Abstract
With the long-term application of pesticides on sugar beet farms in the irrigated perimeter of Tadla in Morocco for over 50 years, pesticide monitoring is necessary to assess soil health. The objective of our study was to monitor multiple pesticide residues in topsoil samples collected from post-harvest sugar beet fields and verify their migration to deep soil layers. Topsoil and deep soil samples were collected from arbitrarily selected sugar beet fields in the IPT. In this study, a target-screening method was applied. All target pesticides were detected in soil samples, with tefluthrin being the most frequently detected pesticide. The residue with the highest concentration in soil samples was DDE. All the soil samples contained a mixture of pesticide residues, with a maximum of 13 residues per sample. The total pesticide content decreased toward more profound layers of soil, except in one field where it reached a concentration of 348 µg/kg at the deeper soil layer. For pesticides detected at the three soil depths, only tefluthrin concentration increased in the deep soil layer. The results provide comprehensive and precise information on the pesticide residue status in sugar beet soils warning against the multiple risks that this contamination can cause. This study indicates the need of regular monitoring of pesticides over a large area of the perimeter to enable decision-makers to pronounce the impacts of the extension and intensification of sugar beet cultivation at the irrigated perimeter of Tadla.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils produce many ecosystem goods and services (Arrouays et al., 2012; de Groot et al., 2012) and their quality is directly linked to food security, human health, and the sustainability of environmental resources (Cheng, 2003; Liu et al., 2013). The assessment of the sanitary quality of soils to ensure food security, to prevent risks to biodiversity, and avoid contamination of groundwater has been considered a priority for many countries (Panico et al., 2022; Schleiffer & Speiser, 2022). Pesticides are a major source of degradation of soil health (Qu et al., 2016). Their widespread and indiscriminate use in intensive agriculture has significantly improved yields but has also resulted in their persistent presence in soils (Kopittke et al., 2019; Patel, 2009). In the irrigated perimeter of Tadla, agriculture is a vital component of the economy, primarily centered on the cultivation of citrus fruits and sugar beets using intensive agricultural practices. The sugar beet crop covers an area of 12,500 hectares in the irrigated region of Tadla, ensuring the production of 880,000 tons. This enables the production of over 110,000 tons of white sugar, contributing 22% to the national production. However, sugar beet crop heavily depends on pesticides to maintain such a high production level in this area (ORMVAT, 2019). Given the challenging climatic conditions of the region, characterized by the increasing scarcity of water, and considering the health and environmental effects of pesticides, an urgent pesticide residue monitoring system is needed for fields of sugar beet crop, which has a long-established presence in this region. This is essential for better managing and controlling pesticide residues in the soil while safeguarding the local environment and human health against potential adverse effects.
Early detection of changes in soil quality is key to maintaining healthy soil (García et al., 2022; Masiá et al., 2015; Souza et al., 2023). Many countries around the world have conducted monitoring programs for pesticide residue in soils: in Europe (Silva et al., 2018, 2019), in Asia (Liu et al., 2016; Murugan et al., 2013; Pan et al., 2019; Picó et al., 2020; Rafique et al., 2016), in America (Primost et al., 2017), and in Africa (Dankyi et al., 2014; Osesua et al., 2017). And this is due to a couple of causes, including (i) their use in proliferation with ignorance of their good handling practices (Bernhardt et al., 2017); (ii) their toxicity, mobility, and bioaccumulation capacities; and (iii) their occurrence in other matrices such as water (Schulze et al., 2019), crops (Medina-Pastor and Triacchini, 2020), and human beings (Bevan et al., 2017). From those soil-monitoring programs and studies with the theme of pesticides monitoring soil, it has been consistently revealed the presence of organochlorines pesticides (OCPs) and their transformation products (TPs) in soil samples. Despite the worldwide ban on their production and trade, these highly persistent contaminants have been found in soil samples across various regions, including Italy (Qu et al., 2019a), China (Pan et al., 2019; Wang et al., 2023; Yao et al., 2023), Argentina (Lupi et al., 2019), Nigeria (Osesua et al., 2017), and India (Kumar et al., 2014). Among the commonly detected OCPs, p,p′-dichlorodiphenyltrichloroethane (DDT) and its metabolite 1-dichloro-2,2-bis (p-chlorophenyl) ethylene (DDE) were frequently observed (Kosubová et al., 2020), and many studies also report the presence of dieldrin, chlordane, and heptachlor (Lupi et al., 2019; Pan et al., 2019; Qu et al., 2019a). The persistence of OCPs residues in the soil can be attributed to their historical use (Yadav et al., 2016). Heptachlor, especially in its oxidized form, continues to linger in the soil long after its discontinuation (Aigner et al., 1998). Dieldrin, an epoxide of aldrin, degrades very slowly in soil, water, and sediment, potentially affecting concentrations in these environmental compartments over time (Shegunova et al., 2007). As a result, even after decades of prohibition, the stability, resistance to degradation, and lipophilic nature of OCPs have led to their significant accumulation in various ecosystem components (Qu et al., 2019a, 2019b). In a complementary context, research focused on monitoring currently used pesticides in agricultural soils consistently identifies several frequently employed pesticides, including boscalid, metalaxyl, glyphosate, and its main derivative aminomethyl-phosphonic acid (AMPA) (Sabzevari & Hofman, 2022). Organophosphates (OPPs) and pyrethroids (PYs), often used in combination to reduce pesticide resistance, have been identified in a soil monitoring study conducted in China (Yao et al., 2023). Triazole compounds have also been frequently detected in European arable soils (epoxiconazole, tebuconazole, tetraconazole, penconazole, difenoconazole), with some reaching high concentrations in soil (epoxiconazole: 160 ng.g−1 and tebuconazole: 190 ng.g−1) (Froger et al., 2023; Silva et al., 2018, 2019). Individual pesticide concentrations have reached recorded levels of 1000 ng.g-1 in European agricultural soils, associated with azoxystrobin (Froger et al., 2023). For both banned and currently used pesticides, soil contamination is a major concern, emphasizing the essential need for monitoring pesticide contamination in agriculture. Moreover, in Africa, especially Morocco, there are limited published monitoring results compared to other regions (Matthews et al., 2011). Conducting monitoring in unexplored Moroccan regions like the irrigated perimeter of Tadla will provide valuable insights into pesticide contamination and its environmental impact.
Studies conducted in agricultural regions worldwide have consistently revealed that agricultural soils are the most pesticide-contaminated (Froger et al., 2023; Geissen et al., 2021; Hvězdová et al., 2018; Riedo et al., 2021). Frequently, multiple residues were detected in agricultural soil samples, resulting in cumulative total measured pesticide concentrations ranging from 300 to 2870 (Froger et al., 2023; Kosubová et al., 2020; Silva et al., 2019). For instance, twenty-five pesticides were still detectable in European soils even after their anticipated degradation, and 45% of applied pesticides remained detectable in Swiss agricultural soils, despite their relatively short dissipation half-life (less than 1 year) (Chiaia-Hernandez et al., 2017; Froger et al., 2023). This persistence is often attributed to the fact that agricultural soils are frequently used for crop cultivation and experience secondary concentration from neighboring areas (Liu et al., 2016). Additionally, the presence of a high number of pesticide residues in cultivated soils (10–20 pesticide residues) amplifies the risks to the agricultural ecosystem particularly given that the effects of pesticide mixtures remain largely unknown (Cáceres et al., 2010; Coronado et al., 2011; Geissen et al., 2021; Villanneau et al., 2011). The presence of these contaminated soils increases the risk of potentially hazardous compounds entering the food chain. Furthermore, extensive research has highlighted the significant consequences of prolonged pesticide use in agriculture on human health (Alavanja & Bonner, 2012; Kumar et al., 2016), biodiversity (Aktar et al., 2009), and aquatic and terrestrial ecosystems (Kapsi et al., 2019; King & Aaron, 2015; Masiá et al., 2015; Qu et al., 2016; Serra et al., 2020; Sidhu et al., 2019). The high levels of pesticide residues in soil, and the contamination risks these pose to surface water and groundwater quality, emphasize the need to understand pesticide dissipation in soil which is influenced by various factors (Kumar et al., 2022). Consequently, monitoring soil pesticide residues remains essential for evaluating the presence of pesticides, notably their persistent mixtures, which can endure, accumulate over time, and impact various ecosystem components.
Sugar beet occupies a strategic position within the irrigated perimeter of Tadla, and its management relies on the application of significant quantities of pesticides (MAPMDREF, 2022). The persistence of pesticide residues in the soil of sugar beet farms has not yet been investigated in this region and constitutes a first step in trying to enforce a sustainable use of pesticides for environmental conservation at the level of the irrigated perimeter of Tadla. The aim of this research was the following: (i) to assess the distribution of residue levels for 20 pesticides across 10 sugar beet fields, (ii) to establish meaningful connections between monitoring results, soil characteristics, and pesticide properties to identify the critical factors influencing pesticide distribution in soils, and (iii) to investigate pesticide migration patterns at three specific sites with depth soils and balanced textures.
Materials and methods
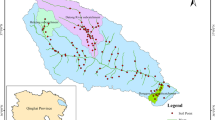
The area selected for the sampling was located in the irrigated perimeter of Tadla, a region in central Morocco where sugar beet is one of the most important crops, accounting for 12,500 hectares of the cultivated area for the agricultural campaign of 2019–2020 (ORMVAT, 2019). The irrigated perimeter is divided into three region areas (Fig. 1): sub-area of the perimeter in the province of Azilal, sub-area of the perimeter in the province of Beni Mellal, and sub-area of the perimeter in the province of Fkih Ben Saleh, with arid to semi-arid climates prevailing, recording an average rainfall annually of approximately 373.8 mm and average minimum and maximum temperatures respectively of 3.32 °C and 24.86 °C. The perimeter is characterized with a dry season from April to October and a wet season from November to March. Temperatures are low in winter, causing morning frosts, and high in summer, characterized by frequent waves of chergui (ORMVAT, 2019). The pursuit of agriculture (sugar beet crops) in this area necessitates irrigation, especially in light of the past two consecutive years of drought. The sampling sites were located to include sugar beet fields with different characteristics across the three sub-areas of the irrigated perimeter particularly with different surface area, different agricultural production of sugar beet crops, and the availability of water resources for irrigation. Three municipalities were designated across two sub-areas of the perimeter: the municipality of Sidi Jaber (sub-area included in the province of Beni Mellal), the municipality of Ouled Gnaou (sub-area included in the province of Beni Mellal), and the municipality of Afourar (sub-area included in the province of Azilal), and the third sub-area was excluded because of scarcity of water which influenced the expansion of sugar beet in this region (Fig. 1). With an irrigated surface of respectively 7209.5 ha, 1989.81 ha, and, 4187.67 ha, the municipalities of Sidi Jabeur, Afourar, and Ouled Gnaou were favorable for the agricultural production of sugar beet. The municipality of Afourar was characterized by an average yield per hectare of approximately 52.9 tons of sugar beet over an area of 400 ha in the five previous normal crop years, with the rural municipality of Sidi Jaber having an agricultural vocation and a large area of sugar beet covering 360 ha, while sugar beet occupied an area of 435.45 ha in the municipality of Ouled Gnaou (ORMVAT, 2019). The predominant soils at the level of these municipalities are subtropical brown isohumic soils in Afourar municipality; favorable to irrigated agriculture because of their depth and balanced texture, with moderately deep red fertialitic soils that are found in the municipalities of Sidi Jaber and Ouled Gnaou and also found in the municipality of Afourar (ORMVAT, 2019). Ten sampling sites were arbitrarily designated across the three municipalities of the two sub-areas of the irrigated perimeter to address the following objectives: (i) determine the distribution of the residues levels of 20 pesticides in soil of sugar beet fields; (ii) relate the monitoring results with soil properties and pesticides properties to identify keys properties affecting the concentration and the distribution for these compounds in the soil; and (iii) determine the residues levels of pesticides in depth layers of soil for three specific sites with a depth soil and balanced texture.
Sampling sites: sugar beet fields
The soil sampling sites were agricultural sites that had previously been cultivated with sugar beet crop. Sugar beet crop need tillage, fertilization, irrigation, and pest and disease control. Table 1 outlines these commonly adopted farming practices with scenarios of pesticide application (date, dose, active ingredient) by beet growers in the irrigated perimeter of Tadla. A total of ten sugar beet fields were arbitrarily designated for the monitoring network and were assigned codes ranging from FP01 to FP10. The area selected for the sampling at each site represents the area where sugar beet crop have been previously cultivated. All the field sites use irrigation, either by utilizing groundwater from wells, surface water, or both. Characteristics related to the farm size, its sugar beet production, the rural municipality of affiliation, and the agricultural campaign are reported for each sampled site in Table 2.
Soil sample collection
Soil physicochemical characterization and soil fertility
Soil samples were taken from the sugar beet fields, and the particle size, organic fertility, and pH were analyzed to characterize these fields. The soil samples were collected after the harvest of sugar beet crop before the beginning of the next agricultural season (in September). Samples were collected using an auger at a depth of 20 cm. To cover the variability and heterogeneity of each of the 10 fields, sampling was carried out according to the Hay method (Sabbe & Marx, 1987), and 13 samples of 100 g were taken from each field. All 13 samples were mixed to obtain a composite sample representative of each field, with a mass of 1 kg. The composite samples were transported in paper bags to the laboratory for physicochemical characterization of the soils.
Characterization of pesticide residues in soils
To analyze pesticide residues in the soil of sugar beet fields, soil samples were taken from the topsoil from 0 to 20 cm. This layer is most concerned with the accumulation of pesticides (Hvězdová et al., 2018). The soil samples were taken from fields in September before the start of the agricultural season. Soil samples were collected after the harvest of sugar beet crop before the beginning of the next agricultural season. A composite sample of 13 samples was collected to ensure the representativeness of each field. Samples were collected using an auger and stored in glass boxes. Soil samples were air-dried, and sieved through a 2 mm sieve and stored at 4 °C. Approximately 1 kg of soil from each field was collected.
Characterization of pesticide migration to deep soil layers
To verify the migration of pesticides, only the fields from the Afourar municipality (FP01, FP02, and FP03), characterized by their depth soils and balanced textures, were sampled. In the rural municipalities of Sidi Jaber and Ouled Gnaou, the selected fields are characterized by soils that are slightly developed rendzines, which limited their sampling to the 0–20 cm surface without extending the sampling to deeper layers. Samples were collected at three soil depths: 0–20 cm, 20–40 cm, and 40–60 cm using an auger and stored in glass boxes. Soil samples were air-dried, and sieved through a 2-mm sieve and stored at 4 °C. The soil samples were collected before the start of the agricultural season.
Physicochemical analysis of soil properties
The pH of the soils (pH-water) was measured in water: soil (1:2.5) ratio, using a pH meter with a glass electrode (Rhoades, 1982). The organic matter content was determined using the method of Walkley and Black by multiplying the % organic carbon by 1.724. The particle size analysis was performed using the Robinson pipette method. The clay and silt contents were determined by sedimentation according to Stocks' law. The physicochemical characteristics of the selected soils were determined and are shown in Table 3. The soil texture was generally sandy clay loam or sandy loam; the soil pH was in the 8.41 to 8.74 range, and the OM content was between 1.539 and 3.53%.
Analysis of pesticide residues in soil
Target pesticides
The physicochemical properties such as soil sorption coefficient (Koc), half-life soil degradation (DT50), solubility in water (Sw), vapor pressure (Vp), GUS leaching potential index (Gus index), and octanol–water partition coefficient (Log P) for the target pesticides are presented in Table 4. Nineteen pesticides and one transformation product were targeted in soils from the studied area. Azoxystrobin, deltamethrin, tebuconazole, tetraconazole, epoxiconazole, tefluthrin, bifenthrin, difenoconazole, ethofumesate, pyriproxyfen, and cypermethrin are pesticides commonly used in sugar beet crop. For those that are prohibited for use such as isopropalin, heptachlor, propiconazole, chlordane, dieldrin, and primiphos-methyl, DDT, and its derivative DDE (IUPAC PPDB Search, n.d.), it is necessary to define their status in the soil of sugar beet fields in the irrigated perimeter of Tadla.
Pesticide extraction technique
Solution preparation for the calibration curve
Twenty standards were prepared individually from 20 formulations with purities of 97–99%. Acetonitrile (90% purity) was used as the solvent to prepare the solutions. From the stock solution prepared at a concentration of 1000 mg/kg, four solutions (1, 2, 3, and 4) were prepared with concentrations respectively of 5, 1, 0.5, and 0.1 mg/kg for each target pesticide. Five concentrations, namely: 10, 20, 50, 100, and 200 µg/kg, were chosen as points for the calibration curve. These concentrations were prepared from solutions 4, 4, 3, 3, and 2 respectively. A blank sample was prepared with acetonitrile, and the fortified control sample was prepared by mixing 0.5 ml of the daughter solution 1 of all target pesticides. Using organic solvents (see sample extraction and analysis), a calibration curve was obtained after injection of the seven solutions (5 concentration points, blank and fortified solutions).
Sample extraction and analysis
Soil samples (25 g wet weight) were transferred to a 500-ml bottle and extracted with 50 ml of acetone and 100 ml petroleum ether. The mix was mixed with 300 ml of ultrapure water and filtrate. The organic phase was transferred to a 250-ml bottle and mixed with anhydrous sodium sulfate. The extract was concentrated and 5 ml of acetonitrile was added prior to vialing. For solvent evaporation, the extract was transferred through a nitrogen flux, and 0.25 ml of acetonitrile was added to the drop. A volume of 2 µL was injected into the gas chromatography-tandem mass spectrometry (GC–MS/MS) system.
The pesticides were analyzed by a CG- MS/MS with an HP–5MS UI column with a migration capacity of 1,1 ml/min under the following instrumental conditions: an initial temperature of 60 °C remains for 1 min, and increases by 10 °C/min up to 250 °C and remains for 5 min; ramp 1 has a temperature of 40 °C/min, up to 170 °C, and stays for 0 min, and ramp 2 increases by 10 °C up to 310 °C and stays 3 min. The injection port was operated at 70 °C, and detectors MS 1 at 150 °C and MS 2 at 150 °C, with the source temperature set at 280 °C. The 2μL injection was carried out with an Agilent autosampler. Helium was used as the carrier gas at 1.2 mL min−1. The MASSHUNPER software was used for sample processing. The limit of quantification of this technique is 1 PPB, and the limit of detection is 0.1%
Statistical analysis
In addition to principal component analysis, Pearson correlations were conducted firstly, to investigate the relationship between the frequency (Freq) and the maximum residual concentration detected for each pesticide (Max), and their physicochemical properties such as Koc, DT50, Sw, Vp, Gus index, and Log P, and secondly, to explore the connection between the total residual quantity for each sugar beet field in the sampling network and the soil properties of selected fields. Pearson correlations were also used to relate the residual concentration of individual pesticides and the soil characteristics. Data of the residual concentration of pesticides in sites FP01, FP02, and FP03 determined at three different sampling depths (0–20 cm, 20–40 cm, and 40–60 cm, respectively) were subjected to a one-way analysis of variance (ANOVA) to assess how sampling depth affects the residual concentration of pesticides. The least significant difference (LSD) test at a confidence level of 95% was used to separate means. IBM SPSS (version 22; USA) statistical software was used.
Results
Pesticide detection in topsoil from sugar beet fields
Up to 2021, over 28 active ingredients from multiple chemical functional categories were commonly used for sugar beet crops, and more than 20 pesticide product formulations were available for this crop in the irrigated perimeter of Tadla (MAPMDREF, 2022). To detect and quantify pesticides, soil analysis was conducted according to a targeted screening of nineteen pesticides and one transformation product. The results of the analysis of the residues demonstrate the presence of pesticides in topsoil samples from the ten selected fields before the start of the agricultural campaign (Table 5). Tefluthrin is the most frequently detected pesticide (DF = 90%). Epoxiconazole is also one of the most frequently detected pesticides, followed by the transformation product DDE. DDE, in addition to being frequent in soil samples (DF = 70%), was present at concentrations greater than 10 µg/kg. Cypermethrin, difenoconazole, and DDT were detected at high frequencies in the tested soils (DF = 50%). Cypermethrin and difenoconazole can reach concentrations greater than 10 µg/kg. Pesticides banned from use, such as isopropalin, heptachlor, chlordane, dieldrin, and pirimiphos-methyl, were detected at frequencies of 10%, 30%, 10%, 20%, and 30%, respectively. Other pesticides most commonly used in sugar beet crop, such as azoxystrobin, deltamethrin, tebuconazole, bifenthrin, ethofumesate, and lenacil, also had low detection frequencies. The Concentrations and detection frequencies of the pesticides varied from one pesticide to another. However, despite the low frequency of detection in the soil, some pesticides can reach concentrations above 10 µg/kg (deltamethrin and pirimiphos-methyl).
The residual concentration of pesticides can provide useful information about their use and their pollution status in topsoil of sugar beet fields. In the topsoil of sugar beet fields, tefluthrin was detected with a mean concentration of 2.910 µg/kg. Five pesticides, namely, epoxiconazole, difenoconazole, tetraconazole, propiconazole, and tebuconazole, belonging to the triazole family, were detected with the mean concentration of 1.40, 4.60, 1.944, 0.40, and 0.30 µg/kg, respectively. Three pyrethroid pesticides (Alpha-cypermethrin, deltamethrin, and bifenthrin) were detected with the mean concentration respectively of 2.20, 2.40, and 0.20 µg/kg. A uniform low concentration was found between other commonly used pesticides in sugar beet crops such as ethofumesate, lenacil, and azoxystrobine, with mean concentration of 0.50, 0.90, and 0.10 µg/kg respectively. DDT is still detected in sampled topsoils and the concentration of DDE observed (37 µg/kg) was higher compared to parent compound. In addition, dieldrin, chlordane, iso-propaline, pyriproxyfen, heptachlore, and pirimiphos-methyl can still detected in topsoil of sugar beet fields with related low mean concentrations of 1.20, 0.10, 0.80, 0.10, 0.50, and 2.30 µg/kg respectively. The results of concentrations of pesticides residues showed variations between residual concentrations of pesticides suggesting variable distribution of pesticides in topsoil of sugar beet fields.
All soil samples from the selected sugar beet fields contained pesticides, and the total residual quantity of pesticides are ranging from 5 to 137 µg/kg. The distribution of the total residual quantity of pesticides in the sugar beet fields selected from the three municipalities is shown in Fig. 2. The soil sample with the highest total concentration was that at site FP03, collected from a sugar beet field in Afourar municipality, with a total residual quantity of pesticides of 137 µg/kg (Fig. 3). Samples taken from FP01 and FP02 in the same municipality showed total residual quantity of pesticides of 128 and 94 µg/kg. The soil sample collected from the municipality of Ouled Gnaou (FP10) had a total residual quantity of 65 µg/kg. In the municipality of Sidi Jaber, the total residual quantity of pesticides ranges from 3 to 100 µg/kg. Four fields in the same municipality (FP07, FP09, FP05, and FP06) had the lowest total residual quantity of pesticides (3, 5, 8, and 17 µg/kg). Thus, large variations were observed among the three municipalities selected in this study. Similarly, this variation in total residual quantities of pesticides even resides between sugar beet fields belonging to the same rural municipality, indicating the variation in the application of pesticides in the different sugar beet fields.
Detected concentration: maximum, median, minimum
The minimum, maximum, mean, and median pesticide residual concentrations are listed in Table 6. DDE had the highest concentration which is 114 µg/kg. The pesticides deltamethrin, difenoconazole, and pirimiphos-methyl also showed significant concentrations of 22, 18, and 18 µg/kg, respectively. Pesticides banned from use are still detected at significant concentrations in the soils; the same is true for pesticides of recent use.
Detected multiple residues of pesticides
The presence of multiple pesticide residues is the result of the application of different pesticide treatments and formulations with several ingredients (Coronado et al., 2011; Li et al., 2023; Lozowicka, 2015). All the soil samples contained multiple pesticide residues, with a maximum of 13 residues per sample. Soil samples with five pesticide residues represented 40%, whereas those with a residue number above five represent 60% of all samples. The profile of multiple pesticide residues varies from one municipality to another and even within selected fields in the same municipality. In the municipality of Afourar, the number of residues in the three selected fields oscillates between 6 and 8, with a mixture of six residues present in the three sites, namely, tefluthrin, epoxiconazole, difenoconazole, DDT, pirimiphos-methyl, and DDE. The number of pesticide residues fluctuated between 3 and 13 for the six fields selected from the municipality of Sidi Jaber. In the municipality of Ouled Gnaou, only one site was selected for soil sampling and contained nine pesticide residues in total. Considering the presence of these large numbers of multiple pesticide residues in the tested soils, the quality of the soils is questioned after the cultivation of sugar beet.
Several types of pesticides are used in sugar beet crop in the irrigated perimeter of Tadla (Table 1). The multiple residues detected in the tested soils were differentiated according to the three classes of pesticides: insecticides, fungicides, and herbicides. Ten insecticides—organochlorines (four insecticides), organophosphates (one insecticide), pyrethroids (four insecticides), and cyclodienes (one insecticide)—were detected in the tested soils. All the sites contained insecticides. Three fields selected for the collection of soil samples have a single pesticide (tefluthrin), 4 fields have two insecticides (tefluthrin and cypermethrin or cypermethrin and deltamethrin), one field has 3 insecticides (tefluthrin, heptachlor, and bifenthrin), and finally 2 fields have 5 insecticides which are respectively tefluthrin, heptachlor, pyriproxyfen, cypermethrin and deltamethrin or tefluthrin, heptachlor, chlordan, DDT and bifenthrin. Of the ten selected fields, only two herbicides were detected (FP06 and FP08). Fungicides were detected in eight sugar beet fields, with FP08 containing five fungicides at a time (teraconazole, propiconazole, tebuconazole, epoxiconazole, and difenoconazole). To improve yield, beet growers tend to apply more insecticides and fungicides, which are more common in the tested soils than herbicides.
Relationship between detected pesticides and their properties
The presence of pesticides in soil depends on the application history, soil properties, pesticide properties, and climatic conditions (Hvězdová et al., 2018). In this study, the relationships between the maximum residual concentration of pesticides (Max), their frequency in soils (Freq), and their properties (Koc, DT50, Sw, Vp, Gus index, and Log P) were explored using PCA (Fig. 4). The first four components in PCA explained 77.973% of the data variability 31.012% in PC1, 18.910% in PC2, 15.279% in PC3, and 12.754% in PC4, reflecting the largest amount of original information. The DT50, detection frequency, and the maximum residual concentration of different pesticides were positively related and constituted the first principal component. The octanol–water partition coefficient, Log P, water solubility, and the Gus index are also positively correlated and constitute the second component, while the vapor pressure of pesticides constitutes the principal component 3, and the organic carbon–water partition coefficient is related to the principal component 4. A positive correlation was found between the maximum residual concentration of pesticides and their frequency in soils (0.532), and between the maximum residual concentration of pesticides and their DT50 (0.434). Similarly, a strong positive correlation exists between the water solubility of pesticides (Sw) and their Log P (0.676), and between the Sw and the Gus index (0.416). The properties of pesticides influence their fate in soil (Aryal et al., 2020), and these results demonstrate the influence of the DT50 property on the frequency of detection of pesticides, as much as the maximum residual concentration of pesticides.
The correlation coefficients between the maximum residual concentration of pesticides in soils and their GUS index were positive and significant (0.798, p < 0.005), indicating that this factor influence the maximum residual concentration of pesticides in soils. The correlation coefficients between the maximal residual concentration of pesticides and their DT50 were positive but not significant (0.434, p > 0.05), indicating that this factor do not influence the maximum residual concentration of pesticides in soils. Insignificant negative correlations were found between the maximum residual concentration of pesticides in soils and their Sw (− 0.231, p > 0.05).
Relationship between detected pesticides and soil properties
Soil properties influence the pesticide residue content of soil (Aryal et al., 2020). The relationships between the total residual quantity of pesticides of the sampled fields and their soil properties were explored using principal component analysis (Fig. 5). The properties of the tested soils are presented in Table 3. The clay content, sand content, and pH were correlated with the total residual quantity of pesticides (Total pesticide content) of the sampled soils. The principal component analysis, by its two main components, explained 79.117% of the variability of the data 42.372% for PC1 and 36.745% for PC2, which reflects the basis of the original information. The pH, clay content, and silt content affect the composition of the first component. The total residual quantity of pesticides of the sampled soils (Total pesticide content) was involved in the composition of component 2. The total pesticide residue content was negatively correlated with the organic matter content (− 0.690), in contrast to clays (0.198).
The correlation coefficients between the total residual quantity of pesticides of the sampling network and the clay content, sand content, and pH were not significant (0.189, 0.189, 0.264 respectively, with p > 0.05), indicating that these factors were not influencing the total residual quantity of pesticides in soils of the sampled fields. Significant negative correlations were found between the total residual quantity of pesticides in soils and their organic matter contents (− 0.690, p < 0.05).
Influence of pesticides and soil properties on pesticides residues in soils
Bivariate correlations were obtained between the soil characteristics and the residual concentrations for each individual compound in all sampled fields in order to verify whether there is a possible relationship between the soil properties responsible for pesticide retention by soils and the residual concentration detected of pesticide. The most significant correlations were obtained between the residual concentration of tetraconazole and the inorganic fraction (sand (r = 0.652, p < 0.04), texture (r = 0.654, p < 0.04)). Other positive significant correlations were obtained between the inorganic fraction and the residual concentration of dieldrin (sand (r = 0.835, p < 0.003)) and the residual concentration of primiphos-methyl (sand (r = 0.817, p < 0.004)). Significant negative correlations were obtained between the inorganic fraction and the residual concentration of difenoconazole (texture (r = − 0.815, p < 0.004)), the residual concentration of tetraconazole (texture (r = − 0.640, p < 0.046)), and the residual amount of pirimiphos methyl (silt (r = − 0.893, p < 0.001)). Negative correlations were obtained between the inorganic fraction and the residual amount of dieldrin (texture (r = − 0.79, p < 0.006), silt (r = − 0.76, p < 0.011)).
The most significant correlations were obtained between the organic fraction and the residual concentration of difenoconazole (soil OM (r = 0.710, p < 0.004)). Significant negative correlations were obtained between the organic fraction and the residual concentration of epoxiconazole (soil OM (r = 0.721, p < 0.019)) and the residual concentration of DDT (soil OM (r = − 0.792, p < 0.006)). The residual concentration of the pesticides bifenthrin, alpha-cypermethrin, azoxystrobin, chlordane, deltamethrin, isopropalin, propiconazole, lenacil, pyriproxyfen, DDE, ethofumesate, and heptachlore was not related to any soil characteristic.
Distribution of pesticides located on a 60 cm deep soil profile from sugar beet fields
Considering the migration capacity of pesticides in the soil, monitoring pesticide residues in deep soil layers is relevant for evaluating the mobility of pesticides and their risks to adjacent superficial groundwater. To verify the migration of pesticides, three selected fields (FP01, FP02, and FP03) from the Afourar municipality were sampled at three soil depths: 0–20 cm, 20–40 cm, and 40–60 cm. In FP01, the total residual quantity of pesticides was 128 µg/kg in the topsoil, 23 µg/kg in the middle soil layer, and 31 µg/kg in the deep soil (Table 7). Five identical molecules were detected in the three soil layers, tefluthrin, tetraconazole, DDE, epoxiconazole, and difenoconazole, indicating their migration through these soil layers. The residual concentration of tefluthrin decreases from the surface to the depth, passes from 8 µg/kg to 6 µg/kg and finally to 1 µg/kg. The pesticide tetraconazole is detected at residual concentrations of 6 µg/kg, traces, and 2 µg/kg in the deep soil. Epoxiconazole was detected in approximately similar residual concentrations in the three soil depths, which are from surface to depth: 2 µg/kg, trace, and 1 µg/kg. The residual concentration of difenoconazole decreased from the surface to the depth of the soil; thus, it goes from 18 µg/kg in the topsoil to 4 µg/kg in the middle soil layer and 1 µg/kg in the deep soil layer. For DDE, its residual concentration decreases from 79 µg/kg in the topsoil, to 13 µg/kg in the middle soil layer, and 22 µg/kg in the deep soil. In this field, the pesticide residue content decreased toward deeper soil.
In FP02, the total residual quantity of pesticides for the three layers was approximately 92 µg/kg in the surface soil, 39 µg/kg in the middle soil layer, and 348 µg/kg in the deep soil (Table 7). Four molecules were found in the three soil layers: tefluthrin, DDE, epoxiconazole, and difenoconazole. The residual concentration of tefluthrin increases from the surface toward the deep soil and increases from topsoil concentrations of 4 µg/kg and 2 µg/kg to 304 µg/kg in deep soil. Epoxiconazole was detected at approximately similar residual concentrations in the three soil layers, which were from surface to the deep soil at 3 µg/kg, 1 µg/kg, and 1 µg/kg. The residual concentration of difenoconazole decreases from the surface to the deep soil; thus, it goes from 13 µg/kg in the topsoil to 8 µg/kg in the middle soil layer and 9 µg/kg in the deep soil. For DDE, its residual concentration decreases from 45 µg/kg in the topsoil, to 26 µg/kg in the middle soil layer, and 25 µg/kg in the deep soil. At this site, the total residual quantity of pesticides varied from one layer of soil to another; however, it was very high in deep soil. Similarly, the pesticides detected at the level of the three layers showed a variable behavior: the pesticides epoxiconazole, difenoconazole, and DDE; their residual concentration decreases toward the depth of the soil, unlike teluthrin, which is more concentrated toward the deep soil layer.
At FP03, the total residual quantity of pesticides decreased from 137 µg/kg in the topsoil, 47 µg/kg in the middle soil layer, and 55 µg/kg in the deep soil (Table 7). Three pesticides were detected in three soil layers tefluthrin: DDE, and difenoconazole. The residual concentration of tefluthrin increased from the surface at 6 µg/kg to deep soil at 51 µg/kg. Unlike tefluthrin, the residual concentration of DDE decreased toward deep soil after being detected at very high concentrations in the topsoil 114 µg/kg. The same trend was observed for difenoconazole, which goes from a residual concentration of 12 µg/kg in topsoil to 2 µg/kg in the middle soil layer, to be detected in trace amounts in deep soil. At this site, it is clear that the total residual quantity of pesticides decreases toward deeper soil and pesticide migration does not take place in the same way; tefluthrin, for example, accumulates and difenoconazole decreases in deep soil.
To statistically compare the distribution of residual concentration of pesticides detected in the different layers of soils, an analysis of variance was carried out. The recorded residual concentrations of pesticides in the different layers of monitored soils were not significantly different for a confidence level of 95% (p > 0.1).
Discussion
Monitoring pesticides in sugar beet fields is essential to understand the current pesticide pollution in this specific crop, which is crucial for raising public awareness and developing strategies to combat soil pollution in the irrigated perimeter of Tadla in Morocco. To detect and quantify these contaminants in the soil, 10 sugar beet fields belonging to three municipalities in the irrigated perimeter of Tadla, in central Morocco, were selected. Analysis of the tested soils highlighted the presence of pesticides on the topsoil and for those monitored in depth; analysis revealed the presence of pesticides residues even in the deep soil layers of these sugar beet fields. In the topsoil samples, pesticide residues were detected at varying concentrations and different detection frequencies from one field to another. Currently used pesticides are detected as frequently as those historically used. The total residual quantity of pesticides per sugar beet field showed variation among fields, municipalities, and even within fields of the same municipality. In fields where soil samples were collected at three different depths, a pattern of diminishing total residual quantity of pesticides in deeper soil layers in most fields was apparent. However, this trend varies depending on the pesticides, with some showing migration to deeper soil layers (40–60 cm). The majority of compounds detected at these three sites were similar, with almost all compounds detected at the three depths. For tefluthrin, its residual concentration decreased toward the depth of the soil in field FP01, while it increased toward deep soil in fields FP02 and FP03, unlike difenoconazole and DDE, whose residual concentrations decreased from topsoil to deep soil in all fields. The analysis of variance was carried out on the individually residual concentration of pesticides analyzed in the different soil layers to compare the distribution of pesticides residues detected in the three layers of soils. The recorded concentrations for pesticides residues in the different layers of monitored soils were not significantly different. Indeed, the homogeneity in the distribution of pesticides, regardless of soil depth, could be explained by the nature of the deep-sampled soils, which are isohumic soils characterized by the homogeneity of organic matter throughout the soil profile. It is highly likely that this homogenous distribution of pesticides is influenced by the homogeneity of organic matter, which acts as a determining factor in the distribution of pesticides in the soil.
In soil samples taken from the topsoil of sugar beet fields, DDE and tefluthrin are the most frequently detected pesticides indicating the frequent use of tefluthrin among sugar beet growers. Difenoconazole, deltamethrin, and pirimiphos-methyl had the highest maximum concentrations after DDE. Pesticides that are now banned from use, such as isopropalin, heptachlor, chlordane, dieldrin, pirimiphos-methyl, and DDT are still detected in sampled topsoil. Previous studies have reported the presence of higher level of DDT in soils compared to the observed mean concentration found in this study (Qu et al., 2019a). Dieldrin was still detected in high concentration (with mean concentration of 1.20 µg/kg) due to highly persistence in soil (long half-life of 1400 days) (IUPAC PPDB Search, n.d.). Significant concentrations of tefluthrin were detected. With the exclusion of usage as a factor, tefluthrin were primarily influenced by its affinity for soil organic matter which might explain the elevated concentrations of this compound in the sampled topsoils (Koc = 112,900 Lkg−1) (IUPAC PPDB Search, n.d.). The three pyrethroids (cypermethrin, delthametrin, and bifenthrin) found in the sampled topsoils were also detected in soil from other regions, frequently at higher concentrations (Bragança et al., 2019; Han et al., 2017; Liu et al., 2016). Except for propiconazole, triazole pesticides (epoxiconazole, tebuconazole, tetraconazole, and difenoconazole) exhibited a common characteristic of significant concentrations and frequent detection, indicating their widespread use in treating sugar beet crops. The pesticides currently in use are detected as frequently as those historically employed. This persistence of residues, whether it encompasses a single growing season for commonly used pesticides or extends over years for historically banned pesticides, can have adverse consequences. This emphasizes the importance of continuous long-term monitoring of these chemicals to minimize their impact on the environment, wildlife, water quality, and human health.
The total residual quantity of pesticides per sugar beet field varied from one field to another, from one municipality to another, and between fields from the same municipality. Sixty percent of the selected sugar beet fields contained multiple residues of more than 5 pesticides. The number of pesticide residues reached 13 in the tested soils. Through PCA, we have shown that the total residual quantity of pesticides is negatively correlated with the organic matter content and positively correlated with clay content. The PCA revealed the relationships between total residual quantity of pesticides and the inorganic properties of the tested soils, while the correlation analysis demonstrated the lack of significant correlation between these inorganic properties, namely, pH, clay content, and sand content, and the total residual quantity of pesticides. However, a significant negative correlation was found between the total residual quantity of pesticides and the organic matter content. The inverse relationship observed between the total residual quantity of pesticides and the organic matter content highlights the significant role of soil organic matter, as demonstrated by numerous studies (Liu et al., 2018; Vangronsveld et al., 2009; Yavari et al., 2015), in the retention, and in degradation of pesticides. Similarly, through PCA, we revealed a relationship between the maximum residual concentration of pesticides and their detection frequency with their DT50, yet the correlation suggests the absence of a significant correlation between these variables. However, note should be taken of the positive but not significant relationship between the maximum residual concentration of pesticides and their DT50 (0.434, p > 0.05). Nevertheless, a significant positive correlation was identified between the maximal detected concentration for pesticides and their Gus index. These findings suggest that factors other than half-life duration may influence the frequency of detected pesticides, and that the Gus index could be a better indicator to assess the maximum residual concentration of pesticides in sampled soils.
To assess the behavior and presence of pesticide compounds in soil samples, a correlation was conducted between the residual concentration of these compounds and the soil characteristics, with the aim of establishing meaningful connections with these soil characteristics. Significant positive correlations have been observed between the mineral fraction of sand in the sampled soils and the residual concentration of the compounds dieldrin, pirimiphos-methyl, and tebuconazole, indicating a specific preference for this mineral fraction over others. This finding implies that the distribution and behavior of these compounds in the soil may be influenced by the presence of the sand fraction. Regarding the organic fraction of the tested soils, a positive correlation has been established with the residual concentration of the pesticide difenoconazole, suggesting a preference for soils rich in organic matter. However, negative correlations have been observed concerning the pesticides DDT and Epoxiconazole. This suggests that these pesticides have a lower affinity for soil organic matter. This result may imply that these compounds are less sensitive to soil organic matter, which, in turn, can influence their mobility and persistence in organic-rich soils compared to other pesticides. The residual concentrations of the pesticides tefluthrin, bifenthrin, alpha-cypermethrin, azoxystrobin, chlordane, deltamethrin, isopropalin, propiconazole, lenacil, pyriproxyfen, DDE, ethofumesate, and heptachlore were not related to any soil characteristic. This highlights the presence of other factors and mechanisms responsible for the distribution, mobility, and persistence of these pesticides in the soil. All these results highlight the variability and complexity of interactions between pesticides and the characteristics of the sampled soils.
Monitoring pesticide residues in agricultural soils provides information on their fate in ecosystems (Sabzevari & Hofman, 2022). This study highlights the presence of pesticide residues in agricultural soils previously cultivated with sugar beet and sampled at the beginning of the next agricultural campaign. The total pesticide residue content varies from one sugar beet field to another, from one municipality to another, and even between fields in the same municipality. The highest total concentrations of pesticides were recorded in soils FP01 (128 µg/kg for eight pesticides), FP02 (94 µg/kg for eight pesticides), and FP03 (137 µg/kg for five pesticides) of the rural municipality of Afourar and FP08 (100 µg/kg for thirteen pesticides) of the rural municipality of Sidi Jaber. This variability in the concentrations detected in soil reflects, on one hand the types and quantities of pesticides used and all the pathway of dispersion that these pesticides are subjected to after application. According to the results obtained in these agricultural soils, the organochlorines isopropalin, heptachlor, chlordane, dieldrin, and DDT were still detected in the soil, at significant concentrations (8, 1, 3, 6, and 6 µg/kg respectively). They all share a Gus index classifying them as non-mobile and DT50 values qualifying them as persistent or very persistent (Table 3). These factors strongly contribute to their continued presence in the agricultural sampled soils. The application of difenoconazole, detected at a maximum concentration of 18 µg/kg in the soil, aims to combat fungal diseases that affect sugar beet crops around the middle of their growth cycle. This high concentration is likely due to recent repeated applications toward the end of the growth cycle. As for deltamethrin, its high level (18 µg/kg) could result from recent and frequent applications to combat pests affecting sugar beet. Highly persistent DDE is largely responsible for its concentration in the sampled fields (114 µg/kg). It should be noted that the high level of DDE in soils could come from the degradation of the DDT. The pesticide tefluthrin, which recorded the highest concentration at depths of 40–60 cm (304 µg/kg), is classified with a DT50 rating as moderately persistent and a Gus index indicating low mobility. Its high concentration could be attributed to other factors such as repeated use over the years or the impact of certain agricultural practices like irrigation. The quantities of pesticides present in the soil after the end of the sugar beet agricultural season raise questions about the quantities taken up by the roots and leaves of sugar beet. Several studies have revealed the presence of contaminants at the beginning of the next agricultural campaign (Dankyi et al., 2014; Hvězdová et al., 2018; Primost et al., 2017; Scherr et al., 2017). These can persist after several vegetative periods and pose a threat to non-target plants, such as rotational crops. Similarly, the migration of certain pesticides to deep soil layers (tefluthrin) increases the potential risk of groundwater contamination. Prado-Lu and Leilanie (2015) conducted deep soil sampling and detected the following five pesticides profenofos, triazophos, chlorpyrifos, cypermethrin, and malathion at 100 cm soil deep. The literature is rich in studies confirming the contamination of groundwater resources by pesticides. In Africa, research studies, such as Khreit et al. (2020), Olisah et al. (2020), Rimayi et al. (2018), and Sorensen et al. (2015), reported the presence of the pesticides chlorpyrifos, dieldrin, imidacloprid, acetamiprid, atrazine, azadirachtin, profenofos, carbofuran, cypermethrin (Rimayi et al., 2018), and 2,4-D (Khreit et al., 2020) in groundwater. Thus, this work provides the first report on pesticide contamination in sugar beet fields and underlines the risk of contamination of superficial groundwater by highlighting the migration of some of the detected pesticides. Groundwater is a crucial component for the survival and maintenance of the entire ecosystem and the production of drinking water. Taking into account all these considerations and the importance of groundwater in the climatic, agricultural, and social contexts of the irrigated perimeter of Tadla, groundwater need to be protected through pesticide residue monitoring programs.
Agricultural intensification has already caused a significant loss of biodiversity due to the unsustainable use of pesticides (Shackelford et al., 2015; Topping & Lagisz, 2012). The literature is rich in terms of studies and reports that confirm that the injudicious use of pesticides in agriculture can cause damage to non-target organisms, which has led to a significant decline in biodiversity (Hallmann et al., 2017; Pereira et al., 2009). This study revealed the presence of multiple pesticide residues, with a minimum of 3 and a maximum of 13 residues per sample. Mixtures of 5 pesticides were the most common, found in 50% of the samples. The validation procedure for pesticides for their marketing is based on the evaluation of their effect on a limited number of microorganisms, and the new procedure put in place by EFSA for the evaluation of the effects of mixtures (Committee et al., 2019) is no longer applicable because data and procedures relating to the long-term effects of multiple pesticide residues on non-target and native species and communities are not yet available (Geissen et al., 2021). Pesticides can negatively affect various soil microorganisms (Uwizeyimana et al., 2017; Van Bruggen et al., 2018), while others thrive, sometimes resulting in an imbalance between beneficial and pathogenic microorganisms (Van Bruggen et al., 2018). Earthworms and microorganisms play a key role in soil fertility; however, the consequences of multiple pesticide-contaminated soils remain unclear (Geissen et al., 2021). Since pesticide pollution of soils raises concerns about soil biodiversity, soil functions, and food security, this study represents the first investigation of pesticides in the soils of sugar beet fields that provides comprehensive and accurate information on the status of 20 pesticides for risk assessment in the context of the expansion and intensification of sugar beet cultivation at the irrigated perimeter of Tadla.
Conclusion
This study provides the first report on pesticide contamination in sugar beet fields in irrigated perimeter of Tadla and highlights the migration of some detected pesticides and their risk of groundwater contamination. The quantities of pesticides that remain in the soil before the start of the next agricultural season raise questions regarding their effects on rotational crops. The effects of the multiple pesticide residues highlighted in this study are unknown and need to be studied for native non-target species of irrigated perimeter of Tadla, particularly for soil organisms. Finally, to provide comprehensive information on the extent of pesticide contamination in irrigated perimeter of Tadla, this study needs to be replicated to include more sugar beet fields. Soil monitoring can be performed simultaneously with sugar beet and groundwater monitoring. This study provides comprehensive and precise information on pesticides in the soils and can be used for designing soil monitoring programs for sugar beet crop in irrigated perimeter of Tadla.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aigner, E. J., Leone, A. D., & Falconer, R. L. (1998). Concentrations and enantiomeric ratios of organochlorine pesticides in soils from the U.S. corn belt. Environmental Science and Technology, 32, 1162–1168. https://doi.org/10.1021/es970750h
Aktar, W., Sengupta, D., & Chowdhury, A. (2009). Impact of pesticides use in agriculture: Their benefits and hazards. Interdisciplinary Toxicology, 2, 1–12. https://doi.org/10.2478/v10102-009-0001-7
Alavanja, M. C. R., & Bonner, M. R. (2012). Occupational pesticide exposures and cancer risk: A review. Journal of Toxicology & Environmental Health Part b: Critical Reviews, 15, 238–263. https://doi.org/10.1080/10937404.2012.632358
Arrouays, D., Antoni, V., Bardy, M., Bispo, A., Brossard, M., Jolivet, C., Le Bas, C., Martin, M., Saby, N., Schnebelen, N., Villanneau, E., & Stengel, P. (2012). Soil fertility: Conclusions of the report on the state of the soils in France. Innovations Agronomiques, 21, 1–11.
Aryal, N., Wood, J., Rijal, I., Deng, D., Jha, M. K., & Ofori-Boadu, A. (2020). Fate of environmental pollutants: A review. Water Environment Research, 92, 1587–1594. https://doi.org/10.1002/wer.1404
Bernhardt, E. S., Rosi, E. J., & Gessner, M. O. (2017). Synthetic chemicals as agents of global change. Frontiers in Ecology and the Environment, 15, 84–90. https://doi.org/10.1002/fee.1450
Bevan, R., Brown, T., Matthies, F., Sams, C., Jones, K., Hanlon, J., & La Vedrine, M. (2017). Human biomonitoring data collection from occupational exposure to pesticides. EFSA Support. Publ., 14, 1185E. https://doi.org/10.2903/sp.efsa.2017.EN-1185
Bragança, I., Lemos, P.C., Delerue-Matos, C., Domingues, V.F. (2019). Assessment of pyrethroid pesticides in topsoils in northern Portugal. Water, Air, & Soil Pollution, 230.
Cáceres, T., Megharaj, M., Venkateswarlu, K., Sethunathan, N., & Naidu, R. (2010). Fenamiphos and related organophosphorus pesticides: environmental fate and toxicology. Reviews of Environmental Contamination and Toxicology, 205, 117–62. https://doi.org/10.1007/978-1-4419-5623-1_3
Cheng, S. (2003). Heavy metal pollution in China: Origin, pattern and control. Environmental Science and Pollution Research International, 10, 192–198. https://doi.org/10.1065/espr2002.11.141.1
Chiaia-Hernandez, A. C., Keller, A., Wächter, D., Steinlin, C., Camenzuli, L., Hollender, J., & Krauss, M. (2017). Long-term persistence of pesticides and TPs in archived agricultural soil samples and comparison with pesticide application. Environmental Science and Technology, 51, 10642–10651. https://doi.org/10.1021/acs.est.7b02529
Committee, E. S., More, S. J., Bampidis, V., Benford, D., Bennekou, S. H., Bragard, C., Halldorsson, T. I., Hernández-Jerez, A. F., Koutsoumanis, K., Naegeli, H., Schlatter, J. R., Silano, V., Nielsen, S. S., Schrenk, D., Turck, D., Younes, M., Benfenati, E., Castle, L., Cedergreen, N., … Hogstrand, C. (2019). Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal, 17, e05634. https://doi.org/10.2903/j.efsa.2019.5634
Coronado, G. D., Holte, S., Vigoren, E., Griffith, W. C., Faustman, E., & Thompson, B. (2011). Organophosphate pesticide exposure and residential proximity to nearby fields: Evidence for the drift pathway. Journal of occupational and environmental medicine, 53, 884–891. https://doi.org/10.1097/JOM.0b013e318222f03a
Dankyi, E., Gordon, C., Carboo, D., & Fomsgaard, I. S. (2014). Quantification of neonicotinoid insecticide residues in soils from cocoa plantations using a QuEChERS extraction procedure and LC-MS/MS. Science of the Total Environment, 499, 276–283. https://doi.org/10.1016/j.scitotenv.2014.08.051
de Groot, R., Brander, L., van der Ploeg, S., Costanza, R., Bernard, F., Braat, L., Christie, M., Crossman, N., Ghermandi, A., Hein, L., Hussain, S., Kumar, P., McVittie, A., Portela, R., Rodriguez, L. C., ten Brink, P., & van Beukering, P. (2012). Global estimates of the value of ecosystems and their services in monetary units. Ecosystem Services, 1, 50–61. https://doi.org/10.1016/j.ecoser.2012.07.005
EU Pesticides Database - MRLs [WWW Document], n.d. URL https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed 6.9.23).
Froger, C., Jolivet, C., Budzinski, H., Pierdet, M., Caria, G., Saby, N. P. A., Arrouays, D., & Bispo, A. (2023). Pesticide residues in French soils: Occurrence, risks, and persistence. Environmental Science and Technology, 57, 7818–7827. https://doi.org/10.1021/acs.est.2c09591
García, M. G., Sánchez, J. I. L., Bravo, K. A. S., Cabal, M. D. C., & Pérez-Santín, E. (2022). Review: Presence, distribution and current pesticides used in Spanish agricultural practices. Science of the Total Environment, 845, 157291. https://doi.org/10.1016/j.scitotenv.2022.157291
Geissen, V., Silva, V., Lwanga, E. H., Beriot, N., Oostindie, K., Bin, Z., Pyne, E., Busink, S., Zomer, P., Mol, H., & Ritsema, C. J. (2021). Cocktails of pesticide residues in conventional and organic farming systems in Europe – Legacy of the past and turning point for the future. Environmental Pollution, 278, 116827. https://doi.org/10.1016/j.envpol.2021.116827
Hallmann, C. A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., Stenmans, W., Müller, A., Sumser, H., Hörren, T., Goulson, D., & de Kroon, H. (2017). More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE, 12, e0185809. https://doi.org/10.1371/journal.pone.0185809
Han, Y., Mo, R., Yuan, X., Zhong, D., Tang, F., Ye, C., & Liu, Y. (2017). Pesticide residues in nut-planted soils of China and their relationship between nut/soil. Chemosphere, 180, 42–47. https://doi.org/10.1016/j.chemosphere.2017.03.138
Hvězdová, M., Kosubová, P., Košíková, M., Scherr, K. E., Šimek, Z., Brodský, L., Šudoma, M., Škulcová, L., Sáňka, M., Svobodová, M., Krkošková, L., Vašíčková, J., Neuwirthová, N., Bielská, L., & Hofman, J. (2018). Currently and recently used pesticides in Central European arable soils. Science of the Total Environment, 613–614, 361–370. https://doi.org/10.1016/j.scitotenv.2017.09.049
IUPAC PPDB Search [WWW Document]. (n.d.). https://sitem.herts.ac.uk/aeru/iupac/search.htm. Accessed 6.9.2023.
Kapsi, M., Tsoutsi, C., Paschalidou, A., & Albanis, T. (2019). Environmental monitoring and risk assessment of pesticide residues in surface waters of the Louros River (N.W. Greece). Science of the Total Environment, 650, 2188–2198. https://doi.org/10.1016/j.scitotenv.2018.09.185
Khreit, O. I. G., Awamy, I. O. E., & Abduljalil, O. A. (2020). Development, validation, and application of a method based on reverse-phase HPLC for the simultaneous determination of six organochlorine pesticides in surface and groundwater samples collected from northeast Libya. Al-Mukhtar Journal of Sciences, 35, 116–129. https://doi.org/10.54172/mjsc.v35i2.325
King, A. M., & Aaron, C. K. (2015). Organophosphate and carbamate poisoning. Emerg. Med. Clin. North Am. Management of Hazardous Material Emergencies, 33, 133–151. https://doi.org/10.1016/j.emc.2014.09.010
Kopittke, P. M., Menzies, N. W., Wang, P., McKenna, B. A., & Lombi, E. (2019). Soil and the intensification of agriculture for global food security. Environment International, 132, 105078. https://doi.org/10.1016/j.envint.2019.105078
Kosubová, P., Škulcová, L., Poláková, Š, Hofman, J., & Bielská, L. (2020). Spatial and temporal distribution of the currently-used and recently-banned pesticides in arable soils of the Czech Republic. Chemosphere, 254, 126902. https://doi.org/10.1016/j.chemosphere.2020.126902
Kumar, B., Verma, V. K., Mishra, M., Gaur, R., Kumar, S., & Sharma, C. S. (2014). DDT and HCH (organochlorine pesticides) in residential soils and health assessment for human populations in Korba, India. Human and Ecological Risk Assessment: An International Journal, 20, 1538–1549. https://doi.org/10.1080/10807039.2013.858563
Kumar, S., Kaushik, G., & Villarreal-Chiu, J. F. (2016). Scenario of organophosphate pollution and toxicity in India: A review. Environmental Science and Pollution Research, 23, 9480–9491. https://doi.org/10.1007/s11356-016-6294-0
Kumar, G., Lal, S., Soni, S. K., Maurya, S. K., Shukla, P. K., Chaudhary, P., Bhattacherjee, A. K., & Garg, N. (2022). Mechanism and kinetics of chlorpyrifos co-metabolism by using environment restoring microbes isolated from rhizosphere of horticultural crops under subtropics. Frontiers in Microbiology, 13. https://doi.org/10.3389/fmicb.2022.891870
Li, H., Gong, W., Lv, W., Wang, Y., Dong, W., & Lu, A. (2023). Target and suspect screening of pesticide residues in soil samples from peach orchards using liquid chromatography quadrupole time-of-flight mass spectrometry. Ecotoxicology and Environmental Safety, 253, 114664. https://doi.org/10.1016/j.ecoenv.2023.114664
Liu, D., Keesing, J. K., He, P., Wang, Z., Shi, Y., & Wang, Y. (2013). The world’s largest macroalgal bloom in the Yellow Sea, China: Formation and implications. Estuarine, Coastal and Shelf Science, 129, 2–10. https://doi.org/10.1016/j.ecss.2013.05.021
Liu, Y., Li, S., Ni, Z., Qu, M., Zhong, D., Ye, C., & Tang, F. (2016). Pesticides in persimmons, jujubes and soil from China: Residue levels, risk assessment and relationship between fruits and soils. Science of the Total Environment, 542, 620–628. https://doi.org/10.1016/j.scitotenv.2015.10.148
Liu, Y., Lonappan, L., Brar, S. K., & Yang, S. (2018). Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Science of the Total Environment, 645, 60–70. https://doi.org/10.1016/j.scitotenv.2018.07.099
Lozowicka, B. (2015). Health risk for children and adults consuming apples with pesticide residue. Science of the Total Environment, 502, 184–198. https://doi.org/10.1016/j.scitotenv.2014.09.026
Lupi, L., Bedmar, F., Wunderlin, D. A., & Miglioranza, K. S. B. (2019). Levels of organochlorine pesticides in soils, mesofauna and streamwater from an agricultural watershed in Argentina. Environmental Earth Sciences, 78, 569.
Masiá, A., Campo, J., Navarro-Ortega, A., Barceló, D., & Picó, Y. (2015). Pesticide monitoring in the basin of Llobregat River (Catalonia, Spain) and comparison with historical data. Sci. Total Environ. Towards a Better Understanding of the Links between Stressors, Hazard Assessment and Ecosystem Services under Water Scarcity, 503–504, 58–68. https://doi.org/10.1016/j.scitotenv.2014.06.095
Matthews, G., Zaim, M., Yadav, R. S., Soares, A., Hii, J., Ameneshewa, B., Mnzava, A., Dash, A. P., Ejov, M., Tan, S. H., & van den Berg, H. (2011). Status of legislation and regulatory control of public health pesticides in countries endemic with or at risk of major vector-borne diseases. Environmental Health Perspectives, 119, 1517–1522. https://doi.org/10.1289/ehp.1103637
Medina-Pastor, P., & Triacchini, G. (2020). The 2018 European Union report on pesticide residues in food. EFSA Journal, 18, e06057. https://doi.org/10.2903/j.efsa.2020.6057
Ministry of Agriculture, Maritime Fisheries, Rural Development and Water and Forests (MAPMDREF). 2022. Index phytosanitaire [Phytosanitary Index]. https://www.agrimaroc.ma/index-phytosanitaire-maroc/ (accessed 11.1.23).
Muir, D., Zhang, X., de Wit, C. A., Vorkamp, K., & Wilson, S. (2019). Identifying further chemicals of emerging arctic concern based on ‘in silico’ screening of chemical inventories. Emerging Contaminants, 5, 201–210. https://doi.org/10.1016/j.emcon.2019.05.005
Murugan, A. V., Swarnam, T. P., & Gnanasambandan, S. (2013). Status and effect of pesticide residues in soils under different land uses of Andaman Islands India. Environmental Monitoring and Assessment, 185, 8135–8145. https://doi.org/10.1007/s10661-013-3162-y
Olisah, C., Okoh, O. O., & Okoh, A. I. (2020). Occurrence of organochlorine pesticide residues in biological and environmental matrices in Africa: A two-decade review. Heliyon, 6(3). https://doi.org/10.1016/j.heliyon.2020.e03518
Osesua, B. A., Tsafe, A. I., Birnin-Yauri, U. A., & Sahabi, D. M. (2017). Determination of pesticide residues in soil samples collected from Wurno irrigation farm, Sokoto State Nigeria. Cont. Journal of Agricultural Science, 11, 40–52. https://doi.org/10.5281/zenodo.546475
Pan, H., Lei, H., He, X., Xi, B., & Xu, Q. (2019). Spatial distribution of organochlorine and organophosphorus pesticides in soil-groundwater systems and their associated risks in the middle reaches of the Yangtze River Basin. Environmental Geochemistry and Health, 41, 1833–1845. https://doi.org/10.1007/s10653-017-9970-1
Panico, S. C., van Gestel, C. A. M., Verweij, R. A., Rault, M., Bertrand, C., Menacho Barriga, C. A., Coeurdassier, M., Fritsch, C., Gimbert, F., & Pelosi, C. (2022). Field mixtures of currently used pesticides in agricultural soil pose a risk to soil invertebrates. Environmental Pollution, 305, 119290. https://doi.org/10.1016/j.envpol.2022.119290
Patel, R. (2009). Food sovereignty. The Journal of Peasant Studies, 36, 663–706. https://doi.org/10.1080/03066150903143079
Pereira, J. L., Antunes, S. C., Castro, B. B., Marques, C. R., Gonçalves, A. M. M., Gonçalves, F., & Pereira, R. (2009). Toxicity evaluation of three pesticides on non-target aquatic and soil organisms: Commercial formulation versus active ingredient. Ecotoxicology, 18, 455–463. https://doi.org/10.1007/s10646-009-0300-y
Picó, Y., Alvarez-Ruiz, R., Alfarhan, A. H., El-Sheikh, M. A., Alshahrani, H. O., & Barceló, D. (2020). Pharmaceuticals, pesticides, personal care products and microplastics contamination assessment of Al-Hassa irrigation network (Saudi Arabia) and its shallow lakes. Science of the Total Environment, 701, 135021. https://doi.org/10.1016/j.scitotenv.2019.135021
Prado-Lu, D., & Leilanie, J. (2015). Insecticide residues in soil, water, and eggplant fruits and farmers’ health effects due to exposure to pesticides. Environmental Health and Preventive Medicine, 20, 53–62. https://doi.org/10.1007/s12199-014-0425-3
Primost, J. E., Marino, D. J. G., Aparicio, V. C., Costa, J. L., & Carriquiriborde, P. (2017). Glyphosate and AMPA, “pseudo-persistent” pollutants under real-world agricultural management practices in the Mesopotamic Pampas agroecosystem. Argentina. Environ. Pollut. Barking Essex, 229, 771–779. https://doi.org/10.1016/j.envpol.2017.06.006
Qu, C., Albanese, S., Chen, W., Lima, A., Doherty, A. L., Piccolo, A., Arienzo, M., Qi, S., & De Vivo, B. (2016). The status of organochlorine pesticide contamination in the soils of the Campanian Plain, southern Italy, and correlations with soil properties and cancer risk. Environmental Pollution, 216, 500–511. https://doi.org/10.1016/j.envpol.2016.05.089
Qu, C., Albanese, S., Li, J., Cicchella, D., Zuzolo, D., Hope, D., Cerino, P., Pizzolante, A., Doherty, A. L., Lima, A., & De Vivo, B. (2019a). Organochlorine pesticides in the soils from Benevento provincial territory, southern Italy: Spatial distribution, air-soil exchange, and implications for environmental health. Science of the Total Environment, 674, 159–170. https://doi.org/10.1016/j.scitotenv.2019.04.029
Qu, C., Albanese, S., Lima, A., Hope, D., Pond, P., Fortelli, A., Romano, N., Cerino, P., Pizzolante, A., & De Vivo, B. (2019b). The occurrence of OCPs, PCBs, and PAHs in the soil, air, and bulk deposition of the Naples metropolitan area, southern Italy: Implications for sources and environmental processes. Environment International, 124, 89–97. https://doi.org/10.1016/j.envint.2018.12.031
Rafique, N., Tariq, S. R., & Ahmed, D. (2016). Monitoring and distribution patterns of pesticide residues in soil from cotton/wheat fields of Pakistan. Environmental Monitoring and Assessment, 188, 695. https://doi.org/10.1007/s10661-016-5668-6
Rhoades, J. D. (1982). Soluble salts. In A. L. Page et al. (Eds.), Methods of soil analysis: part 2: chemical and microbiological properties. Monograph Number 9 (2nd Ed., pp 167–179). ASA, Madison, WI. https://doi.org/10.2134/agronmonogr9.2.2ed.c10
Riedo, J., Wettstein, F. E., Rösch, A., Herzog, C., Banerjee, S., Büchi, L., Charles, R., Wächter, D., Martin-Laurent, F., Bucheli, T. D., Walder, F., & van der Heijden, M. G. A. (2021). Widespread occurrence of pesticides in organically managed agricultural soils—The ghost of a conventional agricultural past? Environmental Science and Technology, 55, 2919–2928. https://doi.org/10.1021/acs.est.0c06405
Rimayi, C., Odusanya, D., Weiss, J. M., de Boer, J., & Chimuka, L. (2018). Seasonal variation of chloro-s-triazines in the Hartbeespoort Dam catchment. South Africa. Sci. Total Environ., 613–614, 472–482. https://doi.org/10.1016/j.scitotenv.2017.09.119
Sabbe, W. E., Marx, D. B. (1987). Soil sampling: spatial and temporal variability. In J. R. Brown (Ed.), Soil testing: sampling, correlation, calibration, and interpretation. https://doi.org/10.2136/sssaspecpub21.c1
Sabzevari, S., & Hofman, J. (2022). A worldwide review of currently used pesticides’ monitoring in agricultural soils. Science of the Total Environment, 812, 152344. https://doi.org/10.1016/j.scitotenv.2021.152344
Scherr, K. E., Bielská, L., Kosubová, P., Dinisová, P., Hvězdová, M., Šimek, Z., & Hofman, J. (2017). Occurrence of Chlorotriazine herbicides and their transformation products in arable soils. Environmental Pollution, 222, 283–293. https://doi.org/10.1016/j.envpol.2016.12.043
Schleiffer, M., & Speiser, B. (2022). Presence of pesticides in the environment, transition into organic food, and implications for quality assurance along the European organic food chain – A review. Environmental Pollution, 313, 120116. https://doi.org/10.1016/j.envpol.2022.120116
Schulze, S., Zahn, D., Montes, R., Rodil, R., Quintana, J. B., Knepper, T. P., Reemtsma, T., & Berger, U. (2019). Occurrence of emerging persistent and mobile organic contaminants in European water samples. Water Research, 153, 80–90. https://doi.org/10.1016/j.watres.2019.01.008
Serra, A.-A., Bittebière, A.-K., Mony, C., Slimani, K., Pallois, F., Renault, D., Couée, I., Gouesbet, G., & Sulmon, C. (2020). Local-scale dynamics of plant-pesticide interactions in a northern Brittany agricultural landscape. Science of the Total Environment, 744, 140772. https://doi.org/10.1016/j.scitotenv.2020.140772
Shackelford, G. E., Steward, P. R., German, R. N., Sait, S. M., & Benton, T. G. (2015). Conservation planning in agricultural landscapes: Hotspots of conflict between agriculture and nature. Diversity and Distributions, 21, 357–367. https://doi.org/10.1111/ddi.12291
Shegunova, P., Klánová, J., & Holoubek, I. (2007). Residues of organochlorinated pesticides in soils from the Czech Republic. Environmental Pollution, 146, 257–261. https://doi.org/10.1016/j.envpol.2006.03.057
Sidhu, G. K., Singh, S., Kumar, V., Dhanjal, D. S., Datta, S., & Singh, J. (2019). Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Critical Reviews in Environment Science and Technology, 49, 1135–1187. https://doi.org/10.1080/10643389.2019.1565554
Silva, V., Montanarella, L., Jones, A., Fernández-Ugalde, O., Mol, H. G. J., Ritsema, C. J., & Geissen, V. (2018). Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Science of the Total Environment, 621, 1352–1359. https://doi.org/10.1016/j.scitotenv.2017.10.093
Silva, V., Mol, H. G. J., Zomer, P., Tienstra, M., Ritsema, C. J., & Geissen, V. (2019). Pesticide residues in European agricultural soils – A hidden reality unfolded. Science of the Total Environment, 653, 1532–1545. https://doi.org/10.1016/j.scitotenv.2018.10.441
Sorensen, J. P. R., Lapworth, D. J., Nkhuwa, D. C. W., Stuart, M. E., Gooddy, D. C., Bell, R. A., Chirwa, M., Kabika, J., Liemisa, M., Chibesa, M., & Pedley, S. (2015). Emerging contaminants in urban groundwater sources in Africa. Water Res., Occurrence, fate, removal and assessment of emerging contaminants in water in the water cycle (from wastewater to drinking water). Water Research, 72, 51–63. https://doi.org/10.1016/j.watres.2014.08.002
Souza, M. C. O., Cruz, J. C., Cesila, C. A., Gonzalez, N., Rocha, B. A., Adeyemi, J. A., Nadal, M., Domingo, J. L., & Barbosa, F. (2023). Recent trends in pesticides in crops: A critical review of the duality of risks-benefits and the Brazilian legislation issue. Environmental Research, 228, 115811. https://doi.org/10.1016/j.envres.2023.115811
Tadla Agricultural Development Office (ORMVAT). (2019) Tadla Perimeter Monograph, ed 2019, Ministry of Agriculture, Maritime Fisheries, Rural Development and Water and Forests, Morocco.
Topping, C. J., & Lagisz, M. (2012). Spatial dynamic factors affecting population-level risk assessment for a terrestrial arthropod: An agent-based modeling approach. Human and Ecological Risk Assessment: An International Journal, 18, 168–180. https://doi.org/10.1080/10807039.2012.632292
Uwizeyimana, H., Wang, M., Chen, W., & Khan, K. (2017). The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environmental Toxicology and Pharmacology, 55, 20–29. https://doi.org/10.1016/j.etap.2017.08.001
Van Bruggen, A. H. C., He, M. M., Shin, K., Mai, V., Jeong, K. C., Finckh, M. R., & Morris, J. G. (2018). Environmental and health effects of the herbicide glyphosate. Science of the Total Environment, 616–617, 255–268. https://doi.org/10.1016/j.scitotenv.2017.10.309
Vangronsveld, J., Herzig, R., Weyens, N., Boulet, J., Adriaensen, K., Ruttens, A., Thewys, T., Vassilev, A., Meers, E., Nehnevajova, E., van der Lelie, D., & Mench, M. (2009). Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environmental Science and Pollution Research, 16, 765–794. https://doi.org/10.1007/s11356-009-0213-6
Villanneau, E. J., Saby, N. P. A., Marchant, B. P., Jolivet, C. C., Boulonne, L., Caria, G., Barriuso, E., Bispo, A., Briand, O., & Arrouays, D. (2011). Which persistent organic pollutants can we map in soil using a large spacing systematic soil monitoring design? A case study in Northern France. Science of the Total Environment, 409, 3719–3731. https://doi.org/10.1016/j.scitotenv.2011.05.048
Wang, L., Zhang, Z.-F., Liu, L.-Y., Zhu, F.-J., & Ma, W.-L. (2023). National-scale monitoring of historic used organochlorine pesticides (OCPs) and current used pesticides (CUPs) in Chinese surface soil: Old topic and new story. Journal of Hazardous Materials, 443, 130285. https://doi.org/10.1016/j.jhazmat.2022.130285
Yadav, I. C., Devi, N. L., Li, J., Zhang, G., & Shakya, P. R. (2016). Occurrence, profile and spatial distribution of organochlorines pesticides in soil of Nepal: Implication for source apportionment and health risk assessment. Science of the Total Environment, 573, 1598–1606. https://doi.org/10.1016/j.scitotenv.2016.09.133
Yao, R., Yao, S., Ai, T., Huang, J., Liu, Y., & Sun, J. (2023). Organophosphate pesticides and pyrethroids in farmland of the Pearl River Delta, China: Regional residue, distributions and risks. International Journal of Environmental Research and Public Health, 20, 1017. https://doi.org/10.3390/ijerph20021017
Yavari, S., Malakahmad, A., & Sapari, N. B. (2015). Biochar efficiency in pesticides sorption as a function of production variables—A review. Environmental Science and Pollution Research, 22, 13824–13841. https://doi.org/10.1007/s11356-015-5114-2
Author information
Authors and Affiliations
Contributions
Majda Ouhajjou: conceptualization and investigation process, methodology, validation, writing—review and editing; Hanaa Hachimi: conceptualization and investigation process, methodology, validation, writing—review and editing; Mohamed Edahbi: conceptualization and investigation process, methodology, validation, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ouhajjou, M., Edahbi, M. & Hachimi, H. First surveillance of pesticides in soils of the perimeter of Tadla, a Moroccan sugar beet intensive area. Environ Monit Assess 196, 28 (2024). https://doi.org/10.1007/s10661-023-12182-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-12182-w