Abstract
Air pollution is the change in air composition that disrupts human health and environmental balance. Although natural and anthropogenic processes include crustal movements, photosynthesis, and plant and animal emissions, other sources of contamination also include industrial operations, transportation activities, household resources, and the chemical and metal industries. Thus, biomonitoring can be employed as a quick, affordable, and efficient method for estimating air pollution. In this study, some inorganic pollutants were detected using olive trees (Olea europaea L.) at eleven different points, depending on the traffic density in Artvin, Turkey. Trace element concentrations (Cr, Ti, Fe, Ni, Co, Cu, Zn, Pb, Al, and Mn) were measured in soil once a year and seasonally in plant samples with ICP-OES. Furthermore, basic component analyses total carbon (TC), total nitrogen (TN), total hydrogen (TH), and total sulfur (TS) were done with an elemental analyzer, total chlorophyll contents with a portable chlorophyll meter, and morphological and particle-based plant analyses with SEM–EDS. The pollution levels of these metals were calculated using the enrichment factor (EF) and geoaccumulation index (Igeo) parameters. Furthermore, the accuracy and validity tests of the analyses for trace metals were tested by applying certified reference materials (CRM) (ERM-CD281) for the plant samples and CRM (LGC-6187) for soil samples. Results indicated that soil trace element pollution distributions were ranked according to the following descending order: Fe (37,873.33 mg/kg) > Al (13,300 mg/kg) > Mn (1101.33 mg/kg) > Ti (353.5 mg/kg) > Zn (252.86 mg/kg) > Cu (87.77 mg/kg) > Cr (30.52 mg/kg) > Pb (19.65 mg/kg) > Ni (17.07 mg/kg) > Co (7.65 mg/kg). Moreover, air pollution from anthropogenic sources substantially increased average trace metal concentrations and sulfur emissions in autumn and winter. The average highest values of Fe (321.08 mg/kg) > Al (304.05 mg/kg) > Mn (32.75 mg/kg) > Zn (31.01 mg/kg) > Cu (17.92 mg/kg) > Ti (11.07 mg/kg) Cr (2.57 mg/kg) > Ni (17.07 mg/kg) were found in leaf samples taken from the roadside in autumn and winter. According to the EF and Igeo values, the main polluting trace elements in the soil were Zn, Cu, and Pb, while in the plant, these were detected as Fe, Al, Ti, Cr, Ni, and Cu. Kruskal–Wallis and correlation analysis statistically supported this relationship among metals. Results show that olive leaves are an effective bioindicator for detecting urban air pollution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the advancement of technology, environmental pollution has become a significant concern with ancient roots. These pollutants are caused by human activities, such as uncontrolled wastes, emissions from rapidly growing cities, chemical fertilizers, and discharges (Briffa et al., 2020). A specific pollutant causing concern is trace metals.
Trace metals may be defined as metals present in the earth’s crust at concentrations of 1000 mg/kg or less. Such metals can be classified as heavy or light, depending on their density. In general, a metal with a density greater than 5 g/cm3 is called heavy metals and plays a significant role among all pollutants (Osuji & Onajake, 2004). It has been revealed that metals accumulate in plant and animal cells, leading to serious damaging effects, such as cardiovascular disease, lung cancer, kidney disease, and inflammation-related diseases (Ali et al., 2019).
Various sources contribute to the spread of trace metals throughout the environment, including industrial processes, motor vehicles, mining activities, agricultural pesticides, and urban wastes (Singh et al., 2023). The participation of these trace metals, which are directly involved in biological processes, is regarded as either necessary or toxic. Necessary trace metals are present in certain concentrations in biological systems and are among the basic building blocks of organisms. On the other hand, toxic trace metals that can be absorbed through food, the atmosphere, and clothes have a toxic effect on living organism. For example, while copper is engaged in the oxidation, reduction, and formation of red blood cells in living organisms, traces of mercury can bind to sulfur-containing enzymes and harm many organs, including the brain and kidneys (Bingham et al., 2001).
Damaging trace metals is a critical source of pollution that adversely affects human health and environmental contamination and air pollution. Various factors influence the rise of air pollution, including motor vehicle emissions and thermal power plants. Due to the steadily increasing use of motor vehicles, vehicular emissions have been recognized as the primary source of air pollution. Motor vehicles emit exhaust gases with significant amounts of COx, NOx, HC synthetic derivatives, and trace metals (Cr, Cu, Cd, Pb, Cd, Al, and Fe) (Ali et al., 2019). In accordance with to a 1999 World Health Organization report, particulate emissions from vehicles in three European countries indicated that there was more deaths related to particle emissions than there was to traffic accidents (Sawidis et al., 2011). Furthermore, some heavy metals in the soil and air can be traced back to living organisms through the ecosystem chain or through plants used as biomonitors. Plants in this area absorb some trace metals through their roots as concentrations in the soil increase. Concentrations of these trace metals then also build up in other parts of the plant, such as leaves, fruits, and flowers (Bondada et al., 2004). Countless species, which include fungi, lichens, mosses, annuals, and perennials, can be utilized as biomonitors (Szczepaniak & Biziuk, 2003). Among these species, evergreen and perennial plant species are commonly preferred to be utilized as biomonitors, particularly in urban areas and along highways. In this study, these perennial plant species and their geographical location were studied in Artvin, Turkey, and as olive has a wide distribution area, it was chosen as a biomonitor. Using this species as a biomonitor, the effects of traffic density on some inorganic and organic pollution accumulation in leaves of L. Olea Europaea trees and associated soils were assessed.
Material and methods
Study area
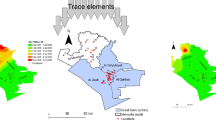
The study site was located in Artvin, Turkey. The region has a Black Sea climate and receives precipitation throughout the year. The province is located between the terrestrial and oceanic regimes due to its geographical location. Increases and decreases in temperature can be observed and are similar to the humid continental climate. The average annual temperature is 12.3 °C, and the average annual precipitation is 690.4 mm (1949–2021). In addition, the wettest month is December (860 mm), while the driest is August (29.3 mm (MGM, 2021). It has a total population of 169.403 people and covers a 7359 km2 area (TUİK, 2022). According to 2021 data, the annual average number of vehicles in Artvin city center is 2842, and the annual air particulate material rate (PM10) from vehicle exhaust emissions is 20.4 (KGM, 2021; Orak & Ozdemir, 2021). The sampling areas in Artvin were chosen from ten points due to the gradual increase in traffic density. Samples have been classed as a function of the traffic intensity in three categories: high traffic points (H): N1, N2, N4, N5, N6, N7, N8, and N10; medium traffic points (M): N3 and N9; and low traffic points (L): N11. Furthermore, the location with the least traffic-related pollution was preferred as the control point, as seen in Fig. 1.
The map of the study area in Fig. 1 was designed using ArcGIS (10.2) interpolated maps.
Laboratory studies
Chlorophyll counting
The olive tree grows abundantly in Artvin province and was selected as a biomonitor because these trees are highly resistant to climatic changes and are evergreen. Olive leaves of the same age in months were sampled from the designated points in the city center of Artvin; approximately 50 g of each sample was collected. Insect infestation, pesticide residue, rough and abnormal dust cover, and other factors that could result in inaccurate results were managed to avoid contamination in the leaf samples.
The amount of chlorophyll in each selected leaf sample was measured in the field with a portable chlorophyll meter (Opti-Sciences CCM-300), and the average value was calculated for each point. After that, all samples were placed in plastic bags with sliders and taken to the laboratory.
Plant and soil sampling analysis
Olive tree leaves were collected in every month from March 2019 to April 2020, washed three times in the laboratory, and dried to constant weight at 40 °C. The samples were placed in polyethylene bags and stored in the refrigerator at 4 °C after being dried and powdered in a homogenizer (Sawidis et al., 2011). 0.3 g of homogenized plant material was accurately weighed on an analytical balance and placed in Teflon vessels for plant analysis.
Then, 2 mL concentrated HNO3 and 3 mL H2O2 were added to each tube, and the mixture was left in a fume hood for 20 min. The digestion program (room temperature to 145 °C in 5.5 min, then 15 min at 180 °C in the microwave) was then applied (speedwave™ MWS-2+, Berghof). Following digestion, 1 mg/L internal standard yttrium was added and the sample volume was filled to 50 mL using ultrapure water. The heavy metal concentrations of Cr, Ti, Fe, Ni, Co, Cu, Zn, Pb, Al, and Mn in these final solutions were measured using ICP-OES (Perkin Elmer Optima 8000), and their dry weights were reported in milligram per kilogram.
Eleven soil samples were collected from 0 to 10 cm depth under the canopy of each selected olive tree (one from each side) and pooled to generate one composite sample. The eleven samples were air-dried at room temperature after being transferred to the laboratory. Samples of soil were taken from 11 traffic density points and dried indoors at room temperature before passing through a 2-mm sieve to remove any stones and root debris. The pH and conductivity were measured using a multi-pH meter after adding 25 mL of distilled water to 10-g soil samples. (water to soil ratio, 2.5/1). Then, the Bouyoucos hydrometer method was applied to assess the soil texture (Bouyoucos, 1962). Fifty grams of dried and sieved (< 2 mm) soil samples was weighed on an analytical balance and added to 50 mL of 25 g/L sodium hexametaphosphate solution. The mixture was left to stand overnight. The next day, the solution was mechanically shaken for 5 min, then placed in glass cylinders, and filled with distilled water until the final volume was 1 L. Hydrometer measurements were taken 40 s after shaking and repeated three times. The last measurement was made after 2 h. The results were calculated based on the percentages of sand, silt, and clay on the basis of the main mass (Stevenson et al., 2023). Microwave-assisted digestion methods US-EPA (2007) were applied to analyze soil samples for trace elements (Cr, Ti, Fe, Ni, Co, Cu, Zn, Pb, Al, and Mn) followed by ICP-OES (Perkin Elmer Optima-8000). For this, a 5-g sample of air-dried ground soil was weighed and placed in Teflon vessels. Then, 9 mL of concentrated HNO3 and 3 mL of HCl were added to each tube, and the mixture was left in a fume hood for 20 min before the digestion program was applied. The digestion program consisted of minutes at room temperature to 175 °C and 15 min at 180 °C in the microwave (speedwave™ MWS-2+, Berghof). Finally, a clear solution was obtained, and 1 mg/L internal standard (Yttrium) was added to the sample and filled to 50 mL with ultrapure water before analysis. The trace metal concentrations in these final solutions were measured using ICP-OES and expressed in milligram per kilogram based on dry weights. The digestion procedure for the plant and soil samples was first applied to the certified reference materials (CRM), and the method that yielded the best recovery data was ascertained. Then, using this method, actual samples were analyzed.
The elemental composition of 132 olive leaves and 11 soil samples was determined using an elemental analyzer (Elementar Vario Macro Cube) and sulfanilamide as a standard. Each 100 mg of each dried sample of plant and soil was weighed and pressed in a tin capsule. The capsule was placed in the autosampler and combustion in an atmosphere of pure oxygen (99.999%). Gas composition by this combustion was detected using a thermal conductivity detector. The result data were derived from weight to atomic percentage (Fernandes et al., 2021).
Electron microscopy
Scanning electron microscopy–energy-dispersive spectrometer (SEM–EDS) analysis was carried out on olive leaves and soil samples collected from site N8, which is the point based on traffic density. For this, washed and dried samples were mounted on separate 12-mm aluminum stubs with double-sided carbon adhesive protrusions and these stubs were sputter-coated with gold particles (Cressington Sputter Coater 108 Auto). The surface morphology of the leaf samples was analyzed using SEM (20 keV, Zeiss LS EVO-10). The elemental content in deposited trace metals was analyzed by an EDS detector (Bruker XFlash 6).
Pollution index
The enrichment factor (EF) and geoaccumulation index (Igeo) are frequently used to assess the level of anthropogenic contamination in soil. EF is also used to detect anthropogenic metal contamination in plants. Here, the geoaccumulation index (Igeo) and enrichment factor (EFsoil) were calculated to analyze metal pollution in soil. In contrast, enrichment factors (EFplant) were calculated for the metal uptake potential of plant species.
Igeo (geoaccumulation index)
Igeo indicates the degree of contamination of soil. In 1979, Müller developed an equation to quantitatively measure the intensity of contamination in aquatic sediments, and it has been widely used in many scientific studies (Muller, 1979).
The geoaccumulation index is signified as described in the following equation:
where \({C}_{n}\) stands for the measured concentration of the metal in the soil, and \({B}_{n}\) represents the geochemical background value of the metal in the soil. Factor 1.5 is the background matrix factor that results from the lithogenic effects.
Müller has defined seven classes of Igeo and Table 1 provides the pollution levels as measured by the soil accumulation index (Wedepolh, 1995). The Igeo cases seen in Table 1 range from class 0 (Igeo = 0, unpolluted) to class 6 (Igeo > 5, extremely polluted). The highest class reflects at least a 100-fold enrichment factor above background values (Muller, 1979).
Enrichment factor
The enrichment factor (EF) determines the level of anthropogenic contamination in the soil. EF values were obtained by dividing the measured metal concentration by a reference or background metal concentration value. In most cases, the background values are metals that are unaffected by contaminating inputs, i.e., the most abundant geochemical metals in the earth’s crust, such as Al and Fe, or rare metals, like Sc and Co (Bourennane et al., 2010; Brumsack, 2006; Hu et al., 2013). Many experimental results and studies have been applied for contaminants made of Fe-normalized metal (Bhuiyan et al., 2010; Ghrefat et al., 2011; Schiff & Weisberg, 1999). Fe- was therefore employed in this study’s background or Xc value since it is very abundant in the earth’s crust and hence mostly unaffected by contaminants brought on by humans.
The EF of soil is expressed as follows:
The EF can be divided into five contamination categories, as shown in Table 2 (Sutherland, 2000).
In addition, anthropogenic metal contamination was calculated by the following equations:
\({(M)}_{\mathrm{Lithogenic}}\)= \({(\mathrm{Fe})}_{\mathrm{sample}}\)× ( \({\frac{M}{\mathrm{Fe}})}_{\mathrm{Lithogenic}}\)where \({(M)}_{\mathrm{Lithogenic}}\) is lithogenic trace metal concentration, \({(\mathrm{Fe})}_{\mathrm{sample}}\) is the total Fe concentration in soil samples, and \({(\frac{M}{\mathrm{ Fe}})}_{\mathrm{Lithogenic}}\) is the ratio in the earth’s crust.
where \({M}_{\mathrm{Anthropogenic}}\) represents the anthropogenic trace metal concentration and \({M}_{\mathrm{total}}\) is the total concentration of heavy metals determined in the soil (Hernandez et al., 2003).
The enrichment factor of plants EFplant was calculated as follows:
where the enrichment factor \({\mathrm{EF}}_{\mathrm{plant}}\) is the relative measured concentration of the metal in the plant, and \({M}_{\mathrm{plant}}\) composed the relative measured concentration of the metal in the plant in local control site \({M}_{\mathrm{control}}\). (Mingorance et al, 2007)
Statistical analysis
SPSS 19.0 was applied for statistical analysis of the collected plant and soil data. Kruskal–Wallis tests were used to identify the differences among groups, and p values < 0.05 were considered to indicate a significant difference between the compared groups. In addition, correlation analysis was used to quantitatively describe and analyze the degree of correlation among trace metal relationships.
Quality control
A certified reference material, blank samples, and replicate samples were used for quality assurance and control of plant and soil samples. Ten blank samples were analyzed, and the limit of detection (LOD) value for each analyte was calculated as the mean of the ten blanks plus three standard deviations (Montoro-Leal et al., 2020). For plants, three samples were weighed from a certified reference material (ERM-CD 281, Rye Grass) and three soil samples were also weighed from a certified reference material (LGC-6187, river sediment). These were then digested and analyzed and contained ten elements. The recovery values, with their standard deviations, are given in Table 9 (by averaging the three sample values).
Result and discussion
Physico-chemical characteristics of soil samples
The soil texture analysis allowed classifying the investigated soils into two texture classes: sandy loam and loam (USDA, 1951). The percentage of sand ranged from 53.97 to 94.22%, silt from 6.66 to 32.20%, and clay from 1.12 to 13.82%. The atomic percentages of TN%, TC%, TH%, and TS% ranged from 0.2 to 0.55, 2.76–7.77, 0.67–1.32, and 0.03–0.12 in surface samples. In the analyzed samples, pH(H2O) ranged from 6.94 to 8.026. The EC values also varied between samples, ranging from 182.9 to 524 µS/cm (Table 3).
The concentration of heavy metals in the soil and the tendency of metals to absorb substances are significantly affected by soil pH, organic carbon content, and clay content (Davis, 1984; Jung, 2008). Hence, soil component analysis is essential for understanding how a metal is distributed among soil elements. According to this study, pH did not significantly vary between the locations; however, EC reached its maximum value to a value of 524 µS/cm at the N6 location. Additionally, the texture analyses revealed that these samples correspond to the sandy silt soil class. Such soils have high permeability and low metal retention capability as structural features. Other parameters used in this study to determine traffic-induced pollution were to determine the soil contents of TC, TH, TN, and TS. The highest concentrations were found at N6, with concentrations of TC, TN, TH, and TS being 7.77%, 0.53%, 1.23%, and 0.12%, respectively. Nevertheless, the total elemental analysis of the control soil sample was determined as TC 3.97%, TN 0.3%, TH 1.09%, and TS 0.03%. The analysis results suggest that the ratio of TC and TN is two times higher, and the ratio of TC and TS is four times higher in regions with increased traffic density than control location. In a recent study by Bandowe et al. (2019), they investigated the chemical (C, N, S, black carbon, soot, and coal) and stable carbon isotope composition and sources of street dust from a major West African metropolis. They observed that samples from industrial zones, markets, and main roads have higher TC, TN, and TS content than those from residential and educational institutions. Also, Leopold et al. (2017) researched traffic-related heavy metal and soil properties in tunnel dust and from roadsides They found that the sulfur results in Eselsberg and Bismarckring tunnels were 0.17 and 0.24%, respectively, which are relatively higher than the value for sulfur found in this study (0.12%).
After the results of the elemental analysis were statistically analyzed using the Spearman correlation test, as illustrated in Table 4, a strong positive correlation was determined between C and N (r = 0.809**, p < 0.01) and between C and H (r = 0.864**; p < 0.01). However, only a reasonable correlation was found between C and S (r = 0.547, p < 0.05) and H and S (r = 0.547, 0.633). Such strong positive correlations highlight the common anthropogenic origin of organic matter, aerosols, fossil fuels, brake wear, asphalt, tires, vehicle exhaust emissions, and anthropogenic origin components accumulating on roads.
Content of trace metals in the topsoil under the olive trees
Figure 2 illustrates the average metal concentrations detected in the topsoil under the olive trees, which were chosen as biomonitors according to the traffic density. The descending average concentrations of heavy metals are Fe > Al > Mn > Ti > Zn > Cu > Cr > Pb > Ni > Co. Although Zn, Co, Ni, Cr, Cu, and Fe had the highest concentrations, the maximum concentrations of other elements were found in various locations. High Pb, Mn, Ti, and Al values were detected at N4, N11, N1, and N6, respectively.
Nevertheless, except for Mn, N11 control soils with low traffic density had the lowest metal concentrations. However, there was no detectable difference in the concentration of Al between the control and sample soils. The analysis results suggested that regions with high traffic densities had higher Zn, Pb, Co, Mn, Cu, and Fe concentrations in the soil sample values. In the literature, countless studies found trace metal values that exceed soil limits for heavy metal pollution brought on by traffic.
According to Amusan et al. (2003), traffic is responsible for the high levels of heavy metals in soil, plants, and roadways. Ullah and Khan (2022) also suggest that the increasing urbanization and traffic density have increased soil concentrations of Pb, Zn, Cu, and Cd. In addition, Vlasov et al. (2022) propose that the soil samples taken from the side of the road in the Western Okrug of Moscow had W, Sb, Mo, Cu, Cd, Sn, Zn, and B concentrations that were above average and at a considerable pollution level. Li et al. (2007) determined that roadside soils in northwest China have substantially higher amounts of Zn, Cd, Hg, Pb, Cu, and Cr than park soils and earth values. Kara (2020) determined the sources and pollution state of trace and toxic elements in street dust in İzmir of Turkey. According to the findings, in street dust samples, potentially toxic elements such as Na, Ba, Cr, Pb, As, Y, Li, Cs, Sb, Dy, Er, Yb, W, Se, Tl, Th, and Ho come from important polluting sources such as traffic and residential heating.
The findings of this study indicate that similar concentrations of Fe, Cu, Ni, Cr, Zn, and Pb were detected in locations with high traffic density when the study area was compared to the literature (Table 5).
Soil enrichment factors
The enrichment factors EFsoil are frequently cited in the literature as evidence for the hypothesis that a specific set of elements has an anthropogenic origin (Sutherland, 2000). The heavy metals in the surface soil samples were classified into five groups based on the data collected. These categories are demonstrated in Table 2 and Fig. 3 with decreasing and increasing EF values.
The EF value of the soil samples from point N9 indicates high enrichment pollution for Zn (7.94). However, the EF values of the soil samples taken from locations N7 and N4 were determined to be at a moderate pollution level for Pb (4.06) and Cu (3.69). Furthermore, the EF values for Co, Cr, and Ni were minimally enriched (EF < 2). In addition, the EF values for Co, Cr, and Ni were determined as minimal enriched (EF < 2). Variations in EF values may result from the difference in input magnitude for each metal in the soil. When EF < 2, values of metal concentrations in soil may indicate weathering by natural processes. However, soil samples with an EF > 2 show that a significant part of the heavy metal originates from anthropogenic inputs (Hernandez et al., 2003). Therefore, soil samples with EF > 2 were calculated as a percentage. The anthropogenic metal ratios with the highest soil sampling values were found to be 79.01% for Zn (N9), 58.97% for Pb (N7), and 54.94% for Cu (N4). Results from the current study demonstrate that under these circumstances, the EF values of soil samples correspond with anthropogenic percentages. Broadly, it is possible to assume that the metals found in soil samples are derived from anthropogenic sources. Among these resources, high traffic, rapid industrialization, agricultural chemicals, and fertilizers can lead to the accumulation of heavy metals.
The Igeo is a tool that can be used, like the EF, to determine the level of soil pollution by heavy metals. In this study, the soil samples were collected from all sampling points and categorized as unpolluted areas according to the Müller (1979), who defined index parameters with negative geoaccumulation indexes for Fe, Mn, Cr, Ni, Al, Ti, and Co. For Zn (1.98) and Pb (1.19), the Igeo accumulation values of soil samples taken from N4, N9, and N10 locations were calculated. These findings categorize Zn as moderately polluted, while Pb has low to moderate pollution levels (Fig. 4).
Statistical analysis of the soil samples
Statistical analysis by SPSS 19 of the soil analysis results of samples taken under olive trees from different locations suggests that the metal distribution among the locations is not homogeneous. For this reason, statistical analysis tests were conducted by applying the Kruskal–Wallis test, a non-parametric test. These results showed significant differences in the trace metal concentrations of samples taken from different locations (p < 0.05). These differences among the groups are also supported by box plot graphs showing the median and scattering of metal concentrations of the trace elements (Fig. 2). These results reveal that metal concentrations in soil samples may vary depending on urban and environmental conditions.
Correlation between heavy metal concentrations
For trace element concentrations in soil samples that did not show normal distribution, Spearman correlation analysis was selected from non-parametric tests. The level of metals among relationship was determined.
The Spearman correlation analysis of surface soil samples indicated that Zn, Ti, Fe, Cu, Cr, Ni, and Zn are positively correlated (r = 0.797** for Zn and Pb, r = 0.809** for Ti and Fe, r = 0.655* for Cu and Cr, and r = 0.627* for Cu and Ni; p < 0.05 and p < 0.01, respectively). This shows that the heavy metals in the soils may have originated from the same reservoir. On the other hand, the surface soil data for the other trace metals Pb, Ni, Ti, and Mn show a negative and weak correlation (r = −0.046 for Pb and Ni, r = −0.027 for Ti and Pb, r = −0.109 for Mn and Ni; p > 0.05) which indicate that the heavy metals in surface soil may be different sources (Table 6).
Plant analysis
Chlorophyll content
In accordance with the control point, the total chlorophyll contents of olive leaves in urban areas were as follows: winter > autumn > summer > spring (Fig. 5). This can be explained by increased pigment with increasing leaf age (Brahmi et al., 2012). At the same time, chlorophyll content in urban site leaves diminished by 17–18% compared to the control point in spring.
Two mosses, Thuidium delicatulum (L.) Mitt., and Thuidium sparsifolium (Mitt.) Jaeg, as well as the leafy liverwort Ptychanthus striatus, were studied by Shakya et al. (2008) to determine how the heavy metals Cu, Zn, and Pb affected chlorophyll concentration. The author discovered that greater Cu concentrations significantly inhibited the total chlorophyll concentrations in leafy liverwort and these mosses. In other study, MacFarlane and Burchett (2001) examined six month-old Avicennia marina (Forsk) tree seedlings of the grey mangrove. They aimed to determine the impact of the heavy metals Cu, Zn, and Pb on the total chlorophyll concentration once the Cu concentration was 200 mg/g and the Zn concentration was 500 mg/g. They noticed a considerable decrease in the total chlorophyll levels measured in leaf tissues. However, the leaf total chlorophyll content did not change significantly due to the increasing lead concentration.
Chlorophyll concentrations may serve as reliable indicators of the presence of heavy metals associated with traffic in urban environments. The lowered pigment levels and the harmful effects of heavy metals harm plant cell membranes and reduce chlorophyll levels (Monni et al., 2001; Bačkor & Váczi, 2002). The findings of this investigation, represented in Fig. 5, indicate that changes in total chlorophyll in olive leaves that vary upon that time of year and the amount of traffic density have similar effects to those reported in the literature.
Elemental analysis in plants
Figure 6 shows the elemental analysis percentages of bioindicators monitored at regular intervals from urban to rural areas. TN, TC, TH, and TS concentrations in leaves ranged from 3.06 to 8.06%, 41.75–50.04%, 6.14–7.40%, and 0.09–0.0.26, respectively (Fig. 6). Comparing the TN, TC, TH, and TS concentrations between seasons, the highest and lowest values were determined in autumn and winter. The lowest TN and TC concentrations in leaves were found at N1 (3.06%) and N10 (%41.75), respectively, while the highest TS content was recorded as N1 (0.26%). On the other hand, the control data for the point N11 were measured as 4.10%, 46.64%, and 0.15% for TN, TC, and TS, respectively. This decrease in TN and TC concentrations can be explained by the seasonal variation of soil plant nutrients (Schulze et al., 1991). Moreover, the change in TS concentrations in autumn and winter may be associated with increased SO2 and NOx concentrations in the atmosphere due to traffic density and fossil fuel use (CSB, 2021).
Spearman correlation results for elemental analysis components revealed weak negative correlations between TN and TS (r = −0.300) and TC and TN (r = −0.236). In addition, it was observed that correlations between TC and TS (r = 0.491) and TC and TH (r = 800**) were positively weakly correlated. The weak and negative correlations between N and C suggest that plants used as biomonitors have mechanisms for storing TN and TC from several sources, such as leaf age, fertilization, traffic density, and industrial processes. For instance, passive stomatal uptake and cultural diffusion can frequently absorb N species with anthropogenic or agricultural origins (NO2, HNO3, NH4, NH3, and RO2NH2) into plant leaves (Baldantoni et al., 2014; Calanni et al., 1999; Gebauer & Schulze, 1991).
Xu et al. (2018) used Cinnamomum camphora leaves, twig bark, and bark as biomonitors to analyze the atmospheric N pollution in Guiyang (SW China). They concluded that urban N accumulation is mainly caused by traffic and heavy industry and that vascular plant leaves and bark can be used to pollute with N. Baldantoni et al. (2014) studied the levels of heavy metals and polycyclic aromatic hydrocarbons produced as a result of anthropogenic activities in industrial, urban, and rural environments. They observed that the lowest %N concentration was determined at the control area (1.27%), while the highest was found in the cement plant area (2.04%). The control and industrial areas’ carbon concentrations did not significantly differ from one another.
Trace metal monitoring in olive leaves can be an excellent tool for modeling atmospheric metal mapping. In the urban environment, trace elements can be distributed into the atmosphere from different anthropogenic sources, including the plastic industry, automobile workshops, and vehicle emissions. Therefore, in this study, trace element concentrations in plants were measured simultaneously in each season and locations (Fig. 7).
Nevertheless, Pb and Cd amounts were calculated in plant trace element analyses. Unfortunately, all data remained below the detection limit since Pb uptake in olives is stored in the roots. There is no significant accumulation of Cd due to its passive transport from the roots to the plant (Chaney, 1989; Madejón et al., 2006). In the comparison of the concentration of trace elements among location area, differences were observed. Differences were observed in comparing the concentration of trace elements between locations. The highest trace metal concentration was detected at N7 for Zn and Ti, at N4 for Fe and Al, at N1 for Ni and Cu, at N2 for Cr, and at N1 for Cu, while the lowest concentration was observed at the N11 control location for Fe, Al, Zn, Cu, Ti, Ni, and Cr (Fig. 7).
Different trace element concentrations were detected in the leaves during the year of study, depending on the season. The highest concentrations were typically determined to be Fe > Al > Zn > Mn > Cu > Ti > Ni > Cr in decreasing order for the autumn and winter (Fig. 8). Trace element concentrations in plant species also vary according to species, geological structure, environmental conditions, and soil structure (Marschner, 2011). Therefore, toxic and limiting values for metal concentrations in plant species are presented in Table 7.
The results from this study revealed that the samples from various locations with a high traffic density had trace element concentrations below the levels deemed toxic for plants. Results further revealed that the trace element concentrations of the samples taken from different areas with high traffic density were below the toxic values determined for plants. However, trace element concentrations in different areas with high traffic density revealed that Zn, Ni, Mn, Cr, Cu, Fe, Al, and Ti, were 1.77, 3.86, 1.29, 4.52, 8.13, 4.54, 2.41, and 5.17 times the background levels in control area leaves, respectively. Furthermore, the EF as a parameter to monitor the accumulation and concentration of trace elements, varied depending on soil structure and anthropogenic factors. The values for EFplant are displayed in Fig. 9.
According to Fig. 9, the highest EF values were determined for each element in different seasons. Moreover, Fe, Al, Ti, and Cr had significant enrichment values, whereas Ni, Cu, Zn, and Mn showed slow enrichment values; the EF limit value adopted was 2 (Mingorance et al., 2007). Additionally, these results were statistically examined using the Kruskal–Wallis test, one of the non-parametric tests. A significant difference at p < 0.05 was detected among trace element concentrations in different locations. The disparities between rural and urban and industrial areas reveal the consequences of anthropogenic pollution.
Basic elements of the earth’s crust include Fe, Al, and Mn. Fe is the fourth most common element in the earth’s crust; soil consists 1–5%. Fe is an essential element for the plant life cycle; however, when plants absorb excess amounts, it causes toxic effects by reducing carbon metabolism, enzyme activities, respiration, and photosynthetic efficiency (Onyango et al., 2018). Typically, soil contains Fe in the low-resolution form (Fe 3+). However, with improper irrigation procedures, soil characteristics change and in anaerobic conditions, Fe3+ in the soil solution is changed to Fe2+ and their levels rise. As a result of this rise, plant absorption quickens and hazardous amounts of excess Fe are stored in the leaves (Audebert & Fofana, 2009).
Another trace element that restricts plant growth and progress is Al. Root length, number, and nutritional requirements are all decreased in plants exposed to high levels of Al Barceló and Poschenrieder (1990). Additionally, in high concentrations, Al has toxic consequences for humans. Acute symptoms brought on by these high concentrations include vomiting, diarrhea, arthritic pain, mouth ulcers, skin ulcers, skin rashes, and nausea. Furthermore, long-term Al toxicity results in coordination loss, poor balance, and memory lapses (Krewski et al., 2007). Many human sources, such as industrial processes (producing building materials, paints, and metal alloys), corrosion of metal parts, and automobile emissions, can release large amounts of Al into the atmosphere (Polizzi et al, 2007). This research implies that Al concentration was 3.86 times higher than the control value and that anthropogenic factors influenced this condition.
One of the crucial nutrients necessary for plant growth and metabolism is Mn. It is essential for the biosynthesis of lignin, phenol, and photosynthesis, among many other processes (Graham & Webb, 1991). The human body needs Mn for skeletal growth and the metabolism of amino acids, lipids, and carbohydrates. If levels of Mn in the brain exceed dangerous levels, Parkinson’s-type illnesses may arise (Aschner, 2000). Several usage areas, including industrial activities, iron and steel production, alloys, battery cathodes, electronic devices, and chemicals for water treatment, are also applied (Calvo & Valero, 2022) Because of its presence in the geological earth crust, Mn in this study has progressive enrichment values. As seen from the control point, fewer anthropogenic effects could be seen among locations.
Zn is among the eight trace elements (Mn, Cu, B, Fe, Zn, Cr, Mo, and Ni) necessary for the healthy growth of plants. Plant roots absorb Zn as a divalent cation (Zn2+),and it is employed in a variety of processes, including seed production, protein synthesis, enzyme activation, and photosynthesis. Additionally, Zn, a vital micronutrient for humans, is utilized significantly, particularly in DNA synthesis, proteins, and other enzyme structures. For adults, the recommended daily consumption of Zn, essential for human health, should not exceed 40 mg. If higher amounts of Zn than this dose are consumed, acute side effects that may be experienced include nausea, vomiting, and appetite loss (Nriagu, 2019). Identifying the origins of Zn emissions is thus crucial in this condition. Zn metal plating alloys are found in a variety of industries, including the steel industry, cooling systems, battery manufacturing, the automobile industry, cosmetics, rubber, paint, and tire manufacturing, as well as the production of dry batteries, electrical equipment, and tires for cars and vehicles. Because of this broad usage of Zn, pollution from industry and traffic is increasing. In this study Zn values indicated slow enrichment However, high Zn concentrations found in urban environments and areas with significant traffic can be linked to anthropogenic sources.
Cr is not found among the nutrients in the plant; therefore, its uptake in plants does not occur by a special mechanism. High Cr concentrations have adverse effects on seed germination, root development, stem development, and leaf growth (Rout et al., 2000; Vajpayee et al., 2001). This transition metal, which has seven oxidation states, is most frequently found in its Cr3+ and Cr6+ forms. Cr3+, a naturally occurring element in rocks and soil, can be oxidized to form Cr6+. The group of trace metals that exhibit severe toxicity to humans includes Cr6+. Chromium is classified as carcinogenic and refers to the first group in the classification provided by the International Agency for Research on Cancer (IARC). Likewise, Cr6+ can damage DNA, chromosome breakage, proteins, lipids, and cellular lipids (Salnikow & Zhitkovich, 2008; DesMarias & Costa, 2019). As a result, it is crucial to monitor the levels of Cr in the environment. The metal plating industry, metal alloys, paint pigments, rubber, cement, paper, wood preservatives, and leather tanning are just a few examples of the environmental sources of Cr. The effects of anthropogenic sources of Cr increased 2.99 times compared to rural areas in this study due to traffic and urbanization, and Cr had considerable enrichment values. Breathing polluted air may result in the anthropogenic release of the hexavalent form of Cr into the environment; thus, monitoring this with a biomonitor is essential.
Ti, typically regarded as inert in the human organism, is the ninth most prevalent element in the earth’s crust. However, since the 1930s, its advantageous effects on plants have been recognized. Specifically, it might boost the amount of certain nutrients, enzymes, and chlorophyll in plant tissues (Hrubý et al., 2002). While TiO2 powder is inert to human metabolism, its extensive use has increased studies into its toxicity (Grande & Tucci, 2016). TiO2 nanoparticle toxicity has been reported in the literature in both cultured human cells and animal models (Kim et al., 2019). Cosmetics, paints, culinary items, medicines, and medical supplies are just a few industries that use titanium in their products (Skocaj et al., 2011). Ti is 2.88 times more abundant than the control region in this investigation and has a substantial enrichment value.
One of the crucial elements in plant physiology is Cu which plays a role in enzymes responsible for the cell wall, respiration, and the production of seeds. Cu is an essential element of plant nutrition, although deficiencies are caused at quantities of less than 5 mg/L and toxicities at levels higher than 20 mg/L. Cu is also used manufacturing f automobiles, water purification systems, pesticides, fertilizers, the energy industry, and many other industrial fields (Bradl, 2005). While plants, animals, and humans are not toxically affected by Cu intake from these sources within ordinary limits, high concentration intakes do occur. Due to the exposure to high concentrations, the early consequence of chronic Cu toxicity initiates in the liver and causes the onset of hepatic cirrhosis (Winge & Mehra, 1990). Consequently, monitoring trace elements in risky areas is essential, and biomonitors are an excellent indicator in this regard. In this study, high concentrations in cities with high traffic rates were considered under hazardous limits even though a slow enrichment factor for Cu was estimated, similarly to that for Zn.
One element that makes up the fundamental building blocks of plants and provides them the vital micronutrients is Ni. It is also found in the metal part of the urease enzyme, which is required for N metabolism in higher plants, and is particularly vital for plant growth. Additionally, Ni is related into the environmental from a broad range of sources due to its widespread usage in contemporary technologies like the production of heat or electricity, nickel mining, the manufacture of steel, and the metal industry. Nickel accumulation in the environment can severely affect human health, causing lung fibrosis, skin allergies, and various degrees of kidney and cardiovascular system poisoning (Denkhaus & Salkinow, 2002). This necessitates its follow-up, even when detected in low environmental concentrations. Based on this study, Ni enriched slowly and was not hazardous. However, given that its concentration was 3.86 times higher than the control point, it might have been affected by environmental conditions.
When the trace element concentrations of olive leaves collected from anthropogenic and natural sources are compared, results revealed that they have different degrees of accumulation, similar to that found in previous literature. For instance, Antoniadis et al. (2022) investigated the impact of environmental pollution trace using trace element concentrations in soil and leaves of olive trees grown in areas affected by silver/lead mining in Lavrio, Greece. According to their findings, Ni, Co, Zn, Pb, and Mn concentrations were higher in the silver/lead mine site in Lavrio, Greece, while the Cr, Fe, Cu, and Al concentrations were higher in the Artvin study area. In addition, Guarino et al. (2021) determined air and heavy metal pollution in different regions of Italy through Olea europaea L. According to the results, the average concentration values for Cr, Fe, and Cu were higher in the Artvin study area (Table 4).
Statistically analysis of plant samples
With the assistance of the Kruskal–Wallis test, trace element analysis of olive leaves collected during different seasons was analyzed statistically. For Mn, Cu, Fe, Al, Cr, and Ti concentrations among locations, a significant difference at the p < 0.05 level was detected. The relationship among the variables of the measurements taken for the plant sample was found using Spearman correlation (Table 8).
Results of the Spearman correlation analysis for the trace metals and assessed parameters are presented in Table 8. There was a significant positive correlation between Ti and Fe (r = 0.843, p < 0.01); Al and Ti (r = 0.815; p < 0.01), and Al and Fe (0.962; p < 0.01). These findings suggest that the trace metals in the plants may have been anthropogenic and/or derived from the earth’s crust. In literature studies, similar high correlation matrices for these trace elements in plants were observed (Turan et al., 2011; Varrica et al., 2022).
For the correlation coefficients between trace element and total chlorophyll content of the analyzed plant samples, a weak positive correlation was observed between Zn, Al, Cu, Fe, Ti, and chlorophyll. On the other hand, a significant negative weak correlation (r = −0.359*; p < 0.05) was found between Cr and chlorophyll and a significant and a significant positive weak correlation (r = 0.307*; p < 0.05) between Mn and chlorophyll was found. These findings indicate frequent weak negative correlations between metal species and chlorophyll compared to other literature research (Baycu et al., 2006; Chetia et al., 2021).
Precision and accuracy
The accuracy and precision results for the plant and soil CRM (Certificated Reference Material) are displayed in Table 9. The result of the accuracy and precision of soil analysis for CRM was in the range of 71.12–115.71%. The analysis of accuracy and precision of plants for CRM was calculated with values ranging from 87.41 to 123.95% (Table 9). These results show the measurement results with the reference material. For each metal in the CRM, the LOD values not within the quantitative limits are still reported at a certain confidence level. The smallest determination value within the quantitative limits in the linear operating range is given for the LOD.
SEM–EDS analysis of plant and soil
SEM images showed mainly inorganic amorphous or crystalline constituents. SEM–EDS analysis of particles adhered to the leaf surface revealed percentages of Ca 1.14%, Si 2.01%, K 1.38%, S 2.11%, and some potential toxic heavy metals such as Al 1.29%, Mg 0.51%, and Fe 1.18% (Fig. 10). On the other hand, SEM–EDS results of the samples taken from the control point were Al 2.52%, Si 10.18, and Ca 7.71% (Fig. 11).
Al, Si, Ca, Ni, Fe, and Pb are typically found among the trace elements produced by fossil fuels when analyzing SEM–EDS results regarding the chemical characteristics of the particles deposited on the leaves. Particles of anthropogenic origin are characterized by their spherical shape and smooth surfaces. They are typically formed as a result of combustion at high temperatures. On leaf surfaces, these particles can be found either singly or as a part of a clustered group (Tomašević et al., 2005).
Conclusion
The results indicated that depending on the element’s chemical properties, its proximity to anthropogenic sources and the morphological qualities of the leaves all affect the trace element concentrations deposited on the axial surfaces of the leaves.
First-line analyses of the study’s findings concluded that soil and plant samples taken from polluted environments had higher trace metal concentrations than samples taken from unpolluted environments. At the same time, trace metal analyses, elemental analyses, chlorophyll measurements, and SEM–EDS images support the results of anthropogenic contamination. Furthermore, the fossil fuel use, petroleum derivatives, and motor vehicle emissions from Artvin’s industrial and urban activities may be used to interpret the increase in these concentrations.
Monitoring the seasonal variations of trace metals using biomonitors is the second step in the analysis. The findings suggest that anthropogenic contamination is higher in autumn and winter than in spring and summer. This study also revealed the potential of olive tree leaves as biomonitors since they are evergreen It also suggests that olive tree leaves can be inexpensive, quick, and reliable indication of environmental pollution. However, the conditions that affect their potential as biomonitors necessitate research, specifically the urban climate, air circulation, and emission levels.
Data availability
Most of the datasets used in the study are freely available. The trace metal measures, elemental analysis, and SEM–EDS results are available from the corresponding author upon reasonable request.
References
Ali, M. U., Liu, G., Yousaf, B., Ullah, H., Abbas, Q., & Munir, M. A. M. (2019). A systematic review on global pollution status of particulate matter-associated potential toxic elements and health perspectives in urban environment. Environmental Geochemistry and Health, 41, 1131–1162.
Amusan, A. A., Bada, S. B., & Salami, A. T. (2003). Effect of traffic density on heavy metal content of soil and vegetation along roadsides in Osun state, Nigeria. West African Journal of Applied Ecology, 4(1).
Antoniadis, V., Thalassinos, G., Levizou, E., Wang, J., Wang, S. L., Shaheen, S. M., & Rinklebe, J. (2022). Hazardous enrichment of toxic elements in soils and olives in the urban zone of Lavrio, Greece, a legacy, millennia-old silver/lead mining area and related health risk assessment. Journal of Hazardous Materials, 434, 128906.
Aschner, M. (2000). Manganese: Brain transport and emerging research needs. Environmental Health Perspectives, 108(suppl 3), 429–432.
Audebert, A., & Fofana, M. (2009). Rice yield gap due to iron toxicity in West Africa. Journal of Agronomy and Crop Science, 195(1), 66–76.
Bačkor, M., & Váczi, P. (2002). Copper tolerance in the lichen photobiont Trebouxia erici (Chlorophyta). Environmental and Experimental Botany, 48(1), 11–20.
Baldantoni, D., De Nicola, F., & Alfani, A. (2014). Air biomonitoring of heavy metals and polycyclic aromatic hydrocarbons near a cement plant. Atmospheric Pollution Research, 5(2), 262–269.
Bandowe, B. A. M., Nkansah, M. A., Leimer, S., Fischer, D., Lammel, G., & Han, Y. (2019). Chemical (C, N, S, black carbon, soot and char) and stable carbon isotope composition of street dusts from a major West African metropolis: Implications for source apportionment and exposure. Science of the Total Environment, 655, 1468–1478.
Barceló, J. U. A. N., & Poschenrieder, C. (1990). Plant water relations as affected by heavy metal stress: A review. Journal of Plant Nutrition, 13(1), 1–37.
Baycu, G., Tolunay, D., Özden, H., & Günebakan, S. (2006). Ecophysiological and seasonal variations in Cd, Pb, Zn, and Ni concentrations in the leaves of urban deciduous trees in Istanbul. Environmental Pollution, 143(3), 545–554.
Bhuiyan, M. A., Parvez, L., Islam, M. A., Dampare, S. B., & Suzuki, S. (2010). Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. Journal of Hazardous Materials, 173(1–3), 384–392.
Bingham, E., Cohrssen, B., & Powell, C. H. (2001). Toxicological issues related to metals: Neurotoxicology and radiation metals and metal compounds. Wiley-Interscience.
Bondada, B. R., Tu, S., & Ma, L. Q. (2004). Absorption of foliar-applied arsenic by the arsenic hyperaccumulating fern (Pteris vittata L.). Science of the total environment, 332(1–3), 61–70.
Bourennane, H., Douay, F., Sterckeman, T., Villanneau, E., Ciesielski, H., King, D., & Baize, D. (2010). Mapping of anthropogenic trace elements inputs in agricultural topsoil from Northern France using enrichment factors. Geoderma, 157(3–4), 165–174.
Bouyoucos, G. J. (1962). Hydrometer method improved for making particle size analyses of soils 1. Agronomy Journal, 54(5), 464–465.
Bradl, H. (Ed.). (2005). Heavy metals in the environment: origin, interaction and remediation. Elsevier.
Brahmi, F., Mechri, B., Dabbou, S., Dhibi, M., & Hammami, M. (2012). The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Industrial Crops and Products, 38, 146–152.
Briffa, J., Sinagra, E., & Blundell, R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon, 6(9), e04691.
Brumsack, H. J. (2006). The trace metal content of recent organic carbon-rich sediments: Implications for Cretaceous black shale formation. Palaeogeography, Palaeoclimatology, Palaeoecology, 232(2–4), 344–361.
Calanni, J., Berg, E., Wood, M., Mangis, D., Boyce, R., Weathers, W., & Sievering, H. (1999). Atmospheric nitrogen deposition at a conifer forest: Response of free amino acids in Engelmann spruce needles. Environmental Pollution, 105(1), 79–89.
Calvo, G., & Valero, A. (2022). Strategic mineral resources: Availability and future estimations for the renewable energy sector. Environmental Development, 41, 100640.
Chaney, R. L. (1989). Toxic element accumulation in soils and crops: Protecting soil fertility and agricultural food-chains. In Inorganic contaminants in the vadose zone (pp. 140–158). Springer Berlin Heidelberg.
Chetia, J., Gogoi, N., Gogoi, R., & Yasmin, F. (2021). Impact of heavy metals on physiological health of lichens growing in differently polluted areas of central Assam, North East India. Plant Physiology Reports, 26, 210–219.
CSB. (2021). Çevre ve İklim Değişikliği bakanlığı, Artvin ili 2021 yılı çevre durum raporu. Retrieved May 11, 2022, from https://webdosya.csb.gov.tr/db/ced/icerikler/artv-n_-cdr2021-20221104090217.pdf
Davis, J. A. (1984). Complexation of trace metals by adsorbed natural organic matter. Geochimica Et Cosmochimica Acta, 48(4), 679–691.
Denkhaus, E., & Salnikow, K. (2002). Nickel essentiality, toxicity, and carcinogenicity. Critical Reviews in Oncology/hematology, 42(1), 35–56.
DesMarias, T. L., & Costa, M. (2019). Mechanisms of chromium-induced toxicity. Current Opinion in Toxicology, 14, 1–7.
Fernandes, B. C. C., Mendes, K. F., Tornisielo, V. L., Teófilo, T. M. S., Takeshita, V., das Chagas, P. S. F., & Silva, D. V. (2021). Effect of pyrolysis temperature on eucalyptus wood residues biochar on availability and transport of hexazinone in soil. International Journal of Environmental Science and Technology, 1–16.
Galal, T. M., & Shehata, H. S. (2015). Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecological Indicators, 48, 244–251.
Gebauer, G., & Schulze, E. D. (1991). Carbon and nitrogen isotope ratios in different compartments of a healthy and a declining Picea abies forest in the Fichtelgebirge, NE Bavaria. Oecologia, 87, 198–207.
Ghrefat, H. A., Abu-Rukah, Y., & Rosen, M. A. (2011). Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Kafrain Dam, Jordan. Environmental Monitoring and Assessment, 178, 95–109.
Graham, R. D., & Webb, M. J. (1991). Micronutrients and disease resistance and tolerance in plants. Micronutrients in Agriculture, 4, 329–370.
Grande, F., & Tucci, P. (2016). Titanium dioxide nanoparticles: A risk for human health? Mini Reviews in Medicinal Chemistry, 16(9), 762–769.
Guarino, F., Improta, G., Triassi, M., Castiglione, S., & Cicatelli, A. (2021). Air quality biomonitoring through Olea europaea L.: The study case of “Land of pyres”. Chemosphere, 282, 131052.
Hernandez, L., Probst, A., Probst, J. L., & Ulrich, E. (2003). Heavy metal distribution in some French forest soils: Evidence for atmospheric contamination. Science of the Total Environment, 312(1–3), 195–219.
Hrubý, M., Cígler, P., & Kuzel, S. (2002). Contribution to understanding the mechanism of titanium action in plant. Journal of Plant Nutrition, 25(3), 577–598.
Hu, B., Cui, R., Li, J., Wei, H., Zhao, J., Bai, F., & Ding, X. (2013). Occurrence and distribution of heavy metals in surface sediments of the Changhua River Estuary and adjacent shelf (Hainan Island). Marine Pollution Bulletin, 76(1–2), 400–405.
Jung, M. C. (2008). Heavy metal concentrations in soils and factors affecting metal uptake by plants in the vicinity of a Korean Cu-W mine. Sensors, 8(4), 2413–2423.
Kabata-Pendias, A. (2000). Trace elements in soils and plants. CRC Press.
Kadem, D. E. D., Rached, O., Krika, A., & Gheribi-Aoulmi, Z. (2004). Statistical analysis of vegetation incidence on contamination of soils by heavy metals (Pb, Ni and Zn) in the vicinity of an iron steel industrial plant in Algeria. Environmetrics, 15(5), 447–462.
Kara, M. (2020). Assessment of sources and pollution state of trace and toxic elements in street dust in a metropolitan city. Environmental Geochemistry and Health, 42, 3213–3229.
KGM. (2021). 2021 Karayolları Trafik Hacim Haritaları https://www.kgm.gov.tr/Sayfalar/KGM/SiteTr/Trafik/TrafikHacimHaritasi.aspx
Kim, K. T., Eo, M. Y., Nguyen, T. T. H., & Kim, S. M. (2019). General review of titanium toxicity. International Journal of Implant Dentistry, 5(1), 1–12.
Krewski, D., Yokel, R. A., Nieboer, E., Borchelt, D., Cohen, J., Harry, J., & Rondeau, V. (2007). Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. Journal of Toxicology and Environmental Health, Part B, 10(S1), 1–269.
Leopold, K., Wörle, K., Schindl, R., Huber, L., Maier, M., & Schuster, M. (2017). Determination of traffic-related palladium in tunnel dust and roadside soil. Science of the Total Environment, 583, 169–175.
Li, F. R., Kang, L. F., Gao, X. Q., Hua, W., Yang, F. W., & Hei, W. L. (2007). Traffic-related heavy metal accumulation in soils and plants in Northwest China. Soil & Sediment Contamination, 16(5), 473–484.
MacFarlane, G. R., & Burchett, M. D. (2001). Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the Grey mangrove, Avicennia marina (Forsk.) Vierh. Marine pollution bulletin, 42(3), 233–240.
Madejón, P., Marañón, T., & Murillo, J. M. (2006). Biomonitoring of trace elements in the leaves and fruits of wild olive and holm oak trees. Science of the Total Environment, 355(1–3), 187–203.
Malkoc, S., Yazıcı, B., & Savas Koparal, A. (2010). Assessment of the levels of heavy metal pollution in roadside soils of Eskisehir. Turkey. Environmental Toxicology and Chemistry, 29(12), 2720–2725.
Marschner, H. (Ed.). (2011). Marschner’s mineral nutrition of higher plants. Academic press.
MGM. (2021). İllerimize Ait İstatiski Veriler. https://www.mgm.gov.tr/veridegerlendirme/il-ve-ilceler-istatistik.aspx?k=A&m=ARTVIN
Mingorance, M. D., Valdés, B., & Oliva, S. R. (2007). Strategies of heavy metal uptake by plants growing under industrial emissions. Environment International, 33(4), 514–520.
Monni, S., Uhlig, C., Hansen, E., & Magel, E. (2001). Ecophysiological responses of Empetrum nigrum to heavy metal pollution. Environmental Pollution, 112(2), 121–129.
Montoro-Leal, P., García-Mesa, J. C., Cordero, M. S., Guerrero, M. L., & Alonso, E. V. (2020). Magnetic dispersive solid phase extraction for simultaneous enrichment of cadmium and lead in environmental water samples. Microchemical Journal, 155, 104796.
Muller, G. (1979). Schwermettalle in den sedimenten des Rheins-Veranderungen seit 1971. Umschau Wissensch Tech., 79, 778–783.
Nriagu, J. O. (2019). Encyclopedia of environmental health. Elsevier. Second edition), Elsevier, Oxford (2019), 500–508.
Oliva, S. R., & Espinosa, A. F. (2007). Monitoring of heavy metals in topsoils, atmospheric particles and plant leaves to identify possible contamination sources. Microchemical Journal, 86(1), 131–139.
Onyango, D. A., Entila, F., Dida, M. M., Ismail, A. M., & Drame, K. N. (2018). Mechanistic understanding of iron toxicity tolerance in contrasting rice varieties from Africa: 1. Morpho-physiological and biochemical responses. Functional Plant Biology, 46(1), 93–105.
Orak, N. H., & Ozdemir, O. (2021). The impacts of COVID-19 lockdown on PM10 and SO2 concentrations and association with human mobility across Turkey. Environmental Research, 197, 111018.
Osuji, L. C., & Onojake, C. M. (2004). Trace heavy metals associated with crude oil: A case study of Ebocha-8 oil-spill-polluted site in Niger Delta. Nigeria. Chemistry & Biodiversity, 1(11), 1708–1715.
Polizzi, S., Ferrara, M., Bugiani, M., Barbero, D., & Baccolo, T. (2007). Aluminium and iron air pollution near an iron casting and aluminium foundry in Turin district (Italy). Journal of Inorganic Biochemistry, 101(9), 1339–1343.
Rout, G. R., Samantaray, S., & Das, P. (2000). Effects of chromium and nickel on germination and growth in tolerant and non-tolerant populations of Echinochloa colona (L.) Link. Chemosphere, 40(8), 855–859.
Salnikow, K., & Zhitkovich, A. (2008). Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: Nickel, arsenic, and chromium. Chemical Research in Toxicology, 21(1), 28–44.
Sawidis, T., Breuste, J., Mitrovic, M., Pavlovic, P., & Tsigaridas, K. (2011). Trees as bioindicator of heavy metal pollution in three European cities. Environmental Pollution, 159(12), 3560–3570.
Schiff, K. C., & Weisberg, S. B. (1999). Iron as a reference element for determining trace metal enrichment in Southern California coastal shelf sediments. Marine Environmental Research, 48(2), 161–176.
Schulze, E. D., Gebauer, G., Ziegler, H., & Lange, O. L. (1991). Estimates of nitrogen fixation by trees on an aridity gradient in Namibia. Oecologia, 88, 451–455.
Shakya, K., Chettri, M. K., & Sawidis, T. (2008). Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Archives of Environmental Contamination and Toxicology, 54, 412–421.
Singh, S., Maiti, S. K., & Raj, D. (2023). An approach to quantify heavy metals and their source apportionment in coal mine soil: A study through PMF model. Environmental Monitoring and Assessment, 195(2), 306.
Skocaj, M., Filipic, M., Petkovic, J., & Novak, S. (2011). Titanium dioxide in our everyday life; is it safe? Radiology and Oncology, 45(4), 227–247.
Sosa, D., Hilber, I., Buerge-Weirich, D., Faure, R., Escobar, A., & Bucheli, T. D. (2022). Heavy metals in soils of Mayabeque, Cuba: Multifaceted and hardly discernable contributions from pedogenic and anthropogenic sources. Environmental Monitoring and Assessment, 194(6), 441.
Sutherland, R. A. (2000). Bed sediment-associated trace metals in an urban stream, Oahu. Hawaii. Environmental Geology, 39, 611–627.
Stevenson, A., Hartemink, A. E., & Zhang, Y. (2023). Measuring sand content using sedimentation, spectroscopy, and laser diffraction. Geoderma, 429, 116268.
Szczepaniak, K., & Biziuk, M. (2003). Aspects of the biomonitoring studies using mosses and lichens as indicators of metal pollution. Environmental Research, 93(3), 221–230.
Tomašević, M., Vukmirović, Z., Rajšić, S., Tasić, M., & Stevanović, B. (2005). Characterization of trace metal particles deposited on some deciduous tree leaves in an urban area. Chemosphere, 61(6), 753–760.
TUİK. (2022). Adrese Dayalin Nufus Kayit SistemiSonucları. https://data.tuik.gov.tr/Bulten/Index?p=Adrese-Dayali-Nufus-Kayit-Sistemi-Sonuclari-2022-49685
Turan, D., Kocahakimoglu, C., Kavcar, P., Gaygısız, H., Atatanir, L., Turgut, C., & Sofuoglu, S. C. (2011). The use of olive tree (Olea europaea L.) leaves as a bioindicator for environmental pollution in the Province of Aydın. Turkey. Environmental Science and Pollution Research, 18, 355–364.
Ullah, R., & Khan, N. (2022). Xanthium strumarium L. an alien invasive species in Khyber Pakhtunkhwa (Pakistan): A tool for biomonitoring and environmental risk assessment of heavy metal pollutants. Arabian Journal for Science and Engineering, 47(1), 255–267.
USDA. (1951). Soil survey manual. Agricultural handbook No. 18. Washington DC.
US-EPA. (2007). Method 3051A (SW-846): Microwave assisted acid digestion of sediments, sludges, and oils, Revision 1. U.S. Environmental Protection Agency, Washington. https://www.epa.gov/sites/default/files/2015-12/documents/3051a.pdf. Accessed 29 March 2022.
Vajpayee, P., Rai, U. N., Ali, M. B., Tripathi, R. D., Yadav, V., Sinha, S., & Singh, S. N. (2001). Chromium-induced physiologic changes in Vallisneria spiralis L. and its role in phytoremediation of tannery effluent. Bulletin of Environmental Contamination and toxicology, 67(2), 246.
Varrica, D., Lo Medico, F., & Alaimo, M. G. (2022). Air quality assessment by the determination of trace elements in lichens (Xanthoria calcicola) in an industrial area (Sicily, Italy). International Journal of Environmental Research and Public Health, 19(15), 9746.
Vlasov, D. V., Kukushkina, O. V., Kosheleva, N. E., & Kasimov, N. S. (2022). Levels and factors of the accumulation of metals and metalloids in roadside soils, road dust, and their PM10 fraction in the Western okrug of Moscow. Eurasian Soil Science, 55(5), 556–572.
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica Et Cosmochimica Acta, 59(7), 1217–1232.
Winge, D. R., & Mehra, R. K. (1990). Host defenses against copper toxicity. International Review of Experimental Pathology, 31, 47–83.
Xu, Y., Xiao, H., Guan, H., & Long, C. (2018). Monitoring atmospheric nitrogen pollution in Guiyang (SW China) by contrasting use of Cinnamomum Camphora leaves, branch bark and bark as biomonitors. Environmental Pollution, 233, 1037–1048.
Acknowledgements
I would like to thank Dr. Halil Akıncı for his support in the mapping studies.
Author information
Authors and Affiliations
Contributions
Determination of the method, laboratory studies, evaluation of analysis results, article writing, and all other processes within this scope were performed by the author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The author has read and understood and has complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and is aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Konanç, M.U. Monitoring trace element concentrations with environmentally friendly biomonitors in Artvin, Turkey. Environ Monit Assess 195, 1001 (2023). https://doi.org/10.1007/s10661-023-11587-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11587-x