of the BADH gene in transgenic plants resulted in the accumulation of GB, which subsequently improved the plant tolerance to salt, oxidative, and cold stresses (Fan et al. 2012; Ahmad et al. 2013; Tang et al. 2014). Researchers have proposed that such improvements in stress tolerance are mediated by induction or activation of ROS scavenging; increased proline accumulation and improved membrane integrity under stress have also been observed. GB has also been shown to counteract the inhibitory effects of salt stress-mediated photoinhibition on the degradation and synthesis of the D1 protein in Synechococcus (Ohnishi and Murata 2006).
The complexity of the maize genome, its relatively long life cycle, and the low transformation efficiency of most maize genotypes make generating transgenic maize difficult. In this study, we used established Agrobacterium-mediated methods to introduce a binary plasmid containing a betaine aldehyde dehydrogenase gene, BADH, from salt-tolerant Atriplex micrantha into maize inbred lines, and subsequently evaluated the stress tolerance of the transgenic plants. Here, we aimed to understand the function of BADH in the tolerance of maize plants to salinity stress, with the ultimate goal of creating new abiotic stress-tolerant maize germplasm.
Materials and methods
Agrobacterium tumefaciens strain and plasmid construct
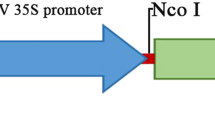
Agrobacterium tumefaciens strain LBA4404 harboring pCAMBIA3300-Ubi-BADH was used to overexpress the BADH gene in the present study (Fig. 1). The 1503-bp coding sequence of the BADH gene (GenBank ID EF208902, and Supplementary Fig. 1) was originally cloned from Atriplex micrantha, a very salt-tolerant plant that grows in salt marshes (Zhu et al. 2007). The BADH gene was then cloned into vector pCAMBIA3300 (Zhu et al. 2007) after appropriate digestion following standard protocols (Sambrook et al. 2000). The recombinant plasmid was transformed into A. tumefaciens strain LBA4404 using the freeze-thaw method.
Schematic diagram of the expression vector p3300-Ubi-BADH. RB, right border; LB, left border; UBI, Ubiquitin promoter, Tnos, Nopaline synthase terminator; BADH, betaine aldehyde dehydrogenase gene; Bar, Bialaphos resistance selectable marker gene; CaMV35S, Cauliflower mosaic virus 35S promoter. HindIII, BamHI, SacI, EcoRI, and KpnI are restriction endonuclease recognition sites
Plant materials and maize transformation
The elite maize inbred lines Zheng58 and Qi319 were used as the genetic materials in this study. The maize transformation procedure was performed according to the method published by Li et al. (2008). Seeds of Zheng58 and Qi319 were surface sterilized and were incubated on autoclaved solid MS medium at 28 °C in darkness until the etiolated seedlings had grown to 3.0–4.0 cm in length. Maize shoot tips with exposed meristems were immersed in the suspension of transformed Agrobacterium tumefaciens that had been grown to log phase then diluted to OD600 = 0.8 for 10 min under 0.05 MPa pressure, and were then co-cultivated on modified solid MS medium in the dark at 24 °C for 3 days. The plantlets were then transplanted into pots and cultivated in the greenhouse. The seedlings that survived spraying with the herbicide Basta at a concentration of 0.1 % were assayed for the presence of the transgene by PCR, and seeds were harvested from the positive T0 plants. T1 plants were grown from the harvested seed and self-pollinated to produce the T2 generation, followed by repeated herbicide screening and PCR assays until the T4 generation. Southern blotting and RT-PCR were carried out on PCR-positive plants in the T4 generation to confirm the integration and stability of the BADH gene, as described below. Experiments were conducted at the Molecular Breeding Experimental Base of Northeast Agricultural University located in Harbin City, Heilongjiang Province, China. Seeds for these plants were stored in a secure room and the plants were incinerated when the experiments were finished, in compliance with laws related to the handling and disposal of transgenic plants in China.
PCR analysis and Southern blotting
Genomic DNA was extracted from the two youngest leaves of each plant using the CTAB protocol (Murray and Thompson 1980). PCR assays were performed using the following BADH gene-specific primers: F, 5′-TCCAAATCCACTACGC-3′; and R, 5′-TTCTGCTGCCCAACTA-3′. Southern blotting was performed with a digoxigenin (DIG)-labelled probe consisting of the gene fragment amplified using the BADH cDNA as template, as described in the DIG System Manual (Roche, Inc., Basel, Switzerland). Genomic DNA from each of the PCR-positive maize plants was digested with BamHI, a restriction enzyme that cuts a single site in the T-DNA and cuts no sites within the BADH sequence. Restriction enzyme digestion was followed by electrophoresis of 20 μg of digested genomic DNA on 0.8 % agarose gels and subsequent transfer onto a positively charged nylon membrane (Roche, USA). After drying at 80 °C for 2 h, the membrane was hybridised to digoxigenin (DIG)-labelled probe, as described in the DIG System Manual.
RNA isolation and RT-PCR
Total RNA was extracted using Trizol reagent (Tiangen Biotech, Beijing, China) from 100 mg of young maize seedling leaves or roots, and 500 ng DNase-treated RNA was reverse transcribed using the RT Reagent Kit (Transgene, Beijing, China). The DNase-treated RNA samples were diluted fivefold to serve as templates for the subsequent PCR. The gene-specific primers from the conserved region of the target gene were used to amplify a 661-bp BADH fragment. Seedling leaves subjected to salt stress as described below were collected for RNA extraction and semi-quantitative RT-PCR. The β-Actin gene was used as an internal control for RT-PCR, and three replications were performed for each of the RT-PCR experiments in this study.
Salt stress treatment during seedling growth
T4-generation transgenic seeds and WT seeds were germinated and planted in pots containing vermiculite in the greenhouse (22 °C, humidity 40–50 %, with 16-h light and 8-h darkness). The plants were grown under well-watered conditions with 0.5× Hoagland’s nutrient solution applied once per day until the three-leaf stage. Plants for the salt treatment were then irrigated with 0.5× Hoagland’s nutrient solution containing 300 mmol NaCl once per day for a 7-days period. Control plants were watered at the same time with 0.5× Hoagland’s solution containing no NaCl. All plants in the experiment, both salt-treated and control, were then irrigated with 0.5× Hoagland’s, every 3 days to prevent the accumulation of excess NaCl in the vermiculite.
Detection of physiological and biochemical parameters and phenotypic changes related to salt tolerance
Plant height, fresh weight, BADH activity, relative electrical conductivity, malondialdehyde (MDA) content, chlorophyll content, phenotypic changes, and physiological parameters were measured for the transgenic and WT seedlings that had been stressed for 3 days. Each measurement was repeated three times. Data were analyzed using the statistical analysis system (SAS).
Plants heights from the first basal leaf to the next leaf immediately above were measured. Seedling fresh weight was scored immediately after removal from vermiculite. Fresh leaves of the transgenic and WT seedlings that had been stressed for 3 days were collected for biochemical parameter assays.
BADH enzyme activity was measured according to previously published procedures (Liu et al. 1997). Relative electrical conductivity was detected using a conductivity meter (DDS-11A) by comparing the electrical conductivity of exudates from the transgenic plant leaves (L1) to those from control leaves after boiling leaf samples (L2) (Li 2000).
Glycinebetaine was extracted according to a published method (Moghaieb et al. 2004). Malondialdehyde (MDA) and chlorophyll contents were measured according to the methods of Bates et al. (1973) and Arnon et al. (1974), respectively.
Maize field trials
Seeds of T4 transgenic lines and their receptor lines were planted in three-row plots in a simulated saline-alkaline field containing soil taken from natural saline-alkaline land in Heilongjiang Province, China. The principle composition of the soil is described in Table 1. The trial plots were arranged in a random complete block design with three replications. Each plot was 2.0 m in length, and 0.60 m wide, with an interval of 0.20 m between plants. The plants were thinned at the five-leaf stage, resulting in 10 plants per plot. Plant and ear heights were measured after fluorescence. Mature ears were harvested, dried, and threshed to determine hundred-grain weight, the number of rows per ear, and the number of grains per row from ten randomly selected ears for each plot.
Results
Molecular characterization of transgenic maize plants
Transgenic maize plants overexpressing the BADH gene from Atriplex micrantha, a plant that is extremely resistant to salt, were successfully generated using Agrobacterium-mediated transformation of binary vector pCAMBIA3300-Ubi-BADH (Fig. 1) into the inbred lines Qi319 and Zheng58. More than 30 T0 independent transgenic plant lines were established and propagated in the greenhouse. A 661-bp PCR product amplified from these lines with BADH gene-specific primers is shown in Fig. 2. Four transgenic maize lines (BZ-136, BQ-2, BQ-12, and BQ-20) with single BADH integration events were verified via Southern blot analysis by hybridising BamHI-digested genomic DNA samples with a BADH probe. The probe did not produce a signal in wild-type plants. Two copies of BADH were detected in the line BQ-16 (Fig. 3). The lines BQ-2, BQ-12, BQ-16, and BQ-20 are transformants of Qi319, and line BZ-136 originated from transformed Zheng58.
Southern blot analysis of transgenic plants and wild-type plants. Total plant DNA was digested with BamHI. M, DNA marker DL15000 (TaKaRa); WT1, wild-type plants of Zheng58; WT2, wild-type plants of Qi319; P, pCAMBIA1301-BADH plasmid; BZ-136, transgenic line derived from Zheng58; BQ-2, BQ-12, BQ-16, and BQ-20, transgenic lines derived from Qi319 transformed with BADH
The expression of the BADH gene in T4 plants leaves was monitored by reverse transcriptase-polymerase chain reaction (RT-PCR), and the results are shown in Fig. 4. The RT-PCR did not produce a product in wild-type plant samples.
RT-PCR analysis of transformed samples using BADH primers. M, DNA marker DL2000 (TaKaRa); WT1, wild-type plants of Zheng58; WT2, wild-type plants of Qi319; P, pCAMBIA1301-BADH plasmid; BZ-136, transgenic line derived from Zheng58; BQ-2, BQ-12, BQ-16, and BQ-20, transgenic lines derived from Qi319 transformed with BADH
BADH activities and GB contents of transgenic plants
BADH enzyme activity measured in the transgenic lines was 5.7-fold higher than that in the WT plants after 3 days of 300 mM NaCl treatment. BADH activities in the transgenic lines were between 10.92 and 12.68 U, and varied slightly among the different transgenic plant lines. Line BQ-2 showed the highest BADH activity (Table 2).
The lines WT1 and WT2 accumulated 1.02 and 1.25 mg GB per g fresh weight, whereas the BADH-transgenic maize lines accumulated 15- to 20-fold more GB in their leaves than did wild-type plants under the 300 mM NaCl treatment. Analyses of variance indicated significant differences between transgenic and wild-type plants in terms of GB accumulation (Table 2).
Over-expressing the BADH gene enhanced the tolerance of maize seedlings to salt stress
Seedlings that had reached the three-leaf stage were treated with a control 0.5× Hoagland’s solution or 300 mM NaCl solution for 7 days, then growth of the transgenic and wild-type lines were compared under control and salt-stressed conditions. Growth of the transgenic maize plants was better than that of the WT plants under NaCl stress. The transgenic plants were 4.2–5.3 cm taller and 9.6–15.8 % heavier in terms of fresh weight per plant compared with WT plants (Table 2; Fig. 5). The seedlings of wild-type lines turned almost entirely yellow after 7 days of salt stress treatment. All transgenic plants were still growing well after the 3rd and 4th days of the NaCl stress treatment; however, the leaves had become paler by the 7th day of salt stress and the lower leaves had turned yellow. The line BZ-136 performed best among all of the transgenic lines (Fig. 5).
During the salt-stress treatment, the membrane integrity of the plants was also measured (Table 2; Fig. 6). MDA contents and the relative electrical conductivities (REC) in all maize plants analysed increased due to salt stress. However, the MDA contents and the REC values of the transgenic plants were significantly lower than those of WT under the 300 mM NaCl treatment, which indicated that the membranes of the transgenic plants suffered less salt-induced damage than those of the WT.
Effects of NaCl stress on REC, MDA, and chlorophyll content of wild-type and the transgenic maize plants. Wild-type (Zheng58 or Qi310) or transgenic maize plants were treated with 300 mM NaCl or 0.5× Hoagland’s (0 mM NaCl) for 4 days, then REC, MDA, and chlorophyll contents were measured. a REC content b MDA content, and c Chlorophyll content. WT1, wild-type plants of Zheng58; WT2, wild-type plants of Qi319; BZ-136, Zheng58 plant transformed with BADH; BQ-2, BQ-12, BQ-16, and BQ-20, transgenic lines from Qi319 plants transformed with BADH. Values shown are mean ± SE of three replicates per plant. *Indicates significantly different at P ≤ 0.05 according to the LSD test
Salt stress often results in degradation of photosynthetic pigments, resulting in reduced of photosynthetic capacity. Measurements indicated that the chlorophyll contents of all lines decreased under salt stress. All of the transgenic plants had higher chlorophyll content than the wild type (Fig. 6).
All of the above results indicate that the expression of the BADH gene in maize seedlings enhanced the salt tolerance of these transgenic plants, and the lines BQ-2 and BZ-136 appeared most tolerant, which was consistent with results of the BADH enzyme activity assays.
Expression of BADH increased grain yield
The transgenic maize lines were planted in natural soda saline-alkaline soil in order to investigate the saline-alkali tolerance. The result showed that the characteristics of rows per ear and grains per row were not significantly different from the WT lines. The plant height and ear height of transgenic lines was significantly higher than WT lines except for BQ-16 and BQ-20. The hundred-grain weight of lines BQ-2 and BZ-136 was 34.7 and 25.7 % higher than that of the WT plants, respectively. These results indicated that expression of BADH increased the tolerance of maize plants to salt stress (Table 3).
Discussion
GB has been detected as an osmoprotectant in many organisms and has been a target of stress-resistance engineering for many years. Heterologous expression of a bacterial choline dehydrogenase (betA) or choline oxidase (codA), or a plant-derived BADH gene, results in increased accumulation of endogenous GB that can enhance stress tolerance (Sakamoto and Murata 2000; Chen and Murata 2008). Interestingly, GB can confer increased tolerance to cold, freezing, heat, drought, and salt stress in BADH transgenic plants even at low levels (Sakamoto and Murata 2002; Chen and Murata 2008). In another study, genetic evidence showed that a GB-deficient mutation reduced the salt tolerance of maize (Saneoka et al. 1995).
GB has proven very effective for enhancing salt-stress tolerance and increasing the productivity of various crop species including tomato (Zhou et al. 2007), sweet potato (Fan et al. 2012), wheat (He et al. 2010), cotton (Zhang et al. 2009), rice (Mohanty et al. 2002), and potato (Zhang et al. 2011).
In the present study, injured shoot tips from sterile germinated maize seeds were used for Agrobacterium-mediated transformation to reduce the likelihood of chimeric transformants. This method has previously been used to generate many transgenic maize lines (Li et al. 2008; He et al. 2013). The effectiveness of this transformation method is demonstrated by the molecular characterization of the transgenic plants recovered in this study (Figs. 2, 3, and 4).
The BADH-transgenic maize plants synthesised and accumulated more GB than did the WT under salt-stressed conditions, as shown in Table 1, and had 5.7-fold higher BADH activity than did WT under salt stress. Our results indicated that the BADH transgene likely enhanced the tolerance of transgenic maize plants to salinity stress through membrane stabilization, as the transgenic plants showed lower relative electrical conductivities and MDA content than did the WT under salt stress (Fig. 6a, b). The BADH transgene also enhanced plant height and fresh weight of transgenic seedlings, and increased grain production parameters of transgenic plants under salt-stressed conditions compared to the WT plants (Tables 1, 3).
In other experiments, expressing the BADH gene together with other stress-protective genes in transgenic plants has shown additive enhanced tolerance against salt and drought stress (Duan et al. 2009; Zhou et al. 2008; Ahmad et al. 2010; Wei et al. 2011). Therefore, increasing GB levels via the technology applied in the present study should be very useful for engineering crop plants with enhanced tolerance to multiple environmental stresses.
In conclusion, these results suggest that the BADH gene can be used to improve the salinity tolerance of maize through genetic engineering. Further research will investigate the effects of introducing the BADH gene into maize on yield and other agronomic characteristics.
References
Ahmad R, Kim YH, Kim MD, Kwon SY, Cho K, Lee HS, Kwak SS (2010) Simultaneous expression of choline oxidase, superoxide dismutase and ascorbate peroxidase in potato plant chloroplasts provides synergistically enhanced protection against various abiotic stresses. Physiol Plant 138:520–533
Ahmad R, Lim CJ, Kwon SY (2013) Glycine betaine: a versatile compound with great potential for gene pyramiding to improve crop plant performance against environmental stresses. Plant Biotechnol Rep 7:49–57
Arnon DI, McSwain BD, Tsujimoto HY, Wada K (1974) Photochemical activity and components of membrane preparation from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochem Biophys Acta 357:231–245
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Chen THH, Murata N (2008) Glycine betaine: an effective protectant against abiotic stress in plants. Trends Plant Sci 13:499–505
Duan X, Song Y, Yang A, Zhang J (2009) The transgene pyramiding tobacco with betaine synthesis and heterologous expression of AtNHX1 is more tolerant to salt stress than either of the tobacco lines with betaine synthesis or AtNHX1. Physiol Plant 135:281–295
Fan WJ, Zhang M, Zhang HX, Zhang P (2012) Improved tolerance to various abiotic stresses in transgenic sweet potato (Ipomoea batatas) expressing spinach betaine aldehyde dehydrogenase. Plos One 7:1–14
He C, Yang A, Zhang W, Gao Q, Zhang J (2010) Improved salt tolerance of transgenic wheat by introducing betA gene for glycine betaine synthesis. Plant Cell Tiss Org Cult 101:65–78
He CM, He Y, Liu Q, Liu TS, Liu CX, Wang LM, Zhang JR (2013) Co-expression of genes ApGSMT2 and ApDMT2 for glycinebetaine synthesis in maize enhances the drought tolerance of plants. Mol Breed 31:559–573
Holmstrom KO, Somersalo S, Mandal A, Palva TE, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot 51:177–185
Li HS (2000) Principles and techniques of plant physiological and biochemical experiment (in Chinese). Higher Education Press, Beijing, pp 261–263
Li B, Wei AY, Song CX, Li N, Zhang JR (2008) Heterologous expression of the TsVP gene improves the drought resistance of maize. Plant Biotechnol J 6:146–159
Liu FH, Guo Y, Gu DM, Xiao G, Chen ZH, Chen SY (1997) Salt tolerance of transgenic plants with BADH cDNA (in Chinese with English abstract). Acta Genet Sin 24:54–58
Moghaieb REA, Saneoka H, Fujita K (2004) Effect of salinity on osmotic adjustment, glycinebetaine accumulation and the betaine aldehyde dehydrogenase gene expression in two halophytic plants, Salicornia europaea and Suaeda maritima. Plant Sci 166:1345–1349
Mohanty A, Kathuria H, Ferjani A, Sakamoto A, Mohanty P, Murata N, Tyagi A (2002) Transgenics of an elite indica rice variety Pusa Basmati 1 harbouring the codA gene are highly tolerant to salt stress. Theor Appl Genet 106:51–57
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Ohnishi N, Murata N (2006) Glycinebetaine counteracts the inhibitory effects of salt stress on the degradation and synthesis of D1 protein during photoinhibition in Synechococcus sp. PCC 7942. Plant Physiol 141:758–765
Park EJ, Jeknić Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, Chen THH (2004) Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J 40:474–487
Quan RD, Shang M, Zhang H, Zhao YX, Zhang JR (2004) Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol J 2:477–486
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Ann Rev Plant Physiol Plant Mol Biol 44:357–384
Sakamoto A, Murata N (2000) Genetic engineering of GB synthesis in plants: current status and implications for enhancement of stress tolerance. Exp Bot 51:81–88
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant, Cell Environ 25:163–171
Sambrook J, Fritsch EF, Maniatis T (2000) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Saneoka H, Nagasaka C, Hahn DT, Yang WJ, Premachandra GS, Joly RJ, Rhodes D (1995) Salt tolerance of glycinebetaine-deficient and -containing maize lines. Plant Physiol 107:631–638
Su J, Hirji R, Zhang L, He C, Selvaraj G, Wu R (2006) Evaluation of the stress-inducible production of choline oxidase in transgenic rice as a strategy for producing the stress-protectant glycine betaine. J Exp Bot 57:1129–1135
Tang W, Sun JQ, Liu J, Liu FF, Yan J (2014) RNAi-directed down regulation of betaine aldehyde dehydrogenase 1 (OsBADH1) results in decreased stress tolerance and increased oxidative markers without affecting glycine betaine biosynthesis in rice (Oryza sativa). Plant Mol Biol 86:443–454
Tester M, Langridge P (2010) Breeding technologies to increase crop production in a changing world. Science 327:818–822
Unesco Water Portal (2007) http://www.unesco.org/water.
Wei A, He C, Li B, Li N, Zhang J (2011) The pyramid of transgenes TsVP and BetA effectively enhances the drought tolerance of maize plants. Plant Biotechnol J 9:216–229
Wood AJ, Saneola H, Rhides D, Joly RJ, Goldbrough PB (1996) Betaine aldehyde dehydrogenase in Sorghum. Plant Physiol 110:1301–1308
Zhang H, Dong H, Li W, Sun Y, Chen S, Kong X (2009) Increased glycine betaine synthesis and salinity tolerance in AhCMO transgenic cotton lines. Mol Breed 23:289–298
Zhang N, Si HJ, Wen G, Du HH, Liu BL, Wang D (2011) Enhanced drought and salinity tolerance in transgenic potato plants with a BADH gene from spinach. Plant Biotechnol Rep 5:71–77
Zhou SF, Chen XY, Xue XN, Zhang XG, Li YX (2007) Physiological and growth responses of tomato progenies harboring the betaine aldehyde dehydrogenase gene to salt stress. J Integr Plant Biol 49:628–637
Zhou S, Chen X, Zhang X, Li Y (2008) Improved salt tolerance in tobacco plants by co-transformation of a betaine synthesis gene BADH and a vacuolar antiporter gene SeNHX1. Biotechnol Lett 30:369–376
Zhu XC, Hu YL, Wang XL, Zhu JB, Lin ZP (2007) Cloning and sequence analysis of betaine aldehyde dehydrogenase in Atriplex (in Chinese with English abstract). Biotechnol Bull 6:88–91
Acknowledgments
We would like to thank Prof. Zhongping Lin of the College of Life Science, Beijing University for his kind gift of the recombinant plasmid pCAMBIA3300-Ubi-BADH for performing these studies. This research was supported by the Major Genetically Modified Organism Breeding Project of China (2014ZX08011003), and the Scientific Research Foundation for Returned Scholars of Heilongjiang Province, China (LC201411).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Di, H., Tian, Y., Zu, H. et al. Enhanced salinity tolerance in transgenic maize plants expressing a BADH gene from Atriplex micrantha . Euphytica 206, 775–783 (2015). https://doi.org/10.1007/s10681-015-1515-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1515-z