Abstract
Widespread use of pesticides in intensive agriculture indicates the need for permanent monitoring of their residues in soil. For this purpose, a total of 22 evenly distributed sampling points of arable land were chosen in the sampling area in Serbia. Soils were divided into groups according to the previous crop (pepper, vegetables, maize, wheat and soybean). Soil properties and residues of 68 pesticides, of which 22 herbicides, 25 insecticides and 21 fungicides, were analysed in the collected soil samples. The obtained data confirm the heterogeneity of soil samples regarding their organic matter content (1.41–3.39%) and pH value (pH 4.27–8.08). The average number of active ingredients detected per type of previous crop was 1–2 herbicides, 14–16 insecticides, and 3–4 fungicides, while the residues of 3 herbicides, 20 insecticides and 9 fungicides were found in total. Insecticides with mainly organochlorine compounds represented the majority of the detected active ingredients.
Although rapid degradability of sulfonylurea herbicides is a well-known fact, the residues of nicosulfuron, ranging from 0.15 to 1.99 μg/kg, were found in three soil samples where maize was grown as a previous crop. Furthermore, triazoles prothioconazole (0.08 ± 0.11 μg/kg), tebuconazole (0.10 ± 0.24 μg/kg) and epoxyconazole (0.13 ± 0.42 μg/kg) were detected in 36%, 18% and 14% soil samples, respectively, while difenoconazole and flusilazole were detected in only one sample. Soil pH value mostly correlated with pesticides. The levels of pesticides detected in agricultural soils should be monitored further, especially in terms of environmental risks posed by their transfer to groundwater and surface waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Application of pesticides for the control of pests and diseases, in order to achieve better quality of products and higher yield, is one of the widely used practices in agriculture. Intensification of agricultural production resulted in the rise of pesticide use (Silva et al. 2019). Despite benefits gained from plant protection products in terms of increasing the economic value of crop production, their intensive and widespread use raises serious concerns regarding their harmful effect to humans and wildlife, and issues brought on by the release of harmful substances into the environment (Hvezdová et al. 2018; Jorfi et al. 2019). Soil, as an essential natural resource, has a high capacity to retain and store chemical substances such as pesticides. The activity of pesticides depends on their susceptibility to degradation, among the many other factors. Once adsorbed onto soil particles, pesticides may rapidly degrade or in the case of persistent chemicals, may be slowly released into the atmosphere, ground and surface waters and living organisms (Soares et al. 2015). Accumulation of environmentally persistent chemical pollutants, including agricultural pesticides, is increasing societal concerns about the sustainability of ecosystems and even more about risks to human health (Holt et al. 2013). These contaminants are known as xenobiotics, which are chemical substances found in the environment but not naturally produced (e.g. pesticides, medicaments, industrial wastes, etc.) (Navarro et al. 2007).
Therefore, the level of soil contamination and environmental risk resulting from the application of pesticides should be monitored through the levels of pesticide residue in agricultural soils. Furthermore, pesticide residues in soils can cause damage to subsequent crops, especially in the case of multiple applications (Grahovac 2016). Additionally, only a small amount of pesticides reaches the targeted organisms, while the rest are potential environmental contaminants with a wide range of possible negative impacts (Hvezdová et al. 2018). For example, approximately 50% of treatments applied to the aerial part of crops reaches the soil, while this ratio is increased in pesticides used for soil treatment (nematicides, disinfectants, and mostly herbicides) (Navarro et al. 2007).
The fate of pesticides in soil depends on many processes responsible for their mobility and persistence (Pérez-Lucas et al. 2018). The interaction of pesticides with soil is complex and controlled by numerous physical, chemical and biological factors, whereas these processes can take place simultaneously in many cases. Pesticide degradation is related to environmental factors, including the abundance and diversity of microorganisms, soil physical properties (texture) and chemical properties (pH, organic matter, and metal ion content), as well as climatic conditions (Yang et al. 2005; Pu et al. 2006). Excessive accumulation of pesticides in soil creates a serious risk of their transfer into the food chain or infiltration into groundwater (Babut et al. 2013).
The total annual import of pesticides in the Republic of Serbia based on the data for the period 2004–2014 increased and reached 13.1 thousand tons per year (both pesticides and active ingredients), whereby the estimated pesticide consumption per year was about 4 kg/ha. Herbicides accounted for almost 68%, fungicides and bactericides 19%, insecticides 10%, and others less than 2% of the estimated pesticide consumption (Anonymus 2015).
Pesticide residues in Serbian soils has been the subject of numerous papers (Pucarević et al. 2007a, 2007b; Nešić et al. 2008; Pucarević et al. 2008; Dugalić et al. 2010; Pucarević et al. 2010; Lazić et al. 2011; Bursić et al. 2012a). Various pesticides, such as triazines (Đurović et al. 2011), dicamba (Bursić et al. 2012b) and clomazone (Bursić et al. 2011), have been detected in Serbian soils. However, these studies were conducted almost ten years ago and were mainly based on organochlorine (OCPs), organophosphorus compounds and atrazine residues. OCPs have a long residual action, persistence and long-term bioaccumulation in the environment without losing their toxicity (Fosu et al. 2016), and their use has been banned or limited in the past few decades. Some of OCPs, such as aldrin, dieldrin, heptachlor and chlordane, were banned in the early seventies in Serbia, while DDT and endrin were allowed for public health purposes almost until the nineties. The use of lindane for wood preservation was forbidden in 2007, while endosulfan was banned in 2008. Based on these facts, monitoring of the OCP levels in soil is essential. The quantities of other pesticides in soil, such as herbicides from the sulphonylurea group and triazol fungicides, have not been measured in Serbia so far.
On the other hand, generally accepted residue level of pesticides and their metabolites defined by law does not exist, but the values vary greatly in different countries (Aaron and Zijian 2014). Pesticide residues in the soils of Serbia are regulated by the Regulation on limit values of pollutants and hazardous substances in the soil (Official Gazette of RS no. 30/2018 and no.64/2019). The regulation covers the level of organochlorine pesticides and their metabolites, as well as atrazine, carbaryl, carbofuran, maneb, organotin pesticides and azinphos-methyl.
Research and analysis of the current level of pollution of agricultural soils could be used for the development of a common system for monitoring ecological and chemical status of soils. This research was a part of Interreg IPA Cross-border project IMPACT ENVI HR-RS 182. The project area covered agricultural land in Northern Serbia (Vojvodina) and Mačva. Therefore, the aim of the study conducted in the scope of the Interreg IMPACT-ENVI project area was to evaluate the current concentrations of pesticides, including banned OCPs and active ingredients allowed for usage in vegetable and field crop production in Serbia. This study was conducted in Serbia (Sremska Kamenica) and Croatia (Osijek), during the year 2017 and 2018.
Materials and methods
Soil sampling
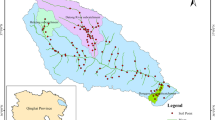
The sampling area covered agricultural land in Northern Serbia (Vojvodina) and Mačva, near the border with Croatia, and included 22 evenly distributed sampling points within the project region (Fig. 1. and supplementary file Table 1a). The Northern and North-Western part of Serbia, the regions of Vojvodina (Pannonian Plain) and Mačva (alluvial fertile flatland between the rivers Drina and Sava), are mainly flatlands. Both regions are known as areas with intensive agricultural production. The soils were sampled (0–30 cm depth) from the last week of October to the first decade of November 2017. The soil samples originated from the production plots varying from 0.03 to 4 ha in size. The plots were 3 greenhouses (two 0.03 ha and one 1.15 ha); 6 plots under 1 ha (0.65 ha on average), and 13 plots from 1 to 4 ha (2.37 ha on average). Soil samples were collected according to ISO procedure 1038 1-4 (2003). The topsoil samples were taken from the depth 0–30 cm. This depth was chosen as the zone of the most active root systems of vegetable crops. The samples were taken using a soil drill agrochemical probes and stored in polyethylene bags. The initial quantity of samples was approximately 1.5 kg. The collected soil samples were stored for maximum two weeks in cold chambers (5–6 °C) in dark conditions, at the Institute of Field and Vegetable Crops, Novi Sad. For the purpose of further analysis, the soil samples were divided into five groups according to the previous crop: vegetables without pepper (6 samples), pepper (2 samples), soybean (7 samples), wheat (3 samples), and maize (4 samples). Soil properties were analysed at the Faculty of Agrobiotechnical Sciences Osijek (Croatia), and pesticide analyses were performed at Educons University (Sremska Kamenica, Serbia) in the period from November 2017 to March 2018.
Soil quality analysis
The following methods were used for the analyses of basic soil properties-suspension of soil and water, as well as 1 M KCl (potassium chloride) (1:2:5), was used for determination of the soil pH value, according to HRN EN ISO 10390 (2005) standards. Calcium carbonate (CaCO3) content was determined volumetrically, using the Schneibler calcimeter (Behrotest, B00607682 SCM1, Germany) (HRN ISO 10693, 2004), and the organic carbon C content was determined in accordance with HRN EN ISO 14235 (2004) standards, by oxidising organic matter using sulfochromic oxidation. AL solution consisting of 0.1 M ammonium lactate and 0.4 M acetic acid, with a pH of 3.75, was used for the extraction of plant available P and K, at a soil to soil ratio of 1:20 (w/v) (AL-method, Egner et al. 1960). Concentrations of P and K were measured by UV–Vis spectrophotometer Cary 50 (Agilent technologies/ Varian Inc. Cary 50 UV–Vis SPV-1 × 0) and by ICP technique (ICP- OS Perkin Elmer, Optima, 2100 DV), and by ICP technique (ICP- OS Perkin Elmer), respectively.
Detection of pesticides
Soil samples were air-dried and ground to a particle size < 2 mm. Pesticides were extracted from the soil by the modified QuEChERS method EN15662 (Wang et al. 2012) using Chromabond (Ref. 730,970) Quechers MIX I citrate extraction kit (4 g MgSO4, 1 g NaCl, 1 g Na citrate, 0.5 g disodium citrate sesqui hydrate). The obtained soil extracts were analysed on a liquid-mass-mass chromatograph TSQ Quantum™ Access MAX Triple Quadrupole Mass Spectrometer and on a GC-MSThermos scientific Trace 1300 ISQ(Termo scientific, USA). The operating conditions on GC/MS were as follows: injection volume 2 ul, MS transfer line temperature 270 °C, ion source temp. 220 °C in electron ionization mode, dwell time 0.2 min with initial oven temperature at 70 °C, for 2 min, heating to 150 °C at a rate 25 °C min−1, then to 200 °C at a rate 3 °C min−1, next to 280 °C at a rate 8 °C min−1with a final 10 min hold time. The LC–MS/MS had the following conditions: injection volume 6 ul, column temp. 40 °C, column flow 0.3 ml/min, mobile phase A (0.1% HCOOH in water + 4 mM HCOONH4) + B (0.1% HCOOH in methanol + 4 mM HCOONH4). The results were quantified using standard pure pesticide substances from the manufacturer Riedel de Haen. Confidence in the results was ensured by determining the extraction recovery and the detection limit for each pesticide.
Limit of detection and ions selected for MS determination are presented in Table 1b (supplementary file). At the beginning of the research, the recovery of pesticide extraction from the soil was examined using a mixture of pesticides of known concentration and quartz sand. The recoveries were from 70 to 110%.
The pesticides were classified into chemical groups according to HRAC (Herbicide resistance committee), IRAC (Insecticide resistance committee), and FRAC (Fungicide resistance committee), and the list is given in Table 1.
Data analysis
The obtained data were represented according to the number of detected pesticides per group of soil samples, percentage of the group of pesticides detected and the number of pesticides below the LOD (limit of detection calculated as three standard deviation of the baseline noise signal) and pesticides which were not detected in soil sample. Descriptive statistics was used for the soil traits. Data normality (for pesticides) was tested by the Shapiro–Wilk test. The difference between the sample groups was tested by the LSD test (p < 0.05). The graphs were shown only for the pesticides where data were normally distributed with significant differences between the sample groups. Correlation coefficients were calculated according to Spearman (p < 0.05 and 0.01). The Principal Component Analysis (PCA) was programmed according to the iterative NIPALS algorithm in order to reduce the complexity of the multivariate system and to highlight potential trends of the whole data. PCA with NIPALS algorithm was carried out (38 variables, 22 cases, fitting method: minimum eigenvalue limit 1.00) to determine whether the variables could be used to differentiate plant groups. Data were analysed by STATISTICA 13.2 (Dell Inc., USA).
Results and discussion
Soil properties
The values of the basic soil properties (phosphorus, calcium and potassium levels, the content of humus and acidity) are shown in the Table 2. The samples were taken from the southern part of the Pannonian plain located in the Central and Southeast part of Europe. The Pannonian plain is a flat area dominated by the Danube River with mostly fertile soils and intense agriculture production. The climate in this region is continental with cold winters and hot summers, and the dominant soil types are camisole, fluvosol, phaeozem and the “flagship” black soil: chernozem. Chernozem is commonly found in semiarid steppe areas, which cover about 1.000.000 ha of the Vojvodina region, with the parental material of calcareous loess, an aeolian sediment with 20–30% CaCO3. In the majority of locations in Serbia, the texture of chernozem is loam characterised by a good, crumbly structure, stable aggregates, and good water permeability, which enables cultivation. The results of the soil chemical properties are in line with the results of other researchers, so Zebec et al. (2017) determined the acidity (pH) of the soil from 4.95 to 8.75 and the content of organic matter from 1.83 to 7.03% in the same area.
Pesticide residues
Pesticide residues were detected in all soil samples. According to the group of crops, there was no difference in the number of active ingredients detected in the soil samples. On average, 1–2 herbicides, 14–16 insecticides, and 3–4 fungicides were detected in one soil sample (Fig. 2). Over 80% of the tested soils originated from EU States contained pesticide residues (25% of samples had 1 residue, while 58% of samples had mixtures of two or more residues) (Silva et al. 2019). Insecticides comprised the majority of the detected pesticides, with 76% active ingredients—mainly OCPs, while fungicides had nearly 17%, and herbicides less than 7% of the total share (Fig. 3).
The minimum and maximum levels of measured pesticide residues are given in Table 3. These data will be commented further for every group of pesticides separately.
The residues of more than a half of active ingredients and metabolites have not been detected in the tested soil samples. These substances were not be present in the soil or they were below the LOD (Fig. 4). Most of the active ingredients from this group belong to herbicides (19 out of 22), fungicides (12 out of 21) and insecticides (5 out of 25). Such ratio of pesticide residues is in agreement with the fact that insecticides are used mostly in the developing countries, while fungicides/herbicides are used in the developed countries (World Health Organization, 2022).
However, only two active ingredients belonging to OCPs were not found in the analysed soils. This finding confirms their classification as persistent organic pollutants (POPs).
The total content of OCPs in analysed soil samples ranged from 5.979 to12.775 mg/kg. Therefore, OCP concentrations in agricultural soils in Serbia are higher than those obtained in other European countries, such as the Czech Republic (4.65–1022.3 µg/kg, Holoubek et al. 2009), Germany (28.3–184.50 µg/kg, Manz et al. 2001), Spain (0.08–448.0 µg/kg, Cabrerizo et al. 2011), the United Kingdom (0.1–100 µg/kg Meijer et al. 2001), and Poland (0.61–1031.64 µg/kg, Ukalska-Jaruga et al. 2020). OC pesticides were forbidden in EU and in Serbia in the same period.
The obtained NCP (non-organochlorine pesticides) concentrations ranged from 2.810 to 7.546 mg/kg. They were higher than those measured in Poland (< 0.01 to 43.92 µg/kg) Ukalska-Jaruga et al. 2020, but not the same active ingredients were measured. High values of NCPs concentration in our case were mainly affected by residues of trifluralin soil incorporated herbicide and chlorpyrifos soil incorporated insecticide, and two samples with fenitrothion content over 1.500 mg/kg as well, while NCP content was below 0.800 mg/kg (Table 3.) in other samples.
Herbicides
In this study, residues of 22 herbicides in soil samples were analysed. Among the tested herbicides, cycloateis were forbidden in 2005, lactofen in 2015, and fluometuron is not registered in Serbia, while others are allowed for application. Residues of three out of the 22 tested herbicides were found in the soil samples. Residues of the active substance trifluralin were determined in all examined soils in the range from 0.316 to 2.907 mg/kg (Table 3). Due to the fact that trifluralin is a selective pre-emergence herbicide registered for weed control (banned in the EU) in soybeans and sunflowers, it means that it could persist in soils for more than one year. These findings are in accordance with those of Chowdhury et al. (2021), who stated that trifluralin has the potential to persist in clay loam soil for several years at ≤ 20 °C in a laboratory model, which could potentially affect the subsequent crops in rotation. Furthermore, findings of Grover et al. (1997) reported that the estimated half-lives of its persistence from 'moderate' to 'persistent'. The statistical difference was observed between fields with pepper and maize as a pre-crops (Fig. 5). Whereby the highest residues were found in the soils with pepper as a pre crop, probably due to an herbicide applied in the transplanting period.
Acetochlor is widely used for the selective pre-emergence weed control of annual grasses and many broadleaf weeds in maize, soybean, and grain. Although acetochlor has been forbidden since 2014, it was found in 6 tested soils. In all of these soils, field crops (soybean, maize, and wheat) were sown as previous crops. The lowest content of carbonates was found in two soil samples containing residues of acetochlor, while it was around 10% in three soil samples. The results of Kucharski et al. (2014) showed that clay and organic carbon content in soil could influence the fate of acetochlor residues. Acetochlor residues were below LOD in all soil samples where pepper and vegetables were grown as previous crops. This pesticide has not been used in these fields due to its phytotoxic effect on vegetable crops.
Residues of nine different active substances from the chemical group of sulfonylurea were tested. Sulfonylurea (SU) herbicides established the beginning of a new era in the technology of chemical weed control. Compared to other herbicides, SUs are environmentally safe due to the low dosage (c. 2–75 g/ha). Furthermore, SUs are broad spectrum herbicides with high efficacy, but selective for cultivated plants. Persistence of nicosulfuron in soil can influence subsequent crops even at low concentrations (Ahmadi et al. 2017).
Nicosulfuron was detected in three out of four soils where the previous crop was maize, with the maximum residue level of 1.988 µg/kg (Table 3). The reason for this could be the pH value of these soils which is 7.5–8. On the other hand, the pH value of the soil samples (maize pre-crop) in which this herbicide was not detected was pH 4.4. According to Brown (1990), sulfonylurea hydrolysis is significantly faster under acidic (pH 5) than alkaline (pH 8) conditions, allowing the use of soil pH as a predictor of soil residual activity. Soil samples were taken at the end of October, while herbicides based on nicosulfuron are usually applied on maize crops in May or June, and the residues were detected in the amount of 0.1–2 μ/kg four to five months later. Ahmadi et al. (2017) reported that the presence of nicosulfuron residues was detected in maize soils 60 days after application, at the depth of 0–15 cm in the amount of 4 μ/kg. However, after that period, herbicide residues were below 1 μ/kg which was a detectable level.
Insecticides
The research covered the detection of 25 insecticide residues, including 20 organochlorine insecticides and their metabolites, two insecticides from the group of organophosphate compounds, and one belonging to pyriproxyfen, etoxazole and pyrethroid groups. Residues of 20 insecticides were found in the tested soil samples (Table 3).
Metabolites of DDT and DDD (0.005–0.111 mg/kg) were present in 18% of the soil samples, while others were below LOD. DDE (0.004–2.402 mg/kg) was present in 63% samples, while DDT (0.003–0.681 mg/kg) was detected in all the tested samples (Table 3). These data are in the accordance with the data obtained by Malusa et al. (2020), who reported that samples from more than 80% of examined organic farm soils in Poland contained detectable amounts of DDT or its metabolites. Also, Ukalska-Jaruga et al. (2020) reported the presence of DDT in all soil samples in Poland. In Serbia, the maximum residue levels of DDT and lindane, plus their metabolites, are 100 mg/kg and 60 mg/kg, respectively (Šovljanski and Ostojić 1989). The residue levels of DDT and γ-HCH in all the soil samples were below these quantities, which is in agreement with the results obtained by Pucarević and Sekulić (2004). These authors analysed over 900 soil samples from the Novi Sad municipality and all the concentrations were below the MRL (maximum residue level) for these compounds. The ratio of total DDT and its isomers indicate the transformation of the parent compound. The ratio of DDE/DDT can be used as an index to identify the fresh and/or aged application of DDT in a research area (Dirbaba et al. 2018). In the current study, the ratio ranged from 0 to 10.68 mg/kg. According to Maliszewska-Kordybach et al. (2014), the obtained data generally indicated the deposition (a ratio value of < 20) of these contaminants in soil is old and that it originates from the pesticide application conducted 15–20 years ago.
Data obtained for insecticide residues revealed that three (β-HCH, γ-HCH and δ-HCH) out of four lindane metabolites, heptachlor, the chemical group of aldrin, dieldrin, endrin, endrin aldehyde and endrin ketone were present in all the tested soil samples in the total content from 5.940 to 10.857 mg/kg. Furthermore, the highest percentage proportion of HCHs consisted of γ-HCH, followed by β-HCH, δ-HCH and α-HCH. An even higher ratio of β-HCH and γ-HCH was over 99.6% (34 and 65% respectively), denoting them as the dominant HCH congeners (Table 3). According to these data, it can be concluded that some residues of insecticides were degraded, but that, in most cases, they are still in γ-HCH as the main component of the commercial pesticide lindane. This is supported by the fact that the proportions of β-HCH/ΣHCHs is below 0.5. A significant difference was found between the content of residues of γ-HCH between pepper and maize as previous crops (Fig. 6a). The obtained data indicated high persistence and very slow degradation in the soil (Maliszewska-Kordybach et al. 2014; Dirbaba et al. 2018). Endosulfan II was found in all tested samples with a significant difference between soils preceded by pepper and maize as a previous crops (Fig. 6b). The total content of endosulfanes per sample ranged from 0.012–0.071 mg/kg. Residues of dieldrin, endrine, endrin aldehyde and endrin ketone were detected in all soil samples, but without a significant difference within the tested groups. Methoxychlor was detected in 82% of the tested samples and the content of methoxychlor in the tested samples ranged from 0.001 to 0.018 mg/kg.
Although OCPs have been forbidden for a long period of time, almost all were found in the tested soils, with differences among the groups of soils and among different previous crops. The fact that these pesticides resist degradation by chemical, physical, microbiological, and biological means raises concerns about the potential of these compounds to damage these ecosystems. They are designated in the list of POPs due to their toxicity and environmental persistence (Shukla et al. 2014). Furthermore, their persistence leads to bioaccumulation in the food chain, posing threats to human health. Many organochlorine pesticides and their metabolites are highly toxic and can cause a wide range of serious health problems such as cancer and birth defects (Taiwo 2019).
Organophosphate insecticide fenitrothion was detected in only 2 samples (vegetables and soybeans as pre-crops), while the organophosphorus insecticide chlorpyrifos was established in all of the tested samples. The highest content of chlorpyrifos was found in soils with vegetables as the previous crop (Fig. 6c). Such results could be explained by the frequent application of this insecticide in the control of various soil pest species, such as wireworms, cutworms and mole crickets, in many crops. Chlorpyrifos has been banned for use in Serbia since 2020.
Fungicides
Fungicides are applied directly to the soil or enter the soil as run-off from treated aerial plant parts. Besides their role in the control of phytopathogenic organisms, they also have a significant effect on the non-pathogenic soil microflora. Nine out of 21 tested fungicides were identified in the examined soils with no difference in fungicide residues among the different previous crops. Phthalimide fungicides, captan and folpet were all identified in the tested samples. Captan and folpet are often used in integrated pest management (IPM) programs in conjunction with other fungicides. Captan was first registered in the first half of twentieth century. It proved to be extremely efficient against many pathogens and is still in use. Captan was detected in an amount of over 2 μg/kg in the soil samples where vegetables and soybeans were grown as previous crops. However, the data provided by Kuthubutheen and Pugh (1979) showed that captan-treated soils were recolonised by soil fungi within no more than 7 days of fungicide application. Residues of folpet were found in all soil samples. Given the fact that folpet is not used in the treatment of soybeans and maize, this indicates that the residues persisted from the previous crops and treatments. Chlorothalonil is a widely used active ingredient both individually and combined in co-formulations. Chlorothalonil residues were present in 10 out of 22 sites, which indicates its persistence.
Triazole fungicides represent one of the most important group of fungicides with an excellent protective, curative and eradicant power towards a wide spectrum of crop diseases. They are applied directly on plants (Li et al. 2013), widely commercially used and known for their persistence in soil. Prothioconazole, tebuconazole and epoxyconazole were detected in 36%, 18% and 14% soil samples, respectively, while in other samples they were below the detection level (Table 3). In laboratory conditions, flutriafol, epoxiconazole and triadimenol were very persistent, with half‐lives greater than two years at 10 °C and 80% water field capacity, while propiconazole was moderately persistent under the same conditions, with a half‐life of about 200 days (Bromilow et al. 1999). Strobilurins, which are relatively new class of fungicides, have become one of the most widely used fungicides. Azoxystrobin was found in 14% soil samples and remained below the detection level in the other 86%.
Correlation pesticide residues and soil traits
Pesticide properties are an important factor in determining their fate in the soil. Furthermore, significant soil properties that are responsible for the fate of pesticides are organic matter content, texture, permeability, pH value and soil depth. Due to their chemical structure, organic matter and clay have a more pronounced adsorption power and they are suitable sorbents of pesticides. The solubility of pesticides is higher at lower pH values of the soil solution, and their sorption is often weaker under such conditions.
The correlation and the effects of soil properties on the level of pesticide residues were analysed by means of Spearman correlation coefficients (Table 4). The obtained correlation indicated the relationship between some pesticides and the pH value, the content of calcium, phosphorus and potassium in the soil. Tebuconazole had a negative correlation coefficient with pH (H2O) and pH (KCl) as well as with CaCO3. In a recent publication, Siek et al. (2021) reported that the adsorption of tebuconazole was inversely correlated with soil pH. The content of humus positively correlated with epoxiconazole. pH measured in KCl was negatively correlated with the content of ACTCL and α-HCH. The content of CaCO3 was positively correlated with γ-HCH, endrin, endosulfan II, while it correlated negatively with tebuconazole. Positive correlation coefficients (significant at 5% level) were obtained between the content of P2O5 and K2O and fungicide azoxystrobin. Additionally, the content of P2O5 positively correlated with the content of heptachlor.
Deeper insight into the relationship between soil physical and chemical properties, pesticide residue concentrations and crops could be obtained by PCA analysis (Fig. 7). With the first two PC explained 31.24% of variation (Table 5). The top 5 most important variables for traits and plant species separation in a PCA biplot were: difenoconazole, DDT, DDD, DDE and nicosulfuron (variable power higher than 0.8). The nicosulfuron, DDD, DDE DDT, folpet and difenoconazole mainly contributed to the PC 1 (loadings > − 0.79). Among all plant species and plant groups, maize had a high negative influence at the PC1 (-0.639). The main soil traits: pH (H2O), pH (KCl), Al (P2O5), Al (K2O) positively influenced at the PC2 (loadings between 0.61 and 0.76).
According to the positioning of the tested active ingredients, soil traits and crops in the PCA biplot, it could be assumed that the fate of the pesticides also depend on a great number of external factors, climate type, number of pesticide applications and the type of associated compounds (solvents, surfactants, other agents) which cannot be controlled. The same conclusion was given by Ukalska-Jaruga et al. 2020. The banned pesticides such as DDT and its metabolites as well as lindane metabolites grouped separately in PCA biplot on a high distance of soil traits. Furthermore, DDT and its metabolite residues did not depend on the type of crop. Endosulfan and its metabolites had close connectivity with calcium content. Aikpokpodion et al. (2010) reported that application of endosulfan significantly reduced the concentration of calcium in the soil. Residues of azoxystrobin are connected with phosphorus and potassium content as it was in the correlation coefficient. A close connection of endrin and the pH values and potassium content could be observed. Tebuconazole residues are closely connected with wheat crops, as it is well known that the fungicides based on tebuconazole have widespread usage in the control of wheat phytopathogenic fungi. On the other hand, a close correlation could be observed between the residues of the organophosphate insecticide fenitrothion and vegetables as the previous crop. This insecticide was widely used in vegetable production until it was banned. The soil residue of lindane, captan and trifloxystrobin was closely connected with humus content in soil (Fig. 7). According to literature data, soil organic matter has been reported to be an important factor in the retention of herbicides and has shown the potential for environmental decontamination (Bonfleur et al. 2010; Tejada and Benítez 2017). On the other hand, some authors reported that organochlorine pesticides tend to bind with soil, which means that the increase in soil organic matter content may lead to the increase in the pesticide residue levels in soil (Tan 2014).
Conclusion
As chemical compounds, pesticides accumulate in soils. Therefore, it is very important to measure pesticide residues in soils, including those pesticides which are not currently in official use, such as OCPs. The current study has shown that the majority of residues belonged to organochlorine pesticides (75%), which are currently banned. This study confirmed the persistence of these compounds. New-generation pesticides were much less frequently detected, which could be explained by lower application doses of new generation pesticides and the limit of their detection. The detected residues of sulphonylurea herbicides and triazole fungicides indicated the necessity of monitoring these groups of pesticides in order to minimize environmental pollution and health risks.
Moreover, the number of active ingredients per sample did not depend on the previous crop, which could indicate similar cultivation practices. Additionally, physical soil properties that may affect pesticide accumulation levels should be taken into consideration. The obtained levels of certain pesticide residues in the soil pointed to a real danger from their transfer to groundwater and surface waters. Pesticide residues thus reach a different environment, their lifespan changes, and they accumulate in non-target organisms, where they are transformed and leave negative consequences. Monitoring pesticide residues in groundwater and surface waters is a mandatory step in the control of pesticide soil residues.
Data availability
Data are available.
Code availability
Not applicable.
References
Aaron AJ, Li Zijian (2014) Scope of the worldwide effort to regulate pesticide contamination in surface soils. J Environ Manage 146:420–443. https://doi.org/10.1016/j.jenvman.2014.07.020
Ahmadi AR, Shahbazi S, Diyanat M (2017) Analysis of nicosulfuron residues in maize field soil by high-performance liquid chromatography. Qual Assur Saf Crop & Foods 9(2):229–235. https://doi.org/10.3920/QAS2015.0741
Aikpokpodion PE, Lajide L, Ogunlade MO, Ipinmoroti R, Orisajo S, Iloyanomon CI, Fademi O (2010) Degradation and residual effects of endosulfan on soil chemical properties and root-knot nematode Meloidogyne incognita populations in cocoa plantation in Ibadan, Nigeria. J ApplBiosci 26: 1640–1646. http://www.m.elewa.org/JABS/2010/26/7.pdf
Anonymous (2015) Import of plant protection products and active substances for their manufacture in Serbia in the period 2000–2014 Ministry of AgricultureRepublic of Serbiahttps://www.uzb.minpolj.gov.rs/attachments/047_UVOZ_SZB_2000-2014. Accessed 12 May 2021
Babut M, Arts GH, Caracciolo AB, Carluer N, Domange N, Friberg N (2013) Pesticide risk assessment and management in a globally changing world—report from a European interdisciplinary workshop. Environ Sci Pollut Res Int 20:8298–8312. https://doi.org/10.1007/s11356-013-2004-3
Bonfleur E, Lavorenti A, Tornisielo VL (2010) Mineralization and degradation of glyphosate and atrazineapplied in combination in a Brazilian oxisol. J Environ Sci Heal Pesticides 46:69–75. https://doi.org/10.1080/03601234.2011.534384
Bromilow R, Evans A, Nicholls P (1999) Factors affecting degradation rates of five triazole fungicides in two soil types: 1. Laboratory incubations. PesticSci 55:1129–1134
Brown HM (1990) Mode of action, crop selectivity, and soil relations of the sulfonylurea herbicides. PesticSci 29:263–281. https://doi.org/10.1002/ps.2780290304
Bursić V, Lazić S, Tarbuk S, Pucarević M, Šunjka D (2011) Validation of method for the analysis of clomazone residues in soil. 18th Young investigators seminar on analytical chemistry, Novi Sad, 28 June-1 July pp 69
Bursić V, Đurović R, Lazić S, Zeremski-Škorić T, Pucarević M (2012a) Comparison of four extraction methods for the multiresidue determination of pesticides in soil by gas chromatography-mass spectrometry detection. 4th International Congress EurosoilFera del Levante, Bari, Italy 2–6 July 2012a. Book of abstracts, pp 2578
Bursić V, Lazić S, Šutonja I, Zeremski-Škorić T, Vuković S, Gvozdenac S, Pucarević M (2012b) Analysis of dicamba residues in soil by QUECHERS and HPLC-DAD. Annual MGPR Meeting International Conference on Food and Health Safety: Moving Towards a Sustainable Agriculture, Belgrade 10–12. October pp 70
Cabrerizo A, Dachs J, Jones KC, Barcel D (2011) Soil-Air exchange controls on background atmospheric concentrations of organochlorine pesticides. AtmosChem Phys 11:12799–12811. https://doi.org/10.5194/acp-11-12799-2011
Chowdhury IF, Rohan M, Stodart BJ, Chen C, Wu H, Doran G (2021) Persistence of atrazine and trifluralin in a clay loam soil undergoing different temperature and moisture conditions. Environ Pollut 276:116687. https://doi.org/10.1016/j.envpol.2021.116687
DirbabaNB Li S, Wu H, Yan X, Wang J (2018) Organochlorine pesticides, polybrominated diphenyl ethers and polychlorinated biphenyls in surficial sediments of the Awash River basin. Ethiopia. PLoS ONE 13(10):e0205026. https://doi.org/10.1371/journal.pone.0205026
Dugalić G, Krstić D, Jelić M, Nikezić D, Milenković B, Pucarević M, Zeremski-Škorić T (2010) Heavy metals, organic and radioactivity in soil of western Serbia. J Hazard Mater 177:697–702. https://doi.org/10.1016/j.jhazmat.2009.12.087
Đurović R, Bursić V, Lazić S, Pucarević M, Zeremski-Škorić T, Guszvany V (2011) Comparison of four Extraction Methods for the Analysis of Triazin and Organophosphorus Pesticides in Soil Samples With Gas Chromatography coupled with Mass Spectrometry. 12th European Meeting on Environmental Chemistry, 7–10 Dec, Clermont-Ferrand France pp 67
Egner H, Riehm H, Domingo WR (1960) Untersuchungenüber die chemischeBodenanalysealsGrundlagefür die Beurteilung des Nahrstoffzustandes der Boden II. ChemischeExtractionsmetodenzu Phosphor – und Kaliumbestimmung. K LantbrHogskAnnlr WR 26:199–215
HRN EN ISO 10390 (2005) Soil quality -- Determination of pH (ISO 10390:2005).HZN Glasilo 1/2005. https://repozitorij.hzn.hr/norm/HRN+ISO+10390%3A2005
HRN EN ISO 14235 (2004) Soil quality -- Determination of organic carbon by sulfochromic oxidation (ISO 14235:1998).Glasilo DZNM 4–6/2004.https://repozitorij.hzn.hr/norm/HRN+ISO+14235%3A2004
Fosu Mensah B, Okoffo E, Darko G, Gordon C (2016) Assessment of organochlorine pesticide residues in soils and drinking water sources from cocoa farms in Ghana. Springerplus 5:869. https://doi.org/10.1186/s40064-016-2352-9
FRAC CODE LIST 1 Fungicides sorted by FRAC Code https://ipm.ifas.ufl.edu/resources/success_stories/t&pguide/pdfs/Appendices/Appendix6-FRAC.pdf.Accessed 10 November 2020
Grahovac N (2016) Monitoring of residues of sulfonylurea in soil under real conditions by using high pressure liquid chromatography. Dissertation, University of Novi Sad, Faculty of Technology (in Serbian) https://hdl.handle.net/21.15107/rcub_nardus_7195
Grover R, Wolt JD, Cessna AJ, Schiefer HB (1997) Environmental fate of trifluralin. Rev Environ ContamToxicol 153:1–64. https://doi.org/10.1007/978-1-4612-2302-3_1 (PMID: 9380893)
HoloubekI Dušek L, Sánka M, Hofman J, Cupr P, Jarkovsky J, Zbíral J, Klánová J (2009) Soil burdens of Persistent organic pollutants-their levels, fate and risk. Part I. Variation of concentration ranges according to different soil uses and locations. Environ Pollut 157:3207–3217. https://doi.org/10.1016/j.envpol.2009.05.031
Holt VL, Trabert BL, Upson K (2013) Endometriosis. In: Goldman MB, Triosi R, Rexrode KM (eds) Women and Health, 2nd edn. Academic Press, San Diego, pp 271–284
HRAC GLOBAL HERBICIDE CLASSIFICATION LOOKUP https://hracglobal.com/tools/classification-lookup. Accessed 15 May 2021
HRN ISO 10693 (2004) Soil quality -- Determination of carbonate content -- Volumetric method (ISO 10693:1995). Glasilo DZNM 4–6/2004. https://repozitorij.hzn.hr/norm/HRN+ISO+10693%3A2004
Hvezdová M, Kosubová P, Košíková M, Scherr K, Šimek Z, Brodský L, Šudoma M, Škulcová L, Sánka M, Svobodová M (2018) Currently and recently used pesticides in central European arable soils. Sci Total Environ 613–614:361–370. https://doi.org/10.1016/j.scitotenv.2017.09.049
IRAC Mode of Action Classification Scheme (2020) Insecticide Resistance Action Committee. https://www.irac-online.org moa-classification.Accessed 12 October 2020
ISO procedure 1038 1–4 (2003) Soil Quality –Sampling- Part 4 Guidance on the procedure for investigation of natural, near-natural and cultivated sites. International Organization for Standardization, Genève, Switzerland
Jorfi F, Atashi Z, AkhbarizadehR KZ, Ahmadi M (2019) Distribution and health risk assessment of organochlorine pesticides in agricultural soils of the Aghili plain. Southwest Iran Environ Earth Sci 78:603. https://doi.org/10.1007/s12665-019-8605-5
Kucharski M, Dziągwa M, SadowskiJ, (2014) Monitoring of acetochlor residues in soil and maize grain supported by the laboratory study. Plant Soil Environ 60:496–500
Kuthubutheen AJ, Pugh GJF (1979) The effects of fungicides on soil fungal populations. Soil BiolBiochem 11:297–303. https://doi.org/10.1016/e0038-0717(79)90075-0
Lazić S, Šunjka D, Pucarević M, Grahovac V, Jakšić S, Vuković S, Bursić V (2011) Presence of atrazine residues in soil and its contents in ground water. 22nd International symposium ”Safe food production”, Trebinje. Bosnia and Herzegovina, 19 - 25 June pp 404–406
Li ZH, Zlabek V, Velisek J, Grabic R, Machova J, Kolarova J, Li P (2013) Multiple biomarkers responses in juvenile rainbow trout, oncorhynchus mykiss, after acute exposure to a fungicide propiconazole. Environ Toxic 28:119–126. https://doi.org/10.1002/tox.20701
Maliszewska-Kordybach B, Smreczak B, Klimkowicz-Pawlas A (2014) Evaluation of the status of contamination of arable soils in Po land with DDT and HCH Residues; national and regional scales. Pol J EnvironStud 23(1):139–148
Malusa E, Tartanus M, Danelski W, Miszczak A, Szustakowska E, Kicińska J, Furmanczyk E (2020) Monitoring of DDT in agricultural soils under organic farming in poland and the risk of crop contamination. Environ Manage 66:916–929. https://doi.org/10.1007/s00267-020-01347-9
Manz M, Wenzel KD, Dietze U, Schüürman G (2001) Persistent organic pollutants in agricultural soils of germany. SciTotal Environ 277:187–198. https://doi.org/10.1016/S0048-9697(00)00877-9
Meijer SN, Halsall CJ, Harner T, Peters AJ, Ockenden WA, Johnston AE, Jones KC (2001) Organochlorine pesticide residues in archived UK Soil. Environ SciTechnol 35:1989–1995. https://doi.org/10.1021/es0000955
Navarro S, Vela N, Navarro G (2007) An overview on the environmental behaviour of pesticide residues in soils. Span J Agric Res 5:357–375
Nešić L, Belić M, Manojlović M, Pucarević M, Sekulć P, Vasin J (2008) Fertility status and hazardous harmful residues in the soils of Srem. Book of Abstracts of EUROSOIL 04–08. August, Viena, Austria, pp 286
Official Gazette of the Republic of Serbia no. 64/2019
Official Gazette of the Republic of Serbia no. 30/2018
Pérez-Lucas G, Vela N, El Aatik A, Navarro S (2019) Environmental risk of groundwater pollution by pesticide leaching through the soil profile. In: Larramendy Marcelo, Soloneski Sonia (eds) Pesticides - Use and Misuse and Their Impact in the Environment. IntechOpen. https://doi.org/10.5772/intechopen.82418
Plant protection products on Serbian market (2017) Plant Doctor 45. Ministry of agriculture, forestry and water management, Republic of Serbia
Pu X, Cutright T (2006) Sorption-desorption behavior of PCP on soil organic matter and clay minerals. Chemosphere 64:972–983. https://doi.org/10.1016/j.chemosphere.2006.01.017
Pucarević M, Sekulić P (2007a) Investigations of pesticide residue content in the soils of Srem region Results of 1992–2006. 5TH MGPR Symposium of Pesticides in Food and the Environment in Mediterranean Countries, 21–24 November, Agadir, Morocco
Pucarević M, Sekulić P, Rajović S (2007b) Organochlorine pesticides in the soils of Serbia. Proceedings of the First PSU-UNS Joint Conference on Bioscience, Food Agriculture and Environment, 17–19 August, Hat Yai, Songkhla, Thailand, pp 275–279
Pucarević M, Miloradov-Vojnović M, Zeremski-Škorić T, Sekulić-Turk M (2008) Persistant organic pollutants in the soil, water and air of Serbia. The Second Joint PSU – UNS International Conference on BioScience: Food, Agriculture and Environment 22 – 24 June, Novi Sad, Serbia
Pucarević M, NešićLj, Belić M, Ćirić V, Bošković S (2010) Organochlorine pesticide residues content in the soils for food production. International eco-conference Safe food 22–25 September Novi Sad, Serbia, pp 81–88
Pucarević M, Sekulić P (2004) Polycyclic aromatic hydrocarbons and pesticides in soil of Vojvodina. Proc Nat SciMaticaSrpska 107:93–100. https://doi.org/10.2298/ZMSPN0417093P
Shukla S, Mangwani N, Subba Rao T, Das S (2014) Biofilm-mediated bioremediation of polycyclic aromatic hydrocarbons. Microbial Biodegradation and Bioremediation. Elsevier, pp 203–232. https://doi.org/10.1016/B978-0-12-800021-2.00008-X
Siek M, Paszko T, Jerzykiewicz M, Matysiak J, Wojcieszek U (2021) Mechanisms of tebuconazole adsorption in profiles of mineral soils. Molecules 26(16):4728. https://doi.org/10.3390/molecules26164728
Silva V, MolH ZP, Tienstra M, Ritsema CJ, Geissena V (2019) Pesticide residues in european agricultural soils-a hidden reality unfolded. Sci Total Environ 653:1532–1545. https://doi.org/10.1016/j.scitotenv.2018.10.441
Soares C, Neves A, Queiroz M, Oliveira A, Costa A, Assisa R, Andradea C (2015) Determination of pesticides in soil using a hyphenated extraction technique. J BrazChemSoc 26:1790–1797. https://doi.org/10.5935/0103-5053.20150155
Šovljanski R, Ostojić Z (1989) Study on uniform criteria for registration of pollutants and water, air and soil pollution in the SAP area. Vojvodina and the urgent protection measures in the places with the most pronounced pollution. Part Land, PIV, Novi Sad
Taiwo A (2019) A review of environmental and health effects of organochlorine pesticide residues in Africa. Chemosphere 220:1126–1140. https://doi.org/10.1016/j.chemosphere.2019.01.001
Tan Kim H (2014) Humic Matter in Soil and the Environment. CRC Press. https://doi.org/10.1201/b17037
Tejada M, Benítez C (2017) Flazasulfuron behavior in a soil amended with different organic wastes. Appl Soil Ecol. https://doi.org/10.1016/j.apsoil.2017.05.009
Ukalska-Jaruga A, Smreczak B, Siebielec G (2020) Assessment of pesticide residue content in polish agricultural soils. Molecules 25:587. https://doi.org/10.3390/molecules2503058
Wang Y, Du L, Zhou X, Tan H (2012) QuEChERS extraction for high performance liquid chromatographic determination of pyrazosulfuron-ethyl in Soils. J ChemSoc Pak 34(1):28–32
World Health Organization (2022) Children's Health and the Environment WHO Training Package for the Health Sector https://www.who.int/ceh/capacity/Pesticides.pdf. Accessed 01 April 2022
Yang H, Wu X, Zhou L, Yang Z (2005) Effect of dissolved organic matter on Chlorotoluron sorption and desorption in soils. Pedosphere 15(4):432–439
Zebec V, Rastija D, Lončarić Z, Bensa A, Popović B, Ivezić V (2017) Comparison of chemical extraction methods for determination of soil potassium in different soil types. Eurasian Soil Sc 50:1420–1427. https://doi.org/10.1134/S1064229317130051
Acknowledgements
This research is supported by Interreg IPA Cross-border project IMPACT ENVI HR-RS 182 and by Ministry of education, science and technological development, Republic of Serbia, grant no.451-03-68/2022-14/ 200032
Funding
Supported by Interreg IPA Cross-border project IMPACT ENVI HR-RS 182 and by Ministry of Education, Science and Technological Development of the Republic of Serbia, grant number: 451–03-68/2020–14/200032.
Author information
Authors and Affiliations
Contributions
Sample collection was done by Sladjana Medić Pap, Dario Danojević and Janko Červenski. Soil analysis was performed by Mira Pucarević, Nataša Stojić and Brigita Popović. Statistics analysis was performed by Dario Danojević and Brigita Popović. The first draft of the manuscript was written by Sladjana Medić Pap, Brigita Popović, Nataša Stojić, Mira Pucarević and Dario Danojević. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Marcela Šperanda was involved in Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Editorial responsibility: Hari Pant.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Medić Pap, S., Popović, B., Stojić, N. et al. The environmental issue of pesticide residues in agricultural soils in Serbia. Int. J. Environ. Sci. Technol. 20, 7263–7276 (2023). https://doi.org/10.1007/s13762-022-04424-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04424-0