Abstract
The present state of iron (Fe) and manganese (Mn) concentration in groundwater of Changchun city located within the Songnen Plain of northeastern China was evaluated in this study. Heavy metal sources, as well as triggering mechanism, were analyzed using a physicochemical, statistical and spatial approach. Results revealed that out of the 2600 samples analyzed, 214 (representing 8.24%) for Fe and 606 wells (representing 23.34%) for Mn exceeded the water standard. Organic matter–rich sediments and Fe-Mn nodules in aquifer and soil serve as sources of Fe and Mn. Organic and inorganic complex formations, as well as long residence time, were found to foster the release of Fe and Mn into groundwater. Additionally, pH and well depth was important in triggering Mn dissolution while groundwater mineralization, depth to the water table and well proximity to the river were found to have minimal/negligible effect on heavy metal mobilization. The removal of Fe and Mn from the water before use was proposed along with the sinking of deeper wells for groundwater exploitation to limit the use of polluted water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Changchun is a large city in Jilin Province, northeastern China, hosting within it a 3.68 million-strong population of Changchun and a total of about 7.534 million people in the ten counties that constitute the study area (Changchun Bureau of Statistics 2017). Groundwater in Changchun constitutes 37% of the total drinking water supply for the vast majority of the population in decentralized water supply areas (Changchun Water Conservancy Bureau 2017). Studies have shown the presence of elevated iron (Fe) and manganese (Mn) in groundwater from the area (Gui-shan 2011; Xi-Jun 2015). The national standard qualities for groundwater are 0.3 mg/l and 0.1 mg/l for Fe and Mn, respectively (National Standardization Administration 2017). The problem of Fe and Mn in groundwater has been extensively studied (Carretero and Kruse 2014; Kshetrimayum and Hegeu 2016; Le Luu 2017; de Meyer et al. 2017). The concentration of Fe and Mn in groundwater is usually related to geology, well depth, proximity to rivers (anthropogenic sources), groundwater age (residence time), the thickness of overburden, groundwater flow pattern and sometimes well age (Beqiraj and Beqiraj 2011; Achary 2014; Demlie et al. 2014; Moyosore and Coker 2014; de Meyer et al. 2017).
Ingestion of water with elevated Fe and Mn can cause chronic intoxication, lung embolism, bronchitis, impotence, nerve damage and parkinsonism (Zoni 2007). Excessive Mn intake has also been observed to influence the intellectual function of children, hyperactive behavior and in other cases causing impaired intellectual development (Wasserman et al. 2005; Bouchard et al. 2007; Bouchard et al. 2010) resulting in health and economic cost (sometimes fatal cost) to consumers of such untreated water (Howard et al. 2006). Despite these effects and the fact that Fe and Mn are notable water quality parameters, studies on these metals are fewer than other trace metals (Huang et al. 2015). Consequently, source water management, as well as treatment, is important for the control of Fe and Mn water intake (Demlie et al. 2014; Tobiason et al. 2016). This is because prevention and remediation strategies for groundwater can successfully be carried out with accurate knowledge of the source of pollution (Huang et al. 2018). Additionally, other heavy metals are usually incorporated in the Fe and Mn oxide matrix as impurities. When oxides are precipitated, it can serve as indicators of conditions that would be conducive to high concentrations of trace elements that have similar chemical characteristics acting as surrogates for other more toxic, trace elements which are released when the Fe and Mn are reduced and dissolved (Berg et al. 2001; Martin 2005; Zachara et al. 2001). In parts of the Songnen Plain of northeast China, some studies have postulated geogenic sources of Fe and Mn pollution (Jia et al. 2018, Zhang et al. 2020). However, anthropogenic contributions have also been identified in large cities like Harbin (Guo et al. 2018). Consequently, the objective of this research is to (1) investigate current levels of Fe and Mn pollution in groundwater; (2) delineate its source (including probable anthropogenic signatures); and (3) examine several factors (HCO3, NH4, turbidity, total hardness, total dissolved solids, depth to the water table, well proximity to river and well depth) that can trigger its dissolution in groundwater. The result is aimed at providing data for better remediation strategies as well as protecting the health of the city’s residents.
Study area
Changchun is located between latitude 43° 05′ N to 45° 15′ N and longitude 124° 18′ E to 127° 05′ E covering an area of about 20,565 km2. Mean annual temperature in the study area is 6.1 °C ranging from 39.5 to −39.8 °C in summer and winter, respectively, with a large temperature difference between day and night. Mean annual precipitation is 577.3 mm with 80% concentrated within May to September. July has the highest precipitation (169.3 mm) while January has the least (3.9 mm). Mean annual humidity, air pressure and evaporation are 61.9%, 986.7 hPa and 62.7 mm respectively (Changchun Water Affairs Bureau 2018).
The study area is located in the east of Songliao Plain. Due to the neo-tectonic movement, the land surface rises slightly and the terrain is higher in the southeast and lowers in the northwest. The overall terrain varies by about 200 m. Geomorphic types in the study area can be divided into three categories, namely low hills, platform plain and valley plain. Low mountains and hills are mainly distributed in the southeast of the study area, and the elevation is 100–200 m higher than that of the northwest river valley as the terrain fluctuates greatly. The table and plain are distributed in most areas of the study area, which is undulating or hilly. The river valley plain is mainly composed of the flood plain and first-level terraces, which are distributed along both sides of Yitong river, Yinma river and Songhua river, and the terrain is relatively flat.
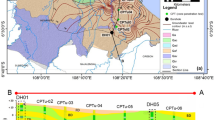
Geologically, the study area is underlain by Holocene sediments which include humus and subclay (Q4), alluvial clay (Q3, Guxiangtun Formation) and loess (Q2, Huangshan Formation); Lower Pleistocene fluvioglacial sandy gravel and clay (Q1, Baitushan Formation); Early Cretaceous glutenitic sandstone, siltstone and mudstone (K1, including Quantou, Qinshangkou, Yaojia and Najing Formations). Other rock formations in the area include Jurassic tuffs, sandstones, clays and pyroclastics grouped as (J3), (J2) and (J1); Early Triassic glutenite with clay (T3) and clastic rock (T1); Permian sandstones and glutenites (P2) and (P1); a transitional rock (CP); limestone (C2, Mopanshan Formation); sandstone with limestone (C1, Lujuantun Formation); and older crystalline rocks which include diorite (δ5), syenite (ξ5), granite (γ4, Varisan), granite (γ 3, Caledonian) and New Proterozoic (Pt3) (Fig. 1). Unconfined aquifers formed from the various geological formations occur in the area, although the silty rock, fine sandstone and gravel stone of Quantou and Qingshankou Formations (K1) often occur as a stratified confined aquifer (Xiao et al., 2009).
Methodology
Sample collection and analysis
A total of two thousand six hundred (2600) groundwater samples were used with data sourced from the Water Resources Bureau of Changchun city. Groundwater samples from different well locations were collected during the fourth quarter in 2018 using plastic bottles. At each location, the bottle was rinsed with the water to be sampled before the water was collected. Sampling was done following the National Environmental Protection Standard (2009) where two water samples each was collected from the two thousand six hundred wells. One sample was acidified for cation analysis in the field by adding a few drops of concentrated HNO3 to achieve a pH of about two, thereby keeping the ions in solution and reduce reaction with the container wall. Both samples were transported to the Laboratory. The non-acidified water samples were used for the determination of anions. Coordinates of each of the wells were taken during the fieldwork. The water samples were tested by Pony Testing International Group in Changchun (http://www.ponytest.com). All analysis was done using standard procedure approved in China (National Standardization Administration of China 2007a; National Standardization Administration of China 2007b; State Environmental Protection Administration 2004). Temperature and pH were measured in situ using EC/pH meter (HANNA, HI99131). TDS was measured by an electric blast-drying oven and an electronic analytical balance (vapor-drying method) while turbidity was determined via formazine scatterometer method (Xinrui, WGZ-200) with detection limit determined to be 0.5NTU. Total hardness (TH) was measured by Na2EDTA titrimetric method; the detection limit was determined to be 1.0 mg/L. Fe and Mn were tested by inductively coupled plasma atomic emission spectrometry (Agilent, 5100ICP-OES) with detection limits determined to be 0.0045 and 0.0005 mg/L respectively. Ammonia nitrogen was analyzed using an ultraviolet-visible spectrophotometer (UNICO, UV-2800) with the detection limit determined to be 0.025 mg/L (National Standardization Administration of China 2007c). The reliability of the water sample analysis data was checked by the relative error of the anion and cation milliequivalent, and the error of all water samples was less than 10%.
Computation of thickness of overburden
Depth to the water table (DTW) often referred to as the thickness of overburden in the literature (Hossain et al. 2015) was estimated from the relationship between the high-resolution (2 m) digital elevation model (DEM) image from National Aeronautics and Space Administration (accessed using BIGEMAP software) and groundwater contours drawn from monitoring 188 observation wells using eq. (1)
where To is overburden thickness (m), Te is the topographic elevation above mean sea level (m) (from high-resolution DEM) and GWe refers to groundwater elevation above mean sea level (m).
Statistical and spatial analysis
One thousand eighty-four (1084) well measurements for Fe (representing 41.69% of the wells) and one thousand four hundred sixty-three (1463) wells for Mn (representing 56.27%) were found to be below detection limit of the measuring instruments. Thus, the two thousand six hundred (2600) data points were reduced to one hundred ninety-seven (n = 197) for correlation and factor analysis. Median and 75th percentile values of whole data were used for comparison as they are least subject to variation amid many non-detects data (Helsel, 1990, Ayotte et al. 1999). Correlation and factor analysis were carried out between Fe, Mn and some related physical and chemical parameters using Microsoft Excel and Statistical Package for Social Sciences (SPSS) software version 20. Varimax rotation was applied to principal components during factor analysis (FA) using Kaiser’s criterion (Kaiser 1960). The well proximity to rivers (WPR) was estimated using the measurement tool in ArcMap 10.2. Data reduction was manually carried out because only distance of wells in the valley of the third-order river (Songhua), fourth-order river (Yinma) and fifth-order rivers (Yitong, Mushi and Wukai) was measured totalling four hundred twenty-two (n = 422) (Fig. 1).
A total of 2596 well data was employed in spatial analysis because four wells had their location coordinates missing. The procedure involved placing Fe and Mn concentration on thematic maps of geomorphology, geology and soil. The total numbers of wells located on the different features of thematic maps were determined. The percentage of Fe and Mn exceedances (concentration in wells above the standard) was subsequently compared.
Results and analysis
Physiochemical parameters
Data obtained from laboratory and fieldwork is presented in Table 1. In terms of Fe concentration in groundwater, 214 samples representing 8.24% out of the 2600 samples of the data were above the 0.3 mg/L national standard, while for Mn, 606 wells (23.34%) were above the 0.1 mg/L standard (National Standardization Administration 2017). Fe concentration had a median of 0.03 mg/L (75th percentile of 0.1 mg/L) while Mn concentration had a median of 0.01 mg/L (75th percentile of 0.08 mg/L). Other measured parameters relating to Fe and Mn occurrence and concentration had median values of 7.63 for pH (75th percentile of 7.83), 0.05 mg/L for NH4 with 75th percentile of 0.07 mg/L, 0.5 NTU for turbidity with 75th percentile of 0.95, 313.86 mg/L for TH (75th percentile of 478.78 mg/L) and 405.55 mg/L for TDS with 75th percentile of 613.21 mg/L. Median depth to water table (DWT) and 75th percentile were, respectively, 7.5 m indicating the phreatic sources of most wells in the area. The measured median proximity of wells in the river valley to the respective rivers was 1265 m while the median depth of wells in the study area was 22.00 m.
Correlation matrix
Pearson’s correlation results are shown in Table 2. A weak significant relationship is seen between Fe and Mn in water (r = 0.145, p < 0.05). The weak relationship between Fe and Mn has similarly been observed in groundwater in Xiangjiang watershed in China (Huang et al. 2015) while Carretero and Kruse (2014) working in Argentina found a negligible correlation between Fe and Mn in groundwater. This weak relationship may be due to the influence of multiple factors on the occurrence of both ions in groundwater or the fact that Fe and Mn contents in the aquifer or soil mineral are not homogenous (Langmuir 1997). Fe has a significant moderate correlation with NH4 and turbidity (r = 0.419 and 0.637 respectively, p < 0.01). Fe relationship with NH4 may be indicative of the redox state of the aquifer system as it infers reducing conditions (Rotiroti 2013; Luu 2017). Fe has a weak significant negative correlation with TH (r = − 166, p < 0.05).
Mn has a weak significant correlation with HCO3 (r = 0.149, p < 0.05) highlighting the effect of organic decomposition on Mn in groundwater (Mapoma et al. 2017). Mn has a weak significant negative correlation with pH (r = − 0.195) indicating that acidic conditions foster Mn dissolution (Jenne 1968; Gilkes and McKenzie 1988; Yamanaka et al. 2011). As was the case with Fe, a weak significant positive correlation of Mn with NH4 was observed (r = 0.181, p < 0.05 respectively). Mn has a weak significant positive correlation with TH (r = 0.143, p < 0.05) indicating the influence of inorganic complexes (Weng et al. 2007).
Factor analysis
The results indicate four factors account for 66.83% of the variation within the data (Table 3). Factor 1 which accounts for 20.13% of the variance has strong loadings of Fe and turbidity, moderate loadings of NH4 and minor loading of Mn. This factor is related to the redox state of the water. Factor 2 explains 18.64% of the variance having high loadings of TDS and TH and moderate loadings of HCO3 and is related to the mineralization of the water as earlier shown by the correlation matrix. Factor 3 with moderate and strong positive loadings of HCO3 and WPR respectively as well as weak negative loading of pH, and a strong negative loading of depth to water table explains 13.07% of the variance. This factor displays the effect of surface-groundwater interaction and how it affects pH and alkalinity. Alkalinity has been used to distinguish acidic organically enriched soil water (surface water) from more alkaline groundwater (Soulsby et al. 2003, Rodgers et al. 2004). In general, when nearness to stream corroborates with shallower DWT, it infers an influent stream (losing stream) regime as it contributes groundwater to the wells. In this study, nearness to stream is negatively loaded with DWT implying that streams that are closer to the river have deeper DWT. Such a scenario implies an effluent stream (gaining stream) regime as wells/aquifer contributes to the streamflow regime which is consistent with field observations of rivers in Changchun (Qiao 2016; Hao 2017). Factor 4 which accounts for 11.78% of the variance has high positive loadings of well depth and pH with moderate negative loadings of Mn indicating the relationship between deep aquifers and pH and its effect on Mn dissolution.
Spatial statistical results
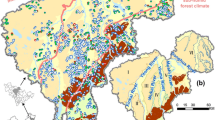
The summary of Fe and Mn concentration distribution as related to the geomorphology, geology and soil of the study area is shown in (Table 4 and Fig. 2). The percentage of water samples above the Fe standard as distributed on respective geomorphological features of the area is in the order alluvial valley plain (13.98%) > erosional hills (10.78%) > denudation accumulation basin (7.28%) > alluvial lacustrine high plain (5.43%) > platform high plain (4.35%) (Fig. 3, Table 4). Mn concentration distribution over the standard in terms of geomorphology is of the order platform high plain (66.67%) > alluvial valley plain (37.28) > denudation accumulation basin (23.34%) > alluvial lacustrine high plain (20.53%) > erosional hill (18.81%) (Fig. 3).
In terms of geology, the percentage of water samples above the Fe standard as distributed in the study shows tuff, clay and sandstone (J2) to be the most abundant (22.22%). Syenite and diorite which have higher percentages (33.30% and 50% respectively) are considered as outliers because of their smaller sample population. Other higher Fe concentration percentages of samples above Fe standard have sandstone and glutenite (P2) (15.22%) > alluvial humus subclay (Q4) (11.33%) > alluvial clay(Q3) (8.41%) > mudstone and siltstone (K1) (6.82%). Mn concentration distribution over the standard (excluding the outliers of syenite and diorite 33.33% and 0.0% respectively) is of the order alluvial humus subclay (Q4) (38.4%) > alluvial clay (Q3) (22.6%) > sandstone and glutenite (P2) (21.7%) > tuffs, sandstone and clays (J2) (22.22%) > mudstone and siltstone (K1) (15.5%). It is worthy to note that lithological formations with the higher Fe and Mn exceedances are analogous to both Fe and Mn, although ranks vary between them (Fig. 4).
The relationship of percentage exceedances of Fe concentration in groundwater to the soil is of the order umbrisols (10.48%) > chernozems (7.63%) > kastanozems 7.55%) > albic soils (5.91%) > arenosols (1.85%) > > solonchaks and solonetzs (90%). In the case of soil relationship to Mn concentration in groundwater, percentage exceedances is of the order arenosols (53.70%) > albic soils (35.86%) > umbrisols (29.64%) > solonchaks and solonetzs (28.57%) > chernozems (18.05%) > kastanozems (10.07%) (Fig. 5).
Source of Fe and Mn in groundwater
The association of elevated Fe with Mn in groundwater in northeastern China has previously been reported (Zhang et al. 2012), and though mobilization mechanism varies, Fe and Mn sources are mostly related to soils, iron-manganese nodules in aquifer clay layers as well as anthropogenic sources (Guo et al. 2018; Zhang et al. 2020). A similar Fe-Mn association is observed in this study as revealed by correlation and factor analysis. The principal factor that affects Fe and Mn occurrence in the groundwater of Changchun is related to the organic matter content of the groundwater as evidenced by the relationship of these ions with ammonia and turbidity both in the correlation and factor analysis results. Reducing conditions at near-neutral pH is known to trigger the dissolution of iron oxides and enrichment in groundwater (Lindsay 1991; Chen et al., 2003; Pezzetta et al. 2011; Molineri et al., 2014). These redox conditions are fostered by a relatively high content of organic carbon and fine-grained sediments because organic matter favor conditions that mobilize Fe and Mn into groundwater (Roychoudhury and Merret 2006; Palmucci 2016) which may be released from soil or aquifer matrix (Erikson and Barnes, 2005; Zachara et al. 2001; Beqiraj and Beqiraj 2011). Spatial distribution of Fe and Mn exceedances on the thematic map of geology and soil corroborate this position.
In the case of geology (excluding the outliers of syenite and diorite which are themselves rich in pyroxenes and amphiboles that may release Fe and Mn into groundwater), the major rocks/aquifers with the highest percentage of Fe and Mn above the standard are rich in organic matter. The tuffs, sands and clays (J2) of Xiajiajie Formation are rich in organic matter to the extent of being interbedded with coal (Annals of Changchun 1995). Other lithologies with high Fe and Mn exceedances like sandstone glutenite (P2), alluvial humus subclay (Q4), alluvial clay (Q3) and mudstone siltstone (K2) are all associated with high organic matter content with some containing loess-like clay with large number Fe and Mn tube nodules. The reductive dissolution of Fe (and Mn) oxides driven by the degradation of peat is thus believed to affect the mechanism of Fe and Mn mobilization in groundwater as has been observed in other aquifer systems (e.g. Caschetto et al. 2014; Rotiroti 2013; Rotiroti et al. 2014).
Additionally, the soil type developed within the study area is also an important source of Fe and Mn in groundwater. Umbrisols, chernozems and kastanozems are usually characterized by the pronounced accumulation of organic matter (IUSS Working Group WRB 2015) which enhance anoxic conditions that promote Fe and Mn mobilization. This is similar to observations in the study area as average humus contents of soils range from kastanozems (2.76%), umbrisols (2.73%), chernozems (2.50%), albic soils (2.08%), arenosols (1.89%), to solonchaks and solontzes (1.82%) (Annals of Changchun 1995). Alternate reducing and oxidizing environments caused by (1) repeated cycles of water logging due to summer rains and permafrost, followed by dry spring period, and (2) alternating oxidation and reduction in soil caused by seasonal rise and fall of groundwater (water level fluctuation) have, respectively, led to the formation of Fe-Mn nodules in soil subsurface layer. This water level fluctuation also serves to trigger Fe and Mn dissolution in groundwater (Hossain et al. 2015; Zhang, 2020). A similar scenario is common with umbrisols as high groundwater level in the valley plain being the main geochemical feature of umbrisol formation (Jenčo et al. 2018).
Anthropogenic influence on Fe and Mn mobilization in groundwater has been inferred from a positive relationship between the proximity of groundwater wells to rivers in some studies (Luu 2017; De Meyer et al. 2017). The negligible correlation observed in this study implies minimal anthropogenic influence on the heavy metal mobilization in contrast to results found elsewhere in northeast China (Guo et al. 2018). Jia et al. (2018) observed wide spread human-influenced mobilization of geogenic groundwater pollutants in parts of China; however, the Fe and Mn concentration in groundwater of Changchun area appears to be mainly geogenic in origin.
A qualitative comparison of the spatial distribution of Mn on the thematic map of geology and soil displays the dual role of geology and soil as sources of Fe and Mn. Mn exceedances in the northwestern portion of the study area are here thought to be as a result of the alluvial humus subclay geology rather than the arenosols (Fig. 3). Alluvial aquifers are at times the most sensitive to contamination as observed from Fe and Mn distribution because of the peculiar characteristics in terms of low permeability and long residence time, for the presence of organic matter (Caschetto et al. 2014; Rotiroti et al. 2014; Kim et al. 2014; Yadav et al. 2015).
Effect of pH and bicarbonate (HCO3) on Fe and Mn
The influence of pH on Fe in the study area is negligible because of the insignificant correlation of Fe to pH. Back and Barnes (1965) noted that little/no correlation between pH and elevated Fe in groundwater is possible and Fe dissolution is lower than Mn at pH of greater than 4.8 (Hem 1963). Mn dissolution decreases with increase in pH (increased alkalinity). This is consistent with other studies which have found that acidic conditions favor Mn dissolution (Martin 2005; Rao 2006; Moyosore et al. 2014; Kshetrimayum and Hegeu 2016). Since HCO3 concentration often defines groundwater alkalinity (Groschen et al. 2009), the significant positive correlation between HCO3 and Mn may be due to the formation of MnHCO3+ complexes which increase Mn solubility (Hem 1963). Factor analysis display of a positive relationship between well depth and pH implies alkalinity increases with the depth of well. This is believed to be related to the lithological composition and groundwater conditions of most of the study area as aquifers are characterized by organic-rich alluvial formations (Demlie et al. 2014). At near surface (shallower depth), availability of dissolved oxygen leads to the oxidation of Fe2+ to ferric form Fe3+ (or Mn2+ to Mn + 4+) state which produces H+ that contributes to acidity (Hem 1985).
Effects of TH on Fe and Mn
TH has strong significant correlation with TDS in this study. It is believed to be related to the mineralization and sometimes salinization associated with alluvial aquifers of the study area (Fan et al. 2011; Wang et al. 2018). However, TDS has no significant effect on Fe and Mn concentration implying that samples affected by Fe and Mn contamination are the minority with respect to the dataset (Palmucci et al. 2016). Positive correlation of TDS with Fe and Mn usually indicates inorganic complex formations as a source of elevated concentrations in groundwater (Kshetrimayum and Hegeu 2016, Zhang et al. 2020). Thus, the significant correlation of Fe and Mn with TH may suggest the slight influence of inorganic complex formations related to CO3 ion because it was CaCO3 hardness that was determined. The significant relationship of Fe and Mn (to a lesser extent Mn) with NH4 and turbidity emphasizes the strong influence of organic complexes on heavy metal dissolution because Fe-binding natural organic matter favors dissolution of Fe in water (Pham et al. 2004; Gerringa et al. 2016; Krupinska 2017). Microbial metabolism of organic carbon in sediments is also known to drive reduction of both Fe hydroxides and Mn oxides (Chapelle 2000).
Effect of geomorphology on Fe and Mn
The prolonged residence time of groundwater in aquifers may result in increased Fe and Mn concentration in groundwater because it tends to be released progressively from the geological materials (Gruett, 1999; Minnesota Pollution Control Agency 1999; Beqiraj and Beqiraj 2011; Moyosore et al. 2014). The relationship between groundwater age and geomorphology has been conceptually shown to be limited in arid regions (Maxwell et al. 2016); however, it has been severally shown to affect groundwater residence time (Hrachowitz et al. 2009; Mcguire et al. 2005; Tetzlaff et al. 2009a; Tetzlaff et al. 2009b). Residence times are estimated on the basis of its inverse proportionality to the gradient of groundwater flow which is dependent on the geomorphic characteristic of the area. Consequently, with a strong influence of geomorphology (inferred residence time) on Fe and Mn concentration in groundwater, it is expected that ideal order of Fe and Mn exceedances should be alluvial valley plain > denudational accumulation basin > alluvial lacustrine plain > platform high plain > erosional plain. Comparison of this assertion with results from this study shows that erosional hills in the case of Fe and platform high plain in the case of Mn are the only odd occurrences to this hypothesized ideal situation. Thus, it is thought that residence time as inferred from geomorphology has an influence on Fe and Mn concentration in groundwater in the study area.
Conclusion
This study determined the present state of Fe and Mn in groundwater Changchun area. Within the 2600 samples analyzed, 214 (representing 8.24%) for Fe and 606 wells (representing 23.34%) for Mn were found to exceed the water standard.
The main source of these heavy metals was found to be related to reducing environments created by organic matter–rich aquifers and soils, along with the Fe and Mn nodules present in them. Mobilization of Fe was enhanced by formation of organic complexes which trigger Fe dissolution. Mn dissolution on the other hand was mostly controlled by pH and formation of inorganic complexes. Long residence time as inferred from geomorphology was found to foster release of Fe and Mn into groundwater. Groundwater mineralization, depth to water table and well proximity to river were found to have minimal/negligible effect on heavy metal mobilization while anthropogenic contribution to heavy metal concentration was also precluded.
Since heavy metal exceedances is spatially spread, the following recommendations are made: (1) The source water removal of Fe and Mn prior to use is advocated to ensure safety of drinking water; (2) sinking of deeper wells is also encouraged as a means of exploiting less polluted water especially in terms of Mn.
References
Achary, G. S. (2014). Studies on ground water pollution due to iron content in Cuttack City, Odisha, India. International Journal of Multidisciplinary and Current Research, 2(1), 86–89 http://ijmcr.com.
Annals of Changchun (1995) Physical geography, Changchun Local Chronicles Compilation Committee . Jilin literature and history press, Changchun. p559.
Ayotte, J.D., Nielson, M.G., Robinson, G.R. Jr. & Moore, R.B. (1999). Relation of arsenic, iron, and manganese in groundwater to aquifer type, bedrock, lithogeochemistry, and land use in New England coastal basin. U.S. Geological Survey:Water-Resources Investigation Report 99–4162. Available at http://water.usgs.gov/pubs/wri/wri994162
Back, W. & Barnes, I. (1965). Relation of electrochemical potentials and iron content to ground-water flow patterns. Hydrology of Aquifer System, 22.
Beqiraj, A., & Beqiraj, E. (2011). Effects of sediment mineralogy to high-iron content in the groundwater of Rrogozhina aquifer (Western Albania). Studia Universitatis Babes-Bolyai, Geologia, 56(2), 25–30 http://scholarcommons.usf.edu/geologia/vol56/iss2/art3/.
Berg, M., Tran, H. C., Nguyen, T. C., Pham, H. V., Schertenleib, R., & Giger, W. (2001). Arsenic contamination of groundwater and drinking water in Vietnam: A human health threat. Environmental Science & Technology, 35(13), 2621–2626.
Bouchard, M., Laforest, F., & Vandelac, L. (2007). Children’s health hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Research, 1, 122–127.
Bouchard, M. F., Sauvé, S., Barbeau, B., et al. (2010). Intellectual impairment in school-age children exposed to manganese from drinking water [J]. Environmental Health Perspectives, 119, 138–143.
Carretero, S., & Kruse, E. (2014). Iron and manganese content in groundwater on the northeastern coast of the Buenos Aires province Argentina. Environment and Earth Science, 73(5), 1983–1995.
Caschetto, M., Barbieri, M., Galassi, D. M. P., et al. (2014). Human alteration of groundwater – Surface water interactions (Sagittario River, Central Italy): Implication for flow regime. Contaminant Fate and Invertebrate Response, 1791–1807.
Changchun Bureau of Statistics (2017). Changchun statistical year book. Chinese Statistics Press, pp 61–64.
Changchun Water Affairs Bureau (2018). Changchun city water resources bulletin. 1122010001382739X6/2019–00497. Available online at: http://zwgk.changchun.gov.cn/zcbm/fgw_98087/gkml/201912/t20191212_2092914.html. Accessed 20th July, 2020.
Changchun Water Conservancy Bureau (2017). Changchun area water resource bulletin. Available online at: www.changchun.gov.cn. Accessed 11 March 2020.
Chapelle, F. H. (2000). The significance of microbial processes in hydrogeology and geochemistry. Hydrogeology Journal, 8, 41–46.
Chen, J., Gu, B., Royer, R. A., & Burgos, W. D. (2003). The roles of natural organic matter in chemical and microbial reduction of ferric iron. Sci. Total Environ., 307(1–3), 167–178.
De Meyer, C. M. C., Rodriquez, J. M., Caprio, E. A., et al. (2017). Arsenic, manganese and aluminum contamination in groundwater resources of Western Amazonia (Peru). Sci. Total Environ., 607–608, 1437–1450. https://doi.org/10.1016/j.scitotenv.2017.07.059.
Demlie, M., Hingston, E., & Mnisi, Z. (2014). A study of the sources, human health implications and low cost treatment options of iron rich groundwater in the northeastern coastal areas of KwaZulu-Natal, South Africa. Journal of Geochemical Exploration, 144(PC), 504–510. https://doi.org/10.1016/j.gexplo.2014.05.011.
Fan, Y., Zhang, G., Yin, X. R., Liu, Z. J., & Huang, G. G. (2011). Study on capillary rise from shallow groundwater and critical water table depth of a saline-sodic soil in western Songnen plain of China. Journal of Environment and Earth Science, 64(8), 2119–2126.
Gerringa, L. J. A., Rijkenberg, M. J. A., Bown, J., Margolin, A. R., Laan, P., & de Baar, H. J. W. (2016). Fe-binding dissolved organic ligands in the oxic and suboxic waters of the Black Sea. Frontiers in Marine Science, 3. https://doi.org/10.3389/fmars.2016.00084.
Gilkes, R. J., & McKenzie, R. M. (1988). Geochemistry of manganese in soils. In R. D. Graham, R. J. Hannam, & N. C. Uren (Eds.), Manganese in soils and plants (pp. 23–35). Dordrecht: Kluwer.
Groschen, G.E., Arnold, T.L., Morrow, W.S. and Warner, K.L. (2009). Occurrence and distribution of iron, manganese and selected trace elements in groundwater in the glacial aquifer system of north United States. U.S. Geological Survey Scientific Investigation Report 2009–5006, 89p.
Gui-shan, H. (2011). The study on the biological removal of iron and manganese in the groundwater of northeast rural area. Unpub. Doctoral dissertation, Jilin University), 55p (in Chinese).
Guo, X., Zuo, R., Meng, L., Wang, J., Teng, Y., Liu, X., & Chen, M. (2018). Seasonal and spatial variability of anthropogenic and natural factors influencing groundwater quality based on source apportionment. International Journal of Environmental Research and Public Health, 15, 279.
Hao, Y. (2017). Study on spatiotemporal evolution of characteristics of groundwater quantity in plain areas of Jilin Province. Unpub. Doctoral dissertation, Jilin University, 83p (in Chinese).
Hem, J. D. (1963). Chemical equilibria affecting the behavior of manganese in natural water. Hydrological Sciences Journal, 8(3), 30–37. https://doi.org/10.1080/02626666309493334.
Hem, J. D. (1985). Study and interpretation of chemical characteristics of natural waters, (3rd Ed). U.S. Geological Survey Water-Supply Paper 2254.
Hossain, D., Islam, M. S., Sultana, N., & Tusher, T. R. (2015). Assessment of iron contamination in groundwater at Tangail municipality, Bangladesh. J. of Environ. Sci. & Natural Resources, 6(1), 117–121.
Howard, G., Bartram, J., Pedley, S., Schmoll, O., Chorus, I., & Berger, P. (2006). Groundwater and public health. In O. Schmoll, G. Howard, J. Chilton, & I. Chorus (Eds.), Protecting groundwater for health: Managing the quality of drinking-water sources (17p). IWA: WHO.
Hrachowitz, M., Soulsby, C., Tetzlaff, D. et al. (2009). Regionalization of transit time estimates in montane catchments by integrating landscape controls. 45(January).
Huang, B., Li, Z., Chen, Z., Chen, G., Zhang, C., Huang, J., Nie, X., Xiong, W., & Zeng, G. (2015). Study and health risk assessment of the occurrence of iron and manganese in groundwater at the terminal of the Xiangjiang River. Environmental Science and Pollution Research, 22(24), 19912–19921.
Huang, L., Wang, L., Zhang, Y., Xing L., Hao Q., Xiao Y., Yang L., Zhu H. (2018). Identification of groundwater pollution sources by a SCE-UA algorithm-based. Water 10(193).
IUSS Working Group WRB (2015). World reference base for soil resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome.
Jenčo, M., Matečný, I., Putiška, R., Burian, L., Tančárová, K., & Kušnirák, D. (2018). Umbrisols at lower altitudes, case study from Borská lowland (Slovakia). Open Sci. De Gruyter, 10, 121–136. https://doi.org/10.1515/geo-2018-0010.
Jenne, E. A. (1968). Controls on Mn, Fe Co, Ni, Cu and Zn concentrations in soils and waters: the significant role of hydrous Mn and Fe oxides. Am Chem Soc 337–387.
Jia, Y., Xi, B., Jiang, Y., Guo, H., Yang, Y., Lian, X., & Han, S. (2018). Distribution, formation and human-induced evolution of geogenic contaminated groundwater in China: A review. Sci. Total. Environ, 643, 967–999.
Kaiser, H.F. (1960). Consequently perhaps. Educational and Psychological Measurement XX(1), 141–51.
Kim, D. M., Yun, S. T., Man, J. K., Mayer, B., & Kim, K. H. (2014). Assessing redox zones and seawater intrusion in a coastal aquifer in South Korea using hydrogeological, chemical and isotopic approaches. Chemical Geology, 390, 119–134.
Krupinska, I. (2017). Effects of organic substances on the efficiency of Fe(II) to Fe(III) oxidation and removal of iron compounds from groundwater in sedimentation process. Civil Env. Eng. Reports, 26(3), 015–029. https://doi.org/10.1515/ceer-2017-0032.
Kshetrimayum, K. S., & Hegeu, H. (2016). The state of toxicity and cause of elevated iron and manganese concentrations in surface water and groundwater around Naga thrust of Assam-Arakan Basin, northeastern India. Environment and Earth Science, 75(7), 1–14.
Langmuir, D. (1997). Aqueous environmental geochemistry. New Jersey: Prentice Hall.
Lindsay, W.L. (1991). Iron oxide solubilization by organic matter and its effect on iron availability. Iron Nutrition and Interactions in Plants. Springer Netherlands, pp 29–36.
Luu, T. (2017). Remarks on the current quality of groundwater in Vietnam. Water Industry: Water-Energy-Health Nexus. Env. Sci. Pollu. Res.. DOI https://doi.org/10.1007/s11356-017-9631-z.
Martin, S. (2005). Precipitation and dissolution of iron and manganese oxides. Environ. Catalysis (September): 61–82. http://www.crcnetbase.com/doi/10.1201/9781420027679.ch3.
Maxwell, R. M., Condon, L. E., Kollet, S. J., Maher, K., Haggerty, R., & Forrester, M. M. (2016). The imprint of climate and geology on the residence times of groundwater. Geophysical Research Letters, 43, 701–708. https://doi.org/10.1002/2015GL066916.
Mcguire, K.J., Mcdonnell, J.J., Weiler, M., Kendall, C., Mcglynn, B.L. & Welker, J.M. et al. (2005). The role of topography on catchment-scale water residence time. Water Resources Research, 41(5), n/a-n/a.
Minnesota Pollution Control Agency (1999) Iron in Minnesota’s ground water. Groundwater monitoring and assessment programme.
Moyosore, J. O., Coker, A. O., Sridhar, M. K. C., & Mumuni, A. (2014). Iron and manganese levels of groundwater in selected areas in Ibadan and feasible. Engr. Solution, 10(11), 137–153.
National Environmental Protection Standard (2009) Technical requirements for water quality sampling scheme design, HJ495.
National Standardization Administration (2017) Standard for groundwater quality. General administration of quality supervision, inspection and quarantine of the People’s Republic of China. GB14848.
National Standardization Administration of China (2007a). Standard examination methods for drinking water: organoleptic and physical parameters (GB/T 5750.4–2006) (1st ed.); China Standard Press: Beijing, China. (In Chinese).
National Standardization Administration of China. (2007b). Standard examination methods for drinking water: Metal parameters (GB/T 5750.6–2006) (1st ed.). China Standard Press: Beijing (In Chinese).
National Standardization Administration of China. (2007c). Standard examination methods for drinking water: Nonmetal parameters (GB/T 5750.5–2006) (1st ed.). China Standard Press: Beijing (In Chinese).
Palmucci, W., Rusi, S., & Di Curzio, D. (2016). Mobilisation processes responsible for iron and manganese contamination of groundwater in central Adriatic Italy. Environmental Science and Pollution Research, 23(12), 11790–11805.
Pezzetta, E., Lutman, A., Martinuzzi, I., Viola, C., Bernardis, G., & Fuccaro, V. (2011). Iron concentrations in selected groundwater samples from the lower Friulian plain, Northeast Italy: Importance of salinity. Environment and Earth Science, 62(2), 377–391. https://doi.org/10.1007/s12665-010-0533-3.
Pham, A. N., Rose, A. L., Feitz, A. J., & Waite, T. D. (2004). The effect of dissolved natural organic matter on rate of removal of ferrous iron in fresh water. Water Supply, 4(4), 213–219. https://doi.org/10.2166/ws.2004.0080.
Qiao, Y. (2016). Study on the spatial and temporal evolution of groundwater in the central plain of Jilin province. Unpub. Doctoral dissertation, Jilin University, 78p (in Chinese).
Rao, N. S. (2006). Seasonal variation of groundwater quality in a part of Guntur District, Andhra Pradesh, India. Environmental Geology, 49(3), 413–429.
Rodgers, P., Soulsby, C., Petry, J., Malcolm, I., Gibbins, C., & Dunn, S. (2004). Groundwater-surface-water interactions in a braided river: A tracer-based assessment. Hydrological Processes, 18, 1315–1332.
Rotiroti, M. (2013). Hydrogeology and hydrogeochemistry of As, Fe, Mn rich groundwater of the multi-layer aquifer in the lower Po Plain, Lombardy region (northern Italy). Doctoral Thesis, Uni. Milano-Bicocca.
Rotiroti, M., Elisa, S., Fumagalli, L., & Bonomi, T. (2014). Origin of arsenic in groundwater from the multilayer aquifer in Cremona (northern Italy). Environmental Science & Technology, 48(10), 5395–5403.
Roychoudhury, A. N., & Merrett, G. L. (2006). Redox pathways in a petroleum contaminated shallow sandy aquifer: Iron and sulfate reductions. Science of the Total Environment, 366(1), 262–274.
Soulsby, C., Rodgers, P., Smart, R., Dawson, J., & Dunn, S. (2003). A tracer based assessment of hydrological pathways at different spatial scales in a mesoscale Scottish catchment. Hydrological Processes, 17, 759–777.
State Environmental Protection Administration. (2004). Methods for monitoring and analyzing water and wastewater (4th ed.). China Environmental Science Press: Beijing, China (In Chinese).
Tetzlaff, D., Seibert, J., Mcguire, K. J., et al. (2009a). How does landscape structure influence catchment transit time across different geomorphic provinces. Hydrological Processes, 23(January), 945–953.
Tetzlaff, D., Seibert, J., & Soulsby, C. (2009b). Inter-catchment comparison to assess the influence of topography and soils on catchment transit times in a geomorphic province; the Cairngorm Mountains, Scotland. Hydrological Processes, 23(May), 1874–1886.
Tobiason, J. E., Arianne, B., & Joseph, G. (2016). Manganese removal from drinking water sources. Current Pollution Reports, 2, 168–177. https://doi.org/10.1007/s40726-016-0036-2.
Wang, Z., Zhuang, J., Zhao, A. & Li, X. (2018). Types, harms and improvements of saline soil in Songnen Plain. International Symposium on Application of Materials Science and Energy Materials (SAMSE 2018) Shanghai, China.
Wasserman, G.A., Liu, X., Parvez, F., Ahsan, H., Levy, D., Factor-Litvak, P. et al. (2005). Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Perspectives.
Weng, H. X., Qin, Y. C., & Chen, X. H. (2007). Elevated iron and manganese concentrations in groundwater derived from the Holocene transgression in the hang-Jia-Hu plain, China. Hydrogeology Journal, 15, 715–726.
Xi-jun, G. (2015). Removal of iron, manganese and ammonia from groundwater of northeast small towns. Unpub. Doctoral dissertation, Jilin University, 54p (in Chinese).
Yadav, I. C., Devi, N. L., & Singh, S. (2015). Reductive dissolution of iron-oxyhydroxides directs groundwater arsenic mobilization in the upstream of Ganges river basin, Nepal. Journal of Geochemical Exploration, 148, 150–160.
Yamanaka, T., Mikita, M., Lorphensri, O., Shimada, J., Kagabu, M., Ikawa, R., Nakamura, T., & Tsujimura, M. (2011). Anthropogenic changes in a confined groundwater flow system in the Bangkok Basin, Thailand, part II: How much water has been renewed? Hydrological Processes, 25, 2734–2741.
Zachara, J. M., Fredrickson, J. K., Smith, S. C., & Gassman, P. L. (2001). Solubilization of Fe ( III ) oxide-bound trace metals by a dissimilatory Fe ( III ) reducing bacterium. Geochimica et Cosmochimica Acta, 65(1), 75–93.
Zhang, B., Song, X., Zhang, Y., Han, D., Tang, C., Yu, Y., & Ma, Y. (2012). Hydrochemical characteristics and water quality assessment of surface and groundwater in Songnen plain, Northeast China. Water Research, 46, 2737–2748.
Zhang, Z., Xiao, C., Adeyeye, O., Yang, W., & Liang, X. (2020). Source and mobilization mechanism of iron, manganese and arsenic in groundwater of Shuangliao city, Northeast China. Water, 12, 534.
Zoni, S. (2007). Neuropsychological testing for the assessment of manganese neurotoxicity: A review and a proposal. American J. of Ind. Machines, 830, 812–830.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adeyeye, O., Xiao, C., Zhang, Z. et al. State, source and triggering mechanism of iron and manganese pollution in groundwater of Changchun, Northeastern China. Environ Monit Assess 192, 619 (2020). https://doi.org/10.1007/s10661-020-08571-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08571-0