Abstract
The present paper reports a detailed study that is based on the monitoring of naproxen, ibuprofen, and diclofenac in Mbokodweni River and wastewater treatment plants (WWTPs) located around the city of Durban in KwaZulu-Natal Province of South Africa. Target compounds were extracted from water samples using a multi-template molecularly imprinted solid-phase extraction prior to separation and quantification on a high-performance liquid chromatography equipped with photo diode array detector. The analytical method yielded the detection limits of 0.15, 1.00, and 0.63 μg/L for naproxen, ibuprofen, and diclofenac, respectively. Solid-phase extraction method was evaluated for its performance using deionized water samples that were spiked with 5 and 50 μg/L of target compounds. Recoveries were greater than 80% for all target compounds with RSD values in the range of 4.1 to 10%. Target compounds were detected in most wastewater and river water samples with ibuprofen being the most frequently detected pharmaceutical. Maximum concentrations detected in river water for naproxen, ibuprofen, and diclofenac were 6.84, 19.2, and 9.69 μg/L, respectively. The concentrations of target compounds found in effluent and river water samples compared well with some studies. The analytical method employed in this work is fast, selective, sensitive, and affordable; therefore, it can be used routinely to evaluate the occurrence of acidic pharmaceuticals in South African water resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Naproxen, ibuprofen, and diclofenac are weak organic acids that belong to the group of non-steroidal anti-inflammatory drugs (NSAIDs) (Table 1). Compounds such as ibuprofen and naproxen are widely used by humans for the treatment of rheumatoid arthritis (Mahkam and Poorgholy 2011). These compounds become part of the human waste, and they are excreted into the environment as un-metabolized parent compounds and metabolites (Koutsouba et al. 2003). High concentration of pharmaceutical compounds enters wastewater treatment plants (WWTPs) daily through urinary or fecal excretion and from pharmaceutical manufacturing facilities (Farre et al. 2001). Due to the polar nature of naproxen, ibuprofen, and diclofenac, they escape the wastewater treatment process easily and contaminate the river water.
Mostly, the environmental monitoring of NSAIDs involves the use of chromatographic tools with solid-phase extraction (SPE) for the reduction of matrix effects and pre-concentration of target compounds. For solid-phase extraction of naproxen, ibuprofen, and diclofenac, sorbents such as multi-walled carbon nanotubes (Dahane et al. 2013), single-template molecularly imprinted polymer (Farrington and Regan 2007), multi-template molecularly imprinted polymer (Madikizela and Chimuka 2016; Duan et al. 2013), Oasis HLB (Zhao et al. 2009), Oasis MCX (Lindqvist et al. 2005), and C18 (Rigobello et al. 2013) have been used. Most of these SPE sorbents except the molecularly imprinted polymers have limited selectivity, and their single usage leads to the production of waste and economic disadvantage. Recent studies are focusing on the application of molecularly imprinted polymer (MIP) for SPE due to its properties that include thermal stability, re-usability, improved selectivity, mechanical strength, etc. (Prasad and Rai 2013).
Development of molecularly imprinted solid-phase extraction (MISPE) for NSAIDs is well documented (Caro et al. 2005; Duan et al. 2013; Zorita et al. 2008). However, such technique is not yet fully exploited for the routine monitoring of selected NSAIDs in wastewater and river water. This is probable due to the slow progress/success in the development of multi-template MIPs for this purpose. This is important in order to employ a cheap analysis method as there is a growing need for the determination of NSAIDs in aqueous samples.
NSAIDs in aqueous samples such as wastewater, river water, and drinking water have been studied extensively in well developed areas such as European and American countries (Carmona et al. 2014; Dahane et al. 2013; Santos et al. 2005; Yu et al. 2006, 2013). However, most African countries including South Africa are lagging behind in this aspect due to limited access to the robust analytical methods and instrumentation. Regardless of these facts, few papers have been published recently that reports on the presence of NSAIDs in South African wastewater and surface water (Agunbiade and Moodley 2014, 2016; Amdany et al. 2014, 2015; Matongo et al. 2015a, b). These papers demonstrated the occurrence of such compounds in low micrograms per liter levels in wastewater and rivers found in South African major cities that includes Durban and Johannesburg. Moreover, in other African countries such as Kenya and Algeria, all three target compounds have been detected in wastewater, river water, and groundwater (Kermia et al. 2016; K’oreje et al. 2016). Transportation of pharmaceuticals from wastewater to drinking water has already been reported in Europe (Carmona et al. 2014). This might be due to compounds being hydrophilic and stable in aqueous medium; hence, the low removal efficiencies have been documented in some cases. The reported removal efficiencies for naproxen, ibuprofen, and diclofenac are in the ranges of 73–100%, 55–100%, and 9–98%, respectively (Kermia et al. 2016; Larsson et al. 2014; Lindqvist et al. 2005; Yu et al. 2006).
As a consequence, it is highly important for South African scientists to develop procedures for the monitoring of NSAIDs in wastewater and rivers as some of these sites are not restricted from the public use. Therefore, this study is designed to focus on the application of multi-template MIP for the extraction of naproxen, ibuprofen, and diclofenac from wastewater and river water. The aim of the study was to determine the concentrations of selected compounds in WWTPs managed by eThekwini Municipality in KwaZulu-Natal Province which is ranked number 3 in terms of pharmaceutical consumption in South Africa (Matongo et al. 2015b). EThekwini Municipality has approximately 28 WWTPs, and there is currently no available data on the simultaneous monitoring of naproxen, ibuprofen, and diclofenac in most of these sewage treatment facilities. Therefore, this is the first detailed study that is based on the occurrence of these acidic pharmaceuticals in the effluent of Amanzimtoti, New Germany, and Umhlatuzana WWTPs. This is also the first study that is aimed to monitor the presence of naproxen, ibuprofen, and diclofenac in Mbokodweni River.
Materials and methods
Analytical reagents
Naproxen (98%), ibuprofen (≥98%), and diclofenac sodium salt were purchased from Sigma-Aldrich (Steinheim, Germany) and used as standards and templates in the synthesis of MIP. 2-Vinylpyridine (97%), 1,1′-azobis-(cyclohexanecarbonitrile) (98%), ethylene glycol dimethacrylate (98%), and toluene (99.7%) purchased from Sigma-Aldrich (Steinheim, Germany) were used in the synthesis of MIP as functional monomer, radical initiator, cross-linking monomer, and porogen, respectively. high-performance chromatography (HPLC)-grade methanol (≥99.9%) from Sigma-Aldrich (Steinheim, Germany), HPLC-grade acetonitrile (≥99.9%), and glacial acetic acid (100%) purchased from Merck (Darmstadt, Germany) were used as solvents. Formic acid (approx. 98%) was purchased from Fluka (Steinheim, Germany) and used in the chromatographic mobile phase.
Sampling and sample pre-treatment
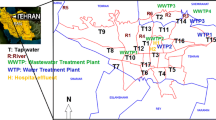
Influent and effluent samples were collected from WWTPs located around the city of Durban in the Province of KwaZulu-Natal, South Africa (Table 2). Samples were also collected from three points in Mbokodweni River (identified as Mbokodweni Rivers A, B, and C in this paper) that is found in South of Durban city. In this case, Mbokodweni River A represents a river sample that was collected approximately 1 km downstream from Amanzimtoti WWTP outfall, whereas Mbokodweni River B and C samples were collected from 1 to 3 km upstream of the WWTP. These samples were collected monthly from January to May in 2016 using glass bottles that were thoroughly cleaned with soap, deionized water, and rinsed in sampling sites with real sample. Samples were immediately protected from light and transported to the laboratory where they were filtered twice with filter papers having pore sizes of 10 and 0.45 μm purchased from Munktell and Filtrak GmbH (Bernstein, Germany) and Millipore (Darmstadt, Germany), respectively. pH in each sample was adjusted to 2.5; thereafter, samples were stored in the refrigerator at 4 °C until further processing.
Synthesis of multi-template molecularly imprinted polymer
Synthetic procedure was adopted elsewhere (Dai et al. 2012; Duan et al. 2013) and modified in the previous work (Madikizela and Chimuka 2016; Madikizela et al. 2016). Synthesis was performed by dissolving 20 mg of 1,1′-azobis-(cyclohexanecarbonitrile) in 50 mL of toluene; thereafter, 1.51 mL of ethylene glycol dimethacrylate was added. The reaction flask was purged with nitrogen for 10 min and sealed. Then, the reaction was allowed to take place with constant stirring in an oil bath set at 70 °C for 8 h. Thereafter, naproxen (76.60 mg), ibuprofen (68.69 mg), and diclofenac (106.04 mg) were dissolved in 25 mL of acetonitrile, followed by the addition of 0.25 mL 2-vinylpyridine, 3.85 mL ethylene glycol dimethacrylate, 60 mg 1,1′-azobis-(cyclohexanecarbonitrile), and 25 mL toluene. These contents were homogenized and transferred to the product obtained in the first step. The resulting mixture was purged with nitrogen gas for 10 min and sealed. The reaction was allowed to polymerize in an oil bath set at 70 °C for 16 h. The obtained polymer was dried at 60 °C, milled, sieved, and particles ranging from 25 to 50 μm were collected. Naproxen, ibuprofen, and diclofenac were removed from the polymer using a mixture of 10% (v/v) acetic acid in acetonitrile.
Multi-template molecularly imprinted solid-phase extraction
Solid-phase extraction cartridge (3 mL) was packed with a slurry that was made with 50 mg of MIP and acetonitrile. Frits were employed below and above the MIP to safeguard against the sorbent loss.
MISPE procedure was adopted from published work (Madikizela and Chimuka 2016). Prior to loading of samples, each MISPE cartridge was conditioned with 2 mL of acetonitrile and equilibrated with 2 mL of acidified deionized water (pH 2.5). With the aid of the vacuum pump, 50 mL of acidified sample (pH 2.5) was percolated at 0.3 mL/min. Thereafter, 2 mL of 10% (v/v) methanol in water was used to wash the cartridge prior to elution of retained compounds with 20% (v/v) acetic acid in acetonitrile (2 mL). The eluted extract (20 μL) was injected into the HPLC system.
Chromatographic separation and quantification
Separation and quantification of target compounds were performed on an HPLC system purchased from Shimadzu Corporation (Kyoto, Japan). HPLC was equipped with an online mobile phase degasser unit (Model: DGU-20A3), 20 μL sample loop, pump (Model: LC-20AB), and photo diode array detector (Model: SPD-M20A). Compounds were separated on a Kinetex C18 HPLC column (150 × 4.6 mm × 2.6 μm) purchased from Phenominex (CA, USA) using a mixture of acetonitrile: 0.2% formic acid in water (60:40, v/v) as mobile phase at a flow rate of 0.8 mL/min. Naproxen was monitored at 230 nm, whereas ibuprofen and diclofenac were both studied at 200 nm. The chromatographic system was equipped with Shimadzu LC solutions software for data collection and processing.
Monitoring of physicochemical parameters
Physicochemical properties such as sample pH, conductivity, salinity, dissolved oxygen and total dissolved solids were measured in sampling sites using a calibrated Bante900P multi-parameter water quality meter that was purchased from Bante instruments (Shanghai, China). The calibration of the multi-parameter water quality meter was performed using the pH calibration buffers (pH 4, 7 and 10) and conductivity calibration solutions (84, 1413, and 12.88 mS/cm) that were provided by the supplier. Single point calibration for dissolved oxygen was carried out in air.
Results and discussion
Performance of analytical method
As shown in Fig. 1, well-resolved peaks within 6 min for all three target compounds were obtained using a reverse-phase chromatographic column. In order to evaluate the performance of the analytical method, figures of merit such as limits of detection (LOD), limits of quantification (LOQ), precision, linearity, and extraction recoveries were determined for each analyte using deionized water that was spiked with 5 and 50 μg/L mixture of target compounds. LOD and LOQ were defined as the concentration where target compounds gave a signal to noise ratio of 3 and 10, respectively. The detection and quantification limits (Table 3) were similar to those reported for the analysis of the same compounds in wastewater using HPLC with photodiode array detection (Payan et al. 2011; Santos et al. 2005). Calibration curves were plotted for each target compound in the concentration range of 30–1000 μg/L. All calibration curves were linear with correlation coefficients greater than 0.99. As shown in Table 3, the extraction recoveries were greater than 80% with relative standard deviation (RSD) values ranging from 4.1 to 10%, which is an indication of accepted accuracy and precision.
Study areas
Wastewater treatment plants sampled in this study have been described in previous studies (Bux and Kasan 1994; Mhlanga et al. 2009; Madikizela et al. 2014; Nzimande 2014). New Germany and Northern WWTPs treat wastewater from the local industries and domestic sources (Bux and Kasan 1994; Nzimande 2014). The treated water is then discharged into the nearest rivers. Shallcross and Mariannridge WWTPs are both located in a site known as Umhlatuzana Works. Their effluent is combined prior to the discharge point. Shallcross receives domestic wastewater only, whereas Mariannridge receives wastewater from industrial (30%) and domestic (70%) sources (Mhlanga et al. 2009). For the purpose of this work, the combined effluent from Shallcross and Mariannridge WWTPs is identified as Umhlatuzana WWTP effluent. Amanzimtoti WWTP receives water from industrial areas and semi-urban areas (Madikizela et al. 2014). Previously, a non-steroidal anti-inflammatory drug known as ketoprofen and triclosan (anti-bacterial agent) was detected in both influent and effluent of Amanzimtoti WWTP (Madikizela et al. 2014). Mbokodweni River was sampled on the upstream (2 points) and downstream (1 point) of Amanzimtoti WWTP outfall. On the upstream of the River, there is an informal settlement on the river banks with poor sanitation system.
Occurrence of naproxen, ibuprofen, and diclofenac in wastewater and river water
Typical chromatograms obtained for environmental analysis are given in Fig. 2. Target compounds were identified in environmental samples based on matching the retention times and photodiode array spectra (Fig. S1) with those of standard solutions. To simplify the presentation of some figures, the use of acronyms as described in Table 4 was applied.
The results obtained for wastewater and river water concentrations are summarized in Fig. 3 and Table 5. The maximum concentrations in wastewater and river water were compared with the data available in literature (Table 6). As can be seen in Fig. 3, the highest concentration of 221 μg/L was obtained for ibuprofen in Northern WWTP influent with the average concentration of 72 μg/L. The maximum concentration of 128 μg/L with an average of 120 μg/L has been reported for Northern WWTP influent located in Johannesburg, South Africa (Amdany et al. 2014). Ibuprofen was also the most frequently detected acidic pharmaceutical in various samples as reported for different matrices in other studies (Bayen et al. 2013; Carmona et al. 2014). This observation was not surprising as ibuprofen has been reported to be the most consumed pharmaceutical in South Africa among the three NSAIDs selected in this study (Matongo et al. 2015a). The average concentration of ibuprofen in Northern WWTP effluent was 10 μg/L, whereas 12 μg/L has been reported previously (Matongo et al. 2015b). Traces of ibuprofen were also detected in river water. At this point, the source of pharmaceuticals in the river could not be traced as the compounds were also detected in the upstream of the river.
Minimum and maximum concentrations of naproxen (a), ibuprofen (b), and diclofenac (c) in environmental samples. Acronyms are explained in Table 4
The concentrations for naproxen were generally lower in all samples except in New Germany WWTP influent, which could be explained by the lower consumption of this drug in South Africa as per the published script lines (Matongo et al. 2015a). Although the consumption of naproxen is low in South Africa, its presence in the wastewater could not be ignored as it has also been found present in other WWTPs located in another South African Province (Amdany et al. 2014). In most cases, there has been a decrease of pharmaceuticals from the raw influent to the final effluent. This may be due to the adsorption of target compounds on solid sludge. The treatment process in all the investigated WWTPs consists of screening, settling tanks, aeration, and chlorination.
Diclofenac was detected in wastewater and river water (Fig. 3; Table 5). The presence of diclofenac in river water was probable due to its poor removal during the wastewater treatment process as reported elsewhere (Rosal et al. 2010; Zorita et al. 2009). The concentration of diclofenac in wastewater and river water is higher than the levels reported for WWTPs and rivers in Europe (Carmona et al. 2014; Gilart et al. 2013; Martin et al. 2012). For instance, the average concentration for diclofenac in wastewater ranges from 3 to 53 μg/L, whereas a mean concentration of 0.72 μg/L has been reported for the same compound in North WWTP located in Spain (Martin et al. 2012). This could be due to variations in pharmaceutical consumption rates from country to country.
aFor simplicity, the concentrations for current study reported in this table are the highest amounts detected during the reported work
Removal of naproxen, ibuprofen, and diclofenac in wastewater treatment plants
The removal efficiency for each compound during the wastewater treatment process was determined based on the average concentrations reported in Table 5, which were detected in the influent and effluent samples. Removal efficiency was not determined for Shallcross and Mariannridge WWTPs, as both treatment facilities have the combined effluent which is identified as Umhlatuzana WWTP. In some cases, high concentrations of the compounds were observed in the effluent rather than in the influent. As stated elsewhere, the situation could be due to the matrix effects being higher in the influents than in the effluents of WWTPs which lead to higher matrix suppression and higher quantification limits in influents (Gracia-Lor et al. 2012). Such cases are common when monitoring these drugs in wastewater (Kermia et al. 2016; Kosma et al. 2014). As shown in Table 7, the removal efficiencies for the studied compounds from wastewater varied broadly. Such wide variations for removal efficiency of NSAIDs from wastewater have been observed in several studies (Sebok et al. 2008; Gros et al. 2010). For example, in a study conducted for WWTPs in Spain, the removal efficiency for diclofenac has varied from no elimination to 100% (Gros et al. 2010). Possible reason for such results could be related to grab sampling uncertainty which might add some variations of pharmaceutical levels and affect the evaluation of the removal rates, which could be overcome by applying high sampling frequency (Ort et al. 2010; Sun et al. 2016). In addition, at times during sampling campaign, some WWTPs were not fully operational which could lead to low removal efficiency or negative values. Overall, high removal efficiencies were achieved in New Germany (NGWWTP) and Northern WWTPs (NWWTP).
Physicochemical parameters of collected samples
Results for physicochemical analysis are given in Table 8. The pH for all the samples was neutral. Hence the pH of collected solutions was reduced to 2.5 in order to allow for the protonation of target compounds prior to MISPE. Salinity was measured as practical salinity unit (psu) and represents the concentration of the dissolved salts in wastewater. Salinity results indicated that the samples contained small amounts of soluble inorganic salts that were not expected to affect the proposed analytical method. High salinity water is known as water with large quantities of soluble inorganic salts and organic compounds (Zhang et al. 2012). On the other hand, a decrease in TDS between the raw influent and effluent was observed. The results of the current study were much lower than those reported in other study, where Anderson et al. (2015) reported a minimum of 981 mg/L for TDS in a wastewater collected from Canada. The conductivity of 703 and 589 μS/cm for wastewater influent and effluent, respectively, has been reported elsewhere (Rosal et al. 2010). In all the investigated WWTPs, there was an increase in dissolved oxygen (DO) between the raw influent and the final effluent, courtesy of aeration process. In this regard, DO increased from 0.85 to 3.02 mg/L in Amanzimtoti WWTP in which case almost similar concentration of 3.56 mg/L was observed in river water sampled in the downstream of the plant. In comparison to Thesis river in Mpumalanga Province (South Africa) where DO of 1.11 mg/L was recorded (Wanda et al. 2016), Mbokodweni river had much higher value. Based on this data, the WWTPs investigated in this work perform in a similar manner as other treatment works in the world.
Conclusion
A rapid analytical method that involves the use of multi-template molecularly imprinted polymer as selective SPE sorbent and HPLC with photodiode array detection for the separation and quantification has been applied for the environmental monitoring of naproxen, ibuprofen, and diclofenac. All three pharmaceutical compounds were detected in wastewater and river water. In all samples (influent, effluent and river water), ibuprofen was detected most frequently with higher concentrations. The occurrence of pharmaceuticals in the upstream of Mbokodweni River was observed which could indicate that human activities play a major role in contamination of water resources. The results of this study demonstrated the necessity to conduct more research on the occurrence of acidic pharmaceuticals in all South African water bodies including lakes and dams. Also, the improvement in the wastewater treatment processes is required in order to reduce the pollution of precious resources such as river water.
References
Agunbiade, F. O., & Moodley, B. (2014). Pharmaceuticals as emerging organic contaminants in Umgeni River water system, KwaZulu-Natal, South Africa. Environmental Monitoring and Assessment, 186, 7273–7291.
Agunbiade, F. O., & Moodley, B. (2016). Occurrence and distribution pattern of acidic pharmaceuticals in surface water, wastewater, and sediment of the Msunduzi River, KwaZulu-Natal, South Africa. Environmental Toxicology and Chemistry, 35(1), 36–46.
Amdany, R., Chimuka, L., & Cukrowska, E. (2014). Determination of naproxen, ibuprofen and triclosan in wastewater using the polar organic chemical integrative sampler (POCIS): a laboratory calibration and field application. Water South Africa, 40(3), 407–414.
Amdany, R., Moya, A., Chimuka, L., & Cukrowska, E. (2015). Optimization of the temperature for the extraction of pharmaceuticals from wastewater by a hollow fiber silicone membrane. Analytical Letters, 48, 2343–2356.
Anderson, J. C., Joudan, S., Shoichet, E., Cuscito, L. D., Alipio, A. E. C., Donaldson, C. S., Khan, S., Goltz, D. M., Rudy, M. D., Frank, R. A., Knapp, C. W., Hanson, M. L., & Wong, C. S. (2015). Reducing nutrients, organic micropollutants, antibiotic resistance, and toxicity in rural wastewater effluent with subsurface filtration treatment technology. Ecological Engineering, 84, 375–385.
Bayen, S., Zhang, H., Desai, M. M., Ooi, S. K., & Kelly, B. C. (2013). Occurrence and distribution of pharmaceutically active and endocrine disrupting compounds in Singapore’s marine environment: influence of hydrodynamics and physical-chemical properties. Environmental Pollution, 182, 1–8.
Bux, F., & Kasan, H. C. (1994). A microbiological survey of ten activated sludge plants. Water South Africa, 20, 61–72.
Carmona, E., Andreu, V., & Pico, Y. (2014). Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water. Science of the Total Environment, 484, 53–63.
Caro, E., Marce, R. M., Cormack, P. A. G., Sherrington, D. C., & Borrull, F. (2005). Selective enrichment of anti-inflammatory drugs from river water samples by solid-phase extraction with a molecularly imprinted polymer. Journal of Separation Science, 28, 2080–2085.
Dahane, S., Gil Garcia, M. D., Martinez Bueno, M. J., Ucles Moreno, A., Martinez Galera, M., & Derdour, A. (2013). Determination of drugs in river and wastewaters using solid-phase extraction by packed multi-walled carbon nanotubes and liquid chromatography-quadrupole-linear ion trap-mass spectrometry. Journal of Chromatography A, 1297, 17–28.
Dai, C., Zhang, J., Zhang, Y., Zhou, X., Duan, Y., & Liu, S. (2012). Selective removal of acidic pharmaceuticals from contaminated lake water using multi-templates molecularly imprinted polymer. Chemical Engineering Journal, 211-212, 302–309.
Duan, Y., Dai, C., Zhang, Y., & Ling-Chen. (2013). Selective trace enrichment of acidic pharmaceuticals in real water and sediment samples based on solid-phase extraction using multi-templates molecularly imprinted polymers. Analytica Chimica Acta, 758, 93–100.
Farre, M., Ferrer, I., Ginebreda, A., Figueras, M., Olivella, L., Tirapu, L., Vilanova, M., & Barcelo, D. (2001). Determination of drugs in surface water and wastewater samples by liquid chromatography-mass spectrometry: methods and preliminary results including toxicity studies with Vibrio fischeri. Journal of Chromatography A, 938, 187–197.
Farrington, K., & Regan, F. (2007). Investigation of the nature of MIP recognition: the development and characterisation of a MIP for ibuprofen. Biosensors and Bioelectronics, 22(6), 1138–1146.
Gilart, N., Marce, R. M., Fontanals, N., & Borrull, F. (2013). A rapid determination of acidic pharmaceuticals in environmental waters by molecularly imprinted solid-phase extraction coupled to tandem mass spectrometry without chromatography. Talanta, 110, 196–201.
Gracia-Lor, E., Sancho, J. V., Serrano, R., & Hernandez, F. (2012). Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere, 87, 453–462.
Gros, M., Petrovic, M., Ginebreda, A., & Barcelo, D. (2010). Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environment International, 36, 15–26.
K’oreje, K. O., Vergeynst, L., Ombaka, D., De Wispelaere, P., Okoth, M., Van Langenhove, H., & Demeestere, K. (2016). Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere, 149, 238–244.
Kermia, A. E. B., Fouial-Djebbar, D., & Trari, M. (2016). Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. Comptes Rendus Chimie, 19(8), 963–970.
Kosma, C. I., Lambropoulou, D. A., & Albanis, T. A. (2014). Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Science of the Total Environment, 466-467, 421–438.
Koutsouba, V., Heberer, T., Fuhrmann, B., Schmidt-Baumler, K., Tsipi, D., & Hiskia, A. (2003). Determination of polar pharmaceuticals in sewage water of Greece by gas chromatography-mass spectrometry. Chemosphere, 51, 69–75.
Larsson, E., al-Hamimi, S., & Jonsson, J. A. (2014). Behaviour of nonsteroidal anti-inflammatory drugs and eight of their metabolites during wastewater treatment studied by hollow fibre liquid phase microextraction and liquid chromatography mass spectrometry. Science of the Total Environment, 485-486, 300–308.
Lindqvist, N., Tuhkanen, T., & Kronberg, L. (2005). Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Research, 39, 2219–2228.
Madikizela, L. M., & Chimuka, L. (2016). Determination of ibuprofen, naproxen and diclofenac in aqueous samples using a multi-template molecularly imprinted polymer as selective adsorbent for solid-phase extraction. Journal of Pharmaceutical and Biomedical Analysis, 128, 210–215.
Madikizela, L. M., Muthwa, S. F., & Chimuka, L. (2014). Determination of triclosan and ketoprofen in river water and wastewater by solid-phase extraction and high performance liquid chromatography. South African Journal of Chemistry, 67, 143–150.
Madikizela, L. M., Mdluli, P. S., & Chimuka, L. (2016). Experimental and theoretical study of molecular interactions between 2-vinyl pyridine and acidic pharmaceuticals used as multi-template molecules in molecularly imprinted polymer. Reactive and Functional Polymers, 103, 33–43.
Mahkam, M., & Poorgholy, N. (2011). Imprinted polymers as drug delivery vehicles for anti-inflammatory drugs. Nature and Science, 9(5), 163–168.
Martin, J., Camacho-Munoz, D., Santos, J. L., Aparicio, I., & Alonso, E. (2012). Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: removal and ecotoxicological impact of wastewater discharges and sludge disposal. Journal of Hazardous Materials, 239-240, 40–47.
Matongo, S., Birungi, G., Moodley, B., & Ndungu, P. (2015a). Pharmaceutical residues in water and sediment of Msunduzi River, KwaZulu-Natal, South Africa. Chemosphere, 134, 133–140.
Matongo, S., Birungi, G., Moodley, B., & Ndungu, P. (2015b). Occurrence of selected pharmaceuticals in water and sediment of Umgeni River, KwaZulu-Natal, South Africa. Environmental Science and Pollution Research, 22, 10298–10308.
Mhlanga, F. T., Brouckaert, C. J., Foxon, K. M., Fennemore, C., Mzulwini, D., & Buckley, C. A. (2009). Simulation of a wastewater treatment plant receiving industrial effluents. Water South Africa, 35, 447–454.
Nzimande, S. B. T. (2014). Treated wastewater effluent as potential source of emerging bacterial pathogens in surface water, MSc thesis, University of KwaZulu-Natal, Durban.
Ort, C., Lawrence, M. G., Reungoat, J., & Mueller, J. F. (2010). Sampling for PPCPs in wastewater systems: comparison of different sampling modes and optimization strategies. Environmental Science and Technology, 44, 6289–6296.
Payan, M. R., Lopez, M. A. B., Torres, R. F., Navarro, M. V., & Mochon, M. C. (2011). Electromembrane extraction (EME) and HPLC determination of non-steroidal anti-inflammatory drugs (NSAIDs) in wastewater samples. Talanta, 85, 394–399.
Prasad, B. B., & Rai, G. (2013). Molecular structure, vibrational spectra and quantum chemical MP2/DFT studies toward the rational design of hydroxyurea imprinted polymer. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 105, 400–411.
Rigobello, E. S., Dantas, A. D. B., Bernardo, L. D., & Vieira, E. M. (2013). Removal of diclofenac by conventional drinking water treatment processes and granular activated carbon filtration. Chemosphere, 92(2), 184–191.
Rosal, R., Rodriguez, A., Perdigon-Melon, J. A., Petre, A., Garcia-Calvo, E., Gomez, M. J., Aguera, A., & Fernandez-Alba, A. R. (2010). Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Research, 44, 578–588.
Santos, J. L., Aparicio, I., Alonso, E., & Callejon, M. (2005). Simultaneous determination of pharmaceutically active compounds in wastewater samples by solid-phase extraction and high-performance liquid chromatography with diode array and fluorescence detectors. Analytica Chimica Acta, 550, 116–122.
Santos, J. L., Aparicio, I., Callejon, M., & Alonso, E. (2009). Occurrence of pharmaceutically active compounds during 1-year period in wastewaters from four wastewater treatment plants in Seville (Spain). Journal of Hazardous Materials, 164, 1509–1516.
Sebok, A., Vasanits-Zsigrai, A., Palko, G., Zaray, G., & Molnar-Perl, I. (2008). Identification and quantification of ibuprofen, naproxen, ketoprofen and diclofenac present in waste-waters, as their trimethylsilyl derivatives, by gas chromatography mass spectrometry. Talanta, 76, 642–650.
Sun, Q., Li, M., Ma, C., Chen, X., Xie, X., & Yu, C. (2016). Seasonal and spatial variations of PPCP occurrence, removal and mass loading in three wastewater treatment plants located in different urbanization areas in Xiamen, China. Environmental Pollution, 208, 371–381.
Wanda, E. M. M., Mamba, B. B., & Msagati, T. A. M. (2016). Determination of the water quality index ratings of water in the Mpumalanga and North West provinces, South Africa. Physics and Chemistry of the Earth, 92, 70–78.
Yu, J. T., Bouwer, E. J., & Coelhan, M. (2006). Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent. Agricultural Water Management, 86, 72–80.
Yu, Y., Wu, L., & Chang, A. C. (2013). Seasonal variation of endocrine disrupting compounds, pharmaceuticals and personal care products in wastewater treatment plants. Science of the Total Environment, 442, 310–316.
Zhang, J., Zhang, Y., & Quan, X. (2012). Electricity assisted anaerobic treatment of salinity wastewater and its effects on microbial communities. Water Research, 46, 3535–3543.
Zhao, J., Ying, G., Wang, L., Yang, J., Yang, X., Yang, L., & Li, X. (2009). Determination of phenolic endocrine disrupting chemicals and acidic pharmaceuticals in surface water of the Pearl Rivers in South China by gas chromatography-negative chemical ionization-mass spectrometry. Science of the Total Environment, 407, 962–974.
Zorita, S., Boyd, B., Jonsson, S., Yilmaz, E., Svensson, C., Mathiasson, L., & Bergstrom, S. (2008). Selective determination of acidic pharmaceuticals in wastewater using molecularly imprinted solid-phase extraction. Analytica Chimica Acta, 626, 147–154.
Zorita, S., Martensson, L., & Mathiasson, L. (2009). Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Science of the Total Environment, 407, 2760–2770.
Acknowledgements
This work is based on the research supported in part by the National Research Foundation (NRF) of South Africa for the Thuthuka grant, Unique Grant No. 93986. NRF is also thanked for funds allocated for lecturer replacement of Lawrence Mzukisi Madikizela. Our sampling team that consisted of Ms. SS Zunngu and Ms. NY Mlunguza is acknowledged. EThekwini Municipality and WWTPs personnel are thanked for sampling arrangements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
(DOCX 91 kb).
Rights and permissions
About this article
Cite this article
Madikizela, L.M., Chimuka, L. Occurrence of naproxen, ibuprofen, and diclofenac residues in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ Monit Assess 189, 348 (2017). https://doi.org/10.1007/s10661-017-6069-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6069-1