Abstract
Glycinebetaine plays an important role in the protection mechanism of many plants under various stress conditions. In this study, genetically engineered maize plants with an enhanced ability to synthesise glycinebetaine (GB) were produced by introducing two genes, glycine sarcosine methyltransferase gene (ApGSMT2) and dimethylglycine methyltransferase gene (ApDMT2), from the bacterium Aphanothece halophytica. Southern blotting and RT-PCR analysis demonstrated that the two genes were integrated into the maize genome and expressed. The increased expression levels of ApGSMT2 and ApDMT2 under drought conditions facilitated GB accumulation in the leaves of transgenic maize plants and conferred improved drought tolerance. Under drought conditions, the transgenic plants showed an increased accumulation of sugars and free amino acids, greater chlorophyll content, a higher photosynthesis rate and biomass, and lower malondialdehyde and electrolyte leakage compared to the wild-type; these results suggest that GB provides vital protection against drought stress. Under normal conditions, the transgenic plants did not show decreased biomass and productivity, which indicated that the co-expression of ApGSMT2 and ApDMT2 in maize plays an important role in its tolerance to drought stress and does not lead to detrimental effects. It was concluded that the co-expression of ApGSMT2 and ApDMT2 in maize is an effective approach to enhancing abiotic stress tolerance in maize breeding programmes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drought is one of the most important abiotic stresses due to its serious effects on plant growth and development and subsequent reduction of global crop yield and quality. Recent patterns of global climate change are likely to further aggravate the impacts of drought in the future (Passioura 2007). Maize is the most important food and animal feed crop worldwide, and it is therefore vital to improve the drought tolerance of maize varieties. Transgenic approaches can be used to create new materials with improved stress tolerance by the introduction of stress-induced genes (Vasil 2007).
Plants often adapt to unfavourable environmental conditions at both the cellular and molecular levels by accumulating osmolytes and proteins (Armengaud et al. 2004; Gupta and Kaur 2005; Liu et al. 2007). Glycinebetaine (N,N,N-trimethyl glycine, GB) is a quaternary ammonium compound that has been found in several different species in response to various types of stress (Rhodes and Hanson 1993). GB has been shown to be a very effective compatible solute that does not perturb metabolic processes or protein structure and function, and it consequently maintains the water content of cells under stress (Boyer 1982). The accumulation of GB in plants could enhance tolerance by maintaining cell membrane integrity and stabilising the quaternary structure of complex proteins under stress conditions (Sakamoto and Murata 2002). GB is also effective in protecting complicated protein complexes, such as the photosystem II complex, by reducing the generation of toxic oxygen species under high light stress (Prasad and Saradhi 2004). Since the first report of the link between GB accumulation and abiotic responses in plants, many studies have supported a strong correlation between increased GB levels and the enhancement of abiotic stress tolerance (Sakamoto and Murata 2002; Shirasawa et al. 2006).
GB may be synthesised by either of two biochemical pathways. The first is a two-step oxidation pathway in which the first and second steps are catalysed by choline mono-oxygenase (CMO) and betaine aldehyde dehydrogenase (BADH), respectively (Rhodes and Hanson 1993). The second pathway is a three-step methylation of glycine in which GB is catalysed by glycine sarcosine methyltransferase and sarcosine dimethylglycine methyltransferase; this pathway is present in the bacteria Aphanothece halophytica and Ectothiorhodospira halochloris (Nyyssola et al. 2000). These GB synthesis pathways have been introduced into various plant species to improve their abiotic tolerance under different stress conditions (Holmström et al. 2000; Sulpice et al. 2003; Kumar et al. 2004; Quan et al. 2004a, b; Waditee et al. 2005; Lv et al. 2007; Yang et al. 2007; He et al. 2010). The majority of these studies introduced the genes of the two-step pathway into the plants, and in most cases the GB levels of these transgenic organisms were still low (although still significantly higher than in non-transgenic control plants).
In 2003, Waditee et al. isolated two novel genes, glycine sarcosine methyltransferase gene (ApGSMT) and dimethylglycine methyltransferase gene (ApDMT), from Aphanothece halophytica, a halotolerant alkaliphilic cyanobacterium that can grow in media of up to 3.0 M NaCl and pH 11, which synthesise betaine from glycine by the three-step methylation pathway. The authors also found that the co-expression of ApGSMT and ApDMT in Synechococcus cells led to the accumulation of a significant amount of GB and conferred sufficient salt tolerance. Moreover, gene function analysis in the higher plant A. thaliana demonstrated that co-expression of the two genes generated an efficient tolerance effect under salt stress (Waditee et al. 2005). Recently, we isolated two N-methyltransferase genes from a strain of A. halophytica that produce enzymes in the glycine-methylation biosynthetic pathway for GB (He et al. 2010). As these genes had approximately 83 and 95 % similarity to ApGSMT and ApDMT, respectively, we named them ApGSMT2 and ApDMT2.
Maize has a complicated genome and a long-term life cycle, and the species has a low efficiency of transformation in most genotypes. As the co-expression of ApGSMT2 and ApDMT2 genes in tobacco conferred enhanced tolerance to drought in comparison with betA transgenic tobaccos (He et al. 2010), we speculated that co-expressing the two genes ApGSMT2 and ApDMT2 in maize might be an effective strategy for the improvement of drought tolerance. Based on the co-expression of these two genes, we could also inspect the possible mechanism and molecular processes of drought tolerance in maize. In the present study, we created a transgenic maize plant that co-expressed the two genes ApGSMT2 and ApDMT2. This survey of the functions of the ApGSMT2 and ApDMT2 proteins and their involvement in maize responses to drought stress is valuable not only for our understanding of the function of these genes in maize plants but also for the creation of new materials for maize abiotic stress breeding.
Materials and methods
Construction of the plant co-expression vector
The co-expression vector pCUE-GSD was constructed from the pGSD vector (He et al. 2010) by substituting the maize ubiquitin promoter for the CaMV35S promoters before the ApGSMT2 and ApDMT2 genes. The selection marker was still the 5-enolpyruvyl-shikimate-3-phosphate synthase (EPSPs) gene, which conferred resistance to the herbicide glyphosate on the transgenic plants.
Gene transfer and plant material
A Chinese elite maize inbred line, Zheng 58, was selected as the starting plant material. The transgenic maize transformation was conducted as described by Li et al. (2008).
Clean maize seeds were selected and surface-sterilized with 70 % ethanol for 8 min, followed by 0.1 % mercuric chloride for 10–12 min. The seeds were rinsed five times with sterilized distilled water before being transferred to sterilized solidified modified MS medium (Li et al. 2000) at 28 °C in darkness. When the coleoptile of the etiolated seedlings was 3.0–4.0 cm in length, it was cut off with a scalpel. The seedlings were prepared as follows: the young leaves were peeled off carefully to expose the shoot tip, and then the shoot tip meristem was wounded with a vertical cut; the wounded seedlings were placed in a Petri dish for transformation. Agrobacterium tumefaciens (harboring the constructed plasmid) in the mid-logarithmic phase was collected and re-suspended in modified MS liquid medium plus 100 μM acetosyringone. The wounded maize shoot tips were dipped in the bacterial suspension for 8–12 min under 0.05 MPa pressure with gentle shaking. After infection, the seedlings were sown in solidified modified MS medium and cultured in the dark at 22–24 °C for 3 days. The seedlings were then transplanted into pots of vermiculite, grown in a greenhouse at 32 °C day/25 °C night (12 h photoperiod), and watered with 1/2 MS medium inorganic solution (Li et al. 1990) every other day. About 10 days later, the seedlings with two to three new green leaves were sprayed with herbicide glyphosate solution (0.51 g/L glyphosate) for primary selection of transgenic plants. Seedlings which survived the selection were transplanted into the field for self-pollination. Herbicide selection was also conducted at T1 and T2 generations. Generally, there were about 200 seedlings inoculated each time. About 160–180 seedlings with shoot tip could be transplanted into pot and grew, and 30–40 could survive the herbicide selection. PCR analysis was not conducted at the T0 generation as most of the transformed T0 seedlings are chimeric. In the T1 generation, seedlings at three-leaf stage were selected by 0.80 g/L glyphosate. About one third to one half of the lines could survive the selection and the PCR-positive rate was about 35–50 % for the lines. The herbicide selection was conducted in the T2 generation as the T1 generation and the majority of seedlings showed herbicide resistance. The overall efficiency of transformation was about 2–5 %, which also depended on the operator’s skill proficiency.
PCR assay and Southern blotting
Genomic DNA was extracted from leaves using the cetyltrimethylamonium bromide (CTAB) protocol. PCR amplifications to detect the two genes and the selection marker gene were conducted in a total volume of 25 μL. The PCR primer sequences for the ApGSMT2, ApDMT2 and EPSPs genes are shown in Electronic Supplementary Material Table 1S. The PCR cycling parameters were 95 °C for 7 min, followed by 35 cycles of 95 °C for 1 min, 56 °C for 1 min and 72 °C for 1 min. The 1-kb, 0.6-kb and 0.9-kb products were separated on 0.8 %-TBE agarose gels.
Southern blotting was performed with a digoxigenin (DIG)-labelled PCR-amplified gene fragment probe (the PCR primers are shown in Table 1S) from either ApGSMT2 or ApDMT2 as described in the DIG System Manual (Roche, Basel, Switzerland). The probe included a fragment of about 600 bp of almost the full length of ApGSMT2 or ApDMT2. To ensure the presence of only one cut site in the T-DNA region, the genomic DNA was digested with BamHI for ApGSMT2 transformants and EcoRV for ApDMT2 transformants, respectively. After BamHI or EcoRV digestion and electrophoresis on 0.8 % agarose gel, 20 μg of DNA was transferred onto a positively charged nylon membrane (Roche, USA). After 2 h of fixation by drying at 80 °C, the membrane was hybridised with digoxigenin (DIG)-labelled probes as described in the DIG System Manual.
Semi-quantitative RT-PCR and real-time qPCR
For semi-quantitative RT-PCR of the two genes, total RNA was extracted using the TRIzol (Sangon, Shanghai, China) Kit, and 500 ng of total RNA was used with the reverse transcription system (Takara, Dalian, China), according to the manufacturer’s protocol. The cDNA samples were diluted 5-fold to serve as the templates for the subsequent PCRs. To confirm that the expression patterns obtained were gene-specific, the primers were designed from sequences outside of the conserved region of the target gene. The ApGSMT2- and ApDMT2-specific primers are also shown in Table 1S.
The sequences of the primers used for real-time qPCR were the same as those used in the RT-PCR, and the relative expression levels of the transgenes were calculated using the actin gene as the inner control. The primers used for the actin gene are shown in Table 1S. The real-time qPCR was performed in an optical 96-well plate using an ABI 7500 real-time qPCR system (ABI, USA) with the SYBR Green I RT-PCR Kit (Takara, Dalian, China) as previously described in Wei et al. (2011). Maize plants from each line were subjected to polyethylene glycol (PEG) solution at the four-leaf stage, and the leaf samples were collected for RNA extraction at 0, 3 and 7 days after PEG-6000 treatment. Three samples from every transgenic line and the non-transgenic plants (WT) were taken, and each sample was derived from the leaves of three individual maize plants. The entire experiment was repeated three times.
Primary drought stress selection and osmotic treatment during seedling growth
To evaluate the tolerance of the plants to drought stress, the seeds of 25 PCR-positive transgenic lines and the WT were sown in plastic plates (length 22 cm, width 16 cm, height 6 cm) with homogeneous loam under natural weather conditions in May in Jinan. The seedlings were watered at the four-leaf stage until water flowed from the bottom of the plates, and no further watering took place until permanent wilting occurred in the WT seedlings. After water was restored for 24 h, the recovery frequencies of the seedlings from each line were counted. Seeds from the WT and the three transgenic lines of the T3 generation from the primary drought stress assay were selected and surface-sterilised and then germinated on plastic plates supplemented with Hoagland nutrient solution at 25 °C in darkness. After germination, the seedlings were transferred into Hoagland nutrient solution and cultured at 32 °C day/25 °C night with a photon flux density (PFD) of 500 μmol m−2 s−1 (14 h light/10 h dark) in a greenhouse. When the seedlings reached the four-leaf stage, they were transferred into Hoagland nutrient solution supplemented with 0 and 12 % (w/v) PEG-6000 for 7 days. Leaves were harvested at 0, 3 and 7 days for RNA extraction and real-time qPCR. Fold changes of the ApGSMT2 and ApDMT2 gene expression levels were calculated using the 2-ΔΔCT method (Livak and Schmittgen 2001).
Analysis of the drought stress tolerance of plants
For the stress treatment, seeds from each transgenic line and the WT were sown in uniform flowerpots (diameter 34 cm, height 32 cm) with 10 kg homogeneous loam and placed in the greenhouse with a photoperiod of 14 h light/10 h dark. When the seedlings reached the four-leaf stage, the plants were thinned. The seedlings were watered every 2 days under normal conditions until the nine-leaf stage. The seedlings at the nine-leaf stage were used for the subsequent stress tolerance assays.
When the drought-stress treatment began, the water content of the soil was controlled at approximately 12–14 % for 7 days. Water was supplied daily over this period according to the weight of the pot, and then sufficient water was given for 1 week. During the stress and recovery period, the content of GB, the photosynthesis rate and other physiological parameters were measured, including the chlorophyll, malondialdehyde (MDA), total soluble sugar and free amino acid contents of leaf cells and their electrolyte leakage. At least three replications were performed for each measurement.
Determination of GB in maize leaves by 1H-NMR
The GB content of the leaves was detected using 1H-NMR spectroscopy as described in Quan et al. (2004a). Briefly, the dried fractions were dissolved in 550 μL D2O, and 50 μL 10 mM 3-(trimethylsilyl) propanesulfonic acid sodium salt (DSS) was then added as an internal reference and method of quantification. 1H spectra were determined using a Bruker AM500 NMR spectrometer.
Measurement of MDA and electrolyte leakage
The MDA content was determined as described by Lv et al. (2007). Approximately 0.2 g of maize leaves was used to determine the MDA concentration. The absorbance values at 450, 532 and 600 nm were determined using a spectrometer, and the concentrations of MDA were calculated using the formula C (μmol/L) = 6.45(OD532 – OD600) – 0.56OD450.
Electrolyte leakage was measured and calculated as described previously (He et al. 2011) and expressed as a percentage.
Chlorophyll determination
Samples (0.1 g) of maize leaves were homogenised with 80 % acetone (v/v), and the total amount of chlorophyll was determined spectrophotometrically according to Arnon (1949).
Assays of total soluble sugar and free amino acids
To determine the soluble sugar content, samples of maize leaves (approximately 0.1 g) were extracted in boiling water for 30 min at different times during the drought treatment. The amounts of total soluble sugar were measured with anthrone reagent using glucose as the standard (Yemm and Willis 1954).
For determination of the free amino acids content, maize leaves were dried at 72 °C and ground into a powder. Approximately 0.2 g of powder was treated with 5 % sulfosalicylic acid and then extracted in boiling water for 10 min. After cooling to room temperature, the extract was centrifuged at 5,000 g at 4 °C for 10 min. The supernatants were then analysed with an amino acid analyser (Hitachi Ltd., Tokyo, Japan).
Measurement of gas exchange and chlorophyll fluorescence
The net photosynthetic rate, stomatal conductance and intercellular CO2 concentration were measured on the second fully expanded leaves from the apex of the seedlings with a portable gas-exchange system (LI-COR 6400, LI-COR, USA). All measurements were performed between 8:00 and 11:00 a.m., and the photosynthetic PFD was maintained at 800 μmol m−2 s−1 using an internal 6400-02B LED source. During these measurements, the actual leaf temperature was between 30.0 ± 1.0 °C, and the ambient CO2 concentration was approximately 400 μmol CO2 mol−1. Chlorophyll fluorescence was measured on dark-adapted, fully expanded young leaves with a pulse amplitude modulation fluorometer (PAM 2000; Walz, Effeltrich, Germany). After 30 min of dark adaptation, the maximum photochemical efficiency of PSII (Fv/Fm) was determined.
Statistical analysis
All data in the figures and tables were analysed using SPSS version 16.0 and are presented as the mean ± standard deviation (SD). An analysis of variance (one-way ANOVA) was used to test for statistical significance, and the differences among the means were tested using Duncan’s multiple comparisons at a significance level of P = 0.05.
Results
Confirmation of transgenic maize co-expressing ApGSMT2 and ApDMT2
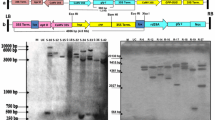
Twenty-five independent T2 transgenic maize lines from about 800 T0 transformed plantlets were identified by resistance to the herbicide glyphosate and PCR assays. Three transgenic lines from the T3 generation, along with the WT plants, were selected for Southern blotting and expression analysis (RT-PCR). The DIG-labelled probes were used for hybridisation and the restriction enzymes BamHI and EcoRV were selected to digest the maize genome according to the T-DNA region map for each probe (Fig. 1a). As shown in Fig. 1b, c, unique bands for the ApGSMT2 or ApDMT2 genes existed in transgenic plant genomes, indicating the integration of the foreign genes and confirming that each plant originated from an independent transformation event. As also shown in these figures, there were two copies of the genes in the L3 transgenic line and only one copy in both the L9 and L11 lines. From these observations, we also confirmed that the two genes were co-integrated in the transgenic maize genome. The co-expression of the two genes in T3 generation plants was determined by RT-PCR, and a part of the results is shown in Fig. 1d. No products similar to the transcripts of ApGSMT2 and ApDMT2 were detected in the RT-PCR for the WT plants. We concluded that the expression levels of the exogenous genes were different across the transgenic lines and that L9 showed a higher expression level than both L3 and L11.
Molecular characterization of transgenic maize plants. a Schematic structure of T-DNA region in plasmid pCUE-GSD. b, c Southern blotting analysis indicated the presence of target genes ApGSMT2 and ApDMT2 in the genomes of the transgenic maize. M, the λDNA/EcoT14 molecular weight marker; L3, L9 and L11 represent three independent transgenic lines; WT, non-transformed maize plant. The probe of (b) was a fragment from the ApGSMT2 gene and the restriction enzyme selected was BamHI; the probe of (c) was a fragment from the ApDMT2 gene and the restriction enzyme selected was EcoRV. d Specific PCR products were detected in the transgenic lines. A 380-bp actin fragment was amplified by RT-PCR as an internal control
Primary drought stress selection and osmotic treatment
Preliminary drought stress studies were conducted on four-leaf stage seedlings to determine the tolerance levels of all lines. Under normal growth conditions, the transgenic lines had no significant differences in phenotype or growth rate compared with the WT. After 3 days of drought stress, the WT plants exhibited early water deficit symptoms. After 6 days of drought treatment, all leaves of WT curled seriously and the old leaves became yellowish or died. However, the leaves of transgenic plants which were regarded as having the highest level of tolerance to drought were hardly affected and curled only slightly. In the end, most of the 25 transgenic lines exhibited more tolerance than the WT. The 6 days of drought treatment had little, if any, adverse effect on eight of the transgenic lines and had only a moderate effect on 12 other transgenic lines. However, five of the transgenic plant lines exhibited tolerance levels only slightly better than or nearly identical to those of the WT. After 6 days of drought treatment without watering, all seedlings were sufficiently re-watered; the WT seedlings did not recover and the five transgenic lines showed little (20–30 %) recovery, but eight transgenic seedling lines showed remarkable (90–100 %) recovery, and another 12 transgenic lines showed moderate (60–70 %) recovery. Three transgenic lines (L3, L9 and L11) from the T3 generation with good performance in the primary drought stress test were selected for the osmotic treatment (Electronic Supplementary Material Fig. 2Sa). As expected, the transgenic lines exhibited greater tolerance to the PEG-osmotic treatment than the WT. The WT plants wilted after 7 days of PEG-6000 treatment, while the three transgenic lines exhibited significantly superior growth (Fig. 2Sb).
Higher expression levels of ApGSMT2 and ApDMT2 resulted in higher GB accumulation in transgenic plants under stress conditions
To compare the expression levels of the exogenous genes, leaves from WT and transgenic lines cultured under normal and osmotic stress conditions were collected for RNA extraction. After reverse transcription, real-time qPCR was performed. As shown in Fig. 2a, b, the expression levels of ApGSMT2 and ApDMT2 in all transgenic lines increased remarkably after 7 days of osmotic stress, and they were significantly higher in L9 than in both L3 and L11.
The relative expression levels of transgenes ApGSMT2 (a) and ApDMT2 (b) under normal and stress conditions. c GB levels in maize leaves under normal (unstressed), drought stress for 7 days (stressed) and watering restored for 7 days (rewatered). L3, L9 and L11: the three independent transgenic lines; WT, non-transformed maize plant. Data are mean ± SD (n = 3). Columns marked with different letters are of different size at 0.01 < P < 0.05 (Duncan’s multiple range test)
Under normal conditions, the GB content of the leaves of WT plants was approximately 0.93 μmol g−1 fresh weight (FW), and it was approximately 2.2–3.6-fold higher in the transgenic lines than in the WT. After 7 days of drought stress, the GB concentration of all lines showed a striking increase, with the greatest increase observed in L9. There was approximately 12.1 μmol g−1 FW GB in the leaves of L9, roughly 3.8-fold the level obtained in the WT. The GB contents of L3 and L11 were slightly lower than that of L9, but they were still 1.9–3.1-fold higher than the content of the WT (3.14 μmol g−1 FW). In summary, there were significant differences between the amount of GB present in the transgenic plants and in the WT (Fig. 2c).
The plants co-expressing ApGSMT2 and ApDMT2 genes had enhanced drought tolerance
There were no significant differences between the growth rates and fresh weights of transgenic and WT seedlings when the plants were cultured in Hoagland nutrient solution (Fig. 2Sa). However, when cultured in Hoagland nutrient solution supplemented with 12 % PEG-6000 for 7 days, the WT plants wilted, while the transgenic lines were less affected (Fig. 2Sb). The transgenic maize lines also had significantly higher total biomass than the WT under these conditions (Table 2S).
To further test the drought stress tolerance of the plants by phenotype and physiological characterisation, watering was withheld from the seedlings of WT and transgenic lines at the nine-leaf stage (with the soil water content at 12–14 %) for 1 week, and then normal watering was restored for 7 days. As shown in Fig. 3a, there were no significant differences in phenotype between the WT and L9/L11; however, the leaves of L3 were slightly wider and the internodes a little shorter than those of the other lines. Nearly all leaves of the WT rolled after 7 days of withheld watering, and the old leaves of the plants became yellow or died. The transgenic plants exhibited more tolerance than the WT, as only a few leaves of these plants rolled, and the youngest leaves seemed green and unaffected. After water restoration for 1 week, the leaf survival rates of the L3, L9 and L11 lines ranged from 80.5 to 93.3 % and were significantly higher than those of WT plants (63.2 %).
Effects of drought stress on the maize growth, the chlorophyll contents, the cell membrane and the contents of total soluble sugars. a Growth of maize plants after 7 days of drought stress treatment; b chlorophyll contents of non-stressed, 7 days water-deficit stressed and rehydrated maize plants; c electrolyte leakage of leaf cells; d total soluble sugars contents; e MDA contents of the leaves in all lines. Maize seedlings at the nine-leaf stage underwent drought stress treatment. After 7 days of drought treatment, almost all leaves of the WT rolled and the old leaves became yellowish or died. However, the transgenic plants exhibited more tolerance than the WT as only a few leaves rolled and the youngest leaf seemed green and unaffected. Chlorophyll and total soluble sugars contents and cell membrane damage were determined under normal and stress conditions. L3, L9 and L11: the three independent transgenic lines; WT, non-transformed maize plant. Data are mean ± SD (n = 3). Columns marked with different letters are of different size at 0.01 < P < 0.05 (Duncan’s multiple range test) when compared with WT plants
Drought stress often destroys the photosynthetic pigments, resulting in the reduction of photosynthetic ability. The chlorophyll content determination indicated that the chlorophyll contents of all lines decreased due to drought stress. The chlorophyll contents in the transgenic lines decreased to approximately 1.6–1.8 mg g−1 FW at the 7th day from a starting level of approximately 2.3 mg g−1 FW (0 days), while it was 1.2–1.3-fold higher than the WT after stress (Fig. 3b). After watering was restored for 1 week, the chlorophyll contents in all plants increased to approximately 70 % of their levels before stress.
During the drought stress treatment, the membrane integrity of the plants was also measured, and the results are shown in Fig. 3c, e. We found that after 7 days of drought stress, the MDA content and the relative electrolyte leakage of the transgenic plants were significantly lower than those of the WT, which indicated that the membranes of the transgenic plants suffered less damage than those of the WT. All of the above results indicated that the co-expression of ApGSMT2 and ApDMT2 genes in maize seedlings enhanced the drought tolerance of these plants.
More soluble sugars and free amino acids accumulated in the transgenic seedlings under drought stress tolerance
Adverse environmental conditions often stimulate plants to accumulate some osmotic protection solutions, such as sugars and free amino acids, into their leaf cells (Gupta and Kaur 2005). Under normal conditions, no significant differences in the contents of soluble sugars were observed between the WT and the transgenic maize plants (Fig. 3d). After water withholding for 7 days, after which most leaves of WT plants had rolled, it was observed that the soluble sugar content of the transgenic lines L3, L9 and L11 were significantly higher than those of the WT. The soluble sugar content that accumulated in these transgenic lines under drought stress was approximately 1.4–1.6-fold higher than that of the WT.
Total free amino acids were determined, and the results are shown in Table 1. There were no significant differences between the WT and the transgenic lines under normal conditions. However, the total free amino acid contents increased greatly after drought stress treatment. The total free amino acids contents were approximately 4-fold higher in all lines after drought stress than before stress treatment; this was partly due to the large accumulation of proline. The proline contents were approximately 0.77–0.79 μmol g−1 dry weight (DW) in all lines before stress treatment and increased to approximately 86.17–97.39 μmol g−1 DW after drought stress for 7 days. The proline contents increased 109–126-fold after drought stress, thus indicating the importance of proline in plant drought resistance.
After drought stress treatment, almost all free amino acids contents increased dramatically, but those of Glu and Met showed obvious decreases. The Glu content in all lines decreased approximately 8-fold from pre-stress levels, and Met was not detected at all after drought stress. After drought stress treatment, the contents of Cys, Leu, Ile, Phe, His, Arg and Ser all exhibited significant differences between transgenic and WT lines. Of these amino acids, only the Ser content was lower in the transgenic lines than in the WT. These results may imply that under drought conditions, the catabolism and anabolism in the leaves of the transgenic lines undergo great changes in comparison with the WT. A portion of the Ser content may be consumed to synthesise glycine (the substrate for glycinebetaine) by methylation in which the Met served as the methyl supplier.
Transgenic maize lines showed reduced changes in photosynthesis and chlorophyll fluorescence under drought stress
To determine the effects of drought stress on the photosynthesis and chlorophyll fluorescence of the WT and transgenic maize plants, the photosynthetic performance of seedlings under normal and drought stress conditions was investigated. Under non-stressed conditions, the maize plants co-expressing ApGSMT2 and ApDMT2 displayed slightly higher photosynthetic rates than the WT (Fig. 4a); the stomatal conductance and intercellular CO2 concentration did not show significant differences between transgenic lines and the WT (Fig. 4b, c). The net photosynthetic rate and stomatal conductance of both WT and transgenic plants were significantly inhibited by drought stress. However, plants from the transgenic lines displayed significantly less inhibition due to drought stress than the WT. After 4 days of drought treatment, the net photosynthetic rates in the transgenic lines were significantly higher than those of the WT. After exposure to drought stress for 4 days or longer, the transgenic lines and the WT had marked decreases in intercellular CO2 concentration from the levels under normal conditions (Fig. 4c).
Photosynthetic parameters of each line under normal and drought stress conditions. a Net photosynthesis; b stomatal conductance; c intercellular CO2 concentration; d Fv/Fm ratios in all lines. L3, L9 and L11: the three independent transgenic lines; WT, non-transformed maize plant. Data are mean ± SD (n = 6). Columns marked with different letters are of different size at 0.01 < P < 0.05 (Duncan’s multiple range test) when compared with WT plants
The Fv/Fm ratio is the most frequently used parameter to indicate injury to the PSII complexes due to stress factors, including drought stress (Prasad and Saradhi 2004). To further investigate the factors limiting the photosynthesis rate in different lines under drought stress, the potential maximum quantum yield of PSII photochemistry, estimated by the Fv/Fm ratio in dark-acclimatised leaves, was measured. No significant differences in the Fv/Fm ratio were observed between transgenic or WT plants under normal conditions (Fig. 4d). However, the Fv/Fm ratio decreased significantly in all lines after drought stress for 7 days, although that of the transgenic lines was significantly higher than that of the WT (Fig. 4d). It appears that short periods of drought stress had relatively minor effects on the reaction centre of PSII in maize plants, whereas long-term drought stress significantly depressed the reaction centre. The maize plants co-expressing ApGSMT2 and ApDMT2 had significantly higher Fv/Fm ratios after exposure to drought stress than the WT.
Discussion
Glycinebetaine is a solute that plays an important role in protecting the stability of the huge molecular complexes of plants under various stress conditions (Sakamoto and Murata 2002). When exposed to abiotic stresses, many plants can accumulate greater levels of compatible osmolytes, such as free amino acids (Armengaud et al. 2004; Liu et al. 2007) and soluble sugars (Gupta and Kaur 2005). These osmolytes function as osmoprotectants in the stress tolerance mechanisms of plants. The relative electrolyte leakage and the MDA content are often used as an index of membrane injury (Morsy et al. 2005). In this study, we produced transgenic maize plants co-expressing two genes, ApGSMT2 and ApDMT2, and found that the transgenic plants were more tolerant to drought stress than the WT. The lower electrolyte leakage and the MDA content of the transgenic plants in comparison with the WT under drought stress, together with their higher GB content (Figs. 2c, 3c, e), supported the hypothesis that the higher accumulation of GB could offer more protection to the cell membranes and enhance the stability of huge molecular complexes in transgenic maize plants. The significantly higher contents of chlorophyll (Fig. 3b) and soluble sugars (Fig. 3d) suggested that enhanced osmoprotection was achieved in the transgenic lines under drought stress. In summary, under the protection of GB and other osmoprotectants, the drought tolerance of the transgenic plants was improved. The total biomass of each line, as shown in Table 2S, supports this claim.
The decline in productivity observed in many plant species subjected to drought stress is often associated with a reduction in photosynthetic capacity (Cornic 2000). Drought, coupled with high temperatures in summer, may indirectly affect growth by decreasing the photosynthesis rate of plants. In this study, drought stress induced higher increases of GB accumulation in the transgenic maize plants, from 1.9- to 3.8-fold that observed in the WT (Fig. 2c). The photosynthetic rate and intercellular CO2 concentration did not show dramatic decreases within 2 days of drought stress (Fig. 4a, b), indicating that drought stress inhibits the photosynthesis rate by decreasing the stomatal aperture during this period (Fig. 4c). Over long-term drought stress, the photosynthetic rate and the stomatal conductance both decreased dramatically, while the intercellular CO2 concentration was not as impacted; these results implied that the photosynthetic apparatus had been injured by the severe dehydration of the leaves. However, significantly higher photosynthetic rates and stomatal conductances were observed in the transgenic plants than in the WT at this time (Fig. 4a, c). The Fv/Fm ratio is often used to determine the degree of damage to the PSII complexes due to abiotic stress (Prasad and Saradhi 2004). Figure 4d shows that the Fv/Fm ratios decreased less in the transgenic lines than in the WT after 7 days of drought stress, suggesting that the PSII complexes of the transgenic plants had enhanced drought tolerance. These results indicated that the accumulation of higher levels of GB could alleviate the production of reactive oxygen species, which would enhance the repair rate of stress-enhanced photoinhibition in the transgenic plants (Yang et al. 2007).
The co-expression of ApGSMT and ApDMT has been suggested to have the potential to improve abiotic stress tolerance in crop plants (Waditee et al. 2005), and we previously found that the co-expression of ApGSMT2 and ApDMT2 in tobacco transgenic lines generated higher drought tolerance than that observed in betA transgenic lines (He et al. 2010). In this study, our results demonstrated that drought stress induced greater increases of GB accumulation in the transgenic maize plants than the WT and that the biomass of the transgenic lines was less affected by drought stress. This observation implied that the yield loss of the transgenic plants would be reduced when experiencing drought stress. Under normal conditions, transgenic plants in both large pots and field conditions had almost the same biomass and grain yield as the WT (data not shown), which indicated that the co-expression of ApGSMT2 and ApDMT2 did not impair the productivity of maize plants. Although there was a slight difference in phenotype between the transgenic L3 and other lines (Fig. 3a), and the content of GB in this line was not the highest among the transgenics, we did not have sufficient evidence to conclude that the co-expression of the two genes changed the phenotype of the maize plants. It is possible that the effects of the transgene insertion position led to the altered phenotype of L3. It was concluded that the high co-expression levels of ApGSMT2 and ApDMT2 contributed to the greater accumulation of GB in the transgenic leaves, which offered them greater stress protection and enhanced drought tolerance; these genes could therefore be used for the genetic improvement of maize.
Maize is a plant that is capable of synthesising a small quantity of GB. Introducing the two-step oxidation synthesis pathway for GB into maize may improve the drought and cold tolerance of the plants (Quan et al. 2004a, b), although this introduction leads to little increased accumulation of GB. The limited choline content (a substrate in the two-step oxidation pathway for GB synthesis) of maize cells may constrain the synthesis of GB (Huang et al. 2000). We therefore hypothesised that it would be more effective to introduce the three-step methylation pathway into maize, as free Gly (a substrate for GB synthesis catalysed by ApGSMT2 and ApDMT2) is abundant as a major amino acid in plant cells. In this study, we produced transgenic maize plants co-expressing the ApGSMT2 and ApDMT2 genes, the sequences for which were originally obtained from the cyanobacterium A. halophytica. Although GB levels increased to 6–12 μmol g−1 FW in the transgenic plants under drought stress, these levels were still much lower than those in natural GB-accumulating species such as spinach (30–40 μmol g−1 FW) (Rhodes and Hanson 1993). This result may be due to the presence of codons in the A. halophytica ApGSMT2/ApDMT2 sequences that are rarely used in maize plants, which could limit the expression of the heterologous protein (Puigbò et al. 2007). It may be necessary to optimise the ApGSMT2/ApDMT2 genes by switching some of their original codons with synonymous codons more prevalent in the maize genome, thereby increasing the expression levels of the transgenes and furthering GB accumulation under abiotic stress.
Under both normal and drought stress conditions, the Gly content in transgenic plants was identical to that of the WT, implying that the consumption of Gly for GB synthesis catalysed by ApGSMT2 and ApDMT2 did not have a significant effect on the Gly level in the transgenic cells. It may be concluded that there are sufficient substrate supplies for GB synthesis in plant cells under natural conditions. After drought stress treatment for 7 days, the GB content increased to higher levels, the Gly content did not show obvious changes and the Ser content was significantly lower in the transgenic lines than in the WT. Ser is known as a precursor in the generation of Gly (Ho and Saito 2001). These results implied that in ApGSMT2/ApDMT2 transgenic plants, considerable amounts of Gly were used for GB synthesis; however, the synthesis of Gly from Ser was rapid enough to meet the demands of consumption. Therefore, it will also be necessary to increase the rate of Ser synthesis and/or methyl supply if we hope to generate greater increases in the GB content. Moreover, after drought stress treatment for 7 days, the contents of free Cys, Leu, Ile, Phe and His in the transgenic lines were significantly higher than in the WT (Table 1), which suggested that the metabolism and allocation of amino acids had different features in comparison with the WT.
In this study, two glycine-methylation genes from A. halophytica, ApGSMT2 and ApDMT2, were introduced into maize plants, and the co-expression of these two genes enhanced the tolerance of the transgenic plants to drought stress. Further understanding of the ApGSMT2/ApDMT2-mediated metabolic pathways and their responses to abiotic stress will be useful for the improvement of drought stress tolerance in maize.
Abbreviations
- BADH:
-
Betaine-aldehyde dehydrogenase
- CMO:
-
Choline mono-oxygenase
- COD:
-
Choline oxidase
- CTAB:
-
Cetyltrimethylammonium bromide
- DIG:
-
Digoxigenin
- EPSPS:
-
5-Enolpyruvyl-shikimate-3-phosphate synthase
- GB:
-
Glycinebetaine
- MDA:
-
Malondialdehyde
- PEG:
-
Polyethylene glycol
- PS II:
-
Photosystem II
References
Armengaud P, Thiery L, Buhot N, Grenier-De March G, Savouré A (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant 120:442–450
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Cornic G (2000) Drought stress inhibits photosynthesis by decreasing stomatal aperture—not by affecting ATP synthesis. Trends Plant Sci 5:187–188
Gupta AK, Kaur N (2005) Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J Biosci 30:761–776
He Y, He C, Li L, Liu Z, Yang A, Zhang J (2010) Heterologous expression of ApGSMT2 and ApDMT2 genes from Aphanothece halophytica enhanced drought tolerance in transgenic tobacco. Mol Biol Rep 38(1):657–666
He CM, Zhang WW, Gao Q, Yang AF, Hu XR, Zhang JR (2011) Enhancement of drought resistance and biomass by increasing glycinebetaine content in wheat seedlings. Euphytica 177:151–167
Ho CL, Saito K (2001) Molecular biology of the plastidic phosphorylated serine biosynthetic pathway in Arabidopsis thaliana. Amino Acids 20(3):243–259
Holmström KO, Somersalo S, Mandal A, Palva ET, Welin B (2000) Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot 51:177–185
Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlindl JK, Keller WA, Selvaraj G (2000) Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol 122:747–756
Kumar S, Dhingra A, Daniell H (2004) Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confer enhanced salt tolerance. Plant Physiol 136:2843–2854
Li SR, Zhang JR, Chen HM (1990) Study on induction of embryogenetic callus and plantlet regeneration in maize. J Shandong Univ 25:116–124 (in Chinese)
Li GS, Yang AF, Zhang JR, Bi YP, Shan L (2000) Genetic transformation of calli from maize and regeneration of herbicideresistant plantlets. Chin Sci Bull 46:563–565
Li B, Wei A, Song C, Li N, Zhang J (2008) Heterologous expression of the TsVP gene improves the drought resistance of maize. Plant Biotechnol J 6:146–159
Liu K, Wang L, Xu Y, Chen N, Ma Q, Li F, Chong K (2007) Overexpression of OsCOIN, a putative cold inducible zinc finger protein, increased tolerance to chilling, salt and drought, and enhanced proline level in rice. Planta 226:1007–1016
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 25:402–408
Lv S, Yang A, Zhang K, Wang L, Zhang J (2007) Increase of glycinebetaine synthesis improves drought tolerance in cotton. Mol Breed 20(3):233–248
Morsy MR, Almutairi AM, Gibbons J, Yun SJ, de Los Reyes BG (2005) The OsLti6 genes encoding low-molecular weight membrane proteins are differentially expressed in rice cultivars with contrasting sensitivity to low temperature. Gene 344:171–180
Nyyssola A, Kerovuo J, Kaukinen P, von Weymarn N, Reinikainen T (2000) Extreme halophiles synthesize betaine from glycine by methylation. J Biol Chem 275(29):22196–22201
Passioura J (2007) The drought environment: physical, biological and agricultural perspectives. J Exp Bot 58(22):113–117
Prasad KVSK, Saradhi PP (2004) Enhanced tolerance to photoinhibition in transgenic plants through targeting of glycinebetaine biosynthesis into the chloroplasts. Plant Sci 166:1197–1212
Puigbò P, Guzmán E, Romeu A, Garcia-Vallvé S (2007) OPTIMIZER: a web server for optimizing the codon usage of DNA sequences. Nucleic Acids Res 7(35):126–131
Quan RD, Shang M, Zhang H, Zhao YX, Zhang JR (2004a) Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci 166:141–149
Quan RD, Shang M, Zhang H, Zhao YX, Zhang JR (2004b) Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol J 2:477–486
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Mol Biol 44:357–384
Sakamoto A, Murata N (2002) The role of glycinebetaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Shirasawa K, Takabe T, Takabe T, Sachie K (2006) Accumulation of glycinebetaine in rice plants that overexpress choline monooxygenase from spinach and evaluation of their tolerance to abiotic stress. Ann Bot 98:565–571
Sulpice R, Tsukaya H, Nonaka H, Mustardy L, Chen TH, Murata N (2003) Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J 36:165–176
Vasil IK (2007) Molecular genetic improvement of cereals: transgenic wheat (Triticum aestivum L.). Plant Cell Rep 26:1133–1154
Waditee R, Tanaka Y, Aoki K, Hibino T, Jikuya H, Takano J, Takabe T, Takabe T (2003) Isolation and functional characterization of N-methyltransferases that catalyze betaine synthesis from glycine in a halotolerant photosynthetic organism Aphanothece halophytica. J Biol Chem 7:4932–4942
Waditee R, Bhuiyan MN, Rai V, Aoki K, Tanaka Y, Hibino T, Suzuki S, Takano J, Jagendorf AT, Takabe T, Takabe T (2005) Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proc Natl Acad Sci USA 102:1318–1323
Wei AY, He CM, Li B, Li N, Zhang JR (2011) The pyramid of transgenes TsVP and BetA effectively enhances the drought tolerance of maize plants. Plant Biotechnol J 9(2):216–229
Yang X, Wen X, Gong H, Lu Q, Yang Z, Tang Y, Liang Z, Lu C (2007) Genetic engineering of the biosynthesis of glycinebetaine enhances thermo tolerance of photosystem II in tobacco plants. Planta 225(3):719–733
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by the anthrone. Biochem J 57:508–514
Acknowledgments
This research was supported by the Hi-Tech Research and Development (863) Program of China (2012AA10A306).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 2S
Maize seedlings growth of each line under normal and osmotic stress conditions. Maize seedlings were cultured in Hoagland solution (a1) and in Hoagland solution containing 12% (w/v) PEG-6000 (a2). Seedlings grown in Hoagland solution were transferred to Hoagland solution supplemented with 12% (w/v) PEG-6000 to be subjected to osmotic stress for 7 d. there are no significant differences between the transgenic lines and the WT seedlings under normal Hoagland solution culture, the WT plants wilted while the three transgenic lines grew much better when cultured under osmotic-stress (JPEG 1210 kb)
Rights and permissions
About this article
Cite this article
He, C., He, Y., Liu, Q. et al. Co-expression of genes ApGSMT2 and ApDMT2 for glycinebetaine synthesis in maize enhances the drought tolerance of plants. Mol Breeding 31, 559–573 (2013). https://doi.org/10.1007/s11032-012-9815-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-012-9815-7