Abstract
Temporal/spatial variations of surface water quality were examined for the Nile River in the Damietta region where it serves as the major source of water for the inhabitants of Damietta Governorate. A total of 32 water quality parameters were monitored at six sampling sites for 12 months from February 2016 to January 2017. Higher values of chemical oxygen demand (COD), biological oxygen demand (BOD), heavy metals, and nutrients were observed upstream. About ~ 70% of the total variance in observations was explained by five main influences using factor analysis. The first factor (24.6% of the variance) was indicative of the mixed sources of natural and anthropogenic inputs. The second (nutritional) and the third (organic) factors were mainly controlled by the discharges from agricultural and domestic sources, respectively. Human activities and natural processes controlled the fourth and fifth factors. Only 11 parameters (K, temperature, COD, HPC, total hardness, DO, NO2, Na, TDS, Cl, and EC) were necessary for distinguishing temporal variations according to Discriminant analysis (DA). Seven parameters (BOD, PO4, SiO3, Al, Turbidity, Fe, and Chlorophyll-a) were the most important variables responsible for spatial variations. Using the results we developed a water quality index (WQI) using only those parameters identified as important. All water quality parameters were below the permissible limits except for turbidity according to the World Health Organization standards, BOD and COD according to the Egyptian regulations. The calculated WQI values ranged between 12.73 and 33.73. According to these values, the Nile River Damietta branch represents a good to an excellent source of drinking water for entering secondary treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Egypt is presently undergoing rapid urbanization to satisfy the country’s population growth that has increased dramatically from 35 million in 1970 to about 100 million in 2017 (Mahmoud & Gan, 2018). The increased population and associated socio-economic activities have resulted in increased water demand and the release of untreated, or poorly treated, effluent that affects water quality (Wahaab et al., 2019).
The Nile ecosystem has changed chemically, physically, and biologically after building the Aswan Dam in 1964 to control the Nile flood (Ismail & Ramadan, 1995). The Damietta branch of the Nile River now extends for 220 km from the Delta barrage to the Farskour dam that controls the river’s flow regime and protects riverine freshwater from the invasion of the Mediterranean seawater entering through the Damietta estuary (El-Tohamy et al., 2018). Despite the great benefit of this dam in the preservation of water supplies, its environmental costs are becoming apparent. By reducing water flow and interrupting the transportation of pollutants, dams ultimately influence the river’s water quality (Wei et al., 2009).
The vital importance of the Nile River in Damietta is manifested in numerous ways. Foremost, it is the only source of drinking water, thereby directly influencing people’s health. Additionally, it is of high economic benefit through major activities such as agriculture, fishing, transportation, and numerous other lesser purposes. All these uses have raised concerns about the river water quality. The monitoring of water quality and an understanding of the various associated physicochemical variables can determine the environmental stress along the river. These have been attributed to both natural processes and human activities, such as climate change, agricultural land use, municipal, and industrial discharges (Abdel-Satar, 2005). During the current decade, water quality in Damietta has improved greatly, especially after July 2010 when the Drainage of Al-Serw discharges switched from the River Nile to Manzalla Lake. This drain was receiving approximately 1900 m3/day of untreated domestic wastewater (Shaban et al., 2010), which was compromising the general health and the environment in the entire Governorate. Although the river is no longer receiving water from municipal sewage, Damietta region has riverside-dwellers that have allowed small drains to open into the river water, influencing the physicochemical and microbiological components of the river.
The study area lies entirely within an ecosystem that permits agricultural activities and is found within both urban and rural areas, that put it at a significant risk from human impact. Many areas along the banks of the river are cultivated all year round. The associated agricultural wastewater from these areas is discharged directly into the river resulting in increased levels of nutrient loading. Confluent to the main river channel, there are numerous branching side channels with small islands between them that often exhibit a significant impact on river environmental characters leading to high spatial and temporal heterogeneity.
Traditionally, to assess surface water quality, a long list of physical, chemical, and biological parameters is compared with relevant threshold values (guidelines). This technique may be effective in identifying parameters that could be responsible for water pollution, but it gives a shallow and deficient view into the general state of water quality (Soltani et al., 2020). Several approaches have been developed to address these shortcomings, particularly multivariate statistical analysis and the determination of a water quality index (WQI). According to many scientific sources (e.g., Kumarasamy et al., 2014; Sharma et al., 2015; Singh et al., 2005; Varol, 2020; Wang et al., 2013), factor analysis (FA), principal component analysis (PCA), and discriminant analysis (DA) can provide meaningful information about pollution sources, thus facilitating the identification of deleterious factors that influence water quality, and offering a valuable toolset for consistent management of water resources. They are well-suited to identify the most significant factors in spatial and temporal variations of surface water quality, and thereby reduce the complexity of large data sets while optimizing monitoring programs by defining a the fewest indicator parameters (Xin et al., 2008). Compared with other data-manipulation methods, multivariate techniques allow the analysis of large datasets without losing original information (Luo et al., 2017).
A WQI, on the other hand, synthesizes a wide range of variables that are important for both scientists and authorities into a single value that is easy to express and understand (Kükrer & Mutlu, 2019). This method, first described by Horton (1965), is considered one of the most successful techniques to assess water quality (Akter et al., 2016). Depending on the goal of water quality monitoring, many WQIs comprised of different variables have been applied all over the world (Koçer & Sevgili 2014). The first step in the development of a WQI is parameter selection. Although the location and sampling periodicity of a water body is essential for that selection, expert opinions are also required for accurate variables selection and assigning their relative importance based on their potential impact on water quality (Lumb et al., 2011). Consequently, the final score of WQI can be highly variable due to the differences in expert opinions. Therefore, a preferable method of variables selection would be one that does not depend mainly on expert opinions and does not prejudice the effectiveness of WQI final results. According to Kachroud et al. (2019), the robustness of WQI results can be increased through the application of multivariate analysis techniques in variable selection. Coletti et al. (2010) used the results of Factor Analysis for variables selection, whereas Tripathi and Singal (2019) used principal component analysis. Moreover, the current study showed a significant difference in parameter selection, which is one of the major steps of WQI development, and therefore, the Discriminant Analysis (DA) was used in this study. The discriminant analysis performs a multivariate test on the explanatory variables of different groups, and, if statistically significant, proceeds to find linear combinations of variables that best discriminate between groups, then it constructs the discriminant functions based on these combinations (Correa-Metrio et al., 2010). DA is next used to identify the most important indicator parameters that are responsible for large variations in water quality (Shrestha & Kazama, 2007).
In this study, we used these approaches to (1) reveal the spatial and temporal variabilities of water quality parameters and to identify the main pollution sources influencing water quality, (2) determine which parameters should be employed in assessing spatial or temporal variations in river water quality, and (3) determine the surface water contamination levels using the World Health Organization's (WHO) standards, Egyptian regulations of Governmental Decree No. 92/2013, and the newly derived water quality index. This aims to provide tools for making better decisions about river water assessment to ensure efficient management, helping to understand contamination sources, and providing insight for the redesign of sampling strategies by focusing on the most effective water quality parameters.
Material and methods
Area of study
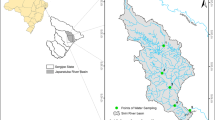
In Damietta Governorate, about 2 million people are served with the drinking water produced from the Damietta branch of the Nile River. Water from the river basin is transported directly to the six main water treatment plants (WTP). The sampling points were selected to be close to the intakes of these plants and cover a length of about 35 km from Al-Adlya, a small village located 2.5 km upstream of Damietta city, to the village of Al-Maysrah, 10 km downstream of Shirbin city (Fig. 1; Table 1).
Samples collection and analysis
Water samples were collected monthly from February 2016 to January 2017 at six sampling sites along the Nile River Damietta Branch (Fig. 1). The samples were collected and manipulated according to the established protocols (American Public Health Association (APHA), (APHA, 2005)) for 32 parameters. Temperature (°C), pH, dissolved oxygen (DO), electrical conductivity (EC), and total dissolved solids (TDS) were measured in the field using portable water quality analyzers. In the laboratory, the determination of turbidity (NTU), alkalinity (CaCO3), total hardness, nitrite (NO2‾), ammonium (NH4+), and phosphate (PO43−) was done based on APHA procedures. Sulphate (SO42−) was measured using the turbidimetric method of Sheen et. al. (1935). The cations (sodium (Na+), potassium (K+), and calcium (Ca2+)) and anions (fluorides (F−) and chlorides (Cl−)) were measured using ion chromatography (Dionex model: ICS-3000). The heavy metals (Al, Fe, Mn, Cd, Cu, Ni, Pb, and Zn) were estimated by atomic absorption spectroscopy (Varian SpectrAA) following the standard acid digestion technique as described by APHA (2005). Also, by following APHA protocols (2005), water organic pollution was investigated using 5-day biological oxygen demand (BOD), chemical oxygen demand (COD), and total organic carbon (TOC). The phytoplankton biomass (Chlorophyll-a) was determined spectrophotometrically (Wetzel & Likens, 2013). The abundance of heterotrophic bacteria was determined according to APHA methods, with 100 ml of each serially diluted water sample transferred to sterilized agar plates in duplicate and incubated at 37 °C for 24–48 h.
Statistical analysis
All data were checked for normality prior to analysis, and non-normal parameters were transformed using log(x + 1). The data transformation and the statistical analyses were performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA) and StatSoft Statistica 8.0 software packages. Variations across the sampling sites were analyzed by one-way analysis of variance (ANOVA) with Tukey’s-b technique. Pearson’s correlation was used to describe the relationship between parameters.
Factor analysis (FA) was used to suggest how many factors are important to explain the variations in water quality (Ouyang et al., 2006). Principal components analysis (PCA) was used for axis extraction (Najafpour et al., 2008). Kaiser–Meyer–Olkin (KMO) and Bartlett’s sphericity tests were applied to examine the suitability of the data for the analysis (Bu et al., 2010). Discriminant analysis (DA) was performed on the original datasets to identify the processes that controlled temporal or spatial variations in surface river water chemistry (Bouguerne et al., 2017), construct linear discriminant functions of the several variables that were used to describe or clarify the differences between groups, and identify the relative contribution of all variables to the separation of the groups (Najafpour et al., 2008). The DA included the determination of a linear equation that will predict to which group the variable belongs (Bouguerne et al., 2017).
Water quality index calculation
The weighted arithmetic index method (Brown et al., 1970) was used for the calculation of WQI in this study. The mathematical formula of this method is given by
where Qi is the quality rating scale of the ith water quality parameters, Wi is the unit weight for the ith parameters, and n is the number of parameters.
Calculating of Qi value as
where Vi is the measured value of ith parameter, Si is the standard permissible value of ith parameter assigned by Egyptian Governmental guidelines (2013), and Vo is the ideal value of ith parameter in pure water.
Calculation of Wi value, as
where \(K = \frac{1}{{\sum\nolimits_{i = 1}^{n} \frac{1}{si} }}\).
Wi is the unit weight for ith parameter and k is proportionality constant for various water quality characteristics.
The suitability of WQI values for human use is rated from excellent to water unfit for use. Accordingly, WQI values are arranged in such a way that 0–25 (Excellent), 26–50 (Good), 51–75 (Bad), 76–100 (Very Bad), and above 100 (Unfit).
Results and discussion
General water quality evaluation
The mean values (± SD) of physio-chemical parameters at various sampling locations were first compared with drinking water guidelines from WHO (2017) and Egyptian guidelines (Table 2). Except for COD and BOD, the measured values of all parameters were within the specifications of the Egyptian standards. According to WHO standards, only turbidity values (in about 66% of all samples) were higher than the acceptable levels for drinking water, but only slightly so.
In general, spatial variability (Fig. 2) was less than temporal variability (Fig. 3) for most of our 32 variables. One-way ANOVA showed significant temporal variability (p < 0.05) except for BOD, PO4, SiO3, SO4, Al, Fe, Cd, Pb, and Ni. On the other hand, only turbidity, BOD, PO4, and SiO3 displayed significant spatial differences.
The overall range of temperature variations reached its minimum in January and maximum in August, following the normal seasonal cycles reported in Egypt. The pH values were on the alkaline side (7.7–8.7), with high seasonal variations, that ranged from a minimum average in March to a maximum in August. The higher values of pH during summer could be due to the photosynthetic activity of the phytoplankton (He et al., 2017), consistent with the high levels of chlorophyll-a during summer months. Also, the positive correlations of pH with temperature (r = 0.37 and P < 0.01) and chlorophyll-a (r = 0.47 and P < 0.001) support this suggestion.
Total dissolved salts (TDS) showed limited spatial variability but high seasonality with an average of 278 mg/L. It was closely tracked by conductivity (EC) values that are usually sensitive to the TDS variations. Chlorides showed the same seasonality with summer-time minima that averaged of 36.4 mg/L over the year being low compared with that of El-Tohamy et al. (2018) (average 163 mg/L), and indicate a considerable decrease in industrial effluents that are rich in chloride since 2013. Calcium and magnesium values ranged between 36.8–41.6 and 14.4–19.2 mg/L, respectively, with high temporal variations (P < 0.001). The observed low levels of calcium and magnesium during some hot months (Fig. 3) could be attributed to adsorption onto clay elements and deposition to the bottom during temperature elevations (Goher et al., 2014). Water hardness showed a downward trend from April to September, then began to increase during winter, with maximum value (177 ± 1.1 mg/L) reached in February. Total alkalinity exhibited nearly the same trend as hardness (Fig. 3). The levels of calcium and magnesium salts that regulated water body hardness are generally associated with carbonates that are the main source of alkalinity (Wruts, 2002). The similar distribution pattern and significant positive correlations between total hardness, magnesium (r = 0.91 and P < 0.001), and alkalinity (r = 0.61 and P < 0.001) revealed the strong association between these parameters. On the other side, the decrease of alkalinity values in the water during hot months likely resulted from the release of hydronium ions from bicarbonate at high temperatures (Thakur & Bais, 1987). The strong negative correlation between temperature and alkalinity (r = − 0.727 and P < 0.001) support the previous argument.
Sodium and potassium values varied in the range, 34–53 and 5.24–6.28 mg/L, respectively. The recorded low values of these ions were likely due to their resistance to disintegration by weathering effects after their arrival with the agricultural effluents from fertilizers (Bouguerne et al., 2017).
DO, BOD, and COD varied in the ranges, 5.10–11.33, 0.7–17.08, and 3.48–177.7 mg/L, respectively. DO values were higher during the winter months and decreased with increasing temperature at most sites. The low DO values with increasing water temperature were likely due to a combination of decreased solubility of oxygen at warmer temperatures and increasing microbial activities that consume oxygen during the decay of organic matter (Varol et al., 2012). Inversely to DO values, BOD reached its highest levels in late spring, summer, and early autumn at sites III and VI, and then fell to its minimum during winter at the site I. The previous observations are supported by temperature’s negative correlation to DO (r = − 0.55 and P < 0.001) and positive correlation to BOD (r = 0.32 and P < 0.01). About 56% of surface water samples, mostly at sites III and VI, during summer and spring, exceeded the permissible BOD maximum of 6 based on the Egyptian Governmental Decree (No 92/2013). The highest COD values were found at site VI, which showed the highest values of BOD and the lowest DO values, strongly indicating that this site was receiving sewage water and agricultural runoffs that were rich in organic pollutants. Nearly 96% of recorded COD values in the study area exceeded the maximum permissible limit of 10 based on Egyptian specifications. The high COD values suggested a high load of dissolved organic matter in the riverine water contributed by domestic and agricultural wastes. Elevated levels of COD lower dissolved oxygen concentrations, thus worsening water quality (Kannel et al., 2007).
For macronutrients, the concentrations of ammonia were consistently high except for February and March, with an average value of 308.8 ± 131.1 µg/L. Nitrite values were found to be in the range of 16 to 28 µg/L. It was at relatively low levels in early spring and winter, then increased in May and reached its maximum value of 25.5 µg/L in June. Nitrite is an intermediate product of the oxidation of ammonia to nitrate, the strong positive correlation between nitrite and ammonia (r = 0.421 and P < 0.001) provides evidence of this process in the study area. The toxicity of nitrate for animals and humans is significantly lower compared with those of ammonia and nitrite (Valencia-Castañeda et al., 2019). In the present study, concentrations of ammonia and nitrite were far below the standards of WHO (2017), so they do not pose a health risk. Phosphorus is a limiting nutrient for phytoplankton growth in freshwater systems and it plays a crucial role in eutrophication (Varol et al., 2012). Phosphate values varied from 110 to 180 µg/L with a mean value of 145.3 ± 21.1. Silicate concentrations fluctuated between 1110 and 1260 µg/L. Generally, upstream sites (IV, V, and VI) recorded the maximum content of nutrients due to the increased discharge of agricultural and domestic effluents.
TOC concentrations ranged between 3.84 and 8.97 with a remarkable seasonal variation (P < 0.001). TOC is directly related to biological factors that are represented in this study by only two parameters, namely, heterotrophic bacteria (Williams et al., 2015) and phytoplankton biomass (Sohrin & Sempéré, 2005). Also, the significant positive correlation between TOC/temperature (r = 0.599 & P < 0.001), TOC/BOD (r = 0.29 and P = 0.012) and the strong negative correlation of TOC with DO (r = -0.52 and P < 0.001) suggest the utilization of dissolved oxygen by microbial pollutants in the decomposition of organic matter during hot months. Sulfate usually enters waters from the use of soap and detergent (Gupta et al., 2014), and their values varied from 26 to 37 mg/L without seasonality. Fluoride was found in the range of 345 to 409 µg/L and also lacked temporal or spatial patterns.
The concentrations of heavy metals were all within the permissible limits by WHO (2017) and Egyptian standards (2013). Although aluminum values were high compared with other metals, aluminum concentrations < 1000 µg/L are considered nontoxic (Fipps, 2003). The heavy metals concentrations increased upstream particularly at sites IV and VI presumably due to domestic sewage and run-offs from extensively farmed areas. Although only Mn, Cu, and Zn showed significant temporal variations (P < 0.05), other metals showed a remarkable seasonal difference (Fig. 3). The maximum concentrations of Fe, Cd, Mn, Cu, Ni, and Pb were found during the hot month period (late spring, summer, and early autumn) in agreement with the results of Ibrahim and Omar (2013) and Goher et al. (2014). The increase of some metals concentrations in surface waters during hot months may be attributed to high evaporation rates during elevated temperatures (Abdel-Satar, 2001), and/or the liberation of some heavy metals from the bottom sediments to the topwater from the fermentation of organic matter at high temperatures (Ali & Abdel-Satar, 2005). A few metals such as Al and Zn showed their maximum values during winter months, which may be attributed to soil leaching from rainfall (Wijngaard et al., 2017). On the other hand, many previous studies (e.g., El-Ameir, 2017; El-Tohamy et al., 2018; Gad & Toufeek, 2010) argued that the fluctuations of effluents from agricultural, sewage, and industrial wastes discharged into the river water are among the main reasons for seasonal differences of metals concentrations.
Chlorophyll-a concentrations in the present work ranged from 1.2 to 17.3 µg/L with an average of 6.6 ± 3.3. The elevated levels of chlorophyll-a are an indicator of poor water quality (Dorgham et al. 2019); values from 1–3 µg/L are mesotrophic, values from 3–5 µg/L are considered as eutrophic, and those above 5 µg/L are polytrophic (Soo et al., 2017). Accordingly, most sites were mesotrophic to eutrophic during late autumn and winter months, while during spring and early autumn months, eutrophic conditions occurred frequently, and the polytrophic levels were reported persistently at all sampling sites during summer months. This intensive phytoplankton production during summer is attributed to the enrichment of nutrients from land runoffs with intensive agricultural activities. This could be clearly deduced from the significant positive correlation of chlorophyll-a with temperature (r = 0.574 and P < 0.001) in the present investigation.
Heterotrophic bacteria naturally inhabit the bodies of humans and animals (Amanidaz et al., 2015). They may comprise up to 90% of all aquatic bacteria detected in surface water (Rheinheimer et al., 1992). In the current study area, the abundance of heterotrophic bacteria was slightly higher at sites IV and VI than other sites (Fig. 2). Temporally, strong seasonal differences were observed (P < 0.001); the highest mean values were found in summer months, whereas the minimum appeared in winter (Fig. 3). According to Cliver and Newman (1987), seasonal temperature variations and sewage effluent into the water system are considered powerful effects that affect the dominance and profiles of bacteria at any given time. The heterotrophic bacteria showed a positive correlation with some of the nutrients as NO2-N (r = 0.27 and P = 0.03) and NH4-N (r = 0.37 and P < 0.01), indicating potential microbial contaminations from sewage.
Factor analysis
Factor analysis (FA) of the 32 variables found structure within the data (KMO and Bartlett’s sphericity tests were 0.665 and 2541.1 (df = 703, P < 0.001), respectively), confirming that FA could be an appropriate and useful tool to provide a significant reduction in data dimensionality (Zhou et al., 2007). The analysis extracted 10 rotated factors but only the first five factors (Fig. 4) had Eigenvalues greater than 1 (Bu et al., 2010) and together explained 69.6% of the total variance (Table 3).
Factor-1, accounting for 24.6% of the total variance, was positively correlated with some major physicochemical sources (EC, TDS, Cl−1, alkalinity, total hardness, and magnesium); temperature (natural) and biological parameters (chlorophyll-a and HPC) were negatively correlated with factor 1. These variables encompassed a seasonal fluctuations factor, representing the changes in total salts concentration along with other chemical changes that provided insight on biological parameters changes in relation to seasonal changes, anthropogenic activities, and temperature variations (LeChevallier, 2003).
Factor-2, encompassed nutritional factor, accounting for 10.6% of the total variance and reflected the level of most macronutrients by a positive correlation with NH4+, NO2‾, PO43−, and potassium. Higher values of potassium, NH4-N, and PO4-P in the study area usually coincided with the local agricultural activities, when farmers planted rice and potato and used nitrogen (e.g., urea), potassium, and phosphate as fertilizers. Such conditions confirm that agricultural pollution from the cultivation reached rivers through soil leaching during rainy seasons (Bu et al., 2010) and/or surface runoffs that correlated with different agricultural activities.
Factor-3 was associated with oxidative/microbial factors: negative DO and positive BOD, TOC, and turbidity, coupled to the processing of organic matter but was only 8.2% of the total variance. The inverse relationship between BOD and DO with remineralization of organic matter is well known (Kannel et al., 2008). This factor can be interpreted as reflecting influences from organic sources such as domestic wastewater discharges.
Factor-4 was associated with metals, having a positive correlation with Cd, Pb, Ni, and Zn that explained 7.2% of the total variance. Industrial and agricultural effluents dispersed along the study area added heavy metals in the riverine water continuously.
Factor-5 was an erosion factor, explaining only 6.1% of the total variance and was positively correlated with Fe, Mn, and SiO32− reflecting the influence of soil erosion through surface runoffs and seasonal effects on water composition (Ojok et al., 2017). It is also possible that surface irrigation systems in the Nile Delta increased the dissociation rate of heavy metals (Shokr et al., 2016) and, accordingly, recycled them back into the riverine water through agricultural runoffs.
The representation of factor scores (1 and 2) in factor analysis (Fig. 5) reveals different pollution sources in the river system on temporal and spatial scales. For the sampling times (Fig. 5a), factor score 1 mostly impacted the water quality during most of the spring and summer months that are coincident with the increase of temperatures and/or agricultural activities. Concerning the sampling sites (Fig. 5b), factor score 2 was most significant to the conditions of sites IV and VI that were strongly polluted by agricultural and domestic runoffs and reflected the influences of both eutrophication and disturbance in the river.
Discriminant analysis
In the present study, the standard discriminant analysis (DA) was applied to the raw data that consisted of the 32 parameters to highlight the spatial and temporal variations in surface water quality across the most significant variables. Spatially, two discriminate functions (DFs) were found to statistically separate the six sampling sites in the study area (Table 4). Accordingly, 95.7% of the total variance was explained by the two DFs. The first function explained 76.5% of the total spatial variance, while the second function explained 19.2%. Only 7 parameters among 32 were required by the two discriminant functions (Table 5). The relative contribution of each parameter was assumed by Eqs. (1) and (2) as the following:
The first function discriminated the key chemical parameters (PO4 > SiO3 > Al > Fe—arranged according to the relative contribution in water quality) and exhibited a strong contribution in discriminating the six sampling sites that were attributed to the differences in exposure to different types of surface runoffs. The second function takes into account the parameters of organic pollution, according to their contribution to discrimination. These parameters can be arranged in the order: BOD > turbidity > chlorophyll-a. It is notable that the seven parameters each represented a category of water quality properties: physical, inorganic chemical, organic chemical, and biological parameters, and heavy metals. BOD and phosphorus showed a stronger contribution to spatial discrimination than other parameters. This can be attributed to the differences in the exposure to the runoffs of phosphate fertilizers from the agricultural activities between the six sampling sites. Also, the organic pollution that is expressed by BOD may have come from many other sources such as anaerobic wastewaters (low dissolved oxygen levels) and anthropogenic runoffs containing poorly degraded organic wastes.
Temporally, the first two DFs explained 86.6% of the total variance (Wilk’s Lambda test) and were also statistically significant (Table 4). The first DF explained 56.5% of the total temporal variance, while the second DF explained 30.3%. A total of 11 among 32 parameters were encompassed by the two functions (Table 5) and the relative contribution of each parameter was given by Eqs. (3) and (4) as follows:
The 11 parameters selected by the temporal DFs can be arranged according to their contribution in the order K > temperature > COD > HPC > total hardness > DO > NO2 > Na > TDS > Cl > EC. Thus, the temporal DA results suggested that K, temperature, COD, and live heterotrophic bacteria were the most significant parameters discriminating the variances between months, with these four variables accounting for most of the observed temporal variations in the river’s surface water quality.
Water quality index
For calculating the WQI index, the results of discriminant analysis led to the reduction of the number of parameters from 32 to 18 indicator parameters that were responsible for large variations in water quality. Since the limits of the Egyptian Decree No 92/2013 were used in the calculation of the WQI scores, eleven parameters were not considered, namely temperature, EC, hardness, Cl, Al, Na, HPC, K, SiO3, turbidity, and chlorophyll-a, where their limits were not established within the Egyptian guidelines (Table 2). Therefore, the final shortlisted 7 parameters were DO, BOD, COD, PO4, Fe, NO2, and TDS.
WQI values in the study area were found to be between 12.7 and 33.7 with an annual mean value of 18.9 ± 3.9. According to these values, the Nile River Damietta branch is classified as having a good to excellent water quality in terms of its WQI. Therefore, the Damietta branch can be safely used for drinking and irrigation purposes after suitable secondary treatment. The values of WQI showed temporal and spatial significant differences (Fig. 6). The highest value was determined at site VI in May, whereas the smallest value was determined at site III in January. Compared with El-Ezaby et al. (2010) and Badr et al. (2013), the WQI results suggest improvement in the Nile water quality at the study area over the last 10 years (El-Ezaby et al., 2010; Badr et al., 2013) during which time Al-Serow drain discharge switched to Manzalla lake (Shaban et al., 2010) and fish cages were removed from the river.
Conclusion
In this study, the spatial and temporal variations in surface water quality were evaluated using multivariate statistical techniques. COD and BOD at most sampling sites exceeded the guidelines of the Egyptian regulations, leading to the concern that organic pollution may threaten the water quality in the Nile River. Temporal variability was significantly greater than spatial variability for most variables. Seasonal fluctuations, nutritious (agricultural discharge), and organic pollution (domestic wastewater) were the main factors influencing the water quality in the Nile River. Spatially, only 7 parameters were required to discriminate between the six sampling sites (BOD > PO4 > SiO3 > Al > Turbidity > Fe > chlorophyll-a—arranged according to their relative contribution in water quality) affording 96% correct assignations in spatial variations in comparison to 11 parameters (K > temperature > COD > HPC > hardness > DO > NO 2 > Na > TDS > Cl > EC—also, arranged by contribution) affording 87% correct assignations in temporal analysis over the year-round. Consequently, a few indicator parameters responsible for most variations in water quality can be used for the environmental monitoring policies in this river. We used this reduction approach to develop a novel water quality index for the Nile River in Egypt. The use of DA led to the reduction of the 32 parameters to 18 that were further reduced to 7 parameters. This simplification will reduce the time, effort, and cost required to conduct water quality monitoring. Except for the above limit turbidity, water quality parameters did not exceed the international (WHO) limits during the study period and Damietta branch has, thus, good water conditions. However, the effects of the anthropogenic activities were determined along the river course, particularly at upstream sites. The upstream site VI is far from the other sites in factor scores analysis and showed the highest WQI values. In general, for the long-term improvement and protection of the current status of water quality in the Nile River Damietta branch, a participatory approach that includes all groups of society should be built. The overuse of fertilizers in Egypt might be reduced through the education of farmers on fertilizer application. The domestic and agricultural wastes must be prevented from being discharged to the river from its banks and adjacent areas through tighter regulatory control. Together these actions will facilitate complement with the recommended standards of effluent discharge set in Egyptian laws for the protection of the Nile River against pollution.
Data Availability
The data is available upon request.
References
Abdel-Satar, M. A. (2001). Environmental studies on the impact of the drains effluent upon the southern sector of Lake Manzalah, Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 5(3), 17–30.
Abdel-Satar, M. A. (2005). Water quality assessment of River Nile from Idfo to Cairo. Egyptian Journal of Aquatic Research, 31(2), 200–223.
Akter, T., Jhohura, F. T., Akter, F., Chowdhury, T. R., Mistry, S. K., Dey, D., et al. (2016). Water Quality Index for measuring drinking water quality in rural Bangladesh: A cross-sectional study. Journal of Health, Population and Nutrition, 35(1), 1–12.
Ali, M. H., & Abdel-Satar, A. M. (2005). Studies of some heavy metals in water, sediment, fish and fish diets in some fish farms in El-Fayoum province, Egypt. Egyptian Journal of Aquatic Research, 31(2), 261–273.
Amanidaz, N., Zafarzadeh, A., & Mahvi, A. H. (2015). The Interaction between heterotrophic bacteria and coliform, fecal coliform, fecal Streptococci bacteria in the water supply networks. Iranian Journal of Public Health, 44(12), 1685.
American Public Health Association. (2005). Standard methods for the examination of water and wastewater (20th ed.). New York: American Public Health Association, American Water Works Association, Water Environment Federation.
Badr, E.-S., El-Sonbati, M., & Nassef, H. (2013). Water quality assessment in the Nile River, Damietta branch, Egypt. Catrina: The International Journal of Environmental Sciences, 8(1), 41–50.
Bouguerne, A., Boudoukha, A., Benkhaled, A., & Mebarkia, A. (2017). Assessment of surface water quality of Ain Zada dam (Algeria) using multivariate statistical techniques. International Journal of River Basin Management, 15(2), 133–143.
Brown, R. M., McClelland, N. I., Deininger, R., & Tozer, R. G. (1970). A water quality index-do we dare? Water Sewage Works, 117, 339–343.
Bu, H., Tan, X., Li, S., & Zhang, Q. (2010). Temporal and spatial variations of water quality in the Jinshui River of the South Qinling Mts., China. Ecotoxicology and Environmental Safety, 73(5), 907–913.
Cliver, D. O., & Newman, R. A. (1987). Drinking water microbiology. Journal of environmental pathology, toxicology and oncology, 7(5–6).
Coletti, C., Testezlaf, R., Ribeiro, T. A. P., de Souza, R. T. G., Pereira, D., & d. A. . (2010). Water quality index using multivariate factorial analysis. Revista Brasileira de Engenharia Agrícola e Ambiental, 14(5), 517–522.
Correa-Metrio, A., Cabrera, K. R., & Bush, M. B. (2010). Quantifying ecological change through discriminant analysis: a paleoecological example from the Peruvian Amazon. Journal of Vegetation Science, 21(4), 695–704.
Dorgham, M. M., El-Tohamy, W. S., Qin, J., Abdel-Aziz, N., & Ghobashy, A. (2019). Water quality assessment of the Nile Delta Coast, south eastern Mediterranean, Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 23(3), 151–169.
Egyptian Governmental Decree (2013). For the protection of the Nile River and its waterways from pollution. Governmental Decree No. 92 of 2013 amending the Ministerial Decree No. 8 of 1982 on the executive Regulations of Law No. 48 of 1982. (in Arabic).
El-Ameir, Y. A. (2017). Evaluation of heavy metal pollution in Damietta branch of Nile River, Egypt using metal indices and phyto-accumulators. Journal of Environmental Sciences, 46(2), 89–102.
El-Tohamy, W. S., Abdel-Baki, S. N., Abdel-Aziz, N. E., & Khidr, A. A. (2018). Evaluation of spatial and temporal variations of surface water quality in the Nile River Damietta branch. Ecological Chemistry and Engineering S, 25(4), 569–580.
Fipps, G. (2003). Irrigation water quality standards and salinity management strategies. Texas farmer Collection: Texas University Agricultural Life Extension.
Gad, N. S., & Toufeek, M. A. (2010). Distribution and accumulation of some trace metals in water and fish from Damietta Branch of River Nile. African Journal of Biological Sciences, 6(1), 95–115.
Goher, M. E., Hassan, A. M., Abdel-Moniem, I. A., Fahmy, A. H., & El-sayed, S. M. (2014). Evaluation of surface water quality and heavy metal indices of Ismailia Canal, Nile River, Egypt. The Egyptian Journal of Aquatic Research, 40(3), 225–233.
Gupta, L., Avtar, R., Kumar, P., Gupta, G. S., Verma, R. L., Sahu, N., et al. (2014). A multivariate approach for water quality assessment of River Mandakini in Chitrakoot, India. Journal of Water Resource and Hydraulic Engineering, 3, 22–29.
He, B., He, J., Wang, J., Li, J., & Wang, F. (2017). Abnormal pH elevation in the Chaobai River, a reclaimed water intake area. Environmental Science: Processes & Impacts, 19(2), 111–122.
Horton, R. K. (1965). An index number system for rating water quality. Journal of Water Pollution Control Federation, 37(3), 300–306.
Ibrahim, A. T. A., & Omar, H. M. (2013). Seasonal variation of heavy metals accumulation in muscles of the African Catfish Clarias gariepinus and in River Nile water and sediments at Assiut Governorate, Egypt. Journal of Biology and Earth Sciences, 3(2), 236–248.
Ismail, S. S., & Ramadan, A. (1995). Characterisation of Nile and drinking water quality by chemical and cluster analysis. Science of the Total Environment, 173, 69–81.
El-Ezaby, H. K., El-Sonbati, M., & Badr, E.-S. (2010). Impact of fish cages on the Nile water quality at Damietta Branch. Journal of Environmental Sciences, 39(3), 329–344.
Kannel, P. R., Lee, S., Kanel, S. R., Khan, S. P., & Lee, Y. (2007). Spatial–temporal variation and comparative assessment of water qualities of urban river system: A case study of the river Bagmati (Nepal). Environmental Monitoring and Assessment, 129(1–3), 433–459.
Kannel, P. R., Lee, S., & Lee, Y. (2008). Assessment of spatial–temporal patterns of surface and ground water qualities and factors influencing management strategy of groundwater system in an urban river corridor of Nepal. Journal of Environmental Management, 86(4), 595–604.
Kachroud, M., Trolard, F., Kefi, M., Jebari, S., & Bourrié, G. (2019). Water quality indices: Challenges and application limits in the literature. Water, 11(2), 361.
Koçer, M. A. T., & Sevgili, H. (2014). Parameters selection for water quality index in the assessment of the environmental impacts of land-based trout farms. Ecological Indicators, 36, 672–681.
Kumarasamy, P., James, R. A., Dahms, H.-U., Byeon, C.-W., & Ramesh, R. (2014). Multivariate water quality assessment from the Tamiraparani river basin. Southern India. Environmental Earth Sciences, 71(5), 2441–2451.
Kükrer, S., & Mutlu, E. (2019). Assessment of surface water quality using water quality index and multivariate statistical analyses in Saraydüzü Dam Lake, Turkey. Environmental Monitoring and Assessment, 191(71), 1–16.
LeChevallier, M. W. (2003). Conditions favouring coliform and HPC bacterial growth in drinking water and on water contact surfaces. Heterotrophic Plate Count Measurement in Drinking Water Safety Management. Geneva, World Health Organization, 1, 177–198.
Lumb, A., Sharma, T. C., & Bibeault, J.-F. (2011). A review of genesis and evolution of water quality index (WQI) and some future directions. Water Quality, Exposure and Health, 3(1), 11–24.
Luo, K., Hu, X., He, Q., Wu, Z., Cheng, H., Hu, Z., & Mazumder, A. (2017). Using multivariate techniques to assess the effects of urbanization on surface water quality: A case study in the Liangjiang New Area, China. Environmental Monitoring and Assessment, 189(174), 1–11.
Mahmoud, S. H., & Gan, T. Y. (2018). Long-term impact of rapid urbanization on urban climate and human thermal comfort in hot-arid environment. Building and Environment, 142, 83–100.
Najafpour, S., Alkarkhi, A. F. M., Kadir, M. O. A., & Najafpour, G. D. (2008). Evaluation of spatial and temporal variation in river water quality. International Journal of Environmental Research, 2(4), 349–358.
Ojok, W., Wasswa, J., & Ntambi, E. (2017). Assessment of seasonal variation in water quality in River Rwizi using multivariate statistical techniques, Mbarara Municipality. Journal of Water Resource and Protection, 9(1), 83–97.
Ouyang, Y., Nkedi-Kizza, P., Wu, Q. T., Shinde, D., & Huang, C. H. (2006). Assessment of seasonal variations in surface water quality. Water research, 40(20), 3800–3810.
Rheinheimer, G., Mayr-Harting, A., & Walker, N. (1992). Aquatic Microbiology (4ed.): Wiley.
Shaban, M., Urban, B., El Saadi, A., & Faisal, M. (2010). Detection and mapping of water pollution variation in the Nile Delta using multivariate clustering and GIS techniques. Journal of Environmental Management, 91(8), 1785–1793.
Sharma, M., Kansal, A., Jain, S., & Sharma, P. (2015). Application of multivariate statistical techniques in determining the spatial temporal water quality variation of Ganga and Yamuna Rivers present in Uttarakhand State, India. Water Quality, Exposure and Health, 7(4), 567–581.
Sheen, R. T., Kahler, H. L., Ross, E. M., Betz, W. H., & Betz, L. D. (1935). Turbidimetric determination of sulfate in water. Industrial & Engineering Chemistry Analytical Edition, 7(4), 262–265.
Shokr, M. S., El Baroudy, A. A., Fullen, M. A., El-Beshbeshy, T. R., Ramadan, A. R., El Halim, A. A., et al. (2016). Spatial distribution of heavy metals in the middle nile delta of Egypt. International Soil and Water Conservation Research, 4(4), 293–303.
Shrestha, S., & Kazama, F. (2007). Assessment of surface water quality using multivariate statistical techniques: A case study of the Fuji River basin, Japan. Environmental Modelling & Software, 22(4), 464–475.
Singh, K. P., Malik, A., & Sinha, S. (2005). Water quality assessment and apportionment of pollution sources of Gomti River (India) using multivariate statistical techniques—A case study. Analytica Chimica Acta, 538(1–2), 355–374.
Sohrin, R., & Sempéré, R. (2005). Seasonal variation in total organic carbon in the northeast Atlantic in 2000–2001. Journal of Geophysical Research: Oceans, 110, C10590.
Soltani, A. A., Bermad, A., Boutaghane, H., Oukil, A., Abdalla, O., Hasbaia, M., et al. (2020). An integrated approach for assessing surface water quality: Case of Beni Haroun dam (Northeast Algeria). Environmental Monitoring and Assessment, 192(10), 1–17.
Soo, C. L., Chen, C. A., & Mohd-Long, S. (2017). Assessment of near-bottom water quality of southwestern coast of Sarawak, Borneo, Malaysia: A multivariate statistical approach. Journal of Chemistry, 2017, 1–12.
Thakur, S., & Bais, V. (1987). Seasonal variation of temperature, alkalinity and dissolved oxygen in the Sagar Lake. Acta Hydrochimica et Hydrobiologica, 15(2), 143–147.
Tripathi, M., & Singal, S. K. (2019). Use of Principal Component Analysis for parameter selection for development of a novel water quality index: A case study of river Ganga India. Ecological Indicators, 96, 430–436.
Valencia-Castañeda, G., Frías-Espericueta, M. G., Vanegas-Pérez, R. C., Chávez-Sánchez, M. C., & Páez-Osuna, F. (2019). Toxicity of ammonia, nitrite and nitrate to Litopenaeus vannamei juveniles in low-salinity water in single and ternary exposure experiments and their environmental implications. Environmental toxicology and pharmacology, 70, 103193.
Varol, M. (2020). Use of water quality index and multivariate statistical methods for the evaluation of water quality of a stream affected by multiple stressors: a case study. Environmental Pollution, 266(3), 1–10.
Varol, M., Gökot, B., Bekleyen, A., & Şen, B. (2012). Water quality assessment and apportionment of pollution sources of Tigris River (Turkey) using multivariate statistical techniques—A case study. River Research and Applications, 28(9), 1428–1438.
Wahaab, R. A., Salah, A., & Grischek, T. (2019). Water quality changes during the initial operating phase of riverbank filtration sites in Upper Egypt. Water, 11(6), 1258–1276.
Wang, Y., Wang, P., Bai, Y., Tian, Z., Li, J., Shao, X., et al. (2013). Assessment of surface water quality via multivariate statistical techniques: a case study of the Songhua River Harbin region, China. Journal of Hydro-Environment Research, 7(1), 30–40.
Wei, G., Yang, Z., Cui, B., Li, B., Chen, H., Bai, J. H., et al. (2009). Impact of dam construction on water quality and water self-purification capacity of the Lancang River, China. Water Resources Management, 23(9), 1763–1780.
Wetzel, R. G., & Likens, G. E. (2013). Limnological analyses (3rd ed.). New York Inc: Springer.
WHO (2017). Guidelines for drinking-water quality (4th edition, incorporating the 1st addendum ed.): World Health Organization.
Wijngaard, R. R., & Marcel, v. d. P., Bas van der, G., & Marc, F. P. B. . (2017). The impact of climate change on metal transport in a lowland catchment. Water, Air, & Soil Pollution, 228(3), 107.
Williams, K., Pruden, A., Falkinham, J. O., & Edwards, M. (2015). Relationship between organic carbon and opportunistic pathogens in simulated glass water heaters. Pathogens, 4(2), 355–372.
Wurts, W. A. (2002). Alkalinity and hardness in production ponds. World Aquaculture-Baton Rouge, 33(1), 16–17.
Xin, X., Lu, W. X., & Gong, L. (2008). Discriminant analysis method application in water quality assessment. Environmental Science and Technology, 31, 113–115.
Zhou, F., Huang, G. H., Guo, H., Zhang, W., & Hao, Z. (2007). Spatio-temporal patterns and source apportionment of coastal water pollution in eastern Hong Kong. Water Research, 41(15), 3429–3439.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taher, M.E., Ghoneium, A.M., Hopcroft, R.R. et al. Temporal and spatial variations of surface water quality in the Nile River of Damietta Region, Egypt. Environ Monit Assess 193, 128 (2021). https://doi.org/10.1007/s10661-021-08919-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-08919-0