Abstract
Municipal wastewater treatment plants (MWWTPs) are considered to reduce the amount of pollutants that enter water reservoirs as a result of wastewater disposal. An assessment of the occurrence and removal of pharmaceutical compounds, mainly nonsteroidal anti-inflammatory drugs (NSAIDs), in wastewater from the Kavoor MWWTP (southwest monsoon region), India, is presented in this paper. The performance of the MWWTP was monitored in the summer (May) and monsoon (September) periods. The highest inlet concentrations of diclofenac, naproxen, ibuprofen, ketoprofen, and acetylsalicylic acid in the wastewater were observed in May and were 721.37, 2132.48, 2109.875, 2747.29, and 2213.36 μg/L, respectively. The ketoprofen content was found to be higher than that of other NSAIDs in the influent in both seasons, whereas the diclofenac content was found to be the lowest. The removal efficiency (RE) of the target NSAIDs in the Kavoor secondary treatment plant varied from 81.82–98.92% during the summer season. During the monsoon season, the influent NSAID concentration level dropped, probably because of infiltration in old sewer pipes. In addition, a 100% RE was achieved for all the target NSAIDs in the wastewater of the MWWTP. The results showed that secondary treatment plants have the potential to remove NSAID compounds from municipal sewage with consistent performance. The environmental hazards caused by the accumulation of such compounds in water reservoirs are due to open discharge. The environmental risk levels of these compounds were also studied by the environmental risk assessment (ERA) using the European Agency for Evaluation of Medicines approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, studies on “emerging contaminants” have received full attention among researchers because of the considerable effects of such compounds upon prolonged exposure. One such contaminant group is pharmaceutical compounds, which are extensively used by animals and humans for medicinal purposes. The probable pathways by which these contaminants enter the environment include (1) animal or human excreta, (2) the discarding of expired or unused medicines, (3) wastewater generated from pharmaceutical industries, and (4) hospital effluents (Lu et al. 2016; Mandaric et al. 2017). In India, approximately 300 companies belong to the pharmaceutical sector and have an annual export growth rate of 30%, boosting the nation’s economy (Balakrishna et al. 2017). Pharmaceutical compounds consist of different prescription classes, such as antibiotics, anti-inflammatory drugs, analgesics, contraceptives, beta-blockers, and antiepileptic drugs (Cardoso et al. 2014). The German company Bayer manufactured the first anti-inflammatory drug, acetylsalicylic acid (aspirin), in 1899. Since then, many other nonsteroidal anti-inflammatory drugs (NSAIDs) have been developed and marketed extensively. It has been estimated that on average, 30 million people per day consume NSAIDs as pain relievers and inflammation reducers (Feng et al. 2013). The incomplete metabolization of these pharmaceuticals in animals and humans causes their excretion into the sewer system either as parent compounds or as derivatives. These compounds cause severe toxicity to the environment even at minor concentrations, i.e., ng/L or μg/L (micropollutants). Due to rapid urbanization, the occurrence of these micropollutants in wastewater has increased drastically, and the compounds can now be found worldwide.
The half-life of these micropollutants causes chronic conditions in aquatic species and endocrine disorders in humans (Sanderson et al. 2003; Sim et al. 2011; Xu et al. 2017). Diclofenac (2-{2-[(2,6-dichlorophenol)amino]phenyl}acetic acid) is considered to be the most consumed NSAID drug, and its presence inhibits the cyclooxygenase (COX) enzyme, which blocks prostaglandin synthesis (Martínez-Alcalá et al. 2017). It has been reported that at the least the observed effect concentration (LOEC) level of 5 μg/L, diclofenac causes the greatest toxicity to aquatic species among all NSAIDs. Ketoprofen (2-(3-benzoylphenyl) propanoic acid) is a crucial NSAID in the aryl propionic acid class that has antipyretic and analgesic properties. It is mainly used to treat arthritis and other painful conditions (Madikizela et al. 2014; Modi et al. 2012). Aspirin (2-acetyloxy benzoic acid) is a widely used painkiller and is estimated to be one of the most frequently consumed pharmaceutical drugs. It is also used to cure fever and to reduce the potential risk of heart attacks (Tewari et al. 2013). Naproxen ((2S)-2-(6-methoxynaphthalen-2-yl) propanoic acid) is another crucial NSAID, mostly used to cure body pains and treat arthritis. Ibuprofen (2-[4-(2-methylpropyl)phenyl]propanoic acid) is a commonly prescribed drug with anti-inflammatory properties. It belongs to the propionic acid derivative group and is considered to be an alternative to aspirin (Martín et al. 2012). Moreover, bioaccumulation of these compounds causes a decrease in the population of certain species, such as the bacterium Aliivibrio fischeri, zebrafish (Danio rerio), and Indian vulture (Gyps indicus) (Dökmeci et al. 2014; Oaks et al. 2004; Praskova et al. 2012, 2014; Sharma and Kaushik 2017). Thus, there is an urgent need for the degradation of NSAIDs in the environment.
Municipal wastewater treatment plants (MWWTPs) serve to curb pharmaceutical compounds, especially NSAIDs such as diclofenac, aspirin, ibuprofen, indomethacin, and oxaprozin, before reaching water reservoirs such as rivers, streams, and lakes(Yuan et al. 2015). Therefore, the NSAID removal performance of a MWWTP should be assessed before discharging treated wastewater into any water reservoir (Samaras et al. 2013). The concentration of these pharmaceutically active compounds has been found to be higher in sewage sludge in MWWTPs, which could be due to the adsorption of micropollutants onto the sludge granules (Ekpeghere et al. 2016). Furthermore, the research literature shows that the removal efficiency of these pharmaceuticals in any MWWTP depends on the operational conditions and applied treatment processes, as well as parameters such as pH, temperature, and seasonal variability (Samaras et al. 2013).
India has four major seasons, and the subcontinent has a diversified climate in different parts of the country. Knowledge regarding pharmaceutical compounds and data about the presence of pharmaceuticals in the environment are still lacking. Few studies have been conducted in the northern, southern, southeastern, and western regions of the country, and significant variations in the concentration levels of these micropollutants have been found in wastewaters from different regions across the country. No preliminary data were found concerning the presence of NSAIDs in urban wastewaters in the present study area (southwest). Therefore, a study on the occurrence of NSAIDs in municipal wastewater in the southwest region of the country is essential.
A secondary treatment plant located in Kavoor, Karnataka, India, which falls within the southwest monsoon region, was selected as the study area for this research. In this research, the occurrence and removal of the NSAIDs diclofenac, naproxen, ibuprofen, ketoprofen, and acetylsalicylic acid, which all belong to prescription-class drugs, were studied because of their high consumption rate and acute toxicity. This study concentrated only on NSAID compounds and not on their derivatives to provide a baseline for future studies. Moreover, the presence of these NSAIDs in the environment poses a significant risk to biodiversity and causes an overall imbalance in terms of environmental sustainability. Therefore, it is imperative to know the risk levels of each NSAID compound to the biotic community at its measured concentrations. This study attempts to give a preliminary assessment of environmental risk in the aquatic environment of the southwest monsoon region of India due to the occurrence of the detected NSAID compounds in both seasons. Table 1 lists the chemical structure and properties of the target pharmaceutical compounds.
Materials and methods
Reagents
Standards for diclofenac, naproxen, ibuprofen, ketoprofen, acetylsalicylic acid, and the derivatization reagent N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) were procured from Sigma-Aldrich Company Ltd. (St. Louis, USA). Phenanthrene d10 obtained from Cambridge Isotope Laboratories Inc. (Andover, USA) was used as an internal standard. Acetone, ethyl acetate, and n-hexane (HPLC grade) were purchased from Thermo Fisher Scientific India Pvt. Ltd. (Mumbai, India). Commercially available 6 cc solid-phase extraction (SPE) (Oasis hydrophilic-lipophilic balance (HLB) sorbent, 200 mg) cartridges (Waters Corporation, Milford, USA) were used in this study. Glass vials with a 2 mL capacity were acquired from Agilent Technologies (Boeblingen, Germany). Milli-Q water was obtained using an ELGA water purification system (Greifswald, UK). Specific standards of all the analytes were prepared by the dissolution of 100 mg in 1 L (1:1 v/v) of an acetone/ethyl acetate stock solution.
Details of the study area and sample collection
Mangalore, located in the Dakshina Kannada district, on the shore of the Arabian Sea in Karnataka, was selected as the study area. Mangalore receives approximately 95% of its rainfall during the southwest monsoon season between May and October, whereas the area remains extremely dry during the other 6 months (Chandramouli 2011). Therefore, the reported NSAID concentrations for other parts of the country may not accurately represent this region, and hence, it is necessary to estimate these compounds in this particular region to understand the distribution of these pharmaceuticals.
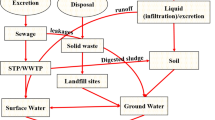
The secondary treatment plant (longitude: 74.826738; latitude: 12.900697) in Kavoor has an installed capacity of 43.5 million liters per day (MLD) with an average operational capacity of 23 MLD and runs throughout the year. Wastewater from various sources, such as household sewage, hospital sewage, and runoff, is received in this MWWTP. Within a radius of approximately 15 km around the Kavoor treatment plant, 20 well-established hospitals are also situated. The treatment units of the secondary treatment plant include a bar screen, grit chamber, upflow anaerobic sludge blanket reactor (UASBR), preaeration step, aeration tank, sludge digester, and sludge drying beds. The flow diagram of the sewage treatment plant is given in Fig. 1. The sampling points selected were the inlet and outlet collection tanks of the wastewater treatment plant. Sample collection was performed at each sampling point as three hourly grab samples over a 24-h period in May and September. The samples were collected 0.5 m below the top surface. Influent and effluent samples were collected as grab samples in 1 L precleaned amber-colored plastic bottles that were prewashed with the corresponding wastewater twice before collection in May and September. The collected samples were stored at 4 °C and transported to the laboratory. The inlet and outlet operational parameters of the sewage plant during the sampling months are listed in Table 2.
Sample extraction
The extraction of nonsteroidal anti-inflammatory drugs from the wastewater samples was performed as per the procedure adopted by Shanmugam et al. (2014). In brief, the wastewater samples were filtered through glass fiber filters with a 0.7-μm pore size to avoid clogging by particles during the extraction procedure. The pH of the samples was maintained at 2 using 3.5 M hydrochloric acid (HCl). Initially, conditioning of the SPE cartridges (HLB) was performed with 1:1 (v/v) ethyl acetate/acetone, acidified ultrapure water (pH 2), and methanol (3 mL each). The vacuum manifold was maintained at a flow rate of 5 mL/min to load the wastewater samples (50 mL) into the SPE cartridges. The cartridges were then washed with 5 mL of a 5% v/v solution of methanol in water and allowed to dry for 1 h under vacuum pressure. The extracts obtained after the elution of analytes in 10 mL of 1:1 (v/v) ethyl acetate/acetone were evaporated under a gentle stream of N2 gas. The addition of anhydrous sodium sulfate to the eluted samples was performed before evaporation to facilitate polar molecule elimination (Kosma et al. 2010).
Then, 500 μL of ethyl acetate was added for the reconstitution of analytes. Finally, 30 μL of the derivatizing agent N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) was added to each sample, followed by incubation for 35 min at 65 °C. MSTFA addition leads to complete derivatization with an increase in the volatilization of the analyte and chromatographic response.
Instrumental analysis
The detection and estimation of the NSAIDs were performed by using gas chromatography (GC) equipped with a 2010 quadrupole (QP) mass spectrometer (Shimadzu Corporation, Japan). The column used for the analysis of target compounds was an SH-Rxi-5MS (30 mL × 0.25 I.D., film thickness: 0.25 μm), and the carrier gas used was 99.99% helium maintained at a column flow rate of 4 mL/min. Temperatures of 250 °C, 270 °C, and 230 °C were maintained at the injection port, interface, and ion source, respectively, of the autosampler. Selected ion monitoring (SIM) mode was used with an ionization energy voltage of 70 eV. The retention times for the target NSAIDs, including ketoprofen, acetylsalicylic acid, ibuprofen, naproxen, and diclofenac, were 17.43 min, 9.91 min, 11.00 min, 15.93 min, and 18.49 min, respectively.
Analytical method validation
The validation of the analytical method adopted was performed by spiking 500 mL of ultrapure water and 500 mL of wastewater (n = 3) with the target analytes. Approximately 100 μg/L concentrations of the target analytes, including ketoprofen, acetylsalicylic acid, naproxen, ibuprofen, and diclofenac, were used for spiking, and the analytes were extracted using the method mentioned in the sample extraction section.
Risk quotient determination
As discussed earlier, the release of these pharmaceutical compounds into the environment is mainly governed by the performance of MWWTPs. Therefore, it is essential to know the vulnerability of various aquatic species to the toxicity caused by incoming compounds in water bodies. According to EMEA (2006), the risk quotient (RQ) method has been employed to find the potential ecological risk of various pharmaceutical compounds. RQ is evaluated for specific test organisms and calculated as the ratio of the measured environmental concentration (MEC) to the predicted no-effect concentration (PNEC) for a given compound. It can be mathematically illustrated as follows:
Risk characterization corresponds to three levels: a low risk for RQ < 0.1, medium risk for 0.1 < RQ < 1.0, and high risk for RQ > 1.0 (Anh et al. 2014). The ecological risk levels were calculated for 3 test species, i.e., a fish, crustacean, and algae, that belong to different trophic levels. The data for the PNEC values of all the target NSAIDs, including ketoprofen, acetylsalicylic acid, naproxen, ibuprofen, and diclofenac, for the test species (fish, crustacean, and algae) were obtained from various studies (Cleuvers 2004; Gamarra Jr. et al. 2015; Praskova et al. 2012, 2014; Sanderson et al. 2003; Zhang et al. 2013). The calculation of PNEC was performed by dividing the EC50 (or LC50) or no observed effect concentration (NOEC) values by an assessment factor of 10 (Singh et al. 2014), and the values are listed below in Table 3.
Results and discussion
Profiling of NSAIDs
All five target NSAIDs were detected in the Kavoor municipal wastewater samples, and the results are depicted in Table 4. The distribution intensities of the target NSAIDs in influent and effluent wastewater from the Kavoor MWWTP during May and September (see supplementary material, fig. S1) varied compared with those in other similar studies. In the present study, ketoprofen was found at higher concentrations in the influent than the other NSAIDs considered for both seasons. Diclofenac and ibuprofen were found to be least abundant during the summer (May) and monsoon (September) seasons, respectively, in influent wastewater. From the analysis, it was clear that the influent concentration of ketoprofen was 2747.29 μg/L and 559.56 μg/L during the summer and monsoon seasons, respectively. After the treatment process, the ketoprofen concentration was reduced to 270.76 μg/L during the summer season, and during the monsoon season, it was nil. According to Nakada et al. (2005), the influent ketoprofen concentration of wastewater ranges from 0.160 to 1.060 μg/L. After treatment, the ketoprofen concentration was reduced to the range of 0.064–0.107 μg/L. Their study achieved a higher ketoprofen removal efficiency through disinfection by UV radiation. A similar trend of lower ketoprofen concentrations in wastewater was also observed by Hashim and Khan (2011), with tertiary-treated wastewater concentrations ranging from 0.0030 to 0.071 μg/L.
Similarly, the ASA concentration in the influent of the MWWTP was found to be 2213.36 μg/L during summer and 351.4 μg/L during the monsoon season, and the corresponding outlet concentrations were 221.86 μg/L and nil, respectively. The acetylsalicylic acid results in the present study showed higher values compared with the study done by Tewari et al. (2013), who found inlet and outlet ASA concentrations in wastewater samples from a Bangkok MWWTP of 4.7 μg/L and 0.261 μg/L, respectively (the highest among the 14 pharmaceuticals considered). Higher ASA concentrations were observed in surface waters (1.36 μg/L in canals and 0.313 μg/L in a river) as a result of sewage disposal into the surface waters. Another study by Sim et al. (2010) revealed the concentration levels of 25 pharmaceuticals in different wastewaters of 10 municipal WWTPs, 1 hospital WWTP, and 5 rivers. They reported a 99% reduction in the concentration of ASA in all MWWTPs, whereas in the hospital WWTP, over 80% removal was achieved by biological processes (conventional activated sludge process and advanced activated sludge process).
The naproxen concentrations at the inlet and outlet of the MWWTP during the summer and monsoon seasons varied, with values of 2132.48 μg/L (influent) and 173.075 μg/L (effluent) in the summer and 115.385 μg/L (influent) and nil (effluent) in the monsoon season. The ibuprofen content in the inlet wastewater was measured to be 2109.875 μg/L, and it was reduced to 22.71 μg/L at the outlet during the summer. During the monsoon season, the ibuprofen concentration was relatively low (43.51 μg/L) at the inlet, and ibuprofen was not detected at the outlet of the MWWTP. The analysis of diclofenac in the wastewater samples revealed that the concentration in the influent was low compared with that of other pharmaceuticals in both May (721.37 μg/L) and September (49.18 μg/L). Previous studies have revealed that attached biomass growth favors diclofenac elimination in wastewater (Vieno and Sillanpää 2014), which may be a possible reason for the reduction in diclofenac concentration in our study. The concentration levels obtained in the present findings were found to be higher than those in the previously reported studies (Hashim and Khan 2011; Langenhoff et al. 2013; Martín et al. 2012; Tewari et al. 2013). The pseudopersistent behavior of these widely consumed prescribed or nonprescribed compounds leads to their permanent presence in municipal wastewaters (Gracia-Lor et al. 2012; Kermia et al. 2016).
NSAID removal efficiency in the MWWTP
The estimated removal efficiency (RE) indicates the performance of a MWWTP in terms of removing target NSAIDs from municipal wastewater (Table 4). The performance was investigated by considering the influent and effluent NSAID concentrations in the system. Many factors, such as the chemical structure of the pharmaceutical compound, its properties, the wastewater retention time, the biodegradability, the hydrophobicity, and the treatment process employed, influence the removal efficiency of each NSAID compound in an MWWTP (Gulkowska et al. 2008; Idder et al. 2013; Li et al. 2009).
During May, the removal efficiency varied from 81.82 to 98.92%, whereas 100% efficiency was achieved for all the target compounds during September. The highest removal efficiency was observed for ibuprofen, and the lowest was observed for diclofenac. The influent and effluent target NSAID concentrations in the MWWTP for two different months (May and September), along with their removal efficiencies, are presented in Table 4. Lin et al. (2009) monitored four WWTPs for the removal of pharmaceuticals in wastewater. It was observed that the NSAID removal efficiency ranged from 72% to 100%, which shows a close resemblance to our data. It was also reported that the secondary treatment process employed in the WWTP helped remove these compounds from the wastewater. Similarly, Kosjek et al. (2007) studied the removal efficiency of pharmaceutical residues (NSAIDs) in a pilot-scale WWTP for a retention period of 12 h. Their results varied from 49% to 87%. In a study performed by Larsson et al. (2013), ibuprofen and naproxen were highly susceptible to biodegradation (97%–100% removal). The removal percentages for ketoprofen and diclofenac were 66% and 67%, respectively, which shows their persistent behavior.
When a wastewater sample has a pH less than the pKa value of the target NSAID, as given in Table 1, acidic compounds become nonionized, causing adsorption through reversed-phase interactions. In this study, the pH values were higher than the pKa values of the target NSAIDs, so no reversed-phase interactions occurred (Madikizela and Chimuka 2017b). Similarly, the solubility of the target compounds also plays a significant role in removal efficiency. The higher the solubility is, the more difficult it is to remove the compound from wastewater. ASA and diclofenac were found to have the lowest removal efficiency during May. ASA possesses a high solubility value, which influences the removal efficiency, whereas diclofenac has the lowest solubility value among the compounds considered but shows a lower removal efficiency. These properties can be justified by the higher half-life (three times greater than those of the other four compounds) (Mlunguza et al. 2019; Zunngu et al. 2017).
The removal mechanism of these NSAID compounds is mainly by biological transformation and partitioning into sludge. Biological transformation contributes a significant fraction to removal, and limited quantities are removed through sludge adsorption (Larsson et al. 2013). Ketoprofen removal can be achieved by physiochemical processes, such as coagulation, sedimentation (15–95%), activated sludge treatment (75%), and membrane filtration (98%). The degradation of acetylsalicylic acid through biodegradation has resulted in 80–98% removal (Szymonik et al. 2017). These removal efficiency data match our present study. Photolysis, ozonation, adsorption on activated sludge, and membrane bioreactors remove the naproxen content by 50–99%. The decomposition of carbonyl and hydroxyl groups in ibuprofen makes this NSAID an efficiently removable pharmaceutical. Sedimentation alone helps in the removal of 45% of compounds from wastewater. The remaining material can be removed by membrane filtration or photocatalysis (with TiO2 as a catalyst). The hydraulic retention time plays a significant role in the degradation process. Compounds with a moderate degradation rate at a longer retention time have better removal efficiencies. Ibuprofen has faster degradation and removal rates in the environment. For diclofenac, a prolonged retention time does not influence the removal rate (Petrovic et al. 2009). In our study, the removal efficiency was lowest for diclofenac, even though its quantity was lower than that of the other target NSAIDs. The low biodegradability of diclofenac is due to its N-H group and Cl atom, which hinder bacterial growth. Approximately 90% removal can be achieved for diclofenac through ozonation, whereas membrane filtration has only 50% removal efficiency. Hence, the removal efficiency in each process in an MWWTP can be evaluated in future work. Thus, the fate of each NSAID in different treatment processes within an MWWTP could be studied more accurately.
Influence of seasonal variability
The variations in concentration in the Kavoor MWWTP in the summer and monsoon seasons were studied. The influent pharmaceutical concentrations during summer were higher than those in the monsoon season. This rise may be due to the reduced consumption of pharmaceuticals, particularly NSAIDs, in the monsoon season than in summer, and also due to the dilution of pollutants by incoming stormwater. The lower total suspended solids (TSS) can justify the lower solids concentration in the influent of the Kavoor MWWTP during the monsoon season. Moreover, the total dissolved solid content was found to be lower, which indicates higher dilution of the influent wastewater. This dilution of the influent is due to high precipitation (2273 mm) during the monsoon season, as reported by the Directorate of Economics and Statistics (2018). Similarly, the rise in dissolved oxygen content from the influent to the effluent of the MWWTP indicated the smooth functioning of the WWTP. The evaluation of MWWTP performance was performed by monitoring pH, total dissolved solids, total suspended solids, chemical oxygen demand, and dissolved oxygen, as shown in Table 2.
Elevated influent concentrations of ketoprofen during the summer and monsoon months suggested that the consumption of this compound was higher than that of the other NSAIDs considered. It was previously reported that ketoprofen consumption increases drastically during spring and summer (Lindholm-Lehto et al. 2016). The lower concentrations of NSAIDs in the influent during the monsoon season are probably due to the dilution effect caused by infiltration in the aging collection system, as stated earlier. Many studies have been performed on factors such as climatic conditions and geographical area, which influence the concentration level of pharmaceutical compounds in effluent treatment plants. During the conveyance of wastewater through sewer lines, seasonal changes in temperature cause the degradation of some compounds, facilitating their removal (Lindholm-Lehto et al. 2016; Loraine and Pettigrove 2006; Sun et al. 2013). In both seasons, the treatment method adopted in the MWWTP is the same, whereas the concentrations of the target NSAIDs entering the treatment plant vary. The lower concentrations of these NSAIDs in the influent wastewater during the monsoon season are due to higher dilution. The lower the pollutant concentration is, the higher the removal efficiency will be (Sun et al. 2013). The results suggested a positive correlation between the dilution ratio and the removal efficiencies of these NSAIDs.
Method validation
The percent recoveries (mean ± standard deviation) of the target NSAIDs (n = 3) obtained for ultrapure water and effluent wastewater ranged from 80.7 ± 14.8 to 95.0 ± 8.3 and 70.4 ± 1.8 to 89.4 ± 3.6, respectively. Table 5 describes the percent recoveries of the target NSAIDs for the method described above. The limit of detection (LOD) using the above method was 0.02 μg/L (ketoprofen), 0.143 μg/L (acetylsalicylic acid), 0.018 μg/L (ibuprofen), 0.030 μg/L (naproxen), and 0.091 μg/L (diclofenac). Similarly, the limit of quantification (LOQ) was 0.03 μg/L (ketoprofen), 0.429 μg/L (acetylsalicylic acid), 0.054 μg/L (ibuprofen), 0.091 μg/L (naproxen), and 0.273 μg/L (diclofenac).
Environmental risk assessment (ERA)
From the calculated RQ value, an environmental risk assessment was performed for the target NSAID compounds in the wastewater of the MWWTP. The assessment was performed under four conditions: 1) untreated MWWTP wastewater during summer, 2) treated MWWTP wastewater during summer, 3) untreated MWWTP wastewater during the monsoon season, and 4) treated MWWTP wastewater during the monsoon season. As provided in Table 6, these results show the potential risk posed by the target NSAIDs to the aquatic environment. A substantial variation in RQ values was obtained for the untreated MWWTP samples for the summer and monsoon seasons. This result is due to variations in the concentrations of the target drugs, as discussed earlier. Ibuprofen showed RQ > 1 for fish and algal bioassays, which implies a higher risk of vulnerability, and for the crustacean, the risk level was medium. In the case of ketoprofen, ASA, naproxen, and diclofenac, the bioassay tests for the fish, crustacean, and algae indicated medium risk (0.1 < RQ < 1.0). This result reveals a potential threat to water bodies if untreated wastewaters containing these compounds are discharged. Such discharge can lead to bioaccumulation and, finally, an increase in the death rate of organisms at all levels of the food chain starting from aquatic species. During the monsoon season, ketoprofen and ibuprofen exhibited a medium risk for fish and algae, respectively, and all other compounds exhibited lower risk. However, after treatment, the concentration of the target NSAIDs was nil in the effluent of the MWWTP, which shows that the treated effluent does not pose an ecological risk to the environment. Similarly, for the treated wastewater, the RQ values were below 0.1 for all the target NSAIDs, which shows that all three bioassay species were at a lower risk.
The surface discharge of effluents including pharmaceutical compounds poses high stress to open aquatic environments and lower stress to closed aquatic environments, based on the dilution phenomenon. The chronic toxicity of these compounds even leads to the slow hatching of zebrafish eggs and retarded population growth (Praskova et al. 2014). The difficulty in the degradation of diclofenac can cause deposition in the environment for months. Upon accumulation, diclofenac causes kidney damage and alteration in the gills. Similarly, the toxicity of naproxen is elevated in the presence of other NSAIDs, such as ibuprofen, acetylsalicylic acid, and diclofenac. Naproxen causes high toxicity levels to phytoplankton (Szymonik et al. 2017). Ideally, to be more precise, a wide variety of bioassay tests would need to be performed to obtain a clear picture of the risk levels at each trophic level. From the present study (Table 7), it can be proven that the Kavoor MWWTP plays a significant role in reducing the environmental risk in Mangalore city from high to low in terms of the five most consumed NSAIDs.
Conclusion
This study elucidates the variation in NSAID concentrations in wastewater in the summer and monsoon seasons, the performance of the MWWTP situated in Kavoor in handling these micropollutants, and the risk involved in the case of untreated sewage being discharged into raw water bodies. From the experimental results, the presence of NSAIDs, viz., ketoprofen, acetylsalicylic acid, naproxen, ibuprofen, and diclofenac, was confirmed, and it was found that their concentration in wastewater from Mangalore city is significantly higher than that determined in most other places. Among the various NSAIDs tested, ketoprofen was found to be present in higher concentrations, i.e., 2747.29 μg/L (summer) and 559.56 μg/L (monsoon), and diclofenac had the lowest concentrations of 721.37 μg/L (summer) and 49.18 μg/L (monsoon). The Kavoor MWWTP was effective in treating all the NSAIDs, with removal efficiencies varying between 81% and 98% in summer and 100% in the monsoon season. It was noticed that diclofenac had the lowest removal efficiency, even under the lower influent concentrations during the summer. Ibuprofen, with its higher removal efficiency, proved to be a readily biodegradable NSAID. We can further conclude that the mechanism behind this removal is probably biodegradation and partly sludge adsorption since there was no tertiary treatment provided.
Risk assessment was performed (by the RQ method) on three species (fish, crustacean, and algae), and it was found that untreated wastewater poses a high to medium risk and medium to low risk during the summer and monsoon seasons, respectively, to these species if it is discharged into water bodies. Furthermore, treatment by the Kavoor MWWTP brings the risk level of the wastewater toward these species to low (summer) or nil (monsoon), thus emphasizing the role of treatment plants and their practical operation in minimizing environmental risk.
Change history
22 August 2020
The original version of this article is unfortunately published online with missing acknowledgment section.
References
Anh, D. V. H., Minh, B. Q., & Nhat, P. H. (2014). Environmental risks of some nonsteroidal anti-inflammatory drugs ( NSAIDs ) in surface water in Ho Chi Minh City. In 3rd World Conference on Applied Sciences, Engineering & Technology (pp. 724–727).
Balakrishna, K., Rath, A., Praveenkumarreddy, Y., Siri, K. G., & Subedi, B. (2017). A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicology and Environmental Safety, 137, 113–120. https://doi.org/10.1016/j.ecoenv.2016.11.014.
Cardoso, O., Porcher, J.-M., & Sanchez, W. (2014). Factory-discharged pharmaceuticals could be a relevant source of aquatic environment contamination: Review of evidence and need for knowledge. Chemosphere, 115, 20–30. https://doi.org/10.1016/j.chemosphere.2014.02.004.
Chandramouli, C. (2011). Census of India 2011 Karnataka. In District census handbook (Vol. 30).
Cleuvers, M. (2004). Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicology and Environmental Safety, 59, 309–315. https://doi.org/10.1016/S0147-6513(03)00141-6.
Directorate of Economics and Statistics. (2018). Annual seasonal rainfall & area coverage during 2017 in Karnataka, Doc. Ref.: DES/10/2018.
Dökmeci, A. H., Dökmeci, I., & Ibar, H. (2014). The determination of single and mixture toxicity at high concentrations of some acidic pharmaceuticals via Aliivibrio fischeri. Environmental Processes, 1, 95–103. https://doi.org/10.1007/s40710-014-0009-7.
Ekpeghere, K. I., Lee, J., Kim, H., Shin, S., & Oh, J. (2016). Determination and characterization of pharmaceuticals in sludge from municipal and livestock wastewater treatment plants. Chemosphere, 168(2), 1211–1221. https://doi.org/10.1016/j.chemosphere.2016.10.077.
Committee For Medicinal Products For Human Use (CHMP) Guideline on the environmental risk assessment of medicinal products for human use, Doc. Ref.: EMEA/CHMP/SWP/4447/00 corr 1, London, 01 June, 2006
Feng, L., Van Hullebusch, E. D., Rodrigo, M. A., Esposito, G., & Oturan, M. A. (2013). Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chemical Engineering Journal, 228, 944–964. https://doi.org/10.1016/j.cej.2013.05.061.
Gamarra Jr., J. S., Godoi, A. F. L., De Vasconcelos, E. C., De Souza, K. M. T., & De Oliveira, C. M. R. (2015). Environmental risk assessment (ERA) of diclofenac and ibuprofen: a public health perspective. Chemosphere, 120, 462–469. https://doi.org/10.1016/j.chemosphere.2014.08.020.
Gracia-Lor, E., Martinez, M., Sancho, J. V., Penuela, G., & Hernandez, F. (2012). Multi-class determination of personal care products and pharmaceuticals in environmental and wastewater samples by ultra-high performance liquid-chromatography-tandem mass spectrometry. Talanta, 99, 1011–1023. https://doi.org/10.1016/j.talanta.2012.07.091.
Gulkowska, A., Leung, H. w., So, M. K., Taniyasu, S., Yamashita, N., Yeung, L. W. Y., et al. (2008). Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen , China. Water Research, 42, 395–403. https://doi.org/10.1016/j.watres.2007.07.031.
Hashim, N. H., & Khan, S. J. (2011). Enantioselective analysis of ibuprofen , ketoprofen and naproxen in wastewater and environmental water samples. Journal of Chromatography A, 1218(29), 4746–4754. https://doi.org/10.1016/j.chroma.2011.05.046.
Idder, S., Ley, L., Mazellier, P., & Budzinski, H. (2013). Quantitative on-line preconcentration-liquid chromatography coupled with tandem mass spectrometry method for the determination of pharmaceutical compounds in water. Analytica Chimica Acta, 805, 107–115. https://doi.org/10.1016/j.aca.2013.10.041.
Kermia, A. E. B., Fouial-djebbar, D., & Trari, M. (2016). Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. Comptes Rendus Chimie, 19(8), 963–970. https://doi.org/10.1016/j.crci.2016.05.005.
Khamis, M., Karaman, R., Ayyash, F., Qtait, A., Deeb, O., & Manssra, A. (2011). Efficiency of advanced membrane wastewater treatment plant towards removal of aspirin, salicylic acid, paracetamol and p -aminophenol. Journal of Environmental Science & Engineering, 5, 121–137.
Kosjek, T., Heath, E., & Kompare, B. (2007). Removal of pharmaceutical residues in a pilot wastewater treatment plant. Analytical and Bioanalytical Chemistry, 387(4), 1379–1387. https://doi.org/10.1007/s00216-006-0969-1.
Kosma, C. I., Lambropoulou, D. A., & Albanis, T. A. (2010). Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. Journal of Hazardous Materials, 179, 804–817. https://doi.org/10.1016/j.jhazmat.2010.03.075.
Langenhoff, A., Inderfurth, N., Veuskens, T., Schraa, G., Blokland, M., Kujawa-roeleveld, K., & Rijnaarts, H. (2013, 2013). Microbial removal of the pharmaceutical compounds Ibuprofen and Diclofenac from wastewater. BioMed Research International, 1–9.
Larsson, E., Rabayah, A., & Jönsson, J. Å. (2013). Sludge removal of nonsteroidal anti-inflammatory drugs during wastewater treatment studied by direct hollow fiber liquid phase microextraction. Journal of Environmental Protection, 4, 946–955.
Li, B., Zhang, T., Xu, Z., & Herbert, H. P. F. (2009). Rapid analysis of 21 antibiotics of multiple classes in municipal wastewater using ultra performance liquid chromatography-tandem mass spectrometry. Analytica Chimica Acta, 645, 64–72. https://doi.org/10.1016/j.aca.2009.04.042.
Lin, A. Y., Yu, T., & Lateef, S. K. (2009). Removal of pharmaceuticals in secondary wastewater treatment processes in Taiwan. Journal of Hazardous Materials, 167, 1163–1169. https://doi.org/10.1016/j.jhazmat.2009.01.108.
Lindholm-Lehto, P. C., Ahkola, H. S. J., Knuutinen, J. S., & Herve, S. H. (2016). Widespread occurrence and seasonal variation of pharmaceuticals in surface waters and municipal wastewater treatment plants in Central Finland. Environmental Science and Pollution Research, 23(8), 7985–7997. https://doi.org/10.1007/s11356-015-5997-y.
Loraine, G. A., & Pettigrove, M. E. (2006). Seasonal variations in concentrations of pharmaceuticals and personal care products in drinking water and reclaimed wastewater in Southern California. Environmental Science & Technology, 40, 687–695.
Lu, M.-C., Chen, Y. Y., Chiou, M.-R., Chen, M. Y., & Fan, H.-J. (2016). Occurrence and treatment efficiency of pharmaceuticals in landfill leachates. Waste Management, 55, 257–264. https://doi.org/10.1016/j.wasman.2016.03.029.
Madikizela, L. M., & Chimuka, L. (2017a). Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environmental Monitoring and Assessment, 189, 348. https://doi.org/10.1007/s10661-017-6069-1.
Madikizela, L. M., & Chimuka, L. (2017b). Simultaneous determination of naproxen, ibuprofen and diclofenac in wastewater using solid-phase extraction with high performance liquid chromatography. Water SA, 43(2), 264–274. https://doi.org/10.4314/wsa.v43i2.10.
Madikizela, L. M., Muthwa, S. F., & Chimuka, L. (2014). Determination of triclosan and ketoprofen in river water and wastewater by solid phase extraction and high performance liquid chromatography. South African Journal of Chemistry, 67, 143–150.
Madikizela, L. M., Mdluli, P. S., & Chimuka, L. (2017). An initial assessment of naproxen, ibuprofen and diclofenac in Ladysmith water resources in South Africa using molecularly imprinted solid-phase extraction followed by high performance liquid chromatography-photodiode array detection. South African Journal of Chemistry, 70, 145–153. https://doi.org/10.17159/0379-4350/2017/v70a21.
Mandaric, L., Diamantini, E., Stella, E., Cano-Paoli, K., Valle-Sistac, J., Molins-Delgado, D., et al. (2017). Contamination sources and distribution patterns of pharmaceuticals and personal care products in Alpine rivers strongly affected by tourism. Science of the Total Environment, 590–591(07), 484–494. https://doi.org/10.1016/j.scitotenv.2017.02.185.
Martín, J., Camacho-mu, D., Santos, J. L., Aparicio, I., & Alonso, E. (2012). Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants : Removal and ecotoxicological impact of wastewater discharges and sludge disposal. Journal of Hazardous Materials, 239–240, 40–47. https://doi.org/10.1016/j.jhazmat.2012.04.068.
Martínez-Alcalá, I., Guillén-Navarro, J. M., & Fernández-lópez, C. (2017). Pharmaceutical biological degradation , sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia , Spain. Chemical Engineering Journal, 316, 332–340. https://doi.org/10.1016/j.cej.2017.01.048.
Mlunguza, N. Y., Ncube, S., Mahlambi, P. N., Chimuka, L., & Madikizela, L. M. (2019). Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. Journal of Environmental Chemical Engineering, 7(3), 103142. https://doi.org/10.1016/j.jece.2019.103142.
Modi, C. M., Mody, S. K., Patel, H. B., Dudhatra, G. B., Kumar, A., & Avale, M. (2012). Toxicopathological overview of analgesic and anti-inflammatory drugs in animals. Journal of Applied Pharmaceutical Science, 2(1), 149–157.
Nakada, N., Komori, K., & Suzuki, Y. (2005). Occurrence and fate of anti-inflammatory drugs in wastewater treatment plants in Japan. Environmental Sciences, 12(6), 359–369.
Oaks, J. L., Gilbert, M., Virani, M. Z., Watson, R. T., Meteyer, C. U., Rideout, B. A., et al. (2004). Diclofenac residues as the cause of vulture population decline in Pakistan. Letters to Nature, 427(02), 630–633.
Petrovic, M., Lopez De Alda, M. J., Diaz-Cruz, S., Postigo, C., Radjenovic, J., Gros, M., & Barcelo, D. (2009). Fate and removal of pharmaceuticals and illicit drugs in conventional and membrane bioreactor wastewater treatment plants and by riverbank filtration. Philosophical Transactions of the Royal Society A, 367, 3979–4003. https://doi.org/10.1098/rsta.2009.0105.
Praskova, E., Sevcikova, M., Živná, D., Štěpánová, S., Ševčíková, M., Blahová, J., et al. (2012). Acute toxicity of acetylsalicylic acid to juvenile and embryonic stages of Danio rerio and embryonic stages of Danio rerio. Neuroendocrinology Letters, 33(3), 71–76.
Praskova, E., Plhalova, L., Chromcova, L., Stepanova, S., Bedanova, I., Blahova, J., et al. (2014). Effects of subchronic exposure of diclofenac on growth, histopathological changes, and oxidative stress in zebrafish (Danio rerio). The Scientific World Journal, 2014, 1–5.
Samaras, V. G., Stasinakis, A. S., Mamais, D., Thomaidis, N. S., & Lekkas, T. D. (2013). Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion. Journal of Hazardous Materials, 244–245, 259–267. https://doi.org/10.1016/j.jhazmat.2012.11.039.
Sanderson, H., Johnson, D. J., Wilson, C. J., Brain, R. A., & Solomon, K. R. (2003). Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicology Letters, 144(3), 383–395. https://doi.org/10.1016/S0378-4274(03)00257-1.
Shanmugam, G., Sampath, S., Selvaraj, K. K., Larsson, D. G. J., & Ramaswamy, B. R. (2014). Non-steroidal anti-inflammatory drugs in Indian rivers. Environmental Science and Pollution Research, 21, 921–931. https://doi.org/10.1007/s11356-013-1957-6.
Sharma, K., & Kaushik, G. (2017). NSAIDS in the environment : From emerging problem to green solution. Annals of Pharmacology and Pharmaceutics, 2(14), 1–3.
Sim, W., Lee, J., & Oh, J. (2010). Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea. Environmental Pollution, 158, 1938–1947. https://doi.org/10.1016/j.envpol.2009.10.036.
Sim, W., Lee, J., Lee, E., Shin, S., Hwang, S., & Oh, J. (2011). Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere, 82(2), 179–186. https://doi.org/10.1016/j.chemosphere.2010.10.026.
Singh, K. P., Rai, P., Singh, A. K., Verma, P., & Gupta, S. (2014). Occurrence of pharmaceuticals in urban wastewater of north Indian cities and risk assessment. Environmental Monitoring and Assessment, 186(10), 6663–6682. https://doi.org/10.1007/s10661-014-3881-8.
Sun, Q., Lv, M., Hu, A., Yang, X., & Yu, C. (2013). Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. Journal of Hazardous Materials, 227, 69–75. https://doi.org/10.1016/j.jhazmat.2013.11.056.
Szymonik, A., Lach, J., & Malińska, K. (2017). Fate and removal of pharmaceuticals and illegal drugs present in drinking water and wastewater. Ecological Chemistry and Engineering, 24(1), 65–85. https://doi.org/10.1515/eces-2017-0006.
Tewari, S., Jindal, R., Kho, Y. L., Eo, S., & Choi, K. (2013). Major pharmaceutical residues in wastewater treatment plants and receiving waters in Bangkok, Thailand, and associated ecological risks. Chemosphere, 91(5), 697–704. https://doi.org/10.1016/j.chemosphere.2012.12.042.
Vieno, N., & Sillanpää, M. (2014). Fate of diclofenac in municipal wastewater treatment plant - a review. Environment International, 69, 28–39. https://doi.org/10.1016/j.envint.2014.03.021.
Xu, J., Wu, L., & Chang, A. C. (2017). Degradation and adsorption of selected pharmaceuticals and personal care products ( PPCPs ) in agricultural soils. Chemosphere, 2009, 1299–1305. https://doi.org/10.1016/j.chemosphere.2009.09.063.
Yuan, X., Qiang, Z., Ben, W., Zhu, B., & Qu, J. (2015). Distribution, mass load and environmental impact of multiple-class pharmaceuticals in conventional and upgraded municipal wastewater treatment plants in East China. Environmental Science: Processes & Impacts, 17(3), 596–605. https://doi.org/10.1039/C4EM00596A.
Zhang, X., Zhang, D., Lin, L., & Yan, C. (2013). Occurrence and risks of pharmaceuticals, personal care products and endocrine disruptors in Jiulongjiang river, South China. In 13th International Conference of Environmental Science and Technology Athens, Greece (pp. 1–7).
Zunngu, S. S., Madikizela, L. M., Chimuka, L., & Mdluli, P. S. (2017). Synthesis and application of a molecularly imprinted polymer in the solid-phase extraction of ketoprofen from wastewater. Comptes Rendus Chimie, 20(5), 585–591. https://doi.org/10.1016/j.crci.2016.09.006.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 186 kb)
Rights and permissions
About this article
Cite this article
Thalla, A.K., Vannarath, A.S. Occurrence and environmental risks of nonsteroidal anti-inflammatory drugs in urban wastewater in the southwest monsoon region of India. Environ Monit Assess 192, 193 (2020). https://doi.org/10.1007/s10661-020-8161-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-8161-1