Abstract
Soil contamination by heavy metals is a serious global environmental problem, especially for developing countries. A large number of industrial plants, which continually pollute the environment, characterize Tuzla Canton, Bosnia and Herzegovina. The aim of this study was to assess the level of soil pollution by heavy metals and to estimate cytotoxicity and genotoxicity of soil leachates from this area. Lead (Pb), cadmium (Cd) and mercury (Hg) were analyzed by ICP-AES and AAS. Soil contamination was assessed using contamination factor, degree of contamination, geoaccumulation index and pollution load index. To determine the connection of variables and understanding their origin in soils, principal component analysis (PCA) and cluster analysis (CA) were used. The results indicate that Cd and Hg originated from natural and anthropogenic activities, while Pb is of anthropogenic origin. For toxicity evaluation, CaCo-2 and HaCaT cells were used. PrestoBlue assay was used for cytotoxicity testing, and γH2A.X for genotoxicity evaluation. Concerning cytotoxicity, Cd and Hg had a positive correlation with cytotoxicity in HaCaT cells, but only Hg induced cytotoxicity in CaCo-2 cells. We also demonstrate that soil leachates contaminated by heavy metals can induce genotoxicity in both used cell lines. According to these results, combining bioassays with standard physicochemical analysis can be useful for evaluating environmental and health risks more accurately. These results are important for developing proper management strategies to decrease pollution. This is one of the first studies from this area and an important indication of soil quality in Southeast Europe.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution caused by a number of anthropogenic activities is a global problem. People are exposed to a variety of potentially harmful agents in the air they breathe, the liquids they drink, the food they eat, the surfaces they touch and the products they use. An important aspect of public health protection is the prevention or reduction in exposure to environmental agents that contribute, either directly or indirectly, to increased rates of premature death, disease, discomfort or disability (WHO 2000).
The soil is one of three major natural but hardly renewable resources, alongside water and air. It is a complex mixture of organic and inorganic materials. Although its primary purpose is the production of food and raw materials, soil is not only a medium for plants to grow or a pool to dispose of undesirable materials but also a transmitter of many pollutants to surface water, groundwater, atmosphere and food (Chen et al. 1997).
Soils are characterized by the presence of naturally occurring trace amounts of heavy metals, which are considered as background values (Christova et al. 2007). However, pollution by heavy metals is mainly due to anthropogenic activities. Global concern regarding soil pollution by heavy metals is related to their non-biodegradable capacity, bioaccumulation and high toxicity (Eja et al. 2003; Likuku et al. 2013). Accumulation of heavy metals in agricultural soil can lead to their bioaccumulation in the crops, and through the food chain, to the exposure of animals and humans with possible adverse effects (Liu et al. 2016; Iqbal et al. 2016). Although all metallic pollutants can cause adverse effects, Cd, Pb and Hg are heavy metals of major public health concerns. They are systemic toxicants and are known to induce multiple organ damage including kidneys, lungs, bones, etc. (Jarup 2003; Fernandez-Luqueno et al. 2013; Tchounwou et al. 2012). According to the United States Environmental Protection Agency (U.S. EPA), Agency for Toxic Substances and Disease Registry (ATSDR) and the International Agency for Research of Cancer (IARC), these metals are also classified as either known or probable human carcinogens. Epidemiological and experimental studies indeed show an association between the exposure and cancer incidence in humans and animals (ATSDR 1999, 2007, 2012; U.S. EPA 2000a, b, 2011; IARC 1993a, b, 2006, 2012).
The present EU and national legislation on soil contamination by heavy metals are based upon their total concentrations. Chemical analyses can provide information about the metal concentration in soil, but they are not sufficient to evaluate their bioavailability and to assess environmental impacts of the combined metal exposure. Combined effects of multiple coexisting metals can be greatly different from the individual ones and can result in additive, supra-additive, synergistic or antagonistic activity (Chen et al. 2005). Bioavailability and bioaccumulation of chemicals as well as their interactions can be evaluated by different bioassays. Various methods are employed to assess the cytotoxic and genotoxic potential of the soil (Bekaert et al. 1999; Watanabe and Hirayama 2001; Lah et al. 2005, 2008; Vidic et al. 2009; Katnoria et al. 2011; Khan et al. 2015; Alimba et al. 2016). Bioavailability of heavy metals is a key factor in the evaluation of cytotoxic, genotoxic and ecotoxic effects of heavy metal-contaminated soils (Alvarenga et al. 2008). Toxicity and bioavailability of heavy metals in soil samples depend not only on the total concentrations of heavy metals but also on their forms. Apart from total concentrations, it is necessary to also analyze the concentrations of labile fractions of heavy metals in soil (Liu et al. 2016). Integration of physicochemical and (eco)toxicological studies is nevertheless recommended for refined evaluation of environmental and human health risk (Bekaert et al. 1999; Chen et al. 2016; Aelion et al. 2009).
Soil contamination by heavy metals is one of the most pressing environmental issues, especially in developing countries. Tuzla Canton is the most populous area in Bosnia and Herzegovina. This area abounds with numerous natural resources, of which coal and salt are most distinguished. Accordingly, the industrialization of the Canton has been developed by exploitation and processing raw materials. Industrial development along with inadequate risk mitigation measures has led to a risk to human health and/or ecosystems. Despite the fact that soil in this area has been continuously contaminated by heavy metals, very few studies have been done so far (Cipurkovic et al. 2011; Cipurkovic et al. 2014a, b; Dellantonio et al. 2008; Djozic et al. 2014; Djozic 2015; Tunjic et al. 2015). Therefore, this work was aimed at investigating the heavy metal contamination of soil in Tuzla Canton area and evaluating in vitro cytotoxic and genotoxic potential of soil leachates by applying an integrated physicochemical–biochemical approach.
Materials and methods
Chemicals, reagents and glassware
All chemicals and reagents for heavy metal analysis were of analytical grade, and all standards were prepared with reagent-grade chemicals. Double-distilled deionized water was used for all dilutions. HNO3 and HCl were of supra pure quality. All glassware and plastic were cleaned by soaking in diluted HNO3 (10% v/v) for 24 h and rinsed with distilled water three times prior to use.
For in vitro cytotoxicity and genotoxicity testing, cell lines HaCaT and Caco-2 were obtained from the Laboratory for Environmental and Life Sciences, University of Nova Gorica, Slovenia. All reagents were of analytical grade. Only sterile glassware, plasticware and pipets were used.
Study area
The Tuzla Canton area is situated in the north-eastern part of Bosnia and Herzegovina. It is an industrial region with a number of heavy metal emission sources. The major sources are Thermal Power Plant Tuzla (TPP) and different chemical and other industries, such as Ytong and Poliochem in Tuzla, former Chlor-alkali Plant Tuzla (HAK), Cement Factory in Lukavac (FCL), Soda Factory in Lukavac (SSL) and Global Ispat Coke Industry Lukavac (GIKIL). The Jala and the Spreca rivers in Tuzla Canton area have been receiving copious amounts of wastes from the industry and from residential wastewaters. Agricultural land was flooded when rivers overflowed their banks after a long period of rain in May 2014. Investigated area is in the region of moderately continental climate, with cold winters and hot summers. Southwest–northeast winds are dominant because of the ground configuration.
Sample collection and chemical analyses
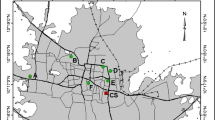
Soil samples were collected at eleven locations, which were selected on the basis of their distance from the energy or industrial facilities. The selected sites represent most representative pollution sites in the Tuzla Canton according to previous studies carried out in this area of Bosnia and Herzegovina. The locations of sampling sites are indicated in Fig. 1. All samples were taken from April 2016 to September 2016. At least three soil samples were collected from each site. Each sample was obtained by collecting 8–10 subsamples from a defined location following a combination of the two approaches: sampling pattern and judgemental sampling. Each of 33 samples from eleven sites was prepared and analyzed in triplicate to determine the contents of heavy metals. The results are presented as mean value of nine measurements for each site.
The methodology of soil sampling and preparation was carried out according to standard procedures ISO 11047, ISO 11466, ISO 11464, ISO 10381 and ISO 10381-5 (ISO 10381-5 2005; ISO 10381-6 2009; ISO 11464 2006).
Topsoils (0–15 cm) were sampled using a plastic tool and transported to the laboratory. Samples were air-dried at room temperature for 7 days. Then, they were ground into fine powder, sieved through a 0.15-mm polyethylene sieve and repacked in transparent plastic bags until analysis.
The total Pb content was determined by Inductive Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES Optima 2100 DV) at Faculty of Mining, Geology and Civil Engineering, University of Tuzla, Bosnia and Herzegovina, and concentrations of Cd and Hg were determined by Atomic Absorption Spectroscopy (Varian SpectrAA 220 GTA 110 and Varian SpectrAA 220 with VGA 77) at Institute for Hygiene and Technology of Meat, Institutions Belgrade, Serbia.
Samples were digested by concentrated acid solutions HCl:HNO3 (3:1). Then, they were filtered and diluted with deionized water in volumetric flasks. A reagent blank for each metal was also prepared and passed through the whole process.
Calibration curves were prepared using analytical-grade metals. A reagent blank was used to zero the instrument. Each sample was analyzed in triplicate. The results were expressed in mg/L of filtrate.
For establishing background values of Pb, Cd and Hg samples were collected from a remote location with no anthropogenic sources of contamination. Three samples per season were collected from the site. Each sample was prepared and analyzed in triplicate. The mean value of all measurements is used as a background concentration.
Particle-size analysis
Particle-size analysis (PSA) was used to evaluate soil texture. The percentage of clay, dust, sand and gravel were measured with this procedure. Approximately 1 kg of each air-dried soil sample was thoroughly mixed and rolled with a wooden rolling pin. Samples were sieved through nine sieves from 8 to 0.045 mm mesh size. The PSA was conducted in Faculty of Mining Geology and Civil Engineering in Tuzla.
Cell lines
Cell lines for in vitro testing were obtained from the Laboratory for Environmental and Life Sciences, University of Nova Gorica, Slovenia. Epithelial colon cancer cells (Caco-2) and Normal Adult Human Primary Epidermal Keratinocytes (HaCaT) were grown in monolayer culture at 37 °C in humidified atmosphere and 5% CO2. We used Dulbecco’s modified Eagle’s medium (DMEM) for Caco-2 and Dulbecco’s modified Eagle medium/Nutrient Mixture F-12 (DMEM F-12) for HaCaT, both supplemented with 10% fetal calf serum (FCS), 1% l-glutamine and antibiotics (100 μg/mL penicillin + 100 μg/mL streptomycin). Single-cell suspension was prepared with 0.25% trypsin–ethylenediaminetetraacetic acid solution (trypsin–EDTA), and cells were finally resuspended in the complete growth medium DMEM or DMEM F-12.
Cell viability: cytotoxicity assay PrestoBlue
Soil leachates (soil: water, 1:2 w/v) were prepared for in vitro testing according to Bekaert et al. (1999). Leaching was performed for 24 h in a linear shaking apparatus at 25 ± 1 °C, and the suspension was allowed to settle for 24 h at 4 °C. The supernatant was used for biotesting the next day.
For cell viability assay, eleven samples were prepared as 5% water soil extract in suitable nutrient medium. As a buffer control, we used 5% deionized water in the medium. Positive control was 30% dimethyl sulfoxide (DMSO) in the nutrient medium, while untreated cells served as a negative control.
The PrestoBlue (PB) assay is a commercially available, ready-to-use, water-soluble preparation. Cell viability assay with PB reagent was performed according to the manufacturer’s protocol (Thermo Fisher Scientific 2010). The cells in suspension were seeded at 1 × 104 cells/well in a 96-well microtiter plate. After 24-h treatment of the cells with 5% water soil extracts, 5% deionized water, positive and negative control, the cells were washed and incubated with PB reagent. Cell viability was evaluated by fluorescence spectroscopy. The fluorescence was measured after 45 min of incubation using a monochromator plate reader (Victor-X2 2030 and program WorkStation 2030) with excitation and emission wavelengths of 570 and 610 nm, respectively. Relative cell viability was expressed as a percentage relative to the untreated control cells
Genotoxicity test
For the assessment of genotoxicity, two soil samples were used. They were chosen on the basis of the same distances from industrial facilities in the opposite directions. Both samples were from the agricultural area, TZ-10 from the lower course of Spreca river, and TZ-5 from the upper course of Jala river.
Indirect immunofluorescence method was used to determine genotoxicity of water soil extracts according to the protocol described by Tanaka et al. (2009). Primary antibody anti-phospho-histone H2A.X was used to identify the damaged DNA chains. Cells were seeded onto glass slides for 24 h at 37 °C. After incubation, the cells were treated for 24 h with 5% water soil extracts in the medium or with the sterile deionized water in the medium as a control. Cells were fixed with 3.7% p-formaldehyde for 15 min at room temperature, permeabilized with 0.5% Triton X-100 for 5 min, blocked with 5% bovine serum albumin (BSA) for 15 min and incubated with anti γH2A.X monoclonal antibody for 1 h at 37 °C. Cells were then washed and incubated with AF488-labeled secondary antibodies for 1 h at 37 °C (Robinson et al. 2009). Nuclei of cells were labeled with 4′,6-diamidino-2-phenylindole (DAPI) dye, which non-specifically binds to DNA molecules. The slides were then mounted in fluorescence mounting medium and visualized using inverted fluorescence microscope (Olympus IX-81) with Olympus Q-Color 5 imaging system. The analysis of images was performed using SlideBook 5.0 digital microscopy software.
Statistical methods
All experiments were set up in triplicate, and the results were expressed as mean values.
A multivariate statistic approach was adopted to assist the interpretation of geochemical data: Principal component analysis (PCA) and cluster analysis (CA) were the statistical tools adopted for data analysis. MS Excel was used to generate descriptive statistics, and IBM SPSS 21 was used for data analysis.
Correlation between heavy metals concentrations and cell viability for both cell lines was established using Pearson’s correlation analysis. The Pearson’s linear correlation was used to determine the associations between concentrations of Pb, Cd or Hg in soils and cytotoxicity values.
The resulting data of heavy metals concentrations and number of identified cells with damaged DNA, for both cell lines, were subjected to significance test, Chi-square analysis. The Chi-square test was used to determine association between two categorical variables, separately concentration of Pb, Cd or Hg in soils and number of identified cells with damaged DNA chain.
The results were considered to be significant at p < 0.05.
Results
Particle-size analysis (PSA)
Grain-size distribution analysis revealed that all eleven studied samples can be classified as sandy soil. Grain-size distribution curves in Fig. S1 show that more than 55% of each sample was in the sand particle size.
Heavy metal content
Three heavy metals were analyzed in samples collected from eleven sampling sites (Fig. 1). The concentrations of Pb, Cd and Hg in the soil samples were measured to estimate the level of pollution and are summarized in Table 1. Obtained values were compared to the permitted limit values (PLVs) of all three metals according to Federal Bosnian and Herzegovinian (F BiH) legislation and ordinance [Law of Agricultural Land (Official Gazette F BiH 52/09 2009) and Ordinance on Identification of the Allowable Amounts of Harmful and Hazardous Substances in Soil and Methods of their Examination (Official Gazette F BiH 72/09 2009)] and to the background values of heavy metals obtained in this study.
According to F BiH legislation and ordinance, the PLVs for Pb were exceeded at sampling locations TZ-2, TZ-3, TZ-5, TZ-6, TZ-10, and TZ-11. For Cd, PLVs were exceeded at two locations TZ-4 and TZ-10, and for Hg at the location TZ-10.
Pb concentrations ranged from 24.33 to 499.59 mg kg−1. The highest concentration of Pb was determined in soil samples TZ-2 and TZ-3, which were sampled near the industrial area and the highway.
Cd was detected in ten samples, in concentrations ranging from 0.08 to 1.29 mg kg−1. The highest concentration of Cd was detected in a sample of agricultural land in the valley of the Spreca River.
Similar to Pb, Hg was detected in all eleven samples, with concentrations ranging from 0.04 to 0.55 mg kg−1. The highest Hg concentration was detected in the sample TZ-10, which had also the highest concentration of Cd. In the sample TZ-2, which is located within industry of HAK, the highest concentration of Pb and the second highest concentration of Hg were detected.
Distribution of soil samples with their respective heavy metal concentrations is shown in Fig. S2. The major contamination source for each sampling site is also provided.
In order to examine relationship among Cd, Hg and Pb concentrations, origin and source of contamination principal component analysis (PCA) and cluster analysis (CA) were applied. Results of the PCA are listed in Table 2. According to the results of the initial eigenvalues, there are two principal components that account for over 93.67% of the total variance. The initial component matrix indicates that Hg and Cd are associated, displaying high values in the first component (F1), while Pb is isolated in the second component (F2).
Hierarchical cluster analysis was performed on heavy metals based on correlation coefficients (Pearson coefficient). The results are shown in Table 3 and Figure S3. According to these results, Hg and Cd are very well correlated with each other. These results confirm the obtained results with the PCA.
Assessment of heavy metal pollution
Assessment of pollution was conducted using contamination factor (CF), contamination degree (Cdeg), geoaccumulation index (Igeo) and pollution load index (PLI) parameters.
Contamination factor is calculated as:
where Cn is the concentration of the heavy metal in contaminated sites and Bn is the median concentration of an element in the background soil sample.
The degree of contamination is calculated as follows:
where N is the number of element analyzed, and CF is the contamination factor.
The results of contamination factor and contamination degree are listed in Table 4. Contamination factor values indicate very high contamination by Pb at TZ-2, TZ-3 and TZ-5 and moderate to considerable contamination for all remaining sampling sites (1.09 < CF < 4.40). Using CF, the soil was classified as very highly contaminated by Cd at TZ-4 and TZ-10, and moderately to considerably contaminated for all remaining sample sites (1.00 < CF < 2.82) except TZ-8 where CF indicates low contamination (CF < 1). Contamination factor values show very high contamination by Hg at TZ-10 and TZ-2, low contamination at TZ-5 and TZ-8 and moderate to considerable contamination for all remaining locations (1 < CF < 3.14).
The contamination degree suggests that the studied area is low to moderately contaminated (1.04 < Cdeg < 10.19).
Geoaccumulation index is calculated as follows:
where Cn is the concentration of the heavy metal in the sample, Bn is the geochemical background value, and 1.5 is the background matrix correction factor due to lithogenic effects.
The results of the geoaccumulation index are shown in Fig. 2. The values of geoaccumulation index reveal that soil samples are highly polluted with Pb at TZ-2 (3.90) and TZ-3 (3.36), moderately to strongly polluted at TZ-5 (2.08), moderately polluted at TZ-6 (1.30) and TZ-11 (1.55), and unpolluted to moderately polluted at TZ-1 (0.48), TZ-7 (0.38) and TZ-8 (0.28). The results show that two samples at TZ-4 (− 0.46) and TZ-9 (− 0.05) are unpolluted by Pb.
The results of geoaccumulation index indicate that soil is strongly polluted with Cd at TZ-10 (3.21), moderately to strongly polluted at TZ-4 (2.97), unpolluted to moderately polluted at TZ-2 (0.35), TZ-3 (0.04) and TZ-5 (0.91). Cd pollution was not detected at TZ-6 (− 0.58), TZ-7 (− 0.04), TZ-8 (− 1.04), TZ-9 (− 0.24) and TZ-11 (− 0.58).
Geoaccumulation index reveals that soil samples are moderate to strongly polluted with Hg at TZ-2 (2.07) and TZ-10 (2.39), moderately polluted at TZ-1 (1.07) and TZ-3 (1.00), unpolluted to moderately polluted at TZ-4 (0.51) and TZ-6 (0.19). The Igeo values show that samples TZ-5 (− 0.81), TZ-7 (− 0.39), TZ-8 (− 1.39), TZ-9 (− 0.58) and TZ-11 (− 0.22) are not polluted with Hg.
Pollution load index (PLI), for a particular site, was evaluated following the method proposed by Tomlinson et al. (1980). This parameter is expressed as:
where n is the number of metals (three in the present study) and CF is the contamination factor. In this study, PLI suggests that soil quality is degraded at all sampling sites except TZ-8 (PLI = 0.99). The results are shown in Fig. 3.
Assessment of cytotoxicity of soil extracts by PrestoBlue assay
The results obtained were expressed as a percentage (%) of survival, using the untreated cells as a reference. As shown in Fig. 4, treatment with soil leachates resulted in a different reduction in cell viability for both cell lines tested. Higher fluorescence values correlate to greater total metabolic activity. Greater metabolic activity indicates greater cell viability and reduced cytotoxicity, and vice versa.
Cell viability of HaCaT and CaCo-2 cell lines after the exposure to soil samples of Tuzla Canton area. Cells were exposed to 5% soil leachates in nutrient medium, positive control and buffer control for 24 h. Cell viability was then measured by PrestoBlue assay and compared to untreated cells (100%)
The cytotoxicity in HaCaT cells did not show any correlation with the total Pb concentrations (Pearson’s correlation coefficient r = − 0.31, p = 0.35). However, total metal concentrations of Cd and Hg showed positive correlation with cytotoxicity in HaCaT cells (Pearson’s correlation coefficient for Cd: r = 0.75, p = 0.01 and for Hg: r = 0.63, p = 0.025).
The cytotoxicity in CaCo-2 cells had a correlation with Hg concentrations (Pearson’s correlation coefficient r = 0.59, p = 0.05). The total metal concentration of Pb and Cd did not correlate with cytotoxicity in CaCo-2 cells (Pearson’s correlation coefficient for Pb: r = − 0.28, p = 0.41 and for Cd: r = 0.60, p = 0.06).
Interestingly, both cell lines showed the highest viability after the exposure to TZ-8, which had the smallest pollution load index, confirming validity of our assay.
Assessment of genotoxicity of soil extracts by immunofluorescence
Genotoxicity of two soil leachates (TZ-5 and TZ-10) in two cell lines was examined by immunostaining using anti-H2A.X antibodies, which mark the DNA double-strand breaks. Total number of cells and number of cells with damaged genetic material were determined by the direct method of cell counting. A representative image of the immunostaining is shown in Fig. 5. We can see that some of the cells have bright green nuclei with dots (positive cells), representing DNA damages, while others show no green signal (negative cells).
For each sample, approximately 150 cells were analyzed and the percentage of positive cells (those with damaged genetic material) was calculated. Both soil leachates in nutrient medium were found to be genotoxic for HaCaT and CaCo-2 cell lines. Leachates showed significant genotoxicity in HaCaT cells compared to untreated cells (statistical significance for TZ-5: p < 0.0001, χ2 = 282.21 and for TZ-10: p < 0.0001, χ2 = 1050.56). CaCo-2 cells treated with soil leachates also showed significant genotoxicity compared to untreated cells (statistical significance for TZ-5: p < 0.0001, χ2 = 4029.26 and for TZ-10: p < 0.0001, χ2 = 3722).
Discussion
The results of heavy metal pollution are summarized as follows: 54.55% of soil samples had higher concentrations of Pb than corresponding PLV, 20% of soil samples exceeded the maximum permissible concentration for Cd, and only one soil sample (9%) had a higher concentration of Hg than corresponding PLV.
For the pollution assessment, four parameters were used: CF, Cdeg, PLI and Igeo. Multivariate analysis can be applied to improve the accuracy of the source identification (Chabukhdaha and Nema 2013; Facchinelli et al. 2001). For this reason and to make the results more easily interpretable, PCA and CA were applied in our study. PCA indicates the degree of pollution by heavy metals from lithogenic action and anthropogenic activities (Facchinelli et al. 2001; Sun et al. 2010; Hu et al. 2013). According to the results of initial eigenvalues, Pb, Cd and Hg concentrations could be grouped into a two-component model, which accounts for 93.67% of all data variation. The initial component matrix that Cd and Hg were associated with displays high values in the first component (F1), while the second component (F2) shows high value of Pb. In the interpretation of PCA patterns, factor loading values higher than 0.71 are typically considered excellent, while those lower than 0.32 are regarded as very poor (Hu et al. 2013). All heavy metals in our study had factor loadings greater than 0.71. Hierarchical cluster analysis was also used to identify the relatively homogenous groups of heavy metals. Table 3 and Fig. S3 show that Cd and Hg significantly correlate with each other and form a cluster. The relationships between heavy metals can provide important information on heavy metal sources and pathways (Manta et al. 2002). Positive correlation was established between Cd and Hg (r = 0.82, p = 0.003). As expected, Pb was separated from other two metals, which indicate slow association of Pb with either Cd or Hg. The results of PCA and CA, combined with Igeo and CF analyses, consistently describe the basic distribution patterns of the studied heavy metals. These results suggest that heavy metals polluting surface soils of Tuzla Canton could be classified into two groups. The first group includes Cd and Hg, which could be considered as a coexistent component originated by both natural and anthropogenic sources. According to Muller’s (1969) classification for the geoaccumulation index, the percentage of soils with Igeo < 0, which are classified as uncontaminated, was 50% in the case of Cd and 45.45% in the case of Hg (Fig. 2). Negative Igeo values suggest that heavy metals from uncontaminated sites originate from natural sources. Similar to Cd, higher values of Hg were also observed in soils from the industrial area and agricultural fields near Spreca river. Combustion of fossil fuels, particularly coal, waste incineration and improper disposal and storage of industrial waste all release relatively large amounts of Cd and Hg into the environment. There are great amounts of mercury in former HAK (chlor-alkali) industrial area, which has leaked into the environment in 2012 due to improper storage in big plastic bottles. There is a thermal power plant near the above-mentioned industrial facility, which disposes of its waste close to the city, with part of this waste disposed also into the river Jala (Dellantonio et al. 2008). The soil analysis near the coal ash landfill indicated increased concentrations of Cd in the soil (Djozic 2015). Results of this study show increased values of Hg and Cd in the soil near these industrial areas, their disposal sites or in the valley of the river Jala. The river has been a disposal site for many years. It is a tributary of the river Spreca, which spreads the contaminants onto the agricultural land nearby during regular flood periods. Accordingly, PCA and CA show that Cd and Hg may originate from a common source of contamination.
On the other hand, Pb can be defined as anthropogenic component and may originate from sewage, industrial and traffic activities. Common sources of lead in soils are sewage sludge, lead-arsenate pesticides, vehicle exhaust and industrial fumes. Tuzla Canton is at the crossroad of many big cities in southeast Europe. Several studies have shown vehicle emissions to be the principal source of Pb in urban environments (Facchinelli et al. 2001; Li et al. 2004; Lee et al. 2006; Yang et al., 2011). Taking into consideration that high levels of Pb were found in areas of heavy traffic and that Igeo values for Pb were relatively high (81.82% of soils that are classified as contaminated by Pb) contamination of soil by Pb in this area very likely originates from both vehicle emission and industrial activities.
To effectively compare a measure of the degree of overall contamination at a sampling site, the pollution load index was used. Relatively high PLI values suggest input from anthropogenic sources attributed to increased human activities and industrial activities or traffic (Mmolawa et al. 2011). The higher PLI values indicated that Pb and Hg are the major contributors to the soil pollution at the location TZ-2, Cd and Hg at location TZ-10, and Pb at the location TZ-3. A large variety of industrial fumes may be responsible for air pollution by heavy metals and consequently soil pollution when they precipitate on the soil surface. Research of the Federal Institute for Agropedology of Bosnia and Herzegovina in period 2008–2010 has pointed to increased concentrations of heavy metals in soil in FBiH (Bukalo et al. 2013). Furthermore, floods of the rivers Spreca and Jala in the investigated area could contribute to soil pollution. Studies conducted in 2014 on soils from Jala and Spreca river banks point to increased levels of Hg around chemical plants, especially in front of the plant Polihem Tuzla where industrial waste was often discharged into the river Jala (Cipurkovic et al. 2014a, b; Djozic et al. 2014). The analyzed soil is characterized as low to moderately contaminated with Pb and Cd and extremely contaminated with Hg. The research conducted on soils near industrial facilities in Tuzla Canton area in 2012 indicated extremely high pollution with Hg that is likely the result of many years of electrolysis in Poliochem industry (Tunjic et al. 2015). Research on soil heavy metals levels in industrial areas and along roads of Tuzla Canton has shown that the soil and in the surrounding factories and highways have significantly increased amounts of heavy metals (Osmanovic et al. 2015; Pasalic et al. 2015). The results obtained in the present study confirmed the previously published data on soil pollution with heavy metals in this area (Cipurkovic et al. 2011, 2014a, b; Djozic et al. 2014; Tunjic et al. 2015).
To provide clearer insight into the impact of metal pollution (Pb, Cd and Hg) on soil quality and consequently on human health, we combined physicochemical analyses with bioassays. Although the total concentration of Pb in more than 50% of samples highly exceeded its PLV (Table 1), cytotoxic effects in HaCaT and CaCo-2 cells did not correlate with the high concentration of Pb in soil samples. It is known, however, that Pb after chronic exposure can induce cytotoxicity in humans (Chen et al. 2002; Jadhav et al. 2000; Steffensen et al. 1994; Tchounwou et al. 2004). This effect is less pronounced in short-term in vitro assays. Low toxicity of Pb in our experiment could be attributed also to a reportedly low water extractability, which has been recognized as a common problem in similar studies (Alvarenga et al. 2008; Aruoja et al. 2004; Kahru et al. 2005; Lah et al. 2005, 2008). Bioavailability of Pb in contaminated soils is usually rather low (Alloway 1995; Kede et al. 2014). Low leaching concentration of Pb is related to low mobility of Pb in soil, and this can also contribute to low cytotoxicity (Zheng et al. 2012). It has been reported that metal concentrations in leachates that were further applied for biotests were lower than the total metal concentration in soils (Knasmuller et al. 1998; Lah et al. 2008). Alimba et al. (2016) have reported a clear decrease in viability of three cell lines after exposure to landfill soil leachates contaminated with Pb and other heavy metals, although using different extraction method. More research is needed to optimize the extraction protocols and to achieve good extraction of heavy metal from soil in a buffer suitable for in vitro cell testing. It has also been shown that filtration of crude leachates through membrane filters used in the leaching procedure can cause reduction in heavy metal contents (Bekaert et al. 1999; Lah et al. 2008; Moucher et al. 2006). It was reported that the extractability of heavy metals with water was lower for soil samples that were more polluted, as low extractability of metals was similar whatever their content in the soil (Alvarenga et al. 2008; Aruoja et al. 2004; Kahru et al. 2005; Vidic et al. 2009). Cadmium shows much higher bioavailability in contaminated soil (Gu et al. 2016). In our study, cytotoxic effect of Cd and Hg in HaCaT cells correlates with their concentration in the particular soil sample. According to IARC classification (IARC 2017), Cd is classified as carcinogenic to humans (Group 1), inorganic Pb as probably carcinogenic (Group 2A) and organic Hg as possibly carcinogenic to humans (Group 2B). All three metals can induce ROS generation, apoptosis, DNA damage and other cytopathological effect (Olivieri et al. 2000; Tsuzuki et al. 1994; Trabelsi et al. 2016; Tchounwou et al. 2012; Unyayar et al. 2006).
We demonstrated that soils leachates contaminated by heavy metals can induce genotoxicity both in HaCaT and in CaCo-2 cell lines. Water-extractable Pb, Cd and Hg are most likely involved in the DNA damage induction. They are potential mutagens, capable of inhibiting DNA synthesis or interfering with DNA repair (Beyersmann and Hartwig 2008; Crespo-Lopez et al. 2009; Unyayar et al. 2006; Villatoro-Pullido et al. 2009). The genotoxicity of metals is a complex issue, particularly in case of coexistence of heavy metals, as is the case in soil leachate. Their cocktail effects on organisms and humans are still poorly understood (Matovic et al. 2015; White and Claxton 2004). We found a clear relationship between genotoxicity of contaminated soil leachates and DNA damage. Namely, the sample TZ-10 with the highest values of Cd and Hg caused a high genotoxicity effect. These results suggest that heavy metals can act jointly to produce mixture effects that are greater than the effects of each toxic metal separately. Combined exposure to metals such as lead, cadmium and mercury may lead to additive or even synergistic effects (Ishaque et al. 2006; Matovic et al. 2015; Silins and Högberg 2011). Lah et al. (2008) reported that soil leachates contaminated with heavy metals can cause genotoxic effects on HepG2 and CaCo-2 cells. Clear relationship between metal contents and genotoxicity was also reported by Villatoro-Pullido et al. (2009).
In real circumstances, rainfall play an important role for the leaching of metals. The leaching of heavy metals can directly influence on groundwater contamination. Heavy metal concentration in groundwater samples was found higher in dry than wet season (Buragohain et al. 2010; Giridharan et al. 2008; Mehrabi et al. 2015; Tiwari et al. 2016; Venugopal et al. 2009). Because of seasonal rainfall, heavy metal concentration was found to be more diluted in the wet season compared with the dry season due to the higher groundwater recharge in the wet season (Huang et al. 2008). It is worth mentioning, however, that soil leachates from different contamination sources and different studies are difficult to compare. In soil, the effects of complex mixtures of pollutants including heavy metals and other organic and inorganic components on cell physiology and morphology are exceedingly difficult to predict.
Conclusion
This study was carried out in order to assess the degree of soil pollution with heavy metals and to estimate cytotoxicity and genotoxicity of soil leachates in Tuzla Canton area. We found that concentrations of heavy metals are significantly higher than the tolerable values in soils near industrial areas and the highway. High concentrations of these toxic metals also occur along the Spreca river banks where local population tends to use water from the contaminated river for irrigation of agricultural land. PCA, CA and correlation between heavy metals indicate that Cd and Hg sources of contamination in soils are controlled by natural and anthropogenic origin. On the other hand, Pb originated mainly from anthropogenic activities including sewage, industrial and traffic activities. Based on this research, the high proportion of samples with heavy metal concentrations above the assessment threshold highlights the need for intensified and continuous monitoring of soil contamination in this area. An appropriate risk assessment of heavy metals should include biological effects of the polluted soil samples. The present results indicate that Cd and Hg from soils can induce cytotoxicity in human cell lines. Low water extractability and low bioavailability of Pb in soil samples could be the reason why Pb concentration in soils did not correlate with cytotoxicity. This study has also demonstrated that soil contaminated with heavy metals can induce genotoxicity in human cell lines. All three examined heavy metals correlated well with the observed DNA damage. In summary, we conclude that heavy metals from soil samples in Tuzla Canton area can cause adverse health effects by inducing cell and DNA damage. In order to identify the possible adverse impact on human health and relationship among different soil pollutants, further studies need to be carried out.
References
Aelion, C. M., Davis, H. T., McDermott, S., & Lawson, A. B. (2009). Soil metal concentrations and toxicity: Associations with distances to industrial facilities and implications for human health. Science of the Total Environment, 407(7), 2216–2223.
Alimba, C. G., Gandhi, D., Sivanesan, S., Bhanarkar, M. D., Naoghare, P. K., Bakare, A. A., et al. (2016). Chemical characterization of simulated landfill soil leachates from Nigeria and India and their cytotoxicity and DNA damage inductions on three human cell lines. Chemosphere, 164, 469–479.
Alloway, B. J. (1995). Soil process and the behavior of metals. In B. J. Alloway (Ed.), Heavy metals in soils (pp. 11–37). London: Blackie Academic & Professional.
Alvarenga, P., Palma, P., Goncalves, A. P., Fernandez, R. M., de Varennes, A., Duarte, V. G. E., et al. (2008). Evaluation of tests to assess the quality of mine-contaminated soils. Environmental Geochemistry and Health, 30, 95–99.
Aruoja, V., Kurvet, I., Dubourguier, H.-C., & Kahru, A. (2004). Toxicity testing of heavy-metal-polluted soils with algae Selenastrum capricornutum: A soil suspension assay. Environmental Toxicology, 19, 396–402.
ATSDR. (1999). Toxicological profile for mercury. Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/toxprofiles/tp46.pdf. Assessed March 16, 2017.
ATSDR. (2007). Toxicological profile for lead. Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf. Assessed March 16, 2017.
ATSDR. (2012). Toxicological profile for cadmium. Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf. Assessed March 16, 2017.
Bekaert, C., Rast, C., Ferrier, V., Bispo, A., Jourdain, M. J., & Vasseur, P. (1999). Use of in vitro (Ames and Mutatox tests) assays to assess the genotoxicity of leachates from contaminated soil. Organic Geochemistry, 30, 953–962.
Beyersmann, D., & Hartwig, A. (2008). Carcinogenic metal compounds: Recent insight into molecular and cellular mechanism. Archive of Toxicology, 82, 493–512.
Bukalo, E., Trako, E., Mitrovic, M., Behlulovic, D., & Rahmani, S. (2013). Soil monitoring in the Federation BiH. In Proceedings of the 48th Croatian and 8th international symposium of agronomist (pp. 65–69).
Buragohain, M., Bhuyan, B., & Sarma, H. P. (2010). Seasonal variation of lead, arsenic, cadmium and aluminium contamination of groundwater in Dhemaji district, Assam, India. Environmental Monitoring and Assessment, 170, 345–351.
Chabukhdaha, M., & Nema, A. K. (2013). Heavy metals assessment in urban soil around industrial clusters in Ghaziabad, India: Probabilistic health risk approach. Ecotoxicology and Environmental Safety, 87, 57–64.
Chen, C. L., Liao, M., & Huang, C. Y. (2005). Effect of combined pollution by heavy metals on soil enzymatic activities in areas polluted by tailings from Pb-Zn-Ag mine. Journal of Environmental Science, 17(4), 637–640.
Chen, G., Straalen, N. M., & Roelofs, D. (2016). The ecotoxicogenomic assessment of soil toxicity associated with the production chain of 2,5-furandicarboxy acid (FDCA), a candidate bio-based green chemical building block. Green Chemistry, 18(16), 4420–4431.
Chen, T. B., Wong, J. W. C., Zhou, H. Y., & Wong, M. H. (1997). Assessment of trace metal distribution and contamination in surface soils of Hong Kong. Environmental Pollution, 96(1), 61–67.
Chen, L., Yang, X., Jiao, H., & Zhao, B. (2002). Tea catechins protect against lead-induced cytotoxicity, lipid peroxidation, and membrane fluidity in HepG2 cells. Toxicological Science, 69(1), 149–156.
Christova, J., Christov, D., & Kuikin, S. (2007). Background contents of some minor and trace elements in the rocks on Bulgarian territory. Geologica Balcanica, 36, 65–76.
Cipurkovic, A., Selimbasic, V., Tanjic, I., Micevic, S., Pelemis, D., & Celikovic, R. (2011). Heavy metals in sedimentary dust in industrial city of Lukavac. European Journal of Scientific Research, 54(3), 347–362.
Cipurkovic, A., Trumic, I., Selimbasic, V., Djozic, A., Tunjic, J., & DjulovicJusic, Z. (2014a). Assessment of mercury pollution in soils along Jala and Spreca river banks in Bosnia and Herzegovina. Journal of Trends in the Development of Machinery and Associated Technology, 18, 183–186.
Cipurkovic, A., Tunjic, J., Selimbasic, V., Djozic, A., & Trumic, I. (2014b). Assessment of heavy metal distribution and contamination in soils at Jala River Banks. European Journal of Scientific Research, 127(4), 392–405.
Crespo-Lopez, M. E., Macedo, G. L., Pereira, S. I. D., Arrifano, G. P. F., Picanco-Diniz, D. L. W., Nascimento, J. L. M., et al. (2009). Mercury and human genotoxicity: Critical considerations and possible molecular mechanisms. Pharmacological Research, 60, 212–220.
Dellantonio, A., Fitz, W., Custovic, H., Repmann, F., Schneider, B. U., Grunewald, H., et al. (2008). Environmental risks of farmed and barren alkaline coal ash landfills in Tuzla, Bosnia and Herzegovina. Environmental Pollution, 153, 677–686.
Djozic, A. (2015). The presence of heavy metals in the soil and locally produced food in settlements around landfills with slag Divkovici/Plane from Thermal Power Plant Tuzla. Tuzla: Center for Ecology and Energy, Off-set.
Djozic, A., Selimbasic, V., Cipurkovic, A., Crnkic, A., Hodzic, Z., & Trumic, I. (2014). Heavy metals in dust deposition in the vicinity of coal ash disposal site Divkovici II. Journal of Life Sciences, 8(5), 461–472.
Eja, C. E., Ogri, O. R., & Arikpo, G. E. (2003). Bioconcentration of heavy metals in surface sediments from the Great Kwa river estuary, Calabar, Southeast Nigeria. Nigerian Journal of Environmental Sociology, 2, 247–256.
Facchinelli, A., Sacchi, E., & Mallen, L. (2001). Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environmental Pollution, 114, 313–324.
Fernandez-Luqueno, F., Lopez-Valdez, F., Gamero-Melo, P., Suarez, S. L., Aguilera-Gonzalez, E. N., Martinez, A. I., et al. (2013). Heavy metal pollution in drinking water—A global risk for human health: A review. African Journal of Environmental Science and Technology, 7(7), 567–584.
Giridharan, L., Venugopal, T., & Jayaprakash, M. (2008). Evaluation of seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai, India. Environmental Monitoring and Assessment, 143(1–3), 161–178.
Gu, Y.-G., Gao, Y.-P., & Lin, Q. (2016). Contamination, bioaccessibility and human health risk of heavy metals in exposed-lawn soils from 28 urban parks in southern China’s largest city, Guangzhou. Applied Geochemistry. https://doi.org/10.1016/j.apgeochem.2016.02.004.
Hu, Y., Liu, X., Bai, J., Shih, K., Zeng, E. Y., & Cheng, H. (2013). Assessing heavy metal pollution in the surface soils of a region that had undergone three decades of intense industrialization and urbanization. Environmental Science and Pollution Research, 20, 6150–6159.
Huang, G. X., Sun, J. C., Jing, J. H., Wang, S., Du, H. Y., Liu, J. T., et al. (2008). Distribution and origin of iron in groundwater of Zhujiang delta. Geology in China, 35, 531–538.
IARC. (1993a). IARC cancer databases. World Health Organization. http://www.iarc.fr. Accessed October 14, 2016.
IARC. (1993b). IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Volume 58. Beryllium, cadmium, mercury and exposures in the glass manufacturing industry. World Health Organization. http://www.iarc.fr. Accessed April 13, 2017.
IARC. (2006). IARC Monographs on the Evaluation of Carcinogenic Risk to Humans. Volume 87. Inorganic and Organic Lead Compounds. World Health Organization. http://www.iarc.fr. Accessed April 13, 2017.
IARC. (2012). Arsenic, metals, fibers, and dust. Volume 100 C. A review of human carcinogens. World Health Organization. http://www.iarc.fr. Accessed April 13, 2017.
IARC. (2017). IARC monographs on the evaluation of carcinogenic risk to humans. Agents classified by the IARC Monograps (Vols. 1–120). World Health Organization. http://monographs.iarc.fr/ENG/Classification/. Accessed Dec 4, 2017.
Iqbal, H., Taseer, R., Anwar, S., Qadir, A., & Shahid, N. (2016). Human health risk assessment: Heavy metal contamination of vegetables in Bahawalpur, Pakistan. Bulletin of Environmental Studies, 1(1), 10–17.
Ishaque, A. B., Johnson, L., Gerald, T., Boucaud, D., Okoh, J., & Tchounwou, P. B. (2006). Assessment of individual and combined toxicities of four non-essential metals (As, Cd, Hg and Pb) in the microtox assay. International Journal of Environmental Research and Public Health, 3(1), 118–120.
ISO 10381-5. (2005). Soil quality-sampling. Part 5: Guidance on the procedure for the investigation of urban and industrial sites with regard to soil contamination. Geneva: International Organization for Standardization.
ISO 10381-6. (2009). Soil quality—Sampling. Part 6: Guidance on the collection, handling and storage of soil under aerobic conditions for the assessment of microbiological processes, biomass and diversity in the laboratory. Geneva: International Organization for Standardization.
ISO 11464. (2006). Soil quality—Pretreatment of samples for physico-chemical analysis. Geneva: International Organization for Standardization.
Jadhav, A. L., Ramesh, G. T., & Gunasekar, P. G. (2000). Contribution of protein kinase C and glutamate in Pb2+-induced cytotoxicity. Toxicology Letters, 115, 89–98.
Jarup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68, 167–182.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants. Florida: CRC Press LLC.
Kahru, A., Ivask, A., Kasemets, K., PollumaaKurvet, I., Francois, M., & Dobourguier, H.-C. (2005). Biotests and biosensors in ecotoxicological risk assessment of fields soils polluted with zinc, lead and cadmium. Environmental Toxicology and Chemistry, 24, 2973–2982.
Katnoria, J. K., Arora, S., Bhardwaj, R., & Nagpal, A. (2011). Evaluation of genotoxic potential of industrial waste contaminated soil extracts of Amritsar, India. Journal of Environmental Biology, 32, 363–367.
Kede, M. L. F. M., Correia, F. V., Conceicao, P. F., Salles Junior, S. F., Marques, M., Moreira, J. C., et al. (2014). Evaluation of mobility, bioavailability and toxicity of Pb and Cd in contaminated soil using TCLP, BCR and earthworms. International Journal of Environmental Research and Public Health, 11(11), 11528–11540.
Khan, A., Khan, S., Khan, M. A., Qamar, Z., & Waqas, M. (2015). The uptake and bioaccumulations of heavy metals by food plants, their effects on plant nutrients, and associated health risk: A review. Environmental Science and Pollution Research, 22, 13772–13799.
Knasmuller, S., Gottmann, E., Steinkellner, H., Fomin, A., Pickl, C., Paschke, A., et al. (1998). Detection of genotoxic effects of heavy metal contaminated soil with plant bioassays. Mutation Research, 420, 37–48.
Lah, B., Avbersek, M., Gorjanc, G., & MarinsekLogar, R. (2005). Toxic and genotoxic potential evaluation of soil samples by bioassays. Actaagriculturae Slovenica, 86(1), 27–38.
Lah, B., Vidic, T., Glasencnik, E., Cepeljnik, T., Gorjanc, G., & Marinsek-Logar, R. (2008). Genotoxicity evaluation of water soil leachates by Ames test, comet assay, and preliminary Tradescantia micronucleus assay. Environmental Monitoring and Assessment, 139, 107–118.
Law of Agricultural Land. (2009). Official Gazette F BiH 52/09. http://www.fbihvlada.gov.ba/bosanski/zakoni/2009/zakoni/32bos.htm. Accessed March 13, 2017.
Lee, C. S., Li, X. D., Shi, W. Z., Cheung, S. C., & Thornton, I. (2006). Metal contamination in urban, suburban, and country park soils of Hong Kong: A study based on GIS and multivariate statistics. Science of the Total Environment, 365, 45–61.
Li, X. D., Lee, S. L., Wong, S. C., Shi, W. Z., & Thornton, I. (2004). The study of metal contamination in urban soils of Hong Kong using GIS-based approach. Environmental Pollution, 129, 113–124.
Likuku, A. S., Mmolawa, K. B., & Gaboutloeloe, G. K. (2013). Assessment of heavy metal enrichment and degree of contamination around the copper-nickel mine in the Selebi Phikwe Region, Eastern Botswana. Environment and Ecology Research, 1(2), 32–40.
Liu, G., Wang, J., Zhang, E., Hou, J., & Liu, X. (2016). Heavy metal speciation and risk assessment in dry land and paddy soils near mining areas at Southern China. Environmental Science and Pollution Research, 23(9), 8709–8720.
Manta, D. S., Angelone, M., Bellance, A., Neriand, R., & Sprovieri, M. (2002). Heavy metals in urban soils: A case study from the city of Palermo (Sicily), Italy. Science of the Total Environment, 30, 229–243.
Matovic, V., Buha, A., Dukic-Cosic, D., & Bulat, Z. (2015). Insight into oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food and Chemical Toxicology, 78, 130–140. https://doi.org/10.1016/j.fct.2015.02.011.
Mehrabi, B., Mehrabani, S., Rafiei, B., & Yaghoubi, B. (2015). Assessment of metal contamination in groundwater and soils in the Ahangaran mining district, west of Iran. Environmental Monitoring and Assessment, 187, 727.
Mmolawa, K. B., Likuku, A. S., & Gaboutloeloe, G. K. (2011). Assessment of heavy metal pollution in soils along major roadside areas in Botswana. African Journal of Environmental Science and Technology, 5(3), 186–196.
Moucher, F., Gauthier, L., Mailhes, C., Jourdain, M. J., Ferrier, V., Triffault, G., et al. (2006). Biomonitoring of the genotoxic potential of aqueous extracts of soils and bottom ash resulting from municipal solid waste incineration using the comet and micronucleus tests on amphibian (Xenopuslaevis) larvae and bacterial assays (Mutatox and Ames tests). Science of the Total Environment, 335, 232–246.
Muller, G. (1969). Index of geo-accumulation in sediments of the Rhine River. Geo Journal, 2(3), 108–118.
Olivieri, G., Brack, Ch., Müller-Spahn, F., Stähelin, H. B., Herrmann, M., & Renard, P. (2000). Mercury induces cell cytotoxicity and oxidative stress and increases β-amyloid secretion and tau phosphorylation in SHSY5Y neuroblastoma cells. Journal of Neurochemistry, 74(1), 231–236.
Ordinance on Identification of the Allowable Amounts of Harmful and Hazardous Substances in Soil and Methods of their Examination. (2009). Official Gazette F BiH 72/09. http://www.uip-zzh.com/files/zakoni/poljoprivreda/72-09.pdf. Accessed February 24, 2017.
Osmanovic, S., Huseinovic, S., & Goletic, S. (2015). Assessment of heavy metals in vegetables grown in the Tuzla area. In Proceedings of the 9th scientific conference with international participation Quality 2015 (pp. 469–473).
Pasalic, A., Bikic, F., & Goletic, S. (2015). The influence of soil pH and the addition of chelating agents on the lead content in certain plants. In Proceedings of the 9th scientific conference with international participation Quality 2015 (pp. 493–497).
Product Information Sheet. (2010). PrestoBlue Viability Reagent Protocol. https://tools.thermofisher.com/content/sfs/manuals/PrestoBlue_Reagent_PIS_15Oct10.Pdf. Accessed September 29, 2016.
Robinson, J. P., Sturgis, J., & Kumar, L. G. (2009). Immunohistochemical staining methods (5th ed.). California: Dako.
Silins, I., & Högberg, J. (2011). Combined toxic exposures and human health: Biomarkers of exposure and Effect. International Journal of Environmental Research and Public Health, 8(3), 629–647.
Steffensen, I.-L., Mesna, O. J., Andruchow, E., Namork, E., Hylland, K., & Andersen, R. A. (1994). Cytotoxicity and accumulation of Hg, Ag, Cd, Cu, Pb and Zn in human peripheral T and B lymphocytes and monocytes in vitro. General Pharmacology, 25(8), 1621–1633.
Sun, Y., Zhou, Q., Xie, X., & Liu, R. (2010). Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang, China. Journal of Hazardous Materials, 174, 455–462.
Tanaka, T., Halicka, D., Traganos, F., & Darzynkiewicz, Z. (2009). Cytometric analysis of DNA damage: phosphorylation of histone H2AX as a marker of DNA double-strand breaks (DSBs). Methods in Molecular Biology, 523, 161–168.
Tchounwou, P. B., Yedjou, C. G., Foxx, D. N., Isnaque, A. B., & Snen, E. (2004). Lead-induced cytotoxicity and transcriptional activation of sress genes in human liver carcinoma (HepG2) cells. Molecular and Cellular Biochemistry, 225, 161–170.
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., & Sutton, D. J. (2012). Heavy metal toxicity and the environment. Molecular, Clinical and Environmental Toxicology, 101, 133–164.
Tiwari, A. K., Singh, P. K., Singh, A. K., & De Maio, M. (2016). Estimation of heavy metal contamination in groundwater and development of heavy metal pollution index by using GIS technique. Bulletin of Environmental Contamination and Toxicology, 96, 508–515.
Tomlinson, D. C., Wilson, D. J., Harris, C. R., & Jeffrey, D. W. (1980). Problem in assessment of heavy metals in Estuaries and the Formation of Pollution Index. HergolandWiss Meeresuniter, 33(1–4), 566–575.
Trabelsi, F., Khlifi, R., Gouw, D., Guillamin, M., Hamya-Chaffai, A., & Sichel, F. (2016). Genotoxic effects of cadmium in human head and neck cell line SQ20B. Environmental Science and Pollution Research, 23, 16127–16136.
Tsuzuki, K., Sugiyama, M., & Haramaki, N. (1994). DNA single-strand breaks and cytotoxicity induced by chromate(VI), cadmium(II), and mercury(II) in hydrogen peroxide-resistant cell lines. Environmental Health Perspectives, 102(3), 341–342.
Tunjic, J., Cipurkovic, A., & Selimbasic, V. (2015). Evaluation of possible applications of soil for agricultural purposes in terms of heavy metals and proximity to industrial facilities. In Proceedings of the third international scientific-expert symposium agricultural production and environmental protection in the development of rural areas (pp. 224–237).
U.S. EPA. (2000a). Cadmium compounds. U.S Environmental Protection Agency. https://www.epa.gov/sites/production/files/2016-09/documents/cadmium-compounds.pdf. Assessed March 16, 2017.
U.S. EPA. (2011). Lead compounds. U.S Environmental Protection Agency. https://www.epa.gov/sites/production/files/2016-09/documents/lead-compounds.pdf. Assessed March 16, 2017.
U.S. EPA. (2000b). Mercury compounds. U.S Environmental Protection Agency. https://www.epa.gov/sites/production/files/2016-09/documents/mercury-compounds.pdf. Assessed March 16, 2017.
Unyayar, S., Celik, A., Cekic, F. Ö., & Gozel, A. (2006). Cadmium-induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Viciafaba. Mutagenesis, 21(1), 77–81.
Venugopal, T., Giridharan, L., Jayaprakash, M., & Periakali, P. (2009). Environmental impact assessment and seasonal variation study of the groundwater in the vicinity of River Adyar, Chennai, India. Environmental Monitoring and Assessment, 149(1–4), 81–97.
Vidic, T., Lah, B., Berden-Zrimec, M., & Marinsek-Logar, R. (2009). Bioassays for evaluating the water-extractable genotoxic and toxic potential of soils polluted by metal smelters. Environmental Toxicology, 24(5), 472–483.
Villatoro-Pullido, M., Font, R., De Haro-Bravo, M. I., Romereo-Jimenez, M., Anter, J., Bailon, A. D. H., et al. (2009). Modulation of genotoxicity and cytotoxicity by radish grown in metal-contaminated soils. Mutagenesis, 24(1), 51–57.
Watanabe, T., & Hirayama, T. (2001). Genotoxicity of soil. Journal of Health Science, 47(5), 433–438.
Wedepohl, H. (1995). The composition of the continental crust. Geochimica and Cosmochimica Acta, 59, 1217–1239.
White, P. A., & Claxton, L. D. (2004). Mutagens in contaminated soil: A review. Mutation Research, 576, 227–345.
WHO. (2000). Environmental Health Criteria 214. Human Exposure Assessment. World Health Organization. http://www.chec.pitt.edu/documents/un-environ-prog.pdf. Accessed February 23, 2017.
Yang, Z., Lu, W., Long, Y., Bao, X., & Yang, Q. (2011). Assessment of heavy metals contamination in urban topsoil from Changchun City, China. Journal of Geochemical Exploration, 108, 27–38.
Zheng, S., Zheng, X., & Chen, C. (2012). Leaching behavior of heavy metals and transformation of their speciation in polluted soil receiving simulated acid rain. PLoS ONE, 7(11), e49664. https://doi.org/10.1371/journal.pone.0049664.
Acknowledgements
This research was partly supported by project of Federal Ministry of Education and Science in the framework of bilateral scientific and technological cooperation between the Republic Slovenia and Bosnia and Herzegovina for 2016–2017 (BI-BA/16-17-036). Furthermore, the financial support of meat industry Natura from Teslic, Bosnia and Herzegovina is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sljivic Husejnovic, M., Bergant, M., Jankovic, S. et al. Assessment of Pb, Cd and Hg soil contamination and its potential to cause cytotoxic and genotoxic effects in human cell lines (CaCo-2 and HaCaT). Environ Geochem Health 40, 1557–1572 (2018). https://doi.org/10.1007/s10653-018-0071-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0071-6