Abstract
In this study, four different plant species, namely Artocarpus heterophyllus, Mangifera indica, Psidium guajava, and Swietenia mahagoni, were selected from seven different locations to assess the feasibility of using them as a cost-effective alternative for biomonitoring air quality. Atmospheric coarse particulate matter (PM10), soil samples, and leaf samples were collected from residential, industrial, and traffic-congested sites located in the greater Dhaka region. The heavy metal concentrations (Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn) in the leaves of the different species, PM10, and soil samples were analyzed. The highest Pb (718 ng/m3) and Zn (15,956 ng/m3) concentrations were found in PM10 of Kodomtoli which is an industrial area. On the other hand, the highest Fe (6,152 ng/m3) and Ni (61.1 ng/m3) concentrations were recorded in the PM10 of Gabtoli, a heavy-traffic area. A significant positive correlation (r = 0.74; p < 0.01) between Pb content in plant leaves and PM fraction was found which indicated that atmospheric PM-bound Pb may contribute to the uptake of Pb by plant leaves. The analysis of the enrichment factor (EF) revealed that soils were contaminated with Cd, Ni, Pb, and Zn. The abaxial leaf surfaces of Psidium guajava growing at the polluted site exhibited up to a 40% decrease in stomatal pores compared to the control site. Saet’s summary index (Zc) demonstrated that Mangifera indica had the highest bioaccumulation capacity. The metal accumulation index (MAI) was also evaluated to assess the overall metal accumulation capacity of the selected plants. Of the four species, Swietenia mahagoni (3.05) exhibited the highest MAI value followed by Mangifera indica (2.97). Mangifera indica and Swietenia mahagoni were also found to accumulate high concentrations of Pb and Cr in their leaves and are deemed to be good candidates to biomonitor Pb and Cr contents in ambient air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is the greatest external threat to human life expectancy on the planet. Outdoor air pollution causes mortality of 4.2 million annually, with over 2 million of those fatalities occurring in South-East Asia (Bulto et al., 2017). Due to the presence of several contaminants, particulate matter (PM) in the atmosphere is of great concern for public health. Furthermore, it facilitates the amalgamation of potentially toxic elements (PTEs), including but not limited to cadmium (Cd), chromium (Cr), iron (Fe), lead (Pb), nickel (Ni), and zinc (Zn). These elements pose significant risks to human well-being, particularly in relation to neurological functioning and the cardiovascular, respiratory, and dermatological systems (Apte et al., 2018; Pope et al., 2004).
It is obvious that unrestrained industrial growth and persistent disregard for laws meant to preserve the quality of the air are the main causes of Dhaka’s quick rise to rank among the most polluted metropolitan areas in the world (Gulshan et al., 2021; Moniruzzaman et al., 2022). Exposure to air pollution caused the premature death of about 24,000 people in Dhaka between 2005 and 2018, the highest among the 46 cities studied (Vohra et al., 2022). Monitoring urban air pollution is a vital research area in discovering existing risks and formulating new planning decisions. It is imperative to develop a sufficient monitoring system that will enable accurate evaluations of the pollution of the environment due to the presence of various contaminants in the air. Numerous studies have successfully shown that different plant species can monitor airborne heavy metals (HMs) contamination as effectively as high-tech instrumentation methods (Baldantoni et al., 2014; Chiarantini et al., 2016; Shahrukh et al., 2023).
Plants are widely regarded as key bioindicators of pollution due to their continuous interaction with all environmental components (Schreck et al., 2020). Trees can filter 60 to 70 times more air pollutants compared to herbs and shrubs (McPherson et al., 1994). Plant parts, particularly leaves, trap and accumulate atmospheric pollutants. According to Fernández and Brown (2013), HMs can penetrate the leaves through a variety of structures, including stomatal pores, cuticular crevices, epidermal cell walls, aqueous pores, and lenticels. PM deposition on plant leaves is influenced by leaf properties such as shape and surface area of leaves, size, arrangement of petiole, and cuticular texture (Beckett et al., 2000; Dzierżanowski et al., 2011). The mechanisms of foliar transfer in plant tissues have been proposed by several researchers (Maisto et al., 2004; Sexton and Roberts, 1982). Heavy metal entry through the cuticle of a leaf consists mostly of four phases (Chamel et al., 1991), namely (1) adhesion to the cuticle, (2) penetration through the cuticle (perhaps via endocytosis), (3) desorption in the apoplast, and (4) absorption by the adjacent cells. Birbaum et al. (2010) reported that coarse particles are adhered to surface wax and diffused into the leaves, while fine particles are taken up by the leaves. Diffusion of HMs in plants is influenced by several variables, including cuticle maturity, leaf shape, and surface area, physicochemical forms of the adsorbed metal, surface texture of the leaves, exposure time to the metal, and environmental factors like gas exchange (Beckett et al., 2000; Nair et al., 2010). Kozlov et al. (2000) obtained a linear relationship between HM concentration in leaves and HMs in the atmosphere, suggesting that the uptake of HMs by foliage may be dose dependent. As various metals accumulate on tree leaves simultaneously, the overall metal accumulation on the leaves of the studied tree species is likely to provide valuable information vis-à-vis their potential as bioindicators (Shahrukh et al., 2023). Consequently, the metal accumulation index (MAI) can be employed to evaluate the overall efficiency of heavy metal accumulation in plants (Hu et al., 2014; Liu et al., 2007; Monfared et al., 2013).
Biomonitoring is an inexpensive and environmentally benign technology that can substitute for expensive and aggressive physical and chemical analytical methods generally used in air pollution monitoring systems (Jashim et al., 2021; Kulkarni et al., 2014). Many higher plants have been employed as biomonitors for evaluating the quality of the environment with respect to pollution from PTEs (Alahabadi et al., 2017; Bilo et al., 2017; Madejón et al., 2013; Majolagbe et al., 2010; Mataruga et al., 2020; Mohammadi et al., 2018; Ozturk et al., 2017; Piczak et al., 2003; Popek et al., 2017). The advantages of utilizing trees as biomonitors include the ease of collection and identification of biological materials, the profusion of biological materials for analysis, and the presence of high biomass for PTE accumulation (Bilo et al., 2017; Sawidis et al., 2011). The danger associated with exposure cannot be sufficiently determined by physical or chemical means, whereas biological techniques directly evaluate exposure risk (Calzoni et al., 2007). The broader goal of this study was to evaluate the performance of some tropical common tree species in urban settings in biomonitoring the concentration of heavy metals in the air. We aimed to identify the metal accumulator higher plants whose metal concentration can best represent the HM concentration in the ambient air. The specific objectives were (i) to determine the concentration of potentially toxic elements (PTEs) in PM10, soil samples, and leaves of four different plant species, (ii) to observe if there are any linear relationships between HM concentration of PM10 of ambient air and that of plant samples, (iii) to compare the stomata pore of epidermis of leaves collected from the control and polluted sites, and (iv) to assess the metal accumulating capacity of the selected plant species using metal accumulation index (MAI).
Materials and methods
Study area
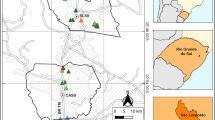
Dhaka, the main study area of this investigation, is the largest city in Bangladesh and home to 21.74 million people (Shahrukh et al., 2023). Numerous motor vehicles, including both public and private transport, ply the roads in the city and serve as a source of air pollutants (Ahmed et al., 2012; Nargis et al., 2022; Rahman et al., 2019). To make the matters worse, the construction of buildings and roads is a common sight in the city (Islam et al., 2015; Rahman et al., 2021). The sampling locations were selected to include traffic-congested sites, industrial sites, and residential sites (Fig. 1). Modonpur, Narayanganj (site 2) and Kodomtoli, Dhaka (site 3) were surrounded by a variety of enterprises. Thus, these two places were categorized as industrial areas. Due to severe traffic on the neighboring roads, Farmgate (site 6) and Gabtoli (site 7) in Dhaka were regarded as traffic-congested areas. Both Doyel Chattar, Shahbag (site 4), and Pallabi, Mirpur (site 5) were chosen as semi-residential and residential areas, respectively. A remote location with limited pollution sources, Hashadah, Chuadanga (site 1) was selected as a control and reference site.
Sampling
PM and soil samples were collected from seven different locations in three replicates. Four different plant species were also selected from those seven locations. For each species, leaf samples were collected from three separate plants. Therefore, a total of 21 PM samples, 21 soil samples, and 84 leaf samples were collected for analysis.
PM sampling
PM samples were collected by a respirable PM10 sampler (model TEI -108 NL) on a 24-h basis. The sampler utilizes inbuilt cyclone separator technology to gather PM from the surrounding air. Particles from 10 µ down to 0.1 µ are deposited on the filter paper mounted on the filter paper holder. The particles collected from the filter paper were further used to analyze the concentration of PM in the ambient air. From the difference between the mass of the empty and loaded filter paper, the mass of the collected particles was measured. After measurement, the filter papers were stored in a desiccator at room temperature.
Leaf sampling
Fully expanded leaves of Artocarpus heterophyllus, Mangifera indica, Psidium guajava, and Swietenia mahagoni were collected from the selected locations. The collected leaves were washed thoroughly with DI water and air-dried. The air-dried leaves were further dried in an oven for 5 min at 105 °C followed by drying at 70 °C for 48 h until they reached a constant weight (Kachenko & Singh, 2006; Zhang et al., 2014). Following weighing, the desiccated leaves were ground in a grinder.
Soil sampling
From each sampling site, soil samples were collected at a depth ranging from 0 to 15 cm using an auger. The collected soil was kept in zip-lock bags and marked properly. Each of the collected soil samples was dried in the air for 7 days. After crushing the aggregates, a portion of the ground samples were subjected to screening, passing through a sieve with a 2-mm aperture and mixed thoroughly. The soil samples were preserved in plastic containers with appropriate labeling.
Morphological analysis of PM
The morphological study of airborne particles was conducted using a scanning electron microscope (SEM) (model JEOL, JSM 6490LA), as depicted in Fig. 2. Double-sided adhesive carbon tape was used to mount 1 mm2 dry and particle-loaded filters on aluminum stubs. Samples were examined and photographed at different magnifications on a stereo SEM with 30 tilt and 10 kV. Four photos at different magnifications were captured for each sample to highlight the morphological features.
The National Institute of Health in Maryland’s ImageJ program was used to analyze the particles. Calculations were made for morphological characteristics such as count, surface area, aspect ratio (AR), circularity, equivalent spherical diameter (ESD), roundness, and solidity.
Measurement of stomatal pore size
Collection of stomatal imprints (nail varnish method)
The stomatal aperture can be measured using the microscope imaging of epidermal imprints of leaf samples by the nail varnish method (Wu & Zhao, 2017). Transparent nail varnish was painted in two places covering an area of about 2 cm2 on the underside of a leaf. It was then allowed to dry out thoroughly for about 5 min over the leaf to avoid denaturing of the imprint. A piece of clear sticky cellophane tape was placed over the painted area to remove the imprint. The cellophane tape was then fully pressed and peeled off. When the sticky tape was peeled off it brought the varnish which had an imprint of the surface of the leaf. It was then taped to a microscope slide, labeled, and taken to the laboratory for microscopic observation. To ensure the validity of this experiment, the test was carried out on leaves with different maturities. Samples of the stomatal pore imprints were taken at the seven sites in question.
Measurement of area of stomatal pore
The imprints were observed using a microscope at a magnification of × 40 (Fig. 2). The area of the stomatal pore was then measured in µm using the ImageJ software. Ten stomatal measurements were recorded per strip attached to the microscopic slide within an hour of strip preparation. Five leaves from each sample location were observed. The image processing program ImageJ was used to analyze these pictures (Rasband, 1997). All data were recorded, and the mean values of pore size were calculated.
Heavy metal analysis
An atomic absorption spectrometer (AAS) (model VARIAN AA-240) was used to identify the HMs in the samples. Soil and plant samples under study were first digested using the wet digestion method (Estefan et al., 2013). Briefly, 1 g of the sample was taken into a 100-mL volumetric flask, and 10 mL of HNO3 was added. The solution was allowed to stand for a few hours before being carefully heated over a sand bath until the brown fumes from the flask ceased completely. After allowing the flask to cool to ambient temperature, 10 mL of perchloric acid was added, and the flask was heated until it was almost dry. After that, it was filtered through a Whatman 42 filter paper, and 100 mL of DI water was added to make up the volume. The PM10 samples were digested with aqua regia (1HNO3:3HCl) (v/v) following Peña-Icart et al. (2011).
Enrichment factor
A common way to study environmental pollution is to look at the enrichment factor (EF) for metal concentration compared to uncontaminated background or reference levels (Callen et al., 2009). By convention, a trace element in PM with an average EF value of 10 comes from the crust, while an EF value greater than 10 means that a large part of the element comes from somewhere other than the crust (Chester et al., 1999). As a reference element, Fe was used to calculate the EF for Dhaka’s urban aerosol with respect to the composition of the earth’s crust.
Enrichment factor can be calculated with the following relationship.
Geoaccumulation index
The geoaccumulation index (Igeo), proposed by Muller (1969), assesses the degree of pollution by comparing the heavy metal contents in the collected samples to the background values (upper continental crust or local soil). The index is determined using the following equation (Khuzestani & Souri, 2013).
Here, Cn represents the concentration of the heavy metals in the soil that was measured. Bn represents the background concentration (upper continental crust) of heavy metal as stated by Wedepohl (1995). A multiplier of 1.5 was employed due to the reduction in background value fluctuations, which can be related to lithogenic differences in the samples.
Contamination factor
The contamination factor (CF) is determined by dividing the concentration of each heavy metal found in soil by the concentration of the same heavy metals found in the background. The calculation of CF was performed using the following equation.
Cn represents the concentration of heavy metals present in the analyzed soil, while Bn represents the background value of heavy metals (Wedepohl, 1995). The categorization of CF is defined as follows: CF < 1 is classified as ‘low contamination’, 1 < CF < 3 is classified as ‘moderate contamination’, 3 < CF < 6 is classified as ‘considerable contamination’, and CF > 6 is classified as ‘very high contamination’ (Gope et al., 2017).
Pollution load index
To evaluate the quality of soil vis-à-vis the concentration of hazardous elements, an integrated method called the pollution load index (PLI) of the eight hazardous elements was employed, as described by Tomlinson et al. (1980). Assigned to the nth root of the multiplication of the contamination factor of metals, the PLI was calculated. A PLI of 1 signifies the presence of baseline levels of pollutants, while a PLI greater than 1 indicates a decline in the quality of the site (Gope et al., 2017).
Metal accumulation index (MAI)
The overall effectiveness of heavy metal accumulation in the plants was evaluated using the metal accumulation index.
\({I}_{j}\) is the sub-index for variable j, and N is the total number of metals examined. To further define \({I}_{j}\), consider the following: \(x\) is the mean concentration of elements, and \(\delta x\) is its standard deviation (El-Amier et al., 2018; Liu et al., 2007).
Saet’s summary index
Saet’s summary index also known as the total pollution index (Zc) was used as criteria for assessing the hazard classified by the hazard degree (Saet & Smirnova, 1983). It is calculated by the formula:

where
 is the correlation coefficient for each element. Kki = Ci/Cib, where Cib is the background concentration (concentration of heavy metals from the control site), Ci is the actual concentration of the i-th element in plant leaves, and n is the number of elements analyzed.
is the correlation coefficient for each element. Kki = Ci/Cib, where Cib is the background concentration (concentration of heavy metals from the control site), Ci is the actual concentration of the i-th element in plant leaves, and n is the number of elements analyzed.
The following critical values enable the total contamination Zc to be characterized by the degree of hazard. For Zc < 16, the contamination is deemed non-toxic; Zc < 32 is considered moderately hazardous; Zc < 128 is considered hazardous; and Zc > 128 is regarded as extremely hazardous (Vodyanitskii, 2010).
Quality assurance and quality control (QA/QC)
To reduce contamination, precautions were taken at every stage of sample collection. The sampler was maintained on a regular basis to ensure that it produced reliable readings. Each filter paper was desiccated and weighed before and after sampling with an analytical microbalance (Sartorius, ME5-F) with a sensitivity of 1 mg. Three plants from each species and nearly 30 leaves from each plant from different branches and heights were collected for homogenization of all leaf samples. Triplicate analyses were performed to verify the repeatability of the leaf sample digestion procedure. Table 1 provides the minimum detection limits of the AAS for the examined elements.
Statistical analysis
Microsoft Excel 2021 and Minitab statistical software (version 21) were used for statistical analysis. For every sample, triplicate analyses were conducted. The concentrations of HMs (Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn) in PM10, soil, and leaf samples were subjected to correlation analysis.
Results and discussion
Physical characterization of PM10
The majority of the particles were not fully spherical, as seen by the circulatory effects, which varied from 0.71 to 0.83 (less than one) (Chang et al., 2022). Irrespective of the size of the particles, the factor of roundness is crucial in defining them. The particles’ roundness ranged from 0.6 to 0.71 (< 1), indicating a range in shape from nearly spherical to irregular (Fig. 3).
The proportionate relationship between an image’s maximum width (Wmax) and maximum height (Lmax) is referred to as the aspect ratio. The cellular absorption of PM is significantly influenced by its aspect ratio. The higher the aspect ratio, the higher the activity of the particles. The diameter of a sphere with an equivalent volume is referred to as the equivalent spherical diameter (ESD) of an irregularly shaped object (Parslow & Jennings, 1986). The respiratory deposition fraction is defined as an ESD value larger than 0.93 (McNaught & Wilkinson, 1997). ESD value (0.73–0.86) of the particles in the current study indicated that these particles were not respiratory fractions that could pass beyond the ciliated airways. No significant difference (p > 0.05) was found in the morphological aspects of PM10 of the studied locations (Table 2).
Stomatal pore size
The morphology of abaxial leaf surfaces of P. guajava exposed to PM collected from different locations was studied. Microscopic examinations of the abaxial (lower) epidermis of leaves revealed that the stomatal pores and stomatal edges in the epidermis of the polluted leaves were clogged with particulate pollutants, thereby preventing the easy exchange of gases. On the other hand, stomata in the control leaves were broadly open and slightly recessed (Fig. 4a). Leaves of the contaminated site had occluded stomatal openings (Fig. 4b).
In previous studies, specific leaf attributes, including the quantity and dimensions of stomata and the length of the leaf lamina, have exhibited their potential as bioindicators of industrial pollution. The length of the foliage was found to exhibit the highest degree of sensitivity (Dimitrova & Yurukova, 2005). In our study, stomatal pore size was decreased up to 40% (Table 3). When compared to the control site, the leaf surface of the plants growing at the contaminated site had smaller stomatal pores. Our results are consistent with the findings of Abdallah and Mejnun (2013), who stated that encrypted and somewhat sunken stomata were seen on the plant leaf in the polluted environment. Compared to healthier leaves, leaves exposed to polluted sites exhibited obvious changes in leaf surface configuration (Alfani et al., 1996).
Stomata are constantly exposed to air pollutants. Therefore, any distinctive or predictable change in stomata caused by pollutants could be used as a diagnostic indicator of exposure to air pollution. According to Eckert and Houston (1982), increased cell permeability induced by pollution may result in the guard cells becoming flaccid and losing water, which closes the stomata. Encrustation or PM deposition on a leaf’s surface may also lessen the amount of light that reaches the leaf, hindering photosynthesis. This could then cause CO2 to build up in the sub-stomatal cavities, causing the stomata to close. A high dose of SO2 also promotes stomatal closure (Morgan et al., 2006). The ambient SO2 concentration was not assessed in the present study. Consequently, the impact of SO2 on leaf stomata was not taken into account in this study.
HM concentration in PM10
PM and airborne HMs have the potential to reach remote areas despite being mostly emitted from urban or industrial areas due to their long-range transportation. After being deposited, the HMs will actively participate in the biogeochemical cycles within various soil–plant-atmosphere systems. Leaf deposition and foliar absorption, in addition to the well-known root assimilation pathway, contribute to the accumulation of HMs in plants.
Lead (Pb)
The Pb concentration varied from 11.57 ng/m3 (Hashadah, Chuadanga) to 718.58 ng/m3 (Kodomtoli, Dhaka) (Fig. 5). The higher value of Pb may be due to industrial activities along with heavy traffic in Kodomtoli area, while the lower value may be attributed to the relatively lower level of traffic and lack of industrial activities. According to Salam et al. (2003), the level of Pb in Bangladesh’s rural areas is below the detection threshold. Our study showed a different scenario. In rural regions, autorickshaws powered by lead-acid batteries may contribute to significant Pb emission. According to the source composition profile of PM2.5 by Begum et al. (2013), the main sources of lead were motor vehicles, soil dust, and fugitive Pb. Anthropogenic sources, primarily from traffic emissions, were responsible for the atmospheric concentration of lead (Basha et al., 2010). Dhaka held the distinction of being the most polluted city globally, as indicated by a recorded lead (Pb) concentration of 463 ng/m3 in PM2.5 (Khaliquzzaman et al., 1997). Steel industries in Kodomtoli area may contribute to significant lead emission in the ambient atmosphere. Lead is used in steel to improve its machinability. It gives rise to a significant release of lead in the atmosphere as 59.98% of input lead ends up in the PM, 0.03% in the stack emissions, and almost no Pb in the slag (Chattopadhyay et al., 2014). Higher concentration of lead in Doyel Chattar area may be due to the nearby lead acid battery industries in Paltan, Gulistan, and Motijheel area (Rahman et al., 2021). A significant portion of lead can also be emitted from the combustion of coal in brick kiln industry (Bhat et al., 2014; Haque et al., 2022; Majumder et al., 2021; Rai et al., 2016). Thus, the brick kiln industry can be accounted for the higher lead concentration in Modonpur. Lead concentration in heavy traffic areas such as Farmgate and Gabtoli is lower than that of Kodomtoli and Modonpour. The progressive decrease in atmospheric Pb concentration in Dhaka is most likely to have been caused by Bangladesh’s prohibition on leaded gasoline (Shahrukh et al., 2023).
Copper (Cu)
The Cu concentrations varied from 7.07 ng/m3 (Hashadah, Chuadanga) to 64.92 ng/m3 (Gabtoli, Dhaka) (Fig. 5). Emission of Cu in rural areas is low. In this study, the highest concentration of Cu was obtained from the area with high traffic volume. Over 90% of the copper (Cu) emissions from vehicles can be attributed to the combined effects of brake wear and exhaust emissions (Johansson et al., 2009). Burning coal in brick kiln can significantly contribute to Cu emission. The primary sources of Cu emissions were the iron industries, smelting, and friction from automobile brakes. According to the findings of Chen et al. (2015), the primary contributors to Cu and Ni emissions are the combustion of coal and oil.
Cadmium (Cd)
The Cd concentration in the rural area was below the detection limit. The highest Cd concentration was recorded in Kodomtoli (22.2 ng/m3) and Doyel Chattar, Dhaka (18.41 ng/m3). The increased Cd concentration may be a result of higher traffic emissions and Cd release from various industrial mechanical operations as documented by Salam et al. (2003) and Salam et al. (2008). Zhao and Zhao (2012) also reported that Cd originated from traffic emissions.
Manganese (Mn)
The Mn concentrations varied from 26.62 ng/m3 (Hashadah, Chuadanga) to 140.5 ng/m3 (Modonpur, Narayanganj). Mn is used in gasoline as it increases the octane number. Thus, traffic emission causes a significant release of Mn in the atmosphere. Begum et al. (2013) reported aged sea salt and fugitive Mn as the main source of Mn. According to Chen et al. (2015), the iron and steel sector, smelting, and friction from car brakes were the main sources of Mn emissions.
Iron (Fe)
The Fe concentration was found to vary from 1086 ng/gm3 (Chuadanga) to 6152 ng/m3 (Gabtoli). This was probably due to the ongoing construction activities of the metro rail, heavy traffic, and industrial activities of the Tejgaon industrial area nearby. The recent Dhaka mass rapid transit development project of the Bangladesh government is responsible for the continual erosion of roads and soils in that area (Moniruzzaman et al., 2022). The lower value of Fe (1086 ng/m3) at Chuadanga was probably due to the lack of industrial activities and less traffic in the area. In comparison to the values reported by Salam et al. (2003), Salam et al. (2008), and Mondol et al. (2014), Fe showed relatively higher concentrations at all seven sites. Begum et al. (2013) reported road dust, brick kilns, motor vehicles, soil dust, and fugitive Fe as the principal source of iron.
Nickel (Ni)
The highest Ni concentration (61.1 ng/m3) was found in Gabtoli, Dhaka, which was probably due to traffic emissions. In the rural area, Ni concentration was below the detection limit. The primary contributors to the abundance of Ni were motor vehicles, as well as the combustion of coal and oil.
Zinc (Zn)
Zn concentrations varied from 455 ng/m3 (Hashadah, Chuadanga) to 15,956 ng/m3 (Kodomtoli). The highest concentration of Zn was found at Kodomtoli which could be attributed to heavy traffic and the industrial release of Zn from the nearby industrial area. The observed minimum value at the rural site may be attributed to less traffic and industrial activities. The mean value of Zn in PM10 in our study was much higher than the value obtained in previous measurements. The current metro rail project may have a significant contribution to this higher level of Zn. Begum et al. (2013) found traffic emission, brick kilns, aged sea salt, and fugitive Zn as the chief source of Zn. Zn in the atmosphere was determined to be a result of vehicle emissions by Basha et al. (2010), who also suggested using Zn as a marker for gasoline engine emissions.
Chromium (Cr)
The highest Cr concentration (138.44 ng/m3) was found in Gabtoli, Dhaka, while in Hashadah, Chuadanga, the concentration was 2.31 ng/m3. The highest concentration was found in heavy-traffic sites. Cr is used in steel which finds its way into vehicles. Car cylinders are also usually covered with a layer of chromium. Cr is also emitted from coal combustion and incinerators, textile, and steel industries as reported by Chen et al. (2015). Cr concentration was higher in Pallabi which was probably owing to the prevalence of textile industries near the sampling location.
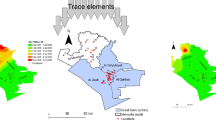
Enrichment factor
The highest enrichment of Pb, Cd, Zn, and Ni was observed in site 3 and site 4 which were industrial and traffic congested sites (Fig. 6) implying that these elements were released into the environment via the anthropogenic sources rather than the geogenic ones. Doyel Chattar is a residential area of the University campus that is intersected by two important roads. Despite the restrictions on heavy duty vehicles, the roads in the area frequently experience significant traffic congestion. In addition, the construction activities associated with the Dhaka Mass Rapid Transit Development Project might have contributed to a significant increase in the concentration of Fe and Zn at this site, as noted by Moniruzzaman et al. (2022). It is possible that the increased levels of lead in the Doyel Chattar area are a result of the presence of lead acid battery industries in the nearby Paltan, Gulistan, and Motijheel areas. A study by Khare and Baruah (2010) in north-east India produced comparable results to the current study. The study concluded that high concentrations of Zn and Cd were caused by vehicular exhaust, and high enrichment of Zn was caused by road dust resuspension (Khare & Baruah, 2010). Near highways, car emissions are the main source of non-crustal components that are related to PM (Weckwerth, 2001). As previously stated by Hailin et al. (2008) and Wu et al. (2020), the higher Pb concentration could be linked to the residue of pre-emitted lead in soil and industrial operations.
HM concentration in soil
HMs of soil from seven different locations were studied (Fig. 7), which revealed that the metal concentration in soil of urban areas was higher than that of rural areas. Cr, Mn, Ni, and Zn content of all the sampling locations met the standard limit set by Sauerbeck (1985). Cd was detected from the soil of Kodomtoli and Modonpur which were industrial areas. Those two locations failed to meet the standard limit. Higher concentrations of Pb were found in the soil of Kodomtoli, Doyel Chattar, Farmgate, and Pallabi that exceeded the standard concentration. However, linear regression analysis between the metal concentration of air and soil revealed no significant correlation (p > 0.05). Significant positive correlation (p < 0.05) was found for Pb (R2 = 0.605) and Cu (R2 = 0.677) contents in soil and air of respective locations.
Geoaccumulation index
The geoaccumulation index (Igeo) of heavy metals in the soil of Dhaka City was evaluated and is presented in Table 4. According to the classification of Kamani et al. (2018), Cr, Cu, Fe, Mn, and Ni were ranked as uncontaminated (Igeo ≤ 0). The concentration of Zn at sites 3, 4, and 7 was ranked as uncontaminated to moderately contaminated (0 < Igeo ≤ 1), whereas in moderately contaminated (1 < Igeo ≤ 2) at site 6 (1.17). Cd was in the heavily contaminated class (3 < Igeo ≤ 4) at site 2 and heavily contaminated to extremely contaminated (4 < Igeo ≤ 5) at site 3. Other sites exhibited low concentration. Pb fell in uncontaminated to moderately contaminated (2 < Igeo ≤ 3) level at sites 5 and 6, moderately contaminated at site 4, and moderately to heavily contaminated (2 < Igeo ≤ 3) at site 3.
Contamination factor
The contamination factor (CF) of heavy metals in the soil of Dhaka City was evaluated and is presented in Table 5. Based on CF classification, Cr, Cu, Fe, Mn, and Ni were ranked as low contamination (CF < 1) for all the sites. Zn (1.17) ranked in the moderate contamination class (1 < CF < 3). Cd was in the heavily contamination class (3 > CF > 6) at sites 3 and 4. Other sites exhibited low contamination. Sites 4, 5, and 6 were moderately contaminated, and sites 3 and 4 were heavily contaminated with Pb.
Pollution load index
The pollution load index (PLI) for different sites was shown in Table 5. All sites showed low contamination according to the PLI classification (Jorfi et al., 2017). PLI found in this study was significantly lower compared to the other studies done in this part of the world (Nargis et al., 2022; Islam et al., 2015). However, as we tried to relate biomonitoring with the metal accumulation index, we collected root-zone soil for this study. Root-zone soil differs significantly from road dust in terms of heavy metal concentration.
HMs in leaf blades of plants
The uptake of HMs by plant leaves from the atmosphere has received less attention in studies than root metal transfer, which has been extensively researched (Pourrut et al., 2011). Markert (1992) proposed a “Reference Plant” that described the mean concentration of all the inorganic elements in plants. Markert (1992) reported that plants originating from uncontaminated environments may exhibit leaf metal concentrations of the following values: 1 mg kg−1 Pb, 0.05 mg kg−1 Cd, 150 mg kg−1 Fe, 1.50 mg kg−1 Cr, 10 mg kg−1 Cu, 200 mg kg−1 Mn, 1.50 mg kg−1 Ni, and 50 mg kg−1 Zn. The plants collected from the control site met the “criteria of reference plant” in terms of inorganic element concentration.
Plants grown in traffic areas accumulated a high amount of Pb (Fig. 8). The soils of those respective locations also contained a high concentration of lead (Fig. 7). But only 3% of the Pb in the root gets transferred to the shoot, according to Zimdahl’s (1976) description of the Pb translocation from roots to tops. The uptake and transport of Pb from atmospheric deposition were examined by Dalenberg and Driel (1990). According to their findings, leaves transmit between 33 and 95% of the total Pb concentration in plants to other plant organs. In the present study, Pb content in plant leaves and PM fraction were observed to significantly correlate, suggesting that Pb bound to PM in the atmosphere may aid in plant leaf uptake of Pb. The result of our investigation aligns with the findings of Shahid et al. (2017) and Gajbhiye et al. (2022).
Concentration (mg/kg) of heavy metals in leaves of four different species grown at different locations. Site 1 = Hashadah, Chuadanga; Site 2 = Modonpur, Narayanganj; Site 3 = Kodomtoli, Narayanganj; Site 4 = Doyel Chattar, Dhaka; Site 5 = Pallabi, Mirpur, Dhaka; Site 6 = Farmgate, Dhaka; Site 7 = Gabtoli, Dhaka
According to Klocek et al. (2003), plants’ essential Cd level should not exceed 0.15 mg kg−1 to be useful for consumptive purposes. Although Cd is not required for metabolic processes, both the root and leaf systems are capable of successfully absorbing it (Kabata-Pendias, 2000). According to Abollino et al. (2002), Cd concentration in soil, PM emission intensity, and physico-chemical characteristics of the soil all affect plant Cd content. The examined plant species had a Cd level that ranged from 0.25 to 1.35 mg kg−1 dry matter. Cd concentration in plants grown in the rural area was below the detection limit. The highest Cd concentration (1.35 mg/kg) was observed in M. indica grown in Farmgate; however, Cd concentration in soils of Farmgate was below the detection limit. Thus, it can be predicted that elevated concentration of Cd can be caused by atmospheric deposition of Cd from heavy traffic and industrial activities. In the current study, Cr, Cu, Fe, Ni, and Zn concentration in plant leaves grown in industrial and roadside locations was greater than that of the plants grown in the rural area (Fig. 8).
Plants quickly and easily absorb Ni from the soil until certain Ni concentrations are reached in plant tissues. Kozlov et al. (2000) examined the movement of Cu and Ni-rich particles in birch trees and hypothesized that stomata may be the route by which particles penetrate plant leaves. On the other hand, Ashton (1972) supported the observation of the present study which states that, in environments with airborne PM bound Ni, plant tops concentrate the most Ni. Roberts (1975) also noted a substantial absorption of foliar-applied Zn and Fe. Thus, Fe, Ni, and Zn accumulation may also be attributed to anthropogenic sources. Although Cr is present in large concentrations in most soils, plants have very little access to it (Kabata-Pendias, 2000). Higher Cr concentration in polluted areas than in rural area (Fig. 8) signifies that plants can absorb Cr from the air.
The Mn pool in soils should directly affect the Mn content of plants because Mn appears to be readily absorbed by plants when it is present in soluble forms (Kabata-Pendias, 2000). Except for M. indica grown in Modonpur and Kodomtoli, Mn concentration in plants did not vary that much which indicated that Mn in particulates did not contribute to plant Mn concentration.
As Fig. 8 depicts, Swietenia mahagoni and Mangifera indica are capable of accumulating substantial amounts of Pb and Cr in their leaves. The result of the current study validates the findings of the earlier research by Intan et al. (2023). Emile and Barde (2020) also revealed that Mangifera indica is a hyperaccumulator plant and could be employed as a biomonitor for pollution studies.
Metal accumulation index
Table 6 depicts the metal accumulation index (MAI) values for the chosen plant species. The local atmospheric chemistry, climatic conditions, height of the tree species, and the genetic features of the plant are some of the variables that affect the index (Hu et al., 2014; Liu et al., 2007). Similar species can have varying MAI values in different locations. In the present study, the MAI value (3.88) was maximum in Mangifera indica at site 1 and the lowest MAI (2.5) was observed for Psidium guajava at site 3. The highest average MAI (3.05) was observed for Swietenia mahagoni (Table 6) and followed the decreasing order of Mangifera indica > Artocarpus heterophyllus > Psidium guajava. All four of the plant species under investigation showed site-specific differences in the current investigation. Hu et al. (2014) and Liu et al. (2007) reported similar findings. Nadgórska-Socha et al. (2017) also demonstrated site-specific differences in MAI values for various plant species. This may be attributed to the different pollution loads in different locations. The findings show that different plant species have varying capacities for removing pollutants and that these capacities depend on several different conditions. Our study suggested that the uptake ability of Swietenia mahagoni was the highest among the four species followed by M. indica. The selection criteria for urban tree species for biomonitoring are based on the measurement of leaf functional traits and leaf surface microstructure characteristics, as these attributes are easily measurable and highly responsive to environmental changes (Rodríguez-Santamaría et al., 2022). High MAI plant species should also be employed as barriers between susceptible regions, like parks, schools, and residential areas, and contaminated areas like heavy traffic and industrial areas (Hu et al., 2014; Nadgórska-Socha et al., 2017).
Saet’s summary index (Zc)
Saet’s summary index (Zc) was calculated for plant leaves of four different plant species collected from different locations and are presented in Fig. 9.
Though Zc index is applied grossly for assessing and classifying soils in terms of heavy metal concentration, we calculated the Zc of plant leaves from varying pollution load. According to the classification of Vodyanitskii (2010), leaves of M. indica collected from all locations fell under the extremely hazardous class in terms of heavy metal concentration. Similar results were found for S. mahagoni except for site 6. The higher Zc index of the plant species may indicate a higher metal accumulation capacity of the plant leaves compared to other species. Thus, assessing the heavy metal concentration of the plant leaves, it can be stated that M indica has the highest accumulative capacity followed by S. mahagoni, P. guajava, and A. heterophyllus.
Conclusions
In this study, four urban plant species were evaluated for their biomonitoring capacities as they can reduce environmental pollution through bioaccumulating contaminants in their tissues. In some sites, the foliar configuration was found to change in the form of a decline in stomatal pore size. Particulate matter was also collected from the selected sites to assess their heavy metal loads. The morphological study of the particles indicated that the particles were nearly spherical to irregular in shape and were not respiratory fractions. The higher values of enrichment factors for Cd, Ni, Pb, and Zn implied that those elements were mostly emitted from anthropogenic sources. The geoaccumulation index (Igeo) and contamination factor indicated extreme pollution of some sites with respect to Cd and Pb. However, no significant correlations were found among the metal concentrations of soil, plant, and PM10. A positive correlation between Pb content in plant leaves and PM10 indicated that plant leaves could be a suitable biomonitor for heavy metal pollution in urban environments. S. mahagoni exhibited the highest MAI value followed by M. indica. These two species were also found to exhibit a build-up of Pb and Cr. As a result, both S. mahagoni and M. indica species could be employed as Pb and Cr bioaccumulators in urban areas. Saet’s summary index (Zc) suggested that M. indica has the highest bio-accumulative property followed by S. mahagoni. Researchers and regulatory agencies may find the species-specific results useful in using trees to monitor environmental health and air quality. The results of our study are likely to provide additional guidance to urban planners in conducting future screening studies for urban greenbelt development.
Data availability
All relevant data has been added to the manuscript.
References
Abdallah, Y. N., & Mejnun, G. E. (2013). Change of the morpho-anatomical structure of leaves of Ligustrum japonicum and Olea europea caused by heavy metal pollution. Caspian Journal of Applied Sciences Research, 2(2).
Abollino, O., Aceto, M., Malandrino, M., Mentasti, E., Sarzanini, C., & Petrella, F. (2002). Heavy metals in agricultural soils from Piedmont, Italy. Distribution, speciation and chemometric data treatment. Chemosphere, 49(6), 545–557.
Ahmed, M. J., Ali, M. K., Hossain, M., Siraj, S., & Ahsan, M. A. (2012). Determination of trace metals in air of Chittagong city-Bangladesh. European Journal of Chemistry, 3(4), 416–420.
Alahabadi, A., Ehrampoush, M. H., Miri, M., Aval, H. E., Yousefzadeh, S., Ghaffari, H. R., ... & Hosseini-Bandegharaei, A. (2017). A comparative study on capability of different tree species in accumulating heavy metals from soil and ambient air. Chemosphere, 172, 459–467.
Alfani, A., Bartoli, G., Rutigliano, F. A., Maisto, G., & Virzo De Santo, A. (1996). Trace metal biomonitoring in the soil and the leaves of Quercus ilex in the urban area of Naples. Biological Trace Element Research, 51, 117–131.
Apte, J. S., Brauer, M., Cohen, A. J., Ezzati, M., & Pope, C. A., III. (2018). Ambient PM2. 5 reduces global and regional life expectancy. Environmental Science & Technology Letters, 5(9), 546–551.
Ashton, W. M. (1972). Nickel pollution. Nature, 237(5349), 46–47.
Baldantoni, D., Cicatelli, A., Bellino, A., & Castiglione, S. (2014). Different behaviours in phytoremediation capacity of two heavy metal tolerant poplar clones in relation to iron and other trace elements. Journal of Environmental Management, 146, 94–99.
Basha, S., Jhala, J., Thorat, R., Goel, S., Trivedi, R., Shah, K., ... & Jha, B. (2010). Assessment of heavy metal content in suspended particulate matter of coastal industrial town, Mithapur, Gujarat, India. Atmospheric Research, 97(1–2), 257–265.
Beckett, K. P., Freer-Smith, P. H., & Taylor, G. (2000). The capture of particulate pollution by trees at five contrasting urban sites. Arboricultural Journal, 24(2–3), 209–230.
Begum, B. A., Hopke, P. K., & Markwitz, A. (2013). Air pollution by fine particulate matter in Bangladesh. Atmospheric Pollution Research, 4(1), 75–86.
Bhat, M. S., Afeefa, Q. S., Ashok, K. P., & Bashir, A. G. (2014). Brick kiln emissions and its environmental impact: A review. Journal of Ecology and the Natural Environment, 6(1), 1–11.
Bilo, F., Borgese, L., Dalipi, R., Zacco, A., Federici, S., Masperi, M., ... & Depero, L. E. (2017). Elemental analysis of tree leaves by total reflection X-ray fluorescence: New approaches for air quality monitoring. Chemosphere, 178, 504–512.
Birbaum, K., Brogioli, R., Schellenberg, M., Martinoia, E., Stark, W. J., Günther, D., & Limbach, L. K. (2010). No evidence for cerium dioxide nanoparticle translocation in maize plants. Environmental Science & Technology, 44(22), 8718–8723.
Bulto, L. N. B., Defo, B. K., Islam, S. M. S., & GBD 2016 Causes of Death Collaborators. (2017). Global, regional, and national age-sex specifc mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. The Lancet, 390(10100), 1151–1210. https://doi.org/10.1016/S0140-6736(17)32152-9
Callen, M. S., Cruz, D. L., Lopez, J. M., et al. (2009). Comparison of receptor models for source apportionment of the PM10 in Zaragoza (Spain). Chemosphere, 76, 1120–1129.
Calzoni, G. L., Antognoni, F., Pari, E., Fonti, P., Gnes, A., & Speranza, A. (2007). Active biomonitoring of heavy metal pollution using Rosa rugosa plants. Environmental Pollution, 149(2), 239–245.
Chamel, A., Pineri, M., & Escoubes, M. (1991). Quantitative determination of water sorption by plant cuticles. Plant, Cell & Environment, 14(1), 87–95.
Chang, P. K., Griffith, S. M., Chuang, H. C., Chuang, K. J., Wang, Y. H., Chang, K. E., & Hsiao, T. C. (2022). Particulate matter in a motorcycle-dominated urban area: Source apportionment and cancer risk of lung deposited surface area (LDSA) concentrations. Journal of Hazardous Materials, 427, 128188.
Chattopadhyay, K., Johnson, D., Young, J., Vieira, J., Bachenheimer, S., & Kumar, S. (2014). Evaluation of air emissions in steel plants with focus on heavy metals emission. https://www.researchgate.net/publication/285598959. Accessed Apr 2024
Chen, P., Bi, X., Zhang, J., Wu, J., & Feng, Y. (2015). Assessment of heavy metal pollution characteristics and human health risk of exposure to ambient PM2.5 in Tianjin China. Particuology, 20, 104–109.
Chester, R., Nimmo, M., & Preston, M. R. (1999). The trace metal chemistry of atmospheric dry deposition samples collected at Cap Ferrat: A coastal site in the Western Mediterranean. Marine Chemistry, 68(1–2), 15–30.
Chiarantini, L., Rimondi, V., Benvenuti, M., Beutel, M. W., Costagliola, P., Gonnelli, C., ... & Paolieri, M. (2016). Black pine (Pinus nigra) barks as biomonitors of airborne mercury pollution. Science of the Total Environment, 569, 105–113.
Dalenberg, J. W., & Van Driel, W. (1990). Contribution of atmospheric deposition to heavy-metal concentrations in field crops. Netherlands Journal of Agricultural Science, 38(3A), 369–379.
Dimitrova, I., & Yurukova, L. (2005). Bioindication of anthropogenic pollution with Plantago lanceolata (Plantaginaceae): Metal accumulation, morphological and stomatal leaf characteristics. Phytologia Balcanica, 11(1), 89–96.
Dzierżanowski, K., Popek, R., Gawrońska, H., Sæbø, A., & Gawroński, S. W. (2011). Deposition of particulate matter of different size fractions on leaf surfaces and in waxes of urban forest species. International Journal of Phytoremediation, 13(10), 1037–1046.
Eckert, R. T., & Houston, D. B. (1982). Foliar peroxidase and acid phosphatase activity response to low level SO2 exposure in eastern white pine clones. Forest Science, 28(3), 661–664.
El-Amier, Y. A., El-Alfy, M. A., & Nofal, M. M. (2018). Macrophytes potential for removal of heavy metals from aquatic ecosystem, Egypt: Using metal accumulation index (MAI). Plant Arch, 18(2), 2131–2144.
Emile, A. F., & Barde, B. G. (2020). Bioaccumulation of heavy metals in mangoes (Mangifera indica L.) found in the vicinity of gold mining sites of Zamfara State, Nigeria. Journal of Environmental Chemistry and Ecotoxicology, 12(1), 45–58.
Estefan, G., Sommer, R., & Ryan, J. (2013). Methods of soil, plants and water analysis, ICARDA, international for agriculture research in the dry areas. 3rd edn. www.icarda.org
Fernández, V., & Brown, P. H. (2013). From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Frontiers in Plant Science, 4, 289.
Gajbhiye, T., Pandey, S. K., & Kim, K. H. (2022). Foliar uptake of toxic metals bound to airborne particulate matter in an urban environment. Aerosol and Air Quality Research, 22, 220050. https://doi.org/10.4209/aaqr.220050
Gope, M., Masto, R. E., George, J., Hoque, R. R., & Balachandran, S. (2017). Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb and Zn) in street dust of Asansol, India. Ecotoxicology and Environmental Safety, 138, 231–241.
Gulshan, J. E., Hossain, S. A., Hossain, M. E., Islam, M. M., Emon, S. Z., Manzum, A. A., ... & Nurul Huda, M. (2021). Seasonal variations of microbes in particulate matter obtained from Dhaka City in Bangladesh. Environmental Pollutants and Bioavailability, 33(1), 122–134.
Hailin, W., Zhuang, Y., Ying, W., Yele, S. U. N., Hui, Y. U. A. N., Zhuang, G., & Zhengping, H. A. O. (2008). Long-term monitoring and source apportionment of PM2. 5/PM10 in Beijing China. Journal of Environmental Sciences, 20(11), 1323–1327.
Haque, S. E., Shahriar, M. M., Nahar, N., & Haque, M. S. (2022). Impact of brick kiln emissions on soil quality: A case study of Ashulia brick kiln cluster Bangladesh. Environmental Challenges, 9, 100640.
Hu, Y., Wang, D., Wei, L., Zhang, X., & Song, B. (2014). Bioaccumulation of heavy metals in plant leaves from Yan’ an city of the Loess Plateau, China. Ecotoxicology and environmental safety, 110, 82–88. https://doi.org/10.1016/j.ecoenv.2014.08.021
Intan, D. Z., Yuniati, R., & Lestari, R. (2023). Comparative study on Pb absorption ability of five shade plant species in industrial estate and urban forests of Bekasi, Indonesia. Biodiversitas Journal of Biological Diversity, 24(2).
Islam, S., Ahmed, K., & Masunaga, S. (2015). Potential ecological risk of hazardous elements in different land-use urban soils of Bangladesh. Science of the Total Environment, 512, 94–102.
Jashim, Z. B., Hossain, S. A., Hossain, M. E., Islam, M., Gulshan, J., & Huda, M. (2021). Effects of air borne particulate matter on the plants grown in different areas of dhaka mega city, bangladesh: an air pollution tolerance study. Environmental Claims Journal, 1–20.
Johansson, C., Norman, M., & Burman, L. (2009). Road traffic emission factors for heavy metals. Atmospheric Environment, 43(31), 4681–4688.
Jorfi, S., Maleki, R., Jaafarzadeh, N., & Ahmadi, M. (2017). Pollution load index for heavy metals in Mian-Ab plain soil, Khuzestan. Iran. Data Brief, 15, 584–590.
Kabata-Pendias, A. (2000). Trace elements in soils and plants. CRC Press.
Kachenko, A. G., & Singh, B. (2006). Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water, Air, and Soil Pollution, 169, 101–123.
Khaliquzzaman, M., Biswas, S. K., Tarafdar, S. A., Islam, A., & Khan, A. H. (1997). Trace element composition of size fractionated airborne particulate matter in urban and rural areas in Bangladesh (p. 10). Dhaka AECD/AED: Atomic Energy Commission.
Khare, P., & Baruah, B. P. (2010). Elemental characterization and source identification of PM2.5 using multivariate analysis at the suburban site of North-East India. Atmospheric Research, 98(1), 148–162.
Khuzestani, R. B., & Souri, B. (2013). Evaluation of heavy metal contamination hazards in nuisance dust particles, in Kurdistan Province, western Iran. Journal of Environmental Sciences, 25(7), 1346–1354.
Klocek, B., Osek, M., & Milczarek, A. (2003). The contamination by cadmium and lead of Leeds produced near the Fast Road. Animal Science, 2, 273–276.
Kozlov, M. V., Haukioja, E., Bakhtiarov, A. V., Stroganov, D. N., & Zimina, S. N. (2000). Root versus canopy uptake of heavy metals by birch in an industrially polluted area: Contrasting behaviour of nickel and copper. Environmental Pollution, 107(3), 413–420.
Kulkarni, S. D., & Ingawale, A. (2014). Biomonitoring of air pollution in by correlating the pollution tolerance of some commonly grown trees in Waluj Industrial area near Aurangabad, India. International Journal Research of Environment Science and Technology, 4(4), 102–106.
Liu, Y.-J., Zhu, Y.-G., & Ding, H. (2007). Lead and cadmium in leaves of deciduous trees in Beijing, China: Development of a metal accumulation index (MAI). Environmental Pollution, 145, 387–390.
Madejón, P., Ciadamidaro, L., Marañón, T., & Murillo, J. M. (2013). Long-term biomonitoring of soil contamination using poplar trees: Accumulation of trace elements in leaves and fruits. International Journal of Phytoremediation, 15(6), 602–614.
Maisto, G., Alfani, A., Baldantoni, D., De Marco, A., & De Santo, A. V. (2004). Trace metals in the soil and in Quercus ilex L. leaves at anthropic and remote sites of the Campania Region of Italy. Geoderma, 122(2–4), 269–279.
Majolagbe, A., Paramole, A., Majolagbe, H., Oyewole, O., & Sowemimo, M. (2010). Concentration of heavy metals in tree barks as indicator of atmospheric pollution in Oyo Town, Southwest Nigeria. Scholars Research Library Archives of Applied Science Research, 2, 170–178.
Majumder, A. K., Al Nayeem, A., Islam, M., Akter, M. M., & Carter, W. S. (2021). Critical review of lead pollution in Bangladesh. Journal of Health Pollution, 11(31), 210902.
Markert, B. (1992). Establishing of ‘Reference Plant’ for inorganic characterization of different plant species by chemical fingerprinting. Water, Air, and Soil Pollution, 64, 533–538.
Mataruga, Z., Jarić, S., Marković, M., Pavlović, M., Pavlović, D., Jakovljević, K., ... & Pavlović, P. (2020). Evaluation of Salix alba, Juglans regia and Populus nigra as biomonitors of PTEs in the riparian soils of the Sava River. Environmental monitoring and assessment, 192, 1–20.
McNaught, A. D., & Wilkinson, A. (1997). Compendium of chemical terminology: IUPAC recommendations
McPherson, E., Nowak, D. J., and Rowntree, R. A. (1994) Chicago’s urban forest ecosystem: Results of the Chicago urban forest climate project, general technical report No. NE-186. Radnor, PA: U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, p 201.
Mohammadi, A., Mokhtari, M., Arani, A. M., Taghipour, H., Hajizadeh, Y., & Fallahzadeh, H. (2018). Biomonitoring levels of airborne metals around Urmia Lake using deciduous trees and evaluation of their tolerance for green belt development. Environmental Science and Pollution Research, 25, 21138–21148.
Monfared, S. H., Matinizadeh, M., Shirvany, A., Amiri, G. Z., Fard, R. M., & Rostami, F. (2013). Accumulation of heavy metal in Platanus orientalis, Robinia pseudoacacia and Fraxinus rotundifolia. Journal of forestry research, 24, 391–395.
Mondol, M. N., Khaled, M., Chamon, A. S., & Ullah, S. M. (2014). Trace metal concentration in atmospheric aerosols in some city areas of Bangladesh. Bangladesh Journal of Scientific and Industrial Research, 49(4), 263–270.
Moniruzzaman, M., Shaikh, M. A. A., Saha, B., Shahrukh, S., Jawaa, Z. T., & Khan, M. F. (2022). Seasonal changes and respiratory deposition flux of PM2. 5 and PM10 bound metals in Dhaka Bangladesh. Chemosphere, 309, 136794.
Morgan, P. B., Mies, T. A., Bollero, G. A., Nelson, R. L., & Long, S. P. (2006). Season-long elevation of ozone concentration to projected 2050 levels under fully open-air conditions substantially decreases the growth and production of soybean. New Phytologist, 170(2), 333–343.
Muller, G. M. M. G. M. G. M. G. P. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2, 108–118.
Nadgórska-Socha, A., Kandziora-Ciupa, M., Trzęsicki, M., & Barczyk, G. (2017). Air pollution tolerance index and heavy metal bioaccumulation in selected plant species from urban biotopes. Chemosphere, 183, 471–482.
Nair, R., Varghese, S. H., Nair, B. G., Maekawa, T., Yoshida, Y., & Kumar, D. S. (2010). Nanoparticulate material delivery to plants. Plant Science, 179(3), 154–163.
Nargis, A., Habib, A., Islam, M. N., Chen, K., Sarker, M. S. I., Al-Razee, A. N. M., ... & Cai, M. (2022). Source identification, contamination status and health risk assessment of heavy metals from road dusts in Dhaka, Bangladesh. Journal of Environmental Sciences, 121, 159–174.
Ozturk, A., Yarci, C., & Ozyigit, I. I. (2017). Assessment of heavy metal pollution in Istanbul using plant (Celtis australis L.) and soil assays. Biotechnology & Biotechnological Equipment, 31(5), 948–954.
Parslow, K., & Jennings, B. R. (1986). Simultaneous size and thickness measurements for heterogeneous micrometre-sized particles. Journal of Physics d: Applied Physics, 19(7), 1233.
Peña-Icart, M., Tagle, M. E. V., Alonso-Hernández, C., Hernández, J. R., Behar, M., & Alfonso, M. S. P. (2011). Comparative study of digestion methods EPA 3050B (HNO3–H2O2–HCl) and ISO 11466.3 (aqua regia) for Cu, Ni and Pb contamination assessment in marine sediments. Marine environmental research, 72(1–2), 60–66.
Piczak, K., Leśniewicz, A., & Żyrnicki, W. (2003). Metal concentrations in deciduous tree leaves from urban areas in Poland. Environmental Monitoring and Assessment, 86, 273–287.
Pope, C. A., III., Burnett, R. T., Thurston, G. D., Thun, M. J., Calle, E. E., Krewski, D., & Godleski, J. J. (2004). Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation, 109(1), 71–77.
Popek, R., Łukowski, A., Bates, C., & Oleksyn, J. (2017). Accumulation of particulate matter, heavy metals, and polycyclic aromatic hydrocarbons on the leaves of Tilia cordata Mill. in five Polish cities with different levels of air pollution. International journal of phytoremediation, 19(12), 1134–1141.
Pourrut, B., Shahid, M., Dumat, C., Winterton, P., & Pinelli, E. (2011). Lead uptake, toxicity, and detoxification in plants. Reviews of Environmental Contamination and Toxicology, 213, 113–136.
Rahman, M. S., Akhter, S., Rahman, R., Choudhury, T. R., Jolly, Y. N., Akter, S., ... & Begum, B. A. (2019). Identification of sources of PM2. 5 at Farmgate Area, Dhaka using reconstructed mass calculation and statistical approaches. Nuclear Science and Applications, 28(1&2).
Rahman, M. S., Bhuiyan, S. S., Ahmed, Z., Saha, N., & Begum, B. A. (2021). Characterization and source apportionment of elemental species in PM2. 5 with especial emphasis on seasonal variation in the capital city “Dhaka”, Bangladesh. Urban Climate, 36, 100804.
Rai, P., Chakraborty, A., Mandariya, A. K., & Gupta, T. (2016). Composition and source apportionment of PM1 at urban site Kanpur in India using PMF coupled with CBPF. Atmospheric Research, 178, 506–520.
Rasband W. S. (1997). ImageJ (Maryland, USA: U.S. National Institutes of Health). Available at: https://imagej.nih.gov/ij/. (Google Scholar)
Roberts, T. M. (1975, October). A review of some biological effects of lead emissions from primary and secondary smelters. In Proc. Int. Conf. on Heavy Metals in the Environment. Ed. TC Hutchinson, Univ. Toronto (Vol. 2, pp. 503–532).
Rodríguez-Santamaría, K., Zafra-Mejía, C. A., & Rondón-Quintana, H. A. (2022). Macro-morphological traits of leaves for urban tree selection for air pollution biomonitoring: A review. Biosensors, 12(10), 812.
Saet, Y. U. E., & Smirnova, R. S. (1983). Geochemical principles of delineation of impact zones in urban agglomerations, Landscape Geochemical Zoning and Protection of the Environment. Mysl’. in Russian.
Salam, A., Bauer, H., Kassin, K., Ullah, S. M., & Puxbaum, H. (2003). Aerosol chemical characteristics of a mega-city in Southeast Asia (Dhaka–Bangladesh). Atmospheric Environment, 37(18), 2517–2528.
Salam, A., Hossain, T., Siddique, M. N. A., & Alam, A. S. (2008). Characteristics of atmospheric trace gases, particulate matter, and heavy metal pollution in Dhaka, Bangladesh. Air Quality, Atmosphere & Health, 1, 101–109.
Sawidis, T., Breuste, J., Mitrovic, M., Pavlovic, P., & Tsigaridas, K. (2011). Trees as bioindicator of heavy metal pollution in three European cities. Environmental pollution, 159(12), 3560–3570.
Schreck, E., Viers, J., Blondet, I., Auda, Y., Macouin, M., Zouiten, C., ... & Darrozes, J. (2020). Tillandsia usneoides as biomonitors of trace elements contents in the atmosphere of the mining district of Cartagena-La Unión (Spain): New insights for element transfer and pollution source tracing. Chemosphere, 241, 124955.
Sexton, R., & Roberts, J. A. (1982). Cell biology of abscission. Annual Review of Plant Physiology, 33(1), 133–162.
Shahid, M., Dumat, C., Khalid, S., Schreck, E., Xiong, T., & Niazi, N. K. (2017). Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. Journal of hazardous materials, 325, 36–58.
Shahrukh, S., Hossain, S. A., Huda, M. N., Moniruzzaman, M., Islam, M. M., Shaikh, M. A. A., & Hossain, M. E. (2023). Air pollution tolerance, anticipated performance, and metal accumulation indices of four evergreen tree species in Dhaka Bangladesh. Current Plant Biology, 35, 100296.
Singh, A., Prasad, S., & Rathore, D. (2022). Monitoring of airborne heavy metal using plants: Perspective and challenges. In New Paradigms in Environmental Biomonitoring Using Plants (pp. 27–44). Elsevier.
Tomlinson, D. L., Wilson, J. G., Harris, C. R., & Jeffrey, D. W. (1980). Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen, 33, 566–575.
Vodyanitskii, Y. N. (2010). Equations for assessing the total contamination of soils with heavy metals and metalloids. Eurasian Soil Science, 43, 1184–1188.
Vohra, K., Marais, E. A., Bloss, W. J., Schwartz, J., Mickley, L. J., Van Damme, M., ... & Coheur, P. F. (2022). Rapid rise in premature mortality due to anthropogenic air pollution in fast-growing tropical cities from 2005 to 2018. Science advances, 8(14), eabm4435.
Weckwerth, G. (2001). Verification of traffic emitted aerosol components in the ambient air of Cologne (Germany). Atmospheric Environment, 35(32), 5525–5536.
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica Et Cosmochimica Acta, 59(7), 1217–1232.
Wu, S., & Zhao, B. (2017). Using clear nail polish to make Arabidopsis epidermal impressions for measuring the change of stomatal aperture size in immune response. Plant pattern recognition receptors: methods and protocols, 243–248.
Wu, S. P., Cai, M. J., Xu, C., Zhang, N., Zhou, J. B., Yan, J. P., ... & Yuan, C. S. (2020). Chemical nature of PM2.5 and PM10 in the coastal urban Xiamen, China: Insights into the impacts of shipping emissions and health risk. Atmospheric Environment, 227, 117383.
Zhang, C., Song, N., Zeng, G. M., Jiang, M., Zhang, J. C., Hu, X. J., ... & Zhen, J. M. (2014). Bioaccumulation of zinc, lead, copper, and cadmium from contaminated sediments by native plant species and Acrida cinerea in South China. Environmental monitoring and assessment, 186, 1735–1745.
Zhao, Y., & Zhao, C. (2012). Concentration and distribution analysis of heavy metals in total suspended particulates along Shanghai-Nanjing expressway. Procedia Environmental Sciences, 13, 1405–1411.
Zimdahl, R. L. (1976). Entry and movement in vegetation of lead derived from air and soil sources. Journal of the Air Pollution Control Association, 26(7), 655–660.
Acknowledgements
We would like to thank the Centre for Advanced Research in Sciences (CARS) at the University of Dhaka, Dhaka 1000, Bangladesh, for providing all the research facilities to carry out this work. We are also grateful to the Department of Soil, Water and Environment, University of Dhaka, for their assistance in metal analysis.
Funding
The first author would like to acknowledge the support from the Ministry of Science and Technology, Bangladesh; and the Centre for Advanced Study and Research in Biological Science, University of Dhaka, Bangladesh. Thanks are also due to the Centre for Advanced Research in Sciences and the Department of Soil, Water, and Environment, University of Dhaka, Bangladesh for providing the support for sample analysis.
Author information
Authors and Affiliations
Contributions
1)Zuairia Binte Jashim: Conceptualization, Methodology, Investigation, Data curation, Writing – Original draft preparation.
2)Saif Shahrukh: Writing – Reviewing and Editing.
3)Shahid Akhtar Hossain: Supervision, Project administration, Funding Acquisition, Resources.
4)Jahan-E-Gulshan: Investigation.
5)Md. Mominul Islam: Supervision, Project administration, Funding Acquisition, Resources.
6)Muhammad Nurul Huda: Supervision, Project administration, Funding Acquisition, Resources.
7)Mohammad Enayet Hossain: Supervision, Validation, Formal Analysis, Writing – Reviewing and Editing.
Corresponding author
Ethics declarations
Ethical approval
No human participants or animals were involved in the study.
Consent to participate
Not applicable.
Consent for publication
Consent has been taken from all the coauthors by the corresponding author.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jashim, Z.B., Shahrukh, S., Hossain, S.A. et al. Biomonitoring potentially toxic elements in atmospheric particulate matter of greater Dhaka region using leaves of higher plants. Environ Monit Assess 196, 468 (2024). https://doi.org/10.1007/s10661-024-12612-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12612-3