Abstract
In this study, organochlorine pesticides (OCP) and heavy metals were analyzed from wastewater- and groundwater- irrigated soils (control samples) by gas chromatography (GC) and atomic absorption spectrophotometry (AAS), respectively. Gas chromatographic analysis revealed the presence of high concentration of pesticides in soil irrigated with wastewater (WWS). These concentrations were far above the maximum residue permissible limits indicating that alluvial soils have high binding capacity of OCP. AAS analyses revealed higher concentration of heavy metals in WWS as compared to groundwater (GWS). Also, the DNA repair (SOS)-defective Escherichia coli K-12 mutant assay and the bacteriophage lambda system were employed to estimate the genotoxicity of soils. Therefore, soil samples were extracted by hexane, acetonitrile, methanol, chloroform, and acetone. Both bioassays revealed that hexane-extracted soils from WWS were most genotoxic. A maximum survival of 15.2 % and decline of colony-forming units (CFUs) was observed in polA mutants of DNA repair-defective E. coli K-12 strains when hexane was used as solvent. However, the damage of polA − mutants triggered by acetonitrile, methanol, chloroform, and acetone extracts was 80.0, 69.8, 65.0, and 60.7 %, respectively. These results were also confirmed by the bacteriophage λ test system as hexane extracts of WWS exhibited a maximum decline of plaque-forming units for lexA mutants of E. coli K-12 pointing to an elevated genotoxic potential. The lowest survival was observed for lexA (12 %) treated with hexane extracts while the percentage of survival was 25, 49.2, 55, and 78 % with acetonitrile, methanol, chloroform, and acetone, respectively, after 6 h of treatment. Thus, our results suggest that agricultural soils irrigated with wastewater from pesticide industries have a notably high genotoxic potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of pesticides has benefitted the modern society by improving the quantity and quality of the world’s sustenance production while the cost of food supply is kept reasonable. Unsurprisingly, the use of pesticides has become an integral and important part of modern agricultural systems. Although many of these chemicals are utilized or destroyed, a high percentage is released into air, water, and soil, representing potential environmental hazards (Alexander 1995; Anwar et al. 2009; Anjum and Malik 2013; Anjum et al. 2014; Yadav et al. 2015). Consequently, earth’s natural resources are not only being depleted but they are also being polluted and hence become increasingly unfit for human use.
The pollution of soils with pesticides is one of the most severe environmental risks (Prakash et al. 2004; Anjum et al. 2011; Anjum and Malik 2012). The major environmental concern arising from the use of pesticides is their capacity to leach from soil causing water contamination (Bhagobaty et al. 2007; Chowdhary et al. 2008). Moreover, soils are known to accumulate potentially toxic elements such as heavy metals like zinc, copper, nickel, lead, chromium, and cadmium associated with a steady pollutant accumulation in the soil. This may result in deteriorated agricultural soil quality, increased phytotoxicity, disturbed microbial processes, and an adverse transfer of zootoxic elements to the human diet from increased crop uptake (Ansari and Malik 2007). Moreover, elevated levels of pesticide pollutants not only decrease soil microbial activities but also threaten human health through the food chain (Prakash et al. 2004; Ansari and Malik 2009; Anjum and Malik 2013). Even agriculture products such as cereals, fruit, and vegetables are often found to be contaminated with residues of persistent pesticides. Matters are complicated further by the fact that the continuous use of pesticides leads to a considerable accumulation to toxic levels in the soil ecosystem. Natural environments are extremely diverse and the majority contains a wide range of microorganisms, reflecting the nature of the habitat and the ability of individual members to compete successfully within a given ecosystem (Aguirre and Lowe 2010). Further, microbes and plants are the most important biological agents that remove and degrade waste materials to enable their recycling in the environment (Anjum et al. 2011). Microorganisms play a significant role in the metabolism of organochlorine pesticides (Nawab et al. 2003).
However, several factors playing a significant role in the dispersal of persistent organic pesticides on a global scale. Firstly, industrial activities are increasingly moving to Asia (Lohmann et al. 2007). Due to that, certain emissions will get transported into the southern hemisphere (Dachs et al. 1999; Semeena et al. 2006). Secondly, persistent organic pollutant (POP) emissions linked to low-temperature combustion processes will increase for Asia, Africa, and S. America (Crutzen and Andrea 1990; Pozo et al. 2006). Thirdly, many currently used pesticides are being increasingly used across the globe, indicating their major use in agricultural areas of S. America, Africa, and Asia (Pozo et al. 2006). Currently, India is the second largest manufacturer of pesticides in Asia, after China. It ranks as the fourth largest pesticide-producing nation in the world after USA, Japan, and China (Yadav et al. 2015). However, organochlorine pesticides (OCP) have been used worldwide to control global agricultural pests and vector-borne diseases (Abhilash and Singh 2009; Zhang et al. 2011). Moreover, besides India, many countries exist being still engaged in the production, usage, and export of lindane (γ-hexachlorocyclohexane; γ-HCH) on a large scale (Zhang et al. 2008; Zhang et al. 2011; Ali et al. 2014). According to a joint report by the World Health Organization (WHO) and the United Nations Environment Programme (UNEP), about 200,000 people die worldwide and around three million are poisoned each year due to an overuse of toxic pesticides (Yadav et al. 2015).
Although OCPs have a long history (over 30 years) of its application, only very scarce information is available for the presence of OCP residues in air, soil, and water systems. Also, health risk-associated studies of how OCPs are impacting the environment are deficient (Sharma et al. 2007; Ali et al. 2014; Yadav et al. 2015). Therefore, the status of the residue level of most persistent OCPs in soil and agricultural crops should be monitored regularly.
Particularly, the assessment of soil toxicity is subjected to some obstacles. The large number of toxic chemicals that may potentially be present at contaminated sites can hinder successful chemical analyses. Also, detailed chemical analyses are limited in its ability to predict the toxicity of organic chemical mixtures. To overcome these problems, many researchers advocate a polyphasic approach by applying bioassays to measure the genotoxic potential of complex environmental samples (Houk and DeMarini 1987). For many years, more than 200 short-term bioassays utilizing microorganisms, insects, or plants have been developed and used to identify agents posing genotoxic hazards (Anjum et al. 2014).
The aim of this study is to assess the genotoxicity of contaminated soils near a pesticide manufacturing industry in India near an industrial area. This study emphasizes on the genotoxicity of cultivated agricultural soils irrigated with wastewater from pesticide factories and groundwater-irrigated soils. Therefore, the survival of DNA repair-defective Escherichia coli K-12 mutant assay and the bacteriophage lambda system were employed.
Materials and methods
Soil sampling
Five soil samples (15 cm depth) were collected from agricultural spots supplied by wastewater from a 200-m-away situated pesticide manufacturing industry (India Pesticide Ltd., Chinhat, Lucknow, U.P. India) as described by Malik and Jaiswal (2000). The sample sites had a relative distance of approximately 50 m from each other. Samples were taken immediately and stored at 4 °C. A single composite soil sample of 1 kg was prepared by mixing all five samples together (WWS). A further control soil sample identically prepared as described above was taken from agricultural fields supplied by groundwater (GWS).
Physical characterization of the soils
All physical soil characteristics (texture, water content) as well as organic carbon contents were determined by a method as described by Gupta (2004) and Ghosh et al. (1983); 10 ml of 1 N potassium dichromate solution was added to 1.0 g of oven-dried soil, then 20 ml of concentrated sulfuric acid was added, gently shaken, and digested for 30 min. The solution was diluted by adding 200 ml of distilled water and 10 ml of phosphoric acid, followed by a diphenylamine indicator step. Finally, the solutions were cooled to room temperature and titrated with a 0.4 N ferrous ammonium sulfate solution until the color changed to brilliant green.
Ten grams of air-dried and sieved (<2 mm) soil samples was taken in a beaker, mixed with 50 ml of distilled water, and shaken continuously for 1 h. The pH of the supernatant was measured as described by Alef and Nannipieri (1985).
Determination of heavy metals in soils
The oven-dried (1 g, 40 °C) soil samples were ground (< 0.1 mm), burnt in a crucible to ashes, and finally transferred to a conical flask. The samples were moistened with double distilled water (ddH2O). Afterwards, HCl (37 %) and HNO3 were successively added (3:1 ratio). The flask was gently heated on a heating plate until the sample was digested, indicated by the formation of a clear supernatant. The mixture was reduced to a volume of 1 ml. The final volume of 100 ml was adjusted by adding ddH2O. The mixture was filtered through a Whatman filter (paper no. 1 and 42). The metal concentrations of all digested samples were analyzed (Table 1) by employing an atomic absorption spectrophotometer (GBC 932 Plus, Australia) as described by Malik and Jaiswal (2000). All metal concentrations measured were above the atomic absorption spectrophotometry (AAS) detection limit as stated by the manufacturer as follows: Cr, Ni—0.003 μg g−1, Cu—0.004 μg g−1, Cd—0.0004 μg g−1, Zn—0.0005 μg g−1, and Fe—0.0007 μg g−1.
Used chemicals were of analytical grade and solutions were prepared in ddH2O. All investigations were determined in triplicates and specified as mean values.
Determination of pesticides in soils
The extraction of pesticides from GWS and WWS soil samples was carried out according to a method as described by Nawab et al. (2003). All extractions were performed in triplicates. The soils (10 g) were mixed with 20 ml of hexane/water solution (4:1, v/v) and shaken vigorously for 1 h (Aleem and Malik 2003). The supernatant was decanted after the mixture was centrifuged at 10,000 rpm for 15 min. This procedure was repeated twice and the extracted volume was decreased to ∼15 ml using a rotary evaporator. Subsequently, the aqueous sample was acidified to a pH of ∼1 with HCl and consecutively triple partitioned with chloroform (high-pressure liquid chromatography (HPLC) grade) using a separatory funnel. The organic phases were again evaporated to dryness, redissolved in 1 ml of n-hexane (HPLC grade), and finally analyzed by gas chromatography (Table 1). Standard solutions were prepared according to the method of Singh et al. (1987) and stored at −20 °C. Prior to the experiments, all samples were filtered through a 0.45-μm membrane filter.
Gas chromatographic analysis of pesticides
Pesticide analyses were conducted by the Central Pollution Control Board (Delhi, India). Analyses of the extracts were performed by using a Perkin Elmer Clarus 500 gas chromatograph equipped with an electron capture detector. Instrument parameters and operating conditions were as follows: column—Elite-5; temperature: injector—250 °C, detector—325 °C, initial oven temperature—170 °C, and then 7 °C min−1 ramp to 220 °C further increased to 250 °C with 5 °C min−1 ramp. The holding time was 5.86 min. The nitrogen carrier flow rate was 1 ml min−1 up to 30 ml min−1. The peaks were identified by comparing their retention times with commercial standards (Sigma-Aldrich, Bangalore, India).
Extraction of soils with different solvents for genotoxicity testing
The extraction of pesticides with hexane, acetonitrile, methanol, chloroform, and acetone from GWS and WWS soils was done according to the method of Knize et al. (1987). Therefore, 10 g of soil was treated with 10 ml of extraction solvent. Obtained extracts were centrifuged at 7000 rpm for 10 min, evaporated to dryness, redissolved in 1 ml of dimethyl sulfoxide (SRL, India), filtered through 0.45-μm filters, and then stored at −20 °C until the genotoxicity testing was completed.
Used bacterial strains and phage λ in genotoxicity assays
SOS gene (recA, lexA, and polA) defective mutants of E. coli K-12 strains (Yale University, E. coli Genetic Stock Center, Department of Biology, New Haven, USA) were fortnightly transferred to nutrient broth agar plates (1.5 %; HiMedia, India). The bacteriophage λ viruses served as vector system (Department of Microbiology and Immunology, Virginia Commonwealth University, Richmond, USA, Prof. E. Christie).
Genotoxicity testing by two different assays
Treatment of DNA repair-defective E. coli K-12 strains with soil extracts
The SOS-defective recA, lexA, and polA mutants of E. coli K-12 strains as well as the isogenic wild-type strains (Tab. 1S) were harvested from an exponentially growing culture (1–3 × 108 colony-forming units (CFUs) viable counts ml−1) by centrifugation. To induce the reaction between soil extracts of WWS and GWS (Fig. 1) and DNA repair-defective mutated E. coli K-12 strains as well as the isogenic wild-type strains, pellets of E. coli K-12 strains were suspended in 0.01 M MgSO4 solution and treated with 5 μl of WWS and GWS soil extracts separately. All samples were withdrawn at regular time intervals (0, 2, 4, 6 h; 37 °C), diluted, and plated on nutrient broth (NB) agar medium plates to assay the colony-forming ability. After an overnight incubation step (37 °C) of plates, all grown colonies were counted. Simultaneously, a negative control was conducted with dimethyl sulfoxide (DMSO; without soil extract).
Extracellular treatment of bacteriophage λ with soil extracts
Five microliters of WWS and GWS soil extracts was incubated with 100 μl of purified bacteriophage λ virus solution (1010 plaque-forming units (PFU) counts ml−1) at 37 °C; 100 μl of phage λ aliquots were suspended at regular time intervals (0, 2, 4, 6 h) in 0.01 M MgSO4 solution (pH 8.0) and allowed to adsorb on the cell surface of DNA repair-defective and wild-type hosts of E. coli K-12 strains (Tab. 1S) at 37 °C. The generated mixtures were plated on NB agar medium by a double-layer method according to Rehana et al. (1996). A solvent control (DMSO without soil extract) was performed simultaneously. Plaques were counted after overnight plate incubation at 37 °C. As phage λ-infected, polA gene-mutated Escherichia coli K-12 strains do not induce required SOS responses, we accordingly omitted corresponding experiments.
Statistics
Variances, mean values, and standard deviations were calculated using the palaeontological statistics software (PAST, version 3.06, 2015, Table 2).
To assess the degree of soil contamination, the pollution index (PI) (Hakanson 1980) was carried out as follows:
where Cn and Cb are the mean concentrations of pollutants (metals and pesticides) of WWS and GWS samples, respectively.
Results
Characteristics of investigated soils and heavy metal analysis
The textures of alluvial soils were of loamy type. The total organic carbon of WWS and GWS was 9.6 and 4.7 % with pH values of 7.6 ± 9.1 and 7.1 ± 1.8, respectively. The percentage of organic matter of WWS and GWS was 4.1 ± 2.4 and 1.3 ± 3.1 %, respectively.
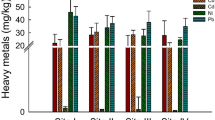
The heavy metal concentrations of WWS were throughout higher as compared to GWS samples (Table 1). WWS covered metal concentrations between 11.2 μg g−1 (Cd) and 241 μg g−1 (Ni), while for GWS, the range was between 0.2 μg g−1 (Cd) and 20.3 μg g−1 (Fe). According to GWS, Cr, Zn, and Fe showed similar concentration ranges (36–43 μg g−1) while Cu and Cd demonstrated comparatively low concentrations (11.2–13.2 μg g−1). For WWS, Cu and Cd were detected at lowest concentrations (0.2–0.3 μg g−1) while the proportions for Ni and Cr were lifted (2.2–2.7 μg g−1). Zn and Fe revealed maximum concentrations (10.8–20.3 μg g−1).
Determination of pesticides in soils
From pesticides focused by this study, only lindane (547 ng g−1), α-endosulfan (422 ng g−1), and β-endosulfan (421 ng g−1) were detected at high concentrations while the remaining pesticide concentrations fell below their detection limit (Table 1). However, the degree of contamination was remarkably increased for WWS samples investigated (>421 ng g−1) compared to GWS (<2.7 ng g−1).
The gas chromatograms were also detecting unidentified peaks for WWS samples indicating the presence of unknown contaminants besides pesticides. Also for GWS, unidentified peaks were observed but with low densities.
In comparison to heavy metals, the calculated PI values of OCPs were increased by the factor 3–130 (Table 1).
Survival of E. coli K-12 strains treated with soil extracts
All mutants revealed various degrees of CFU declines in comparison to their isogenic wild types, while for WWS extracts a significant curve could be observed (Fig. 1). PolA − mutants consistently exhibited a maximum CFU decrease for both WWS and GWS extracts. In particular, polA − mutants treated with hexane extracts of WWS led to a maximum decline in survival by 84.8 % after an exposure of 6 h, while the damage of polA − mutants treated with acetonitrile, methanol, chloroform, and acetone extracts were of 80.0, 69.8, 65.0, and 60.7 %, respectively (Fig. 1). Hence, a relatively weak genotoxic activity was noticed in all mutants of SOS-defective E. coli K-12 when treated with acetonitrile, methanol, chloroform, and acetone extracts, indicating a higher genotoxic potential of hexane extracts as high damage in PolA − mutants was observed in the presence of hexane extracts (Fig. 1).
However, a lower level of genotoxicity was observed for GWS extracts. Thus, a 6-h treatment of polA − mutants with hexane, acetonitrile, methanol, chloroform, and acetone extracts of GWS induced a 34.6, 34, 29.7, 27.6, and 24.3 % loss of polA − mutants of E. coli K-12 strain (Fig. 1). All strains treated with acetonitrile, methanol, chloroform, and acetone extracts revealed a survival of polA − mutants between approximately 70 and 99 % (Fig. 1).
Survival of E. coli K12 strains infected with bacteriophage λ
A decline in PFU was more pronounced in lexA mutants as compared to their wild-type counterparts (Fig. 2). A 6-h incubation of E. coli mutants with hexane extracts from WWS led to the lowest survival of lexA (12 %) while a treatment with acetonitrile, methanol, chloroform, and acetone extracts led to a survival by 25, 49.2, 55, and 78 %, respectively.
Though, compared to the isogenic wild-type strains, the lexA mutants were most sensitive for used solvents. A significant decline of PFUs was observed for lexA mutants when hexane extract was used. According to GWS samples, the survival percentage for hexane, acetonitrile, methanol, chloroform, and acetone extracts was 63.4, 65.0, 68.3, 75.4, and 78.7 %, respectively. Throughout, GWS extracts were less genotoxic compared to WWS (Fig. 2). LexA strains were most sensitive for GWS extracts compared to recA mutants and isogenic wild-type strains.
Discussion
Water is a primary source through which pesticides are transported from an application area to other areas of the environment. Because of their slow decomposition rates, long half-life, and high stability in the environment, OCPs are the most harmful class of pesticides. They can remain and accumulate in the upper trophic levels of food chains (Yadav et al. 2015). To assess the genotoxicity of pesticide-contaminated soils from agricultural fields near a pesticide industry, soil samples were extracted by using various solvents and tested for their genotoxicity. A number of improved analytical methods have been extensively used to identify the organic and inorganic contaminants in polluted water and soils. The contaminants include both organic and synthetic organic compounds. A large group of environmental organic pollutants can be measured by gas chromatography mass spectrometry (GC-MS), high-pressure liquid chromatography (HPLC) and gas-liquid chromatography (USEPA 1983). However, atomic absorption spectrophotometry applied by this study is one of the most extensively used techniques for the determination of trace elements from a wide variety of materials (APHA 1995; Rawat et al. 2003; Anazawa et al. 2004).
Lindane and endosulfan are pesticides mainly used in agriculture to preserve health of crops and to prevent its destruction by diseases and pest infestation (Yadav et al. 2015). Similar to our study, also other researchers (Aleem and Malik 2003; Ansari and Malik 2009; Zhao et al. 2013) reported that lindane and endosulfan are most commonly used pesticides for crop protection.
However, our results revealed high OCP concentrations in agricultural alluvial soils supplied by wastewater, whereas lower levels of OCPs were detected in groundwater-irrigated soils (Table 1). In this regard, it is noticed that other studies (Aleem and Malik 2003; Ansari and Malik 2009) also dealing with agricultural soils irrigated with wastewater from industrial and domestic sewage factories (Aligarh, Ghaziabad, India) revealed more than 1000-fold lower OCP concentrations (e.g., endosulfan 2.2–108.2 pg g−1) as detected by this study. More studies exist, addressing OCP concentrations in soils in which the measured total HCH concentrations (max. 9.0 ng g−1) were 60 times lower (Hong et al. 2005) as compared to our results. Moreover, the OCP residue concentration (esp. lindane) revealed by this study was 10 times above the maximum residue limit (<50 ng g−1) of the National Environmental Standards for agricultural soils for both dichlorodiphenyltrichloroethane (DDT) and lindane (Chahal et al. 2014) and were also far above the defined maximum permissible limits as outlined by the US Environmental Protection Agency (USEPA), respectively.
By this study, alluvial soils were considered which generally contain high concentrations of metals what is due to the fact of a distinct metal-binding capability. Consequently, besides OCPs, also metals were of interest as it is known that heavy metals strongly impact the ecosystem stability, have harmful effects on humans (Steinkellner et al. 1998), and can affect plant growth, phytotoxicity, microbial activities (Lombi et al. 2002), and genotoxicity.
The metal concentrations in WWS recorded by this study were comparably higher than those found in GWS which was above the AAS detection limit. The concentrations of Fe, Zn, Cr, Ni, Cd, and Cu (Table 1) were comparable to reports stating lifted metal concentrations in domestic and industrial wastewater in the vicinity of an industrial area of Aligarh (Malik and Ahmad 1995; Bansal 1998; Rao and Shantaram 1999; Malik and Jaiswal 2000). Moreover, the metal concentrations obtained by this study were about 40-fold higher in comparison to a study of Vanita and Murugesan (2014) who also investigated the presence of Cr, Zn, Ni, Fe, Cu, and Cd in wastewater irrigated soils those may have toxic or genotoxic effects as stated by Masood and Malik (2013). Moreover, in comparison to heavy metals, the calculated PI values of OCPs were 3–130 times higher (Table 1) which proves a high genotoxic potential of OCPs induced their predominant presence.
The present study was mainly conducted for the genotoxicity assessments of contaminated alluvial soils collected from wastewater- and groundwater- irrigated agricultural fields by two different bioassays. The survival of E. coli K-12 mutants and bacteriophage lambda systems revealed notably toxic responses in the presence of pesticide-contaminated soil extracts. Both applied assays revealed polA and lexA mutants, respectively, as most sensitive for the presence of hexane extracts and resulted in significant losses of CFUs and PFUs, respectively (Figs. 1–2).
A maximum damage was most pronounced in polA − mutants in the presence of WWS hexane extracts (15.2 % of survival, Fig. 1) whereas other reports stated most pronounced damages in polA − mutants in the presence of methanol extract from WWS (Aleem and Malik 2003; Ikram and Malik 2009). In their study, the survival of polA − mutant was only 16.5 and 25 %, respectively, whereas our study revealed a survival of polA − mutants by 30.2 % in the presence of methanol extract of WWS. Moreover, Ikram and Malik (2009) detected a survival of polA − mutants by 33 % in the presence of hexane extract (WWS). In comparison to that, our results revealed twice as much capacity to damage polA − mutant in the presence of hexane extract (Fig. 1).
Also, they reported a survival of lexA by only 17.7 and 22 %, respectively, by bacteriophage lambda assay when methanol was used as solvent for industrial wastewater-irrigated soil samples. In contrast to the other studies (Aleem and Malik 2003; Ikram and Malik 2009), hexane extract of WWS revealed highest damage in lexA in our study, therefore only 12 % survival (Fig. 2) was observed. Considering this, our results suggest that alluvial soils have both a high genotoxic potential and high capacity to accumulate OCP (Table 2).
An earlier study (Vargas et al. 1995) demonstrated that the microscreen bacteriophage induction assay is very sensitive for a wide range of DNA alteration suggesting this approach (bacteriophage system) as acutely appropriate for screening of genotoxic compounds present even in low concentrations in environmental samples. Saxena et al. (1997) investigated mutagenic potentials of pesticides by the survival of DNA repair-defective E. coli K-12 mutants, and in their results, a significant decrease in the survival of E. coli K-12 mutants (recA, lexA, and polA) was observed in the presence of pesticides as compared to their isogenic wild-type counter parts those are comparable to our study. Thus, it is assumed that the inducible error-prone repair pathway presumably involving the recA + and lexA + genes could potentially operate on several types of lesions in DNA, triggered by radiation, environmental chemicals, or by other agents (Malik and Ahmad 1995; Rehana et al. 1996; Aleem and Malik 2005).
In conclusion, results obtained by this study revealed that agricultural soils irrigated with wastewater from pesticide industries are rather strongly impacted by exceedingly harmful organochlorine pesticides which might have health hazards to the human beings while the contamination by heavy metals seems to play a minor role. In comparison to other reports, dealing with WWS, a more than 1000-fold higher OCP contamination was detected by this study. Thus, our results suggest that agricultural alluvial soils irrigated with wastewater are highly genotoxic and have also a high capability to accumulate OCPs.
References
Abhilash, P. C., & Singh, N. (2009). Pesticide use and application: an Indian scenario. Journal of Hazardous Materials, 165, 1–12.
Aguirre, S. P., & Lowe, K. L. (2010). Pesticide-tolerant bacteria isolated from agricultural canals in the lower Rio Grande Valley of South Texas. Journal of Biological Sciences, 10, 126–135.
Aleem, A., & Malik, A. (2003). Genotoxic hazards of long-term application of wastewater on agricultural soil. Mutation Research, 538, 145–154.
Aleem, A., & Malik, A. (2005). Genotoxicity of Yamuna River water at Okhla (Delhi), India. Ecotoxicology and Environmental Safety, 61, 404–412.
Alef, K., & Nannipieri, P. (1995). In: Methods in applied soil microbiology and biochemistry London: Academic Press Ltd. ISBN 0–12–513840–7.
Alexander, M. (1995). How toxic are chemicals in soil? Environmental Science and Technology, 29, 2713–2717.
Ali, U., Syed, J. H., Malik, R. N., Katsoyiannis, A., Li, J., Zhang, G., & Jones, K. C. (2014). Organochlorine pesticides in South Asian region: a review. Science in Total Environment, 476–447, 705–717.
Anazawa, K., Kaida, Y., Shinomura, Y., Tomiyasu, T., & Sakamoto, H. (2004). Heavy metal distribution in river waters and sediments around a “firefly village”, Shikoku, Japan: application of multivariate analysis. Analytical Science, 20, 79–84.
Anjum, R., Krakat, N., Reza, M. T., & Klocke, M. (2014). Assessment of mutagenic potential of pyrolysis biochar by Ames Salmonella/mammalian-microsomal mutagenicity test. Ecotoxicology and Environmental Safety, 107, 306–312.
Anjum, R., & Malik, A. (2012). Mutagenicity assessment of contaminated soil in the vicinity of industrial area. Environmental Monitoring and Assessment, 184, 3013–3026.
Anjum, R., & Malik, A. (2013). Evaluation of mutagenicity of wastewater in the vicinity of pesticide industry. Environmental Toxicology and Pharmacology, 35, 285–291.
Anjum, R., Grohmann, E., & Malik, A. (2011). Molecular characterization of conjugative plasmids in pesticide tolerant and multi-resistant bacterial isolates from contaminated alluvial soil. Chemosphere, 84, 175–181.
Ansari, M. I., & Malik, A. (2007). Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with wastewater. Bioresource Technology, 98, 3149–3153.
Ansari, M. I., & Malik, A. (2009). Genotoxicity of wastewaters used for irrigation of food crops. Environmental Toxicology, 24, 103–115.
Anwar, S., Liaquat, F., Khan, Q. M., Khalid, Z. M., & Iqbal, S. (2009). Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by Bacillus pumilus strain C2A1. Journal of Hazardous Materials, 168, 400–405.
APHA (1995). Standard methods for the examination of water and wastewaters (19th ed., ). Washington DC:APHA AWWA WPCF.
Bansal, O. P. (1998). Heavy metal pollution of soils and plants due to sewage irrigation. Indian Journal of Environmental Health, 40, 51–52.
Bhagobaty, R.K., Joshi, S.R., & Malik, A. (2007). Microbial degradation of organophosphorous pesticide: chlorpyrifos (mini-review): The International Journal of Microbiology, 4, no.1.
Chahal, V., Nagpal, A., Pakade, Y., & Katnoria, J. (2014). Ecotoxicological studies of soil using analytical and biological methods: a review. International Journal of Biological Food Veterinary and Agricultural Engineering, 8, 290–306.
Chowdhary, A., Pradhan, S., Saha, M., & Sanyal, N. (2008). Impact of pesticides on soil microbiological parameters and possible bioremediation strategies. Indian Journal of Microbiology, 48, 114–127.
Crutzen, P. J., & Andrea, M. O. (1990). Biomass burning in the tropics—impact on atmospheric chemistry and biogeochemical cycles. Science, 250, 1669–1678.
Dachs, J., Bayona, J. M., Ittekkot, V., & Albaiges, J. (1999). Monsoon-driven vertical fluxes of organic pollutants in the western Arabian Sea. Environmental Science and Technology, 33, 3949–3956.
Ghosh, A. B., Bajaj, J. C., Hassan, R., & Singh, D. (1983). Soil and water testing method: a laboratory manual. New Delhi:Indian Agricultural Research Institute.
Gupta, P. K. (2004). Methods in environmental analysis. In water, soil and air. (pp. pp. 405–pp. 408). India: Jodhpur.
Houk, V. S., & DeMarini, D. (1987). Introduction of prophage lambda by chlorinated pesticides. Mutation Research, 182, 193–201.
Hong, Z., Yonglong, L., Dawson, R. W., Yajuan, S., & Tieyu, W. (2005). Classification and ordination of DDT and HCH in soil samples from the Guanting Reservoir, China. Chemosphere, 60, 762–769.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14, 975–1001.
Knize, M. G., Takemoto, B. T., Lewis, P. R., & Felton, J. S. (1987). The characterization of the mutagenic activity of soil. Mutation Research, 192, 23–30.
Lohmann, R., Breivik, K., Dachs, J., & Muir, D. (2007). Global fate of POPs: current and future research directions. Environmental Pollution, 150, 150–165.
Lombi, E., Zhao, F. J., Wieshammer, G., Zhang, G., & McGrath, S. P. (2002). In situ fixation of metals in soils using bauxite residue: biological effects. Environmental Pollution, 118, 445–452.
Malik, A., & Ahmad, M. (1995). Genotoxicity of some wastewaters in India. Environmental Toxicology and Water Quality, 10, 287–293.
Malik, A., & Jaiswal, R. (2000). Metal resistance in Pseudomonas strains isolated from soil treated with industrial wastewater. World Journal of Microbiology and Biotechnology, 16, 177–182.
Masood, F., & Malik, A. (2013). Cytotoxic and genotoxic potential of tannery waste contaminated soils. Science of the Total Environment, 444, 153–160.
Nawab, A., Aleem, A., & Malik, A. (2003). Determination of organochlorine pesticides in agricultural soil with special reference to γ-HCH degradation by Pseudomonas strains. Bioresource Technology, 88, 41–46.
Pozo, K., Harner, T., Wania, F., Muir, D. C. G., Jones, K. C., & Barrie, L. A. (2006). Toward a global network for persistent organic pollutants in air: results from the GAPS study. Environmental Science and Technology, 40, 4867–4873.
Prakash, O., Suar, M., Raina, V., Pal, C. D. R., & Lal, R. (2004). Residues of hexachlorocyclohexane isomers in soil and water samples from Delhi and adjoining areas. Current Science, 87, 73–77.
Rao, K. J., & Shantaram, M. (1999). Potentially toxic elements in soils treated with urban solid wastes. Indian Journal of Environmental Health, 41, 364–368.
Rawat, M., Moturi, M. C. Z., & Subramanian, V. (2003). Inventory compilation and distribution of heavy metals in wastewater from small-scale industrial areas of Delhi, India. Journal of Environmental Monitoring, 5, 906–912.
Rehana, Z., Malik, A., & Ahmad, M. (1996). Genotoxicity of Ganges water at Narora (UP), India. Mutation Research, 367, 187–802.
Saxena, S., Ashok, B. T., & Mussarat, J. (1997). Mutagenic and genotoxic activities of four pesticides: captan, foltaf, phosphamidon and furadan. Biochemisrty and Molecular Biology International, 41, 1125–1136.
Semeena, V. S., Feichter, J., & Lammel, G. (2006). Impact of the regional climate and substance properties on the fate and atmospheric long-range transport of persistent organic pollutants—examples of DDT and gamma-HCH. Atmospheric Chemistry and Physics, 6, 1231–1248.
Sharma, S., Nagpure, N. S., Panday, S., Srivastava, S. K., Singh, S. K., & Mathur, P. K. (2007). Studies on the genotoxicity of endosulfan in different tissues of fresh water fish Mystus vittatus using the comet assay. Archives Environmental Contamination and Toxicology, 53, 617–623.
Singh, K.P., Takroo, R., & Ray, P.K. (1987). Analysis of pesticide residues in water. ITRC Manual No. 1, Industrial Toxicology Research Centre Lucknow, UP, India.
Steinkellner, H., Mun-Sik, K., Helma, C., Ecker, S., Ma, T. H., & Horak, O. (1998). Genotoxic effects of heavy metals: comparative investigation with plant bioassays. Environmental and Molecular Mutagenesis, 31, 183–191.
USEPA (1983). Health assessment document, United States Environment Protection Agency. NC (USA):Research Triangle Park.
Vanitha, M., & Murugesan, A. (2014). Hydrochemical investigation on ground water quality in and around solid waste dumping site, Chennai City. International Journal of Chemistry and Technology Research, 6, 4352–4358.
Vargas, V. M. F., Guidobono, R. R., Jordao, C., & Henriques, J. A. P. (1995). Use of two short-term tests to evaluate the genotoxicity of river water treated with different concentration/extraction procedures. Mutation Research, 343, 31–52.
Yadav, I. C., Devi, N. L., Syed, J. H., Cheng, J. H., Li, J., Zhang, G., & Jones, K. C. (2015). Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: a comprehensive review of India. Science in Total Environment, 511, 123–137.
Zhang, G., Chakraborty, P., Sampathkumar, J. L. P., Balasubramanian, T., Kathiresan, K., Takahashi, S., Subramanian, A., Tanabe, S., & Jones, K. C. (2008). Passive atmospheric sampling of organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in urban, rural, and wetland sites along the coastal length on India. Environmental Science and Technology, 42, 8218–8223.
Zhang, W. J., Jiang, F. B., & Ou, J. F. (2011). Global pesticide consumption and pollution: with China as a focus. Academy of Ecology and Environmental Sciences, 1, 125–144.
Zhao, L., Dong, Y. H., & Wang, H. (2013). Residues of organochlorine pesticides and polycyclic aromatic hydrocarbons in farm-raised livestock feeds and manures in Jiangsu, China. Science in Total Environment, 450-451, 348–355.
Acknowledgments
N. K. is thankful for grants provided by the German Federal Ministry of Food and Agriculture (BMEL, grant no. 22026411) and the Agency of Renewable Resources (FNR). R. A. is thankful to Prof A. Malik, Department of Agricultural Microbiology, Aligarh Muslim University, India, for his proportional financial support under grants provided by the University Grants Commission, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Esm 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Anjum, R., Krakat, N. Genotoxicity assessments of alluvial soil irrigated with wastewater from a pesticide manufacturing industry. Environ Monit Assess 187, 638 (2015). https://doi.org/10.1007/s10661-015-4830-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4830-x