Abstract
Iron (Fe) and manganese (Mn) are heavy metals ubiquitous in groundwater. High levels of Fe and Mn in groundwater can compromise water quality and pose a risk to human health if the groundwater is used for drinking or irrigation. In the middle region of the Yangtze River Basin, groundwater has been extensively used for domestic and agricultural purposes. However, little is known about the distribution of Fe and Mn in the groundwater in this area. It was found that the 74.4% and 48.9% of the groundwater exceed the China national guideline for Fe (i.e., 0.3 mg/L) and Mn (i.e., 0.1 mg/L), respectively. And 6.38% and 2.13% of the wells had Fe and Mn health risks, respectively. Spatial heterogeneity of Fe and Mn was observed. Notably, the concentrations of Fe and Mn in a plain region located between two major rivers (i.e., the Yangtze River and the Han River) were significantly higher than those in other regions. Modeling using PHREEQC revealed that the Fe-bearing minerals in the plain region were more saturated compared to those in the other regions. Besides, temporal change of Fe and Mn was observed in the plain region, significantly affected by rainfalls and groundwater levels. In addition, the distribution of Fe and Mn was significantly affected by various physicochemical factors. Particularly, Fe was more sensitive to redox potential compared to Mn. Under a reducing condition, organic matter concentration and water residence time also affect the release of Fe from Fe-bearing minerals. Overall, a comprehensive understanding of distribution characteristics of Fe and Mn and affecting factors in the middle area of the Yangtze River Basin can provide guidance for the distribution of industrial water, agricultural water, and drinking water in different regions of the study area. Especially in the plain area between the Yangtze River and the Han River, direct drinking of groundwater shall be reduced since the higher health risk value of Fe and Mn.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the deterioration of surface water worldwide, groundwater is becoming a key source of industrial, agricultural, and domestic water (Aeschbach-Hertig and Gleeson 2012; Huang et al. 2013; Xiao et al. 2014). Therefore, the quality of groundwater has become particularly important. Although groundwater is hidden, many groundwater quality problems have been reported in recent years (Qu and Fan 2010; Zhang et al. 2014). Exceeding of iron and manganese is one of them. Fe and Mn can result in metallic tasting water and staining of clothes and dishes. Excessive Fe and Mn in groundwater may threaten human health if the groundwater is used for drinking or irrigation. Excessive Fe and Mn in drinking water can lead to loss of appetite, vomiting, gastrointestinal disorders, and abnormal stool. More seriously, excessive Fe and Mn in groundwater may be associated with neurological of chronic such as Parkinson-like disease (Homoncik et al. 2010).
Iron (Fe) and manganese (Mn) are heavy metals ubiquitous in groundwater (Carretero and Kruse 2015; Weng et al. 2007; Gan et al. 2014). The occurrence of Fe and Mn in Quaternary pore groundwater has been well documented worldwide (Liu and Wu 2019; Pezzetta et al. 2011; Weng et al. 2007; Zhang et al. 2020). Spatial and temporal distribution of Fe and Mn concentrations has been reported in Western Quebec, Canada (Bondu et al. 2018); Argentina (Carretero and Kruse 2015); Italy (Pezzetta et al. 2011); Scottish (Homoncik et al. 2010); and China (Liu and Wu 2019; Weng et al. 2007; Zhang et al. 2020). For example, the concentration of dissolved Fe and Mn ranged from 0.003–3.499 mg/L and 0.043 to 1.69 mg/L, respectively, in the groundwater in Western Quebe, Canada (Bondu et al. 2018). The concentration of dissolved Fe and Mn ranged from 0.07 to 10.3 mg/L and 0.06 to 2.99 mg/L, respectively, in the groundwater in the Lanyang Plain, Taiwan (Liu and Wu 2019).
The distribution of Fe and Mn in groundwater can be attributed to geological sources. For example, studies showed that Fe and Mn in the groundwater are sourced from pyroxene, amphibole, biotite, iron oxide (e.g., hematite), and hydroxide (e.g., goethite) in sediments (Liu and Wu 2019; Carretero and Kruse 2015). Besides, the distribution of Fe and Mn can be affected by various physicochemical factors. For example, the levels of Fe and Mn in groundwater were greatly affected by redox potential (Homoncik et al. 2010; Bondu et al. 2018). The concentrations of Fe and Mn were positively correlated with the concentration of total dissolved solids and groundwater level fluctuation (Zhang et al. 2020). It appears that the effect of a certain factor on the concentrations of Fe and Mn can be case-specific. For example, a negative correlation was found between pH and Fe concentration in the groundwater in the Songliao plain, China, while there was no correlation between pH and Mn concentration (Zhang et al. 2020). In contrast, a negative correlation was found between pH and Mn concentration in the groundwater in Western Quebec, Canada, while there was no correlation between pH and Fe concentration (Bondu et al. 2018).
In the middle area of the Yangtze River Basin, Quaternary pore groundwater serves as the key source of agricultural and domestic water. There is an increasing concern about the quality of groundwater in the area. The occurrence of various contaminants in the groundwater has been reported, including arsenic (Duan et al. 2015, 2017; Gan et al. 2014), dissolved organic matter (Huang et al. 2015), antibiotics (Yao et al. 2015), ammonium (Du et al. 2017), and nitrate (Niu et al. 2017; Yang et al. 2020). High levels of Fe and Mn were reported in the groundwater in the area between the Yangtze River and Han River and Dongting Plain located in the south of the Yangtze River (Gan et al. 2014; Huang et al. 2021; Liang et al. 2020). The concentrations of dissolved Fe and Mn were up to 48 mg/L and 5.0 mg/L, respectively, in 2011 (Gan et al. 2014; Liang et al. 2020). However, the concentrations of dissolved Fe and Mn were found lower than 1.89 mg/L and 2.35 mg/L in the north of Han River (Chen et al. 2011; Zeng 1997). Thus, to understand the distribution of Fe and Mn in groundwater in the middle area of the Yangtze River Basin, it is essential to investigate potential affecting factors in the north of Han River, the area between the Yangtze River and Han River and the south of Yangtze River.
Previous studies only examined one region in the area and one year. Besides, geological and physicochemical factors affecting the distribution of Fe and Mn were not examined. There is a lack of understanding of the spatial and temporal distribution of Fe and Mn in the area. In addition, potential affecting factors are still unclear. The purpose of this study is to fill a major gap in our understanding of the spatial and temporal variability in groundwater Fe and Mn in the middle area of the Yangtze River Basin and to identify the affecting factors. The results can provide guidance for the distribution of industrial water, agricultural water, and drinking water in different regions of the study area.
Methods and materials
Study area
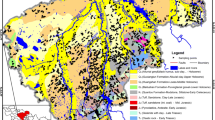
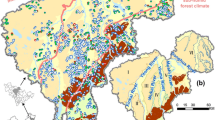
Yangtze River Basin is across the Centra China (Fig. 1a). The study area is located in the middle area of the Yangtze River Basin, containing several rivers and lakes (Fig. 1b). Besides the Yangtze River, the Han River is another major river flowing in the north part of this area (Fig. 1b). The area consists of two main geomorphologies. A low plain with an altitude of 20–30 m and a slope less than 2 degrees is widely distributed in the center of the area and surrounded by hilly grounds with an altitude of 40–170 m and a slope of 2–8° (Gan et al. 2018; Zhou et al. 2013; Du et al. 2017). The area has a subtropical monsoon climate with two distinct seasons. A wet season usually starts from April to August, while a dry season occupies for the rest of the year. The annual rainfall in the area is 1269 mm (Yang et al. 2020). The rainfall is generally lower in the northwest than that in the Southeast. Rainfalls in the wet season contribute over 60% of the total rainfalls (Fig. 2). The annual average temperature is 16.8 °C (Fig. 2).
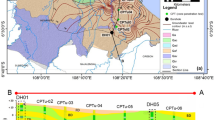
Location and samples distribution of a the Yangtze River Basin, b the middle area of the Yangtze River, c Region 1, d Region 2, e Region 3, and f hydrogeological profile of the study area (Gan et al. 2014)
Rainfalls and temperature in the study area from 2010 to 2020 (Zhou et al. 2013)
The lithology in the middle area of the Yangtze River Basin is mainly Quaternary deposits. The aquifer system can be divided vertically into multiple layers based on lithology and burial conditions. The first layer (0–20 m) is a Holocene (Q4) phreatic aquifer containing silty clay, clayey silt, and silt. The groundwater level in this layer generally ranges from 0.5 to 5 m (Fig. 1f). The second layer (20–100 m) is an Upper Pleistocene (Q3) shallow confined aquifer containing sand, pebbles, and gravel. The thickness of the aquitard in this aquifer is 5–10 m. The confined aquifer is the primary water supply aquifer, with a groundwater level of 10 to 35 m (Gan et al. 2014, 2018; Hu and Zhou 2021).
The groundwater in the area flows slowly from west to east, which is generally consistent with the flow direction of the Yangtze River and the Han River. The groundwater recharge mainly occurs in the wet season, from rainfalls or the downward movement of surface water. Groundwater discharge to surface water, adjacent aquifers, or artificial wells mainly occurs in the dry season. The recharge and discharge of groundwater result in seasonal changes of the groundwater levels (Du et al. 2017).
It has been reported that different sedimentary environments may have an impact on the concentrations of iron and manganese in groundwater (Bondu et al. 2018; Liu and Wu 2019; Pezzetta et al. 2011; Zhang et al. 2020). The area in the north of Han River in the study area belongs to hilly-plain geomorphologie. An alluvial pluvial sedimentary environment was formed by the scouring effect of rainfall and surface runoff in front of the mountain. The area between the Yangtze River and Han River belongs to fluvial alluvial sedimentary environment. The area in the south of Yangtze River is lacustrine environment due to the lacustrine sedimentation of Dongting Lake. Region 1 (R1, located in the north of Han River), Region 2 (R2, located between the Yangtze River and Han River), and Region 3 (R3, located in the south of Yangtze River) were selected as typical areas to compare and analyze the affecting factors of iron and manganese in the three regions (Fig. 1c–e).
Data collection
Data used in this study were collected either from literature or self-tested. The information of data in this study is summarized in Table 1.
A total of four datasets were analyzed in this study. In order to reflect the reality more accurately, samples and data in the phreatic layer and confined layer were sampled or collected respectively. The samples were evenly distributed in the study area as far as possible.
For the first dataset set, 47 confined groundwater samples, which were generally evenly distributed in the whole area, were collected to examine the spatial distribution of Fe and Mn (Table S1). The second dataset includes both groundwater (Table S2; Table S3; Table S4) and sediment samples (Table S5; Table S6) from the three regions described above. The samples in the three areas were sampled and tested with the same method.
For the R1, groundwater samples and sediment samples were all self-tested. The samples were collected in September to October 2016, including 21 phreatic groundwater (PGW) and 77 confined groundwater (CGW). Groundwater samples were taken from hand-pumped wells and motor-pumped wells.
The pH, dissolved oxygen (DO), redox potential (Eh), and electrical conductivity (EC) were measured in situ. Alkalinity (as HCO3−) was measured by HCl titration and using methyl orange as a dye indicator within 24 h of sampling. The remaining 10 physicochemical parameters of NO3-N, NO2-N, Cl−, SO42−, Ca2+, K+, Mg2+, As, Fe, and Mn were analyzed in the laboratory. In order to collect the original groundwater samples, the wells and boreholes were purged by pumping until the temperature, pH, electrical conductivity (EC), and redox potential (Eh) were stable before sampling. Water samples were collected and divided into three 100 mL HDPE bottles after being filtered on site, and the samples for cation analysis were acidified to pH < 2 with concentrated HNO3. Samples were then kept in ice boxes and delivered to the laboratory within 24 h. The samples for anion and cation analysis were tested in the Three Gorges Research Center for Geohazards, China University of Geosciences. Cations (Ca2+, K+, Mg2+, As, Fe, and Mn) were determined using an inductively coupled plasma atomic emission spectrometer (ICP-AES) with the detection limit of 0.001 mg/L and the accuracy within 4%. Anions (NO3-N, NO2-N, Cl−, and SO42−) were determined using an ion chromatograph with the detection limit of 0.001 mg/L and the accuracy within 5%. In addition, 12 sediment samples were collected in 2016, including two samples from the phreatic aquifer and 10 samples from the confined aquifer. After sample collection, both ends of the sampling tubes were covered with PVC and sealed in a vacuum bag for laboratory analysis. The chemical components (total Mn and total Fe) of sediment samples were determined by X-ray fluorescence spectrometer. The samples were tested in Wuhan geological survey center of China Geological Survey. In order to ensure the analysis accuracy of sediment samples, the test center conducted three parallel test and analysis on each sample, and the test error among the three test results was less than 4%. The middle value of the three test results was taken as the final test result.
For the R2 and R3, data were collected from the literature. The spatial distribution of Fe and Mn in both phreatic aquifer groundwater (PGW) and confined aquifer groundwater (CGW) were compared, and affecting factors were analyzed.
The third dataset includes 20 groundwater in the area between the Yangtze River and Han River from 1992 to 2014 (Table S7; Table S8). The temporal distribution of Fe and Mn were examined, and affecting factors were analyzed.
The fourth dataset includes 16 groundwater in the area between the Yangtze River and Han River. Affecting factors (DOC, Opps, water level, and residence time of groundwater) were further examined at a high level of Fe and Mn in the groundwater (Table S9; Table S10; Table S11).
Data analysis
Spatial distribution of Fe and Mn in the groundwater was graphed with ArcGIS (version 10.0). The temporal distribution of Fe and Mn was examined in the area between the Yangtze River and Han River. Health risk assessment of Fe and Mn was done using method proposed by USA Environmental Protection Agency (US EPA 1989) for adults and children respectively based on Eqs. (1) and (2).
where HQ is the health risk index. RfD (mg/kg/day) is the reference dose of iron or manganese as suggested by US EPA (0.7 mg/kg/day for iron and 0.14 mg/kg/day for manganese, respectively). Dw is the proportion of groundwater as drinking water (0.564, based on Hao (2007). CDI is the chronic daily intake of iron or manganese (mg/kg/day). C is the iron or manganese concentration in groundwater (mg/L); IR is the human water ingestion rate in L/day (3.53 L/day for adults and 1.0 L/day for children); ED is the exposure duration in years (70 years for adults and 6 years for children); EF is the exposure frequency in days/year (365 days for adults and children); BW is the average body weight in kg (50 kg for adults and 15 kg for children), and AT is the averaging time (AT = 365 × ED(d)).
The distribution of Fe and Mn in the groundwater in the R1, R2, and R3 was examined using the hydro-geochemical method. PHREEQC was used to calculate the saturation index (SI) of possible minerals in the aquifers. The SI was defined as the logarithm of the ratio of the ion activity product (IAP) of component ions for a solid in solution to the solubility product (Ksp) of the solid (Liu and Wu 2019). SI was used to predict the potential of minerals to precipitate or dissolve. Based on 12 hydrogeochemical parameters of groundwater samples including, pH, redox potential (Eh), HCO3−, Ca2+, Cl−, F−, Fe, Mg2+, Mn, Na+, K+, and SO42− concentrations, the IAP and SI values of possible minerals in the groundwater of the study area were calculated. If SI value is less than zero, it indicates that groundwater is in unsaturation, and the minerals have the tendency to dissolve; if SI value is greater than zero, it indicates that groundwater is in supersaturation, and the minerals tend to precipitate.
Multivariate statistical analysis was conducted to examine affecting factors on the spatial–temporal of Fe and Mn. In order to ensure the analysis results, cation anion balance analysis and nonparametric test were carried out before data analysis. The correlation among Fe, Mn, and other physicochemical factors in the whole area and in each region were analyzed using chemical analysis and correlation analysis method (Cai and Ofterdinger 2016; Cloutier et al. 2008; Florence et al. 2012). All statistics were conducted using IBM SPSS Statistics software (version 20).
Results
Spatial and temporal distribution of Fe and Mn in the groundwater
In the whole area, 74.4% and 48.9% of the groundwater exceed the China national guideline for Fe (i.e., 0.3 mg/L) and Mn (i.e., 0.1 mg/L) (MEP 1994), respectively. The distribution of Fe and Mn was heterogeneous as their concentrations varied at different sampling sites (Fig. 3a, b). The distribution of Mn was less heterogeneous compared to that of Fe. The concentration of Mn in most of the area ranged from 0.1 to 1.5 mg/L. The concentration of Mn in about 40% of the groundwater is below 0.1 mg/L. Comparably, only 2% of the groundwater had a concentration of Mn greater than 1.5 mg/L.
Fe and Mn were detected in all samples in the three regions (Table 2). The concentration of Fe and Mn in the low plain area, particularly in the region between the Yangtze River and Han River, was relatively higher than those in other regions (Fig. 3). The concentration of Fe in the low plain was generally higher than 2.0 mg/L (Fig. 3a). The concentration of Fe in the R2 was significantly higher than those in the R1 and R3 (Table 2). This might indicate that the sedimentary environment or groundwater environmental conditions in the low plain area between the Yangtze River and the Han River were more suitable for the formation and preservation of Fe and Mn.
The vertical distribution of Fe in the groundwater was different among the three regions. For CGW, the median concentration of Fe in the CGW of R2 was 240 and 5.2 times higher than R1 and R3, respectively (Table 2). The result was consistent with the observation in the whole area where the concentration of Fe in the low plain region, between the Han River and Yangtze River, was higher than in other regions. In the R1, the median concentration of Fe of PGW was 4.67 times higher than that of CGW (Table 2). In contrast, the median concentration of Fe of PGW was 1.51 and 1.92 times lower than that of CGW in the R2 and R3, respectively (Table 2). For PGW, the median concentration of Fe of R2 was 34 and 6.6 times higher than R1 and R3, respectively (Table 2). It showed that the formation and preservation of Fe were affected not only by the sedimentary environment but also complex groundwater environment. Overall, the groundwater environment of CGW might be more beneficial to the preservation of Fe.
In all three regions, the concentrations of Mn in the PGW were higher than those in the CGW. The median concentration of Mn of PGW was 139, 4.98, and 1.14 times higher than that of CGW in the R1, R2, and R3, respectively (Table 2). For PGW, the median concentration of Mn in the R1 and R2 was 3.9 and 6.8 times higher than that of R3, respectively. For CGW, the median concentration of Mn in the R2 was 48 and 1.55 times higher than R1 and R3, respectively (Table 2). Different from Fe, the groundwater environment of PGW might be more favorable for Mn.
The concentrations of Fe and Mn changed over time (Fig. 4). The concentrations of Fe in the area between the Yangtze River and Han River were generally stable, ranging from 2.0 to 4.0 mg/L from 1992 to 2014 (Fig. 4a). The concentrations of Mn were stable, ranged from 0.1 to 0.2 mg/L from 1992 to 2010 (Fig. 4b). In addition, significant positive correlations between rainfalls and Fe (p = 0.004) concentrations and between rainfalls and Mn concentrations (p < 0.001) were found in the groundwater (Table 5), which indicated that Fe and Mn in groundwater might be affected by rainfall.
Assessment of human health risks
In this study, only oral ingestion of iron and manganese through drinking wells water were taken into consideration for adults and children health risk assessment. The results of the risk assessment (HQ) are presented in Fig. 5. HQ values of Fe and Mn were higher than 1 in around 6.38% wells (n = 3) and 2.13% wells (n = 1) (Fig. 5a). Wells with HQ values higher than 1 mainly occurred in the area between the Yangtze River and the Han River. This was consistent with the result that the concentration of iron and manganese in groundwater was higher in the area between the Yangtze River and the Han River.
Ninety percent of HQ values of Fe and Mn were lower than 0.6 (Fig. 5b), suggesting no potential non-carcinogenic health concerns. The HQ values for Fe were higher than Mn, suggesting higher health risk of Fe. In addition, health risk of Fe for adults was higher than children.
Saturation indices of Fe and Mn-bearing mineral
Modeling using PHREEQC showed that the main Fe-bearing minerals in the groundwater were goethite, hematite, and siderite (Fig. 6a), while the main Mn-bearing minerals were manganite, pyrolusite, and rhodochrosite (Fig. 6b).
The SI values of goethite (FeOOH) were all greater than zero (Fig. 6a). It indicated that the goethite in most sites of the three regions was super-saturated and trended to precipitate. Similarly, the SI values of hematite (Fe2O3) in all the three regions were greater than zero (Fig. 6a). There was no significant difference in saturation of hematite between PGW and CGW in the three regions (p > 0.05). The SI values of goethite and hematite in the PGW and CGW of R2 and R3 were higher than those of R1, indicating that goethite and hematite were less saturated in R1. Generally, the Fe-bearing minerals in the R2 and R3 were more saturated, as the median SI values of goethite and hematite following the order of R2 > R1 and R3 > R1. Although both hematite and goethite were supersaturated, the SI values of hematite were significantly greater than that of goethite, indicating that the degree of supersaturation of hematite was higher. As goethite was a hydrated iron oxide, it was less prone to supersaturation than hematite (Thompson et al. 2006), resulting in the lower degree of supersaturation. The SI values of siderite in both PGW and CGW in the R1 were all less than zero, indicating that siderite is un-saturated and trended to dissolve. In contrast, siderite was super-saturated in over 50% of the PGW and CGW in the R2 and R3 (Fig. 6a). Particularly, the SI values of siderite in all CGW of R2 were greater than 0, while the SI values of siderite in all CGW of R1 were less than zero.
The SI values of manganite (MnOOH) and pyrolusite (MnO2:H2O) were all less than zero in the three regions, indicating that the two Mn-bearing minerals were un-saturated in the groundwater and trended to dissolve. Comparably, the SI values of rhodochrosite fluctuated around 0 (Fig. 6b). In addition, the median SI values of rhodochrosite in both PGW and CGW of R2 were higher than those in R1 and R3, consistent with higher median concentrations of Mn in the R2.
Unlike the other two types of iron ore or manganese ore, both saturation and unsaturation of siderite and rhodochrosite existed in the study area. Saturation index values of siderite (FeCO3) in R1 were all less than zero, which in R2 fluctuated around zero (Fig. 6a) which was consistent with the finding of Duan (2016). This might due to the partial oxidation of R1 groundwater environment, which would promote the slow dissolution of siderite under aerobic conditions (Duckworth and Martin 2004), resulting in the unsaturated status of siderite (FeCO3) in R1. Under the reduction environment, the biodegradation produced a lot of CO2, which led to the increase of the dissolution of siderite or rhodochrosite, while the alternate redox condition in phreatic groundwater had been shown to increase iron bearing minerals crystallinity (Thompson et al. 2006), which resulted in the precipitation of some iron bearing minerals from water; therefore, the saturation index of iron bearing minerals in phreatic groundwater (PGW) was generally less than confined groundwater (CGW).
Correlation analysis
Fe and Mn were positively correlated with NH4-N in the groundwater (Table 3). A high concentration of NH4-N usually indicates a reducing environment in groundwater. It indicated that a reducing environment was conducive to Fe and Mn. The correlation coefficient between Fe and NH4-N was higher than that between Mn and NH4-N, indicating that Fe was more sensitive to redox conditions. Particularly, the concentration of Fe was significantly negatively correlated with oxidative ions, including Cl−, F−, and SO42− (Table 3). The effect of the redox condition on Fe was further verified in the R1 and R3, as Fe was significantly negatively correlated with Eh (Table S12). In contrast, there was no correlation between Eh and Mn (Table S12). In addition, there was a significant positive correlation between Fe and HCO3− and between Fe and SO42− in groundwater.
The correlation between Fe and Mn and between Fe/Mn and some chemical parameters appears to be region-specific (Table S12). For example, a significant positive correlation between Mn and Fe was observed in the R2 and R3. However, there was no correlation between Fe and Mn in the R1. This indicated that the mineral sources of Fe and Mn in groundwater in different regions may not be the same. In addition, the correlation coefficient between both Fe and HCO3− and Mn and HCO3− in R1 groundwater were negative, which in groundwater of R2 and R3 were all positive (Table S12). It was speculated that the rock mineral reactions in the two of groundwater environments might be different. For another example, NO3-N and pH were only significantly negatively correlated with Fe in R2. Similarly, EC was only significantly positively correlated with Fe in the R3. HCO3− was only significantly negatively correlated with Mn in the R1. The effect of chemical factors on Fe and Mn in the groundwater would be further discussed in the next section.
Discussion
Factors affecting the distribution of Fe and Mn
Sediments and Fe/Mn-bearing minerals
The concentration of Fe and Mn in the sediment in the study area was higher comparable to those in other studies (Liu and Wu 2019; Zhang et al. 2020). Moreover, the concentration of Fe in sediments collected from the phreatic layer was much higher than Mn, ranging from 38,982 to 62,500 mg/kg. In addition, there was no correlation between the concentrations of Fe and Mn, indicating that Fe and Mn might be sourced from different minerals (Fig. 8a–c). In this study, the main Fe-bearing minerals in the groundwater were determined as goethite, hematite, and siderite, while the main Mn-bearing minerals were manganite, pyrolusite, and rhodochrosite. Our finding was consistent with a previous study, while the primary Fe-bearing minerals were hematite and siderite (Duan et al. 2017).
The distribution of ferromanganese nodules in groundwater may directly affect the spatial distribution of Fe and Mn in the groundwater, as they are primary sources of Fe and Mn in the groundwater (Gan et al. 2014; Duan et al. 2017). In the middle area of the Yangtze Basin, ferromanganese nodules are mostly distributed in the upper aquifer (Zhang et al. 2020). A previous study showed that the sediments collected from upper aquifer contain various kinds of heavy minerals rich in Fe and Mn, such as epidote, amphibole, pyroxene, garnet, hematite and limonite, ilmenite, magnetite, and iron stained debris (Gan et al. 2014). In the R2, the averaged concentration of Fe in the sediments from the phreatic layer was higher than those collected from the confined layer (Table 4). In addition, Fe-bearing minerals in both CGW and PGW were found to be super-statured. Therefore, the release of Fe from ferromanganese minerals into groundwater may explain the observed higher concentrations of Fe in the PGW (Duckworth and Martin 2004; Thompson et al. 2006). The sediment samples at a depth of 3.8 m showed a correlation of 43% with Fe bearing silicate and 25% with Fe hydride, while the samples at a depth of 8.0 m appeared a correlation of 94% with Fe bearing silicate and 6% with hematite (Duan et al. 2017). The Fe concentration in shallow clay and silty clay (average of 47 mg/g) was much higher than that in deep sand (average of 34 mg/g) (Duan et al. 2017). Similarly, in the R1 and R2, the averaged concentration of Mn in the sediment collected from the phreatic layer was higher than that of the confined layer, which was consistent with the higher concentrations of Mn in the PGW compared to CGW.
Redox potential and pH
Physicochemical factors in the groundwater may affect the release of Fe and Mn from minerals. No significant correlation between pH and the concentrations of Fe and Mn in groundwater was observed. With the same pH values, the concentrations of Fe and Mn in R2 and R3 were significantly higher than those in R1 (Fig. 7a and b). The result is consistent with a previous finding as pH had no significant effect on the concentration of Fe and Mn in groundwater (Zhang et al. 2020).
Redox potential had a strong effect on the distribution of Fe and Mn in the groundwater (Bondu et al. 2018; Gan et al. 2014; Weng et al. 2007). With the changes of the redox environment, many elements in groundwater have a certain redox order. The values of Eh in groundwater reflect the redox property of groundwater to a certain extent. In this study, 90% of the groundwater in the R2 met Eh < 0, suggesting reducing conditions. In contrast, all the groundwater of R1 met Eh > 0, suggesting oxidized conditions (Fig. 7c–d). The levels of Fe in groundwater when Eh value was negative were significantly higher than that when Eh value was positive. In contrast, there was little difference in Mn levels in groundwater no matter Eh was positive or negative. Compared with Mn, Fe was much more sensitive to Eh values. Our result was consistent with a previous finding (Bondu et al. 2018). When it is within the normal range of pH in groundwater (i.e., 6–8), the state of Mn in groundwater almost never changes with the change of Eh. However, when Eh is greater than 0 and pH is greater than 7, Fe in groundwater will be oxidized to 3 valent and form the rainfall of Fe (OH)3, which will reduce the concentration of Fe in groundwater.
In addition, the pH-Eh diagram of groundwater (Fig. 7e–f) demonstrated that the main valence states of iron were different in groundwater of R1, R2, and R3. Fe in groundwater of R1 might be mainly in trivalent form. This could also be verified by the fact that the pond water often appeared to be maroon during the field survey in R1. Due to the reducibility of the groundwater environment, the iron in groundwater of R2 was mainly in bivalent state which was not easy to precipitate, making the Fe concentration in groundwater higher (Fig. 7e). Fe in groundwater of R3 coexisted in divalent and trivalent forms. Fe in most of PGW appeared to be trivalent form (Fig. 7f). Mn was less sensitive to the redox environment of groundwater than Fe; therefore, the levels of Mn in groundwater of the two regions did not show much difference (Fig. 7e–f).
However, Fe in amount of CGW appeared to be trivalent form, and the distribution of Fe concentrations in R2 was relatively discrete when Eh value was negative, which indicated that other factors affecting the concentration of iron in groundwater in the reduction environment might occur.
HCO 3 −
The concentrations of HCO3− and Fe in groundwater of R1 were significantly lower than that in R2 (Fig. 8d–e). It was speculated that the rock mineral reactions under the groundwater environments of R1 and R2 might be different. There was a large amount of organic matter in the sediments at the central of the plain (R2). Under mildly reducing conditions, the biodegradation of organic matter produced a large amount of CO2, which led to the increase of dissolution of carbonate rocks and iron bearing rocks, thus increasing the concentrations of HCO3− and Fe (Bondu et al. 2018; Liu and Wu 2019). This could be confirmed by the calculation results of PHREEQC. The SI values of siderite (FeCO3) in R2 and R3 fluctuated around zero (Fig. 6a), indicating that siderite might play a role in limiting Fe concentrations under mildly reducing conditions. Similar finding occurred in other research (Bondu et al. 2018). Under the mildly oxidation environment of the R1, siderite was in supersaturated state. The dissolution of CO2 in groundwater increased the concentrations of HCO3− in groundwater, which combined with Fe to form siderite, thus decreasing the Fe levels. Therefore, the correlation between Fe and HCO3− was positive in R2 and R3 but negative in R1.
In the whole area, the groundwater environment in the area where the Yangtze River and Han River met was reduced, and the biodegradation produced a lot of CO2, which led to the increase of the dissolution of siderite (Fig. 6a). Thus, the ion activity product of HCO3− and Fe and the saturation index of siderite increased which led to the super-saturation of siderite. There was a significant correlation between concentration of Fe and HCO3− in groundwater of R1 and R2. The groundwater environment was oxidized in R1, and the concentration of HCO3− of 80% samples were less than 500 mg/L. In R2, the groundwater environment was reducing, and the concentration of HCO3− of 80% of the samples were greater than 500 mg/L. Concentrations of Fe in groundwater of R2 were significantly higher than that of R1. This indicated that reducing groundwater environment and high concentration of HCO3− might be one of the controlling factors for the high concentration of Fe in R2 groundwater.
Other factors affecting the distribution of Fe and Mn in the region between the Yangtze River and the Han River
Organic matter
There was a significant positive correlation between Fe and DOC (Table 5), indicating that microbial degradation of organic matter might promote the release of Fe to groundwater. The variation trend of Fe levels with time was consistent with that of DOC concentrations no matter in 10 m, 25 m, or 50 m groundwater. The organic carbon was degraded into inorganic carbon under the reduction condition, which promoted the dissolution and release of Fe from minerals to groundwater. The values of DOC in groundwater decreased with the increase of depth, suggesting that industrial activities had a greater impact on the Fe and Mn distribution in the groundwater. The total concentrations of organic-Opps pesticides, in different depths of groundwater, did not show great difference (Fig. 9a–c). In addition, the levels of Fe and Mn in groundwater did not show obvious correlation with the total content of organic-Opps pesticides, demonstrating that agricultural activities had a limit effect on the distribution of Fe and Mn in the groundwater.
Water level and rainfalls
The significant fluctuation of surface water level is a significant feature of the hydrological cycle in the study area, which led to the seasonal recharge or discharge of groundwater (Du et al. 2018; Wang et al. 2015). The study showed that the groundwater level had a great influence on the concentration of Fe in the groundwater (Zhang et al. 2020). The variation trend of Fe concentrations in the groundwater of the three holes with time was consistent with that of groundwater levels (Fig. 10; Table 5). The iron concentration decreased significantly after the groundwater level dropped. When the groundwater level decreases, more oxygen in the atmosphere can be dissolved into the groundwater, minimizing the reducibility strength of groundwater, resulting in the decrease of Fe concentration.
Furthermore, the release of Fe and Mn from sediments into groundwater might be affected by rainfall. Rainfall can promote the dissolve of Fe and Mn from miners and increase their concentration in the groundwater (Zhang et al. 2020). A similar result was observed for the Jinzhou (Fig. 4a–b), while a significant correlation between the concentrations of Fe/Mn and rainfall was observed (Table 5).
Residence time of groundwater
A previous study conducted compaction tests to explore the effect of groundwater residence time on Fe and concentration in groundwater in the study area (Xiao 2019). The water release process of Fe and Mn during compaction was shown in Fig. 11. The initial pressure and final pressure of compaction test were set to be 0 MPa and 0.9 MPa, respectively. When the pressurization rate was set to be 0.1 MPa/24 h, the levels of iron and ferrous in pore water increased firstly and then decreased with the change of water release. The Fe-bearing minerals in the aquifer dissolved into the pore water gradually, which enhanced gradually with the reducibility of the groundwater environment. The result suggested that redox condition was the main factor affecting the Fe concentration in groundwater in the initial pressurization stage. When the groundwater environment was reductive sufficiently, other factors appeared to affect the water rock reaction.
Concentration of Fe, Fe(II), and Mn at different amount of water release in pore water a under compaction rate of 0.1 MPa/24 h, b concentration of Fe concentrations vs. water release, and c Fe(II) concentrations and water release in pore water under compaction rates of 0.1 MPa/48 h, 0.1 MPa/24 h, and 0.1 MPa/12 h
When the compaction test was carried out at different pressurization rates, the concentration of iron in pore water decreased with the increase of pressurization rate (Fig. 11a–c), indicating that the residence time of groundwater would also affect the release of iron in sediment. In the process of pore water flowing in the water bearing medium, it needs enough time for groundwater and the Fe-bearing minerals in the medium to react sufficiently to form dissolved iron (Duan et al. 2017).
Similarities with other regions
Fe and Mn was detected frequently in the groundwater from various regions. The concentration of Fe and Mn varied largely at different regions (Table 6).
Fe and Mn in the groundwater was largely controlled by the geological background. Besides, the redox environment played an important role in the migration and transformation process of Fe and Mn from sediment to groundwater. In addition, factors such as organic matter, groundwater level fluctuation, rainfalls, and residence time had certain effects on the concentration of Fe and Mn in groundwater. However, some factors showed mixed effect on the distribution of Fe and Mn concentration in the groundwater. For example, pH has no effect on the distribution of Fe in groundwater in Western Quebec, Canada (Bondu et al. 2018), which is consistent with the finding in this study. However, pH has a negative effect on the distribution of Fe in the groundwater in the Songliao plain, China (Zhang et al. 2020). Unlike Fe, Mn concentrations appear to be affected by the pH conditions because of the important control of Mn-carbonate precipitation on dissolved Mn in groundwater. However, the effect of pH on manganese was not shown in this study area, because the pH fluctuated in a small range not reaching to the alkalinity of manganese precipitation. Low redox potentials greatly affect the high dissolved Fe concentrations in groundwater in Western Quebec, Canada (Bondu et al. 2018). In contrast to Fe, Mn tends to remain dissolved under mildly oxidizing conditions, when most Fe precipitates as oxyhydroxides. This is consistent with the finding in this study that Fe in groundwater were more sensitive on the reducing environment.
Conclusion
In the middle area of the Yangtze River, the concentrations of Fe and Mn in most groundwater exceed the Chinese guideline value. 6.38% and 2.13% of the wells had iron and manganese health risks respectively, and the wells with health risk value (HQ) greater than 1 were mainly distributed in the area between the Yangtze River and the Han River. The distribution of Fe and Mn in groundwater in different sedimentary environments in the middle area of the Yangtze River was quite different. Particularly, the groundwater environment in the plain area between the Yangtze River and Han River was relatively reducing, resulting in high concentrations of Fe in groundwater and the supersaturation of siderite. The concentration of Fe and Mn in PGW was generally higher than that in CGW, which was consistent with the concentration of Fe and Mn in sediment, indicating that Fe and Mn concentration in groundwater was greatly affected by geological background.
The correlation analysis results of the three regions indicated that the rock mineral reactions in groundwater of different regions might be different. Under the reducing conditions, the biodegradation of organic matter produced a large amount of CO2, which led to the increase of dissolution of carbonate rocks and Fe-bearing minerals. Under the oxidizing environment, the dissolution of CO2 in groundwater increased the concentrations of HCO3− in groundwater, which combined with Fe to form siderite precipitation, thus decreasing the Fe and HCO3− levels.
In the geological background of high Fe and Mn in the sediments, the reducibility of the groundwater environment became the main factor affecting the concentrations of Fe and Mn in groundwater. Compared with Mn in groundwater, Fe in groundwater were more sensitive on the reducing environment. However, when the groundwater environment was reductive sufficiently, the concentration of organic matter in groundwater and the residence time of groundwater might be the important factor affecting the release of Fe in sediment.
Data availability
All data can be requested from the authors on reasonable request.
References
Aeschbach-Hertig W, Gleeson T (2012) Regional strategies for the accelerating global problem of groundwater depletion. Nat Geosci 5(12):853–861. https://doi.org/10.1038/NGEO1617
Bondu R, Cloutier V, Rosa E (2018) Occurrence of geogenic contaminants in private wells from a crystalline bedrock aquifer in western Quebec, Canada: geochemical sources and health risks. J Hydrol 559:627–637. https://doi.org/10.1016/j.jhydrol.2018.02.042
Cai Z, Ofterdinger U (2016) Analysis of groundwater-level response to rainfall and estimation of annual recharge in fractured hard rock aquifers, NW Ireland. J Hydrol 535:71–84. https://doi.org/10.1016/j.jhydrol.2016.01.066
Carretero S, Kruse E (2015) Iron and manganese content in groundwater on the northeastern coast of the Buenos Aires Province. Argentina Environ Earth Sci 73:1983–1995. https://doi.org/10.1007/s12665-014-3546-5
Chen Q, Shan B, Ma J, Gao X, Ding S, Liu W, Zhang J (2011) Spatial and temporal variation of Fe and Mn in the stormwater wetland. Environ Sci 32(05):1340–1345 ((in chinese))
Cloutier V, Lefebvre R, Therrien R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydro-geochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353(3–4):294–313. https://doi.org/10.1016/j.jhydrol.2008.02.015
Du Y, Ma T, Deng YM, Shen S, Lu ZJ (2018) Characterizing groundwater/surface-water interactions in the interior of Jianghan Plain, central China. Hydrogeol J 26(4):1047–1059. https://doi.org/10.1007/s10040-017-1709-7
Du Y, Ma T, Deng YM, Shen S, Lu ZJ (2017) Sources and fate of high levels of ammonium in surface water and shallow groundwater of the Jianghan Plain. Central China Environ Sci-Proc Imp 19(2):161–172. https://doi.org/10.1039/c6em00531d
Duan YH, Gan YQ, Wang YX, Deng YM, Guo XX, Dong CJ (2015) Temporal variation of groundwater level and arsenic concentration at Jianghan Plain, central China. J Geochem Explor 149:106–119. https://doi.org/10.1016/j.gexplo.2014.12.001
Duan YH (2016) Seasonal variations of groundwater arsenic concentration in shallow aquifers at Jianghan Plain (Doctoral dissertation). China University of Geosciences, Wuhan, China
Duan YH, Gan YQ, Wang YX, Liu CX, Yu K, Deng YM, Zhao K, Dong CJ (2017) Arsenic speciation in aquifer sediment under varying groundwater regime and redox conditions at Jianghan Plain of Central China. Sci Total Environ 607:992–1000. https://doi.org/10.1016/j.scitotenv.2017.07.011
Duckworth OW, Martin ST (2004) Role of molecular oxygen in the dissolution of siderite and rhodochrosite. Geochim Cosmochim Ac 68(3):607–621. https://doi.org/10.1016/S0016-7037(03)00464-2
Florence PL, Paulraj A, Ramachandramoorthy T (2012) Water quality index and correlation study for the assessment of water quality and its parameters of Yercaud Taluk, Salem District, Tamil Nadu. India. Chem Sci Trans 1(1):139–149. https://doi.org/10.7598/cst2012.100
Gan YQ, Wang YX, Duan YH, Deng YM, Guo XX, Ding XF (2014) Hydrogeochemistry and arsenic contamination of groundwater in the Jianghan Plain, central China. J Geochem Explor 138:81–93. https://doi.org/10.1016/j.gexplo.2013.12.013
Gan YQ, Zhao K, Deng YM, Liang X, Ma T, Wang YX (2018) Groundwater flow and hydrogeochemical evolution in the Jianghan Plain, central China. Hydrogeol J 26(5):1609–1623. https://doi.org/10.1007/s10040-018-1778-2
Hao HY (2007) Research on drinking water safety in the Jianghan plain. Rural Water Conservancy and Hydropower in China 07:31–35 ((in Chinese))
Homoncik SC, MacDonald AM, Heal KV, Dochartaigh BÉÓ, Ngwenya BT (2010) Manganese concentrations in Scottish groundwater. Sci Total Environ 408(12):2467–2473. https://doi.org/10.1016/j.scitotenv.2010.02.017
Huang GX, Sun JC, Zhang Y, Chen ZY, Liu F (2013) Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area. South China Sci Total Environ 463:209–221. https://doi.org/10.1016/j.scitotenv.2013.05.078
Huang SB, Wang YX, Ma T, Tong L, Wang YY, Liu CR, Zhao L (2015) Linking groundwater dissolved organic matter to sedimentary organic matter from a fluviolacustrine aquifer at Jianghan Plain, China by EEM-PARAFAC and hydrochemical analyses. Sci Total Environ 529:131–139. https://doi.org/10.1016/j.scitotenv.2015.05.051
Huang Y, Du Y, Ma T, Deng Y, Tao Y, Xu Y, Leng Z (2021) Dissolved organic matter characterization in high and low ammonium groundwater of Dongting Plain, central China. Ecotoxicol Environ Saf 208:111779
Hu MY, Zhou PJ (2021) Hydro-geochemical evolution characteristics of shallow groundwater in northeast of Jianghan Plain. China Carbonate Evaporite 36:71. https://doi.org/10.1007/s13146-021-00739-0
Liao XP (2016) Study of distribution, oxidation and reduction mechanisms of organophosphorus pesticides in typical area of Jianghan Plain, (Doctoral dissertation). China University of Geosciences, Wuhan, China (in Chinese)
Liu CW, Wu MZ (2019) Geochemical, mineralogical and statistical characteristics of arsenic in groundwater of the Lanyang Plain. Taiwan J Hydrol 577:123975. https://doi.org/10.1016/j.jhydrol.2019.123975
Liu HH, Yang J, Ye M, James SC, Tang ZH, Dong J, Xing TJ (2021) Using t-distributed Stochastic Neighbor Embedding (t-SNE) for cluster analysis and spatial zone delineation of groundwater geochemistry data. J Hydrol 597:126146. https://doi.org/10.1016/j.jhydrol.2021.126146
Liu JF (2017) Technical research of bio-trap and its application for exploring microbial community of degrading naphthalene in groundwater (Doctoral dissertation). China University of Geosciences, Wuhan, China (in Chinese)
Liang Y, Ma R, Wang Y, Wang S, Qu L, Wei W, Gan YQ (2020) Hydrogeological controls on ammonium enrichment in shallow groundwater in the central Yangtze River Basin. Sci Total Environ 741:140350. https://doi.org/10.1016/j.scitotenv.2020.140350
MEP (1994) Ministry of Environmental Protection of the People’s Republic of China, National Quality Standard for Ground Water (GB/T 14848–93) (in Chinese)
Niu BB, Wang HH, Loáiciga HA, Hong S, Shao W (2017) Temporal variations of groundwater quality in the Western Jianghan Plain. China Sci Total Environ 2017:542–550. https://doi.org/10.1016/j.scitotenv.2016.10.225
Pezzetta E, Lutman A, Martinuzzi I, Viola C, Bernardis G, Fuccaro V (2011) Iron concentrations in selected groundwater samples from the lower Friulian Plain, northeast Italy: Importance of salinity. Environ Earth Sci 62:377–391. https://doi.org/10.1007/s12665-010-0533-3
Qu J, Fan M (2010) The current state of water quality and technology development for water pollution control in China. Crit Rev Environ Sci Technol 40(6):519–560
Thompson A, Chadwick OA, Rancourt DG, Chorover J (2006) Iron-oxide crystallinity increases during soil redox oscillations. Geochim Cosmochim Ac 70(7):1710–1727. https://doi.org/10.1016/j.gca.2005.12.005
US EPA (1989) Superfund Public Health Evaluation Manual. Washington DC
Wang H, Jiang XW, Wan L, Han GL, Guo HM (2015) Hydro-geochemical characterization of groundwater flow systems in the discharge area of a river basin. J Hydrol 527:433–441. https://doi.org/10.1016/j.jhydrol.2015.04.063
Weng HX, Qin YC, Chen XH (2007) Elevated iron and manganese concentrations in groundwater derived from the Holocene transgression in the Hang-Jia-Hu Plain. China Hydrogeol J 15:715–726. https://doi.org/10.1007/s10040-006-0119-z
Xiao C (2019) Migration and transformation mechanism of arsenic in variable-permeability clayey aquitard at Jianghan Plain, (Doctoral dissertation). China University of Geosciences, Wuhan, China (in Chinese)
Xiao J, Jin ZD, Wang J (2014) Assessment of the hydrogeochemistry and groundwater quality of the Tarim River Basin in an extreme arid region. NW China Environ Manage 53(1):135–146. https://doi.org/10.1007/s00267-013-0198-2
Yang J, Ye M, Tang ZH, Jiao T, Song XY, Pei YZ, Liu HH (2020) Using cluster analysis for understanding spatial and temporal patterns and controlling factors of groundwater geochemistry in a regional aquifer. J Hydrol 583:124594. https://doi.org/10.1016/j.jhydrol.2020.124594
Yao LL, Wang YX, Tong L, Li YG, Deng YM, Guo W, Gan YQ (2015) Seasonal variation of antibiotics concentration in the aquatic environment: a case study at Jianghan Plain, central China. Sci Total Environ 56-64.https://doi.org/10.1016/j.scitotenv.2015.04.091
Yu K (2016) The sources and influences of dissolved organic matter on temporal variations of groundwater arsenic concentrations: a case study in Jianghan Plain, (Doctoral dissertation). China University of Geosciences, Wuhan, China (in Chinese)
Yuan RQ, Zhong YX, Long XT (2021) Comprehensive evaluation of shallow groundwater quality in the upper plain of the Dongting Lake. Water Resources Protection.1–9 (in Chinese). http://kns.cnki.net/kcms/detail/32.1356.TV.20201209.1237.005.html.
Zeng Z (1997) The background features and formation of chemical elements of groundwater in the region of the middle and lower reaches of the Yangtze River. Acta Geol Sin 71(1):80–89. https://doi.org/10.1111/j.1755-6724.1997.tb00348.x
Zhang X, Qian H, Chen J, Qiao L (2014) Assessment of groundwater chemistry and status in a heavily used semi-arid region with multivariate statistical analysis. Water 6(8):2212–2232
Zhang ZH, Xiao CL, Adeyeye O, Yang WF, Liang XJ (2020) Source and mobilization mechanism of iron, manganese and arsenic in groundwater of Shuangliao City. Northeast China Water 12(2):534. https://doi.org/10.3390/w12020534
Zhou Y, Wang YX, Li YL, Zwahlen F, Boillat J (2013) Hydrogeochemical characteristics of central Jianghan Plain. China Environ Earth Sci 68(3):765–778. https://doi.org/10.1007/s12665-012-1778-9
Acknowledgements
The authors would like to express their gratitude to the National High Technology Research and Development Program of China, the National Natural Science Foundation of China, the National Natural Science Foundation of Hubei province in China, the Open Fund of Hubei Biomass-Resource Chemistry and Environmental Biotechnology Key Laboratory, and the Fund of Eco-environment Technology R&D and Service Center (Wuhan University).
Funding
This work was financially supported by the National High Technology Research and Development Program of China (No. 2007AA06Z418), the National Natural Science Foundation of China (Nos. 20577036, 20777058, 20977070), the National Natural Science Foundation of Hubei province in China (No. 2015CFA137), the Open Fund of Hubei Biomass-Resource Chemistry and Environmental Biotechnology Key Laboratory, and the Fund of Eco-environment Technology R&D and Service Center (Wuhan University).
Author information
Authors and Affiliations
Contributions
Meiyan Hu: acquisition, analysis, and interpretation of data, drafting and revising the manuscript. Peijiang Zhou: revising the manuscript. Chaoqi Chen: revising the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible editor: Xianliang Yi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. High level of Fe was observed in a region between the Yangtze River and the Han River.
2. 6.38% and 2.13% of the wells had Fe and Mn health risks.
3. Fe in the groundwater was more sensitive to redox potential compared to Mn.
4. In a reducing environment, organic matter and residence time affect the Fe release.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, M., Zhou, P. & Chen, C. Spatial and temporal distribution and affecting factors of iron and manganese in the groundwater in the middle area of the Yangtze River Basin, China. Environ Sci Pollut Res 29, 61204–61221 (2022). https://doi.org/10.1007/s11356-022-20253-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20253-7