Abstract
Iron and manganese are two of the most common contaminants that exceed the threshold imposed by international and national legislation. When these contamination occurs in groundwater, the use of the water resource is forbidden for any purposes. Several studies investigated these two metals in groundwater, but research focused in the Central Adriatic area are still lacking. Thus, the objective of this study is to identify the origin of Fe and Mn contamination in groundwater and the hydrogeochemical processes that can enrich aquifers with these metals. This work is based on hydrogeochemical and multivariate statistical analysis of analytical results undertaken on soils and groundwater. Fe and Mn contamination are widespread in the alluvial aquifers, and their distribution is regulated by local conditions (i.e. long residence time, presence of peat or organic-rich fine sediments or anthropic pollution) that control redox processes in the aquifers and favour the mobilisation of these two metals in groundwater. The concentration of iron and manganese identified within soil indicates that the latter are a concrete source of the two metals. Anthropic impact on Fe and Mn contamination of groundwater is not related to agricultural activities, but on the contrary, the contribution of hydrocarbons (e.g. spills) is evident.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deterioration of groundwater quality, connected to both anthropogenic influences and natural processes, is becoming a source of major concern for the wider communities. Greater demands for clean water and the need to protect the environment make good water resource management an important theme for the protection of both groundwater and surface waters.
Iron and manganese are two of the most common elements in the environment, and thus, humans are exposed to significant quantities of these metals that are present in food and water. Some studies show negative health effects, at least on the neonatal population (Ritter et al. 2002; Grazuleviciene et al. 2009).
Iron is considered as non-desirable in water when it reaches concentrations of as little as 0.3 mg/l, due to its tendency to alter the water turbidity, the colour (yellow-reddish) and the taste (WHO 2003, 2006, 2011b). The health-based threshold for manganese is set to 0.4 mg/l, which is significantly higher than the concentration normally found in potable water supply (WHO 2011a). For this reason, a formal maximum concentration has never been set. Manganese alters the taste of water with concentrations as low as 0.1 mg/l. Such small concentrations also stain toilet and sink units and clothes. Therefore, the maximum acceptable concentration for manganese in water is below the health-based threshold of 0.4 mg/l (WHO 2011b).
Iron and manganese are considered contaminant in the international and national legislation (EU 1998, 2000, 2006; IR 2006, 2009; WHO 2011a), and for this reason, Concentration Limits of Contamination (CLCs) are defined for groundwater (Table 1). In particular, for the Italian legislation, when groundwater concentrations for specific parameters exceed the CLC, the site is considered potentially contaminated and the use of the water resource is forbidden for any purposes. In these cases, the site is considered potentially contaminated and additional site investigation is required to identify the origin of the contaminant. Containment and remediation are necessary if the source of contamination is anthropogenic, while they are not required when high contaminant concentrations are naturally occurring. In such cases, the natural background levels should be increased, taking into account the naturally occurring high concentrations at the site
The objective of this study is to identify the origin and the hydrogeochemical processes that are responsible for the elevated concentrations of Fe and Mn detected in groundwater, in order to correctly address the use of water resources and the management of the polluted sites.
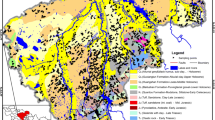
The study focused on a Central Adriatic area of about 5400 km2 in which a large amount of groundwater and soils chemical analysis were observed (Fig. 1). The geological and hydrogeological framework of the study area is similar to several Mediterranean areas; thus, principles and methodology used in this research should be of benefit for them.
Although several studies have investigated the presence of these two metals in groundwater within different geographical and geological contexts (O’Day et al. 2004; Wang et al. 2006; Root et al. 2009; Homoncik et al. 2010; Rotiroti et al. 2014; Upadhyaya et al 2014; Huang et al. 2015), studies focused on this topic, even at regional scale, are still lacking in the study area.
The presence of iron and manganese in the environment has been widely investigated in the literature. These two metals share many chemical properties and in many aspects have similar behaviour in aquatic environments (Giblin 2009). Both of them are redox-sensitive metals that are highly common in nature. Their oxidised forms are not soluble, while the reduced forms have a greater solubility (Schwab and Lindsay 1983; WHO 2003, 2011b). For this reason, iron and manganese in natural waters can be found dissolved, as colloids or as ionic or organic-metal complexes (Waber et al. 1990; Postma and Appelo 2000; Cornell and Schwertmann 2006; Appelo and Postma 2005).
Almost all rocks have some percentage of iron and manganese. Weathering processes mobilise these metals from the primary minerals (Langmuir et al. 1997; Cornell and Schwertmann 2006; Bradl 2005; Gilmour and Riedel 2009) to enrich soils and subsoil as residual phase (Shacklette and Boerngen 1984; Botes 2004; Botes and Van Staden 2007; Alloway 2013; Palmucci and Rusi 2013).
In general, metals are present in soils as free ionic forms, as organic and inorganic soluble complexes, related with organic and inorganic mobile colloidal forms, or as mineral phases. The total content in each metal is the sum of the different form present; thus, iron and manganese mobilisation is related to the correspondent form in pedogenic layer (Bowen 1979; Howe et al. 2004; Vance 1994). When soil leaching occurs, for rainfall seepage or for piezometric level raising in shallow aquifers, the most mobilisable iron and manganese forms move to groundwater (McLean and Bledsoe 1992; Ford et al. 2007).
In the satured zone, redox processes influence the quality of groundwater through the mobilisation of the metal elements that are present in the subsurface (McMahon and Chapelle 2008; Jurgens et al. 2009). Iron and manganese are two of the metals that are commonly involved in the redox reactions, which in turn determines the distribution and concentration of the metals. Reducing conditions are generally favoured by the presence of micro-organisms that facilitate the transfer of electrons between the different ions. The redox processes are heavily influenced by the organic matter oxidation (Lindsay 1991; Chen et al. 2003; Pezzetta et al. 2011; Molinari et al 2014; Rotiroti et al. 2014) and by the complexity of the hydrogeological context, with particular reference to the groundwater residence times within the aquifer (McMahon and Chapelle 2008).
The compounds that produce the largest amount of available energy are reduced first. The least favourable are subsequently reduced based on the following order:
This process continues until the electron donor and acceptor have been completely used (Berner 1981; McMahon and Chapelle 2008; Jurgens et al. 2009).
Human activities can also contribute to the release of iron and manganese into the environment as these metals are commonly used in industry, agriculture or landfilling activities (Ferraz et al. 1988; Ruijten et al. 1994; Mikac et al. 1998; WHO 2003). Further, hydrocarbon spills also contribute to the mobilisation of iron and manganese in waters as the degradation of the organic compounds favours the redox reactions that cause their mobilisation (Tucillo et al. 1999; Berbenni et al. 2000; Atekwana et al. 2005).
Geological and hydrogeological framework
The litho-structural characteristics of the study area support the identification of three distinct types of aquifers (Fig. 1).
The Apennine Chain carbonatic aquifers lie to the west. Moving to the east, these are followed by the alluvial aquifers of the intramontane basins and, finally, the alluvial aquifers of the fluvial plains that extend towards the Adriatic Sea (Desiderio and Rusi 2004; Desiderio et al. 2010; Desiderio et al. 2012; Fiorillo et al. 2015) (Fig. 1).
The limestone aquifers are constituted by limestone-dolomitic and limestone-marly successions belonging to various paleogeographic domains. These aquifers, which are dominated by secondary permeability connected to fracturing and karst, supply high-yield springs or spring lines. These springs generally show little seasonal variability and thus prove that they are fed by extensive recharge areas. The main springs are located at the margins of the aquifers where these come into contact with the aquicludes, represented by the silico-clastic deposits of the Adriatic foredeep, or with the aquitards, represented by quaternary continental deposits (Celico 1983; Boni et al. 1986; Nanni and Rusi 2003; Fiorillo et al. 2015). The main springs are generally either contact springs or depression springs, and many are captured for public water supply or hydroelectric power generation.
The intramontane alluvial aquifers are located within tectonic depressions and surrounded by limestone structures. The aquifers are constituted by terrigenous, detritic, lacustrian and alluvial deposits that originated from the weathering of the surrounding mountains and which filled the tectonic depressions since the Pliocene (Burri and Petitta 2004; Desiderio et al. 2010, 2012) (Fig. 1).
The deposits that fill the depressions are lithologically and granulometrically heterogeneous. This gives rise to multi-layered aquifers which are delimited by low permeability sediments and can locally determine semi-confined conditions (Desiderio et al. 2012). Recharge to the intramontane aquifers is from discharge from the surrounding mountains and from overlying surface water bodies. These aquifers are exploited through boreholes for civil, industrial and agricultural use.
The alluvial valley aquifers are made up of alluvial bodies constituted by levels and lenses of gravels and sands that are immersed in a silty-clayey or silty-sandy matrix. The plio-pleistocence bedrock deposits underlie and border the alluvial valley aquifers (Fig. 1). In general, these aquifers can be considered shallow phreatic aquifers; however in some areas and particularly in the coastal areas, the aquifers can become multi-layered. These aquifers can locally be semi-confined due to thick and laterally extensive low permeability deposits (Desiderio et al. 1999, 2003, 2010). The alluvial bodies are permeable due to primary porosity, which varies based on the granulometric properties of the deposits. The hydraulic conductivity of the more permeable deposits varies between 10−3 and 10−4 m/s, while hydraulic conductivities in the silty and silty-clayey deposits vary between 10−5 and 10−6 m/s (Celico 1983; Desiderio et al. 2007). Therefore, aquifer yields vary as a function of the permeability and of the thickness of the deposits, which increases moving downstream to thicknesses of up to 50 m (Desiderio et al. 1999). The alluvial valley aquifers are frequently used for civil, industrial and agricultural purposes.
The hydrochemical classification (Fig. 2) shows that samples from the Apennine limestone aquifers have a Ca-HCO3 facies with frequent enrichments of Mg and SO4. The main facies of the intramontane aquifers is Ca-HCO3 with enrichments of Na, Mg and Cl. The groundwater hydrochemistry of the alluvial valley aquifers is more variable and complex. The main hydrochemical facies is Ca-HCO3, but Ca-SO4 and Na-Cl facies are frequent.
Iron and manganese are among the most common contaminants in this hydrogeological framework. Approximately 14 % of the samples show iron contamination (i.e. concentration above the CLC), with maximum concentrations reaching 45.8 mg/l. Manganese is even more widespread with 30 % of the samples showing exceedance of the CLC and maximum concentrations of 13.6 mg/l.
Materials and methods
The groundwater chemistry was assessed through the data made available by the Regional Environment Protection Agency (Agenzia Regionale per la Tutela dell’Ambiente, ARTA, Progetto regionale “Inquinamento Diffuso”, ARTA - Abruzzo, unpublished data, 2011–2012) and by the Abruzzo Region (Piano di Tutela delle Acque, Regione Abruzzo, unpublished data, 2010–2011). Groundwater samples were collected in a monitoring network of 653 points distributed within the principal aquifer of the study area.
Approximately 2000 samples, collected between 2010 and 2012 and analysed at the ARTA laboratories, were assessed as part of this work. The electrical conductivity (EC), pH and ORP (using Ag/AgCl reference) were measured in the field with a portable multi-parameter unit. Water samples were filtered in field at 0.45 μm in nitrogen flux, collected in polyethylene bottles and stored in refrigerated containers. One fraction was acidified with HNO3 in the field after filtration for major cations and trace elements determination. Subsequently, the samples were analysed to the standard methods set out in the Italian legislation (APAT 2003). In particular, the major anions (Cl, SO4) were analysed by ion chromatography, the major cations (Ca, Mg, Na, K) by optical emission spectroscopy (ICP-OES), the bicarbonates in laboratory by HCl titration, the dissolved oxygen by iodometric titration and the trace elements, including Fe and Mn, by inductively coupled plasma mass spectrometry (ICP-MS). Finally, total hydrocarbons were analysed by IR spectroscopy, while pesticides by Gascromatografy.
A representative set of results was selected and used for charts in this study for those locations that had more than one set of results. The representative set of results was selected on the basis of the completeness of the analytical set, the ionic balance (<10 %), the timing of the sampling and the level of contamination.
A set of 360 analytical results from soils taken at 302 locations (whose distribution is shown in part 4.4) were also assessed. The soil sampling sites and the groundwater monitoring points do not overlap; however, they were executed in the same areas.
The analytical results were made available by the regional agency of agriculture (Agenzia Regionale per l’ Agricoltura). The soil samples were collected as part of an assessment programme on soil fertility (Studio regionale della fertilità dei suoli, ARSSA - Agenzia Regionale per l’ Agricoltura, unpublished data, 1999).
The soil samples were collected at depths between 40 and approximately 150 cm. The study assessed various properties of the soil and also several minor inorganic elements like iron and manganese, in terms of total and solubilisable concentration. Total concentrations were assessed through solubilisation in a heated nitric-hydrochloride solution and determination with a plasma emission spectrometer (ICP-AES). Solubilisable concentrations were also assessed with a ICP-AES following the extraction process with diethylene triamine pentacetic acid (DTPA). The iron and manganese concentrations from soils were correlated to the concentrations of the two metals within groundwater.
The large quantity of available data suggested an explorative statistical approach to address the analysis. This was undertaken by means of a principal component analysis (PCA) that was chosen, as it is one of the most powerful and used techniques to analyse the interrelationship among different sets of groundwater (Briz-Kishore and Murali 1989; Voudouris et al. 1997; Dragon 2006; Furi et al. 2012; Belkhiri and Narany 2015).
PCA was undertaken using the Varimax criteria (Kaiser 1958), and components were extracted in agreement with both the Kaiser criteria (eigenvalue >1) and with the Scree plot criteria (Cattel 1966). The parameters introduced in the factorial analysis were selected after assessing the availability, spatial frequency and role within the hydrogeochemical processes that occur in the aquifer. Temperature was not considered as its variability is related to the seasons rather than the hydrochemical processes that occur in the aquifer. The pH was excluded because this parameter, in this particular hydrogeological context, is strongly dependent on hydrochemical facies. Therefore, the statistical method used would not have allowed to individuate significant correlation with iron and manganese contamination, which is less common than water-rock interaction and salinisation processes. Furthermore, in these hydrogeological context, the pH values show a small variability (mean 7.1–7.7; standard deviation 0.3–0.4).
Subsequently, the spatial distribution of iron and manganese in groundwater was assessed to identify the most contaminated aquifers and the possible local conditions influencing Fe and Mn presence in groundwater. Groundwaters were classified, according to the criteria set out by Berner (1981), McMahon and Chapelle (2008) and Jurgens et al. (2009), in order to verify the role of redox processes in Fe and Mn contamination.
The classification permitted organising the samples on the basis of the dominating redox process (Table 2).
Given that soils are generally considered an important source of iron and manganese, a comparison between the presence of these two metals in soils and in groundwater was performed. Further, seasonal effects due to the rising of groundwater levels were analysed, in order to assess the influence of soil leaching on iron and manganese concentrations. This analysis was performed on 33 monitoring points selected for redox classification of groundwater (mixed or anoxic), water table shallower than 3 m (comparable with soil thickness) and sampling results available over different periods of the year (wet and dry season).
The possible effects of human activities on the contamination were assessed by identifying possible correlations with other contaminants that may originate from human activities. In particular, correlation with the presence of total hydrocarbons (THC) and total pesticides (TPs) in groundwater was considered.
Hydrocarbons do not include iron or manganese in their molecule. However, hydrocarbons were selected for their mostly organic nature that favour reducing conditions able to mobilise iron and manganese (Holliger and Zehnder 1996; Tucillo et al. 1999; Berbenni et al. 2000; Roychoudhury and Merrett 2006).
Pesticides were analysed due to the fact that the presence of iron and manganese can be correlated to the use of plant protection products and fertilisers.
A total of 54 different pesticides are analysed in the monitoring network, but none of them include iron and/or manganese in its molecules. However, the analysed pesticides are the most commonly used within the study area; therefore, it is believed that total pesticides can be representative of agricultural impact.
Being unable to exclude the use of agricultural products containing iron and manganese in the agricultural land use area, it was decided to carry out a qualitative comparison between the sum of the analysed pesticides (TPs) that are considered to be representative of the agricultural impact, and the content of iron and manganese.
To confirm the connection between hydrocarbons, pesticides and human activities, their presence was compared with the land use using data from Corine Land Cover project (EEA 2006).
Results and discussion
Explorative statistical approach
Given the significant amount of data involved, statistical approach was undertaken to assess the main chemistry and its relationship with iron and manganese. The physico-chemical parameters of the groundwater and the concentration of major ions are characterised by significant variability that is related to the hydrogeological context (Table 3).
The lower values of electrical conductivity (EC) are found in the limestone aquifers (327 μS/cm on average), while the highest concentrations are recorded in the alluvial valley aquifers (996 μS/cm on average) with a maximum of 8440 μS/cm. The variability of the electrical conductivity is justified by the concentration of the major ions that tend to increase from the Apennine carbonatic aquifers towards the Adriatic alluvial aquifers.
The redox potential (ORP) varies between −277 and +487 mV with negative values being present in all three types of aquifers. However, negative values are mostly found in the alluvial aquifers. The pH values are relatively constant in the limestone and intramontane aquifers, while they tend to be more variable in the alluvial valley aquifers. Temperature is an extremely variable parameter and varies as a function of water table depth and period of the year in which the sampling is undertaken.
The correlation matrix (Table 4), calculated on the basis of the chemical data described in Table 3, shows a significant positive ratio (0.80 < r < 0.82) between EC, Cl and Na. Additionally, the strong correlation between Na and Cl (r = 0.86) indicates the chemical relationship of these two analytes. Another significant correlation is that between HCO3, Mg, Ca, and SO4, with r values between 0.4 and 0.63. The correlation matrix does not show a clear relationship between iron and manganese with the other parameters because the samples affected by Fe and Mn contamination are the minority with respect to the whole dataset. Therefore, the Pearson coefficient values are low.
The PCA allows to identify three principal components (PCn) (Fig. 3) that explain almost 59 % of the total variance (Table 5). The PC1 component explains 34 % of the original variance of the dataset. It is characterised by extreme positive loadings for HCO3, Mg, Ca and SO4, and thus, it is representative of the bicarbonate-calcium groundwater which is the most common hydrochemical facies in the study area. This component can be interpreted as being representative of the groundwater-aquifer matrix interaction. The PC2 component explains 13.3 % of the variance and shows extremely high positive loadings for Na, Cl and EC. It can be interpreted as the mineralisation of the groundwater with chloride and sodium enrichment due to salinisation process that is common in the Adriatic alluvial aquifers (Nanni and Vivalda 1999; Desiderio and Rusi 2004; Desiderio et al. 2010; Palmucci and Rusi 2013, 2014; Palmucci et al. 2016).
Projection of the factor loading for the different components. PC1 vs PC2 to the left PC1 vs PC3 to the right. The black circles indicate the PC1, the black crosses indicate the PC2 and the grey squares indicate the PC3. Potassium (empty circle) is a parameter that is characterised by low loadings for all three components
The PC3 component explains 11.4 % of the variance, and it is characterised by moderately high positive loadings for Fe and Mn and significant negative loadings for NO3 and ORP. This component highlights the inverse correlation between these parameters that was not highlighted by mean of the correlation matrix.
The correlations highlighted by the PCA indicate that Fe and Mn mobility is independent from other elements, but rather, it is regulated by redox processes that occur within the aquifers.
Spatial distribution and redox zonation
The distribution of iron (Fig. 4) indicates that this element is present in all the hydrogeological domains of the study area. Concentrations in the limestone aquifers are modest (max 0.17 mg/l), and there are never exceedances of the CLC. On the contrary, iron is widespread with high concentrations (up to 45.8 mg/l) in the intramontane and alluvial valley aquifers. However, some of the Adriatic alluvial aquifers (e.g. Vibrata, Salinello, Tordino and Vomano) show iron concentration that are generally low, and only rarely, there are exceedances of the CLC. The distribution of manganese (Fig. 4) is similar to that of iron, although it is more widespread, with exceedances of legislative limits occurring even in the limestone aquifers (Table 1). Manganese reaches significant concentrations (up to 13.6 mg/l) in all alluvial bodies of the intramontane and alluvial valley aquifers. Generally, the alluvial aquifers of the Adriatic area are the ones that are mostly contaminated, and the contamination tends to be more sever towards the coastal areas of the aquifer.
Distribution of iron and manganese concentration in groundwater within the study area. Colour scheme of lithology is referred to Fig. 1
A deeper analysis of iron and manganese spatial distribution (Fig. 4) shows a high incidence of samples that exceed the CLC as well as a high percentage of sample with very low concentration (i.e. non-detects). Therefore, the percentage with intermediate concentrations, between the CLC and the limit of detection, is small. The great variability of Fe and Mn in groundwater suggests that there are local conditions controlling redox processes and the mobilisation of the two metals within the aquifers.
Iron and manganese distribution indicates that the alluvial aquifers are the most sensitive to contamination. This hypothesis is supported by the peculiar characteristics of the alluvial aquifers in terms of low permeability and long residence time, for the presence of organic matter (e.g. peats, fine sediments rich in organic matter) and because these areas are the most exposed to possible anthropogenic pressure (Desiderio et al. 2000, 2002; Caschetto et al. 2014; Rotiroti et al. 2014; Kim et al. 2014; Yadav et al. 2015).
The classification of the redox processes agrees with the spatial distribution of iron and manganese. In fact, the results shown in Table 6 indicate that the majority of the samples are in a condition of oxidation, with maximums recorded in the Apennine limestone aquifers. On the other hand, the less common conditions are the anoxic conditions recorded in less than 8 % of the samples. The samples that are in a “mixed” condition are relatively common. Amongst these, two distinct anoxic forms coexist in approximately 12 % of the samples, while in the remaining samples, there is coexistence of both oxidised and anoxic forms.
The classification of the main redox processes (Table 6) indicates that the Apennine aquifers are the least heterogeneous with a net dominance of oxygen reduction processes. On the contrary, oxygen reduction processes within the alluvial aquifers are equally spread with reduction processes of other redox species (NO3, Mn4+, Fe3+). Redox processes distribution confirms that the local hydrogeological setting influences the mobilisation of iron and manganese, and as a consequence, the groundwater contamination.
There is a net dominance of the reducing processes of NO3 and Mn4+ compared to the reducing processes of Fe3+ in both the intramontane and in the Adriatic alluvial aquifers. The dominance is due to the hydrogeologic characteristics of the assessed aquifers. These are generally water bodies of modest size, with a shallow (<25 m) water table, and it is quite uncommon that iron reduction can be established in such conditions.
Iron and manganese in soils
The analytical results indicate that all samples have total iron and manganese concentrations above 8000 and 800 mg/kg, respectively, and this confirm that these two metals are always present in the soils of the study area. However, these concentrations are not representative of the true iron and manganese quantities that can be leached to groundwater under neutral conditions.
The solubilisable concentrations are more significant. In fact, after the metal has been released into solution, it can freely move and be considered available for dissolution (Lindsay and Norvell 1978). The solubilisable fraction was determined using an extraction solution that dissolves both the exchangeable fraction (the fraction that is not soluble in water but easily absorbed by plants) and the more soluble fraction associated with organic matter present in the soil.
Soil analysis, with regard to the solubilisable concentrations, highlights that iron and manganese are commonly present in soils with maximum concentrations exceeding 20 mg/kg. In some areas, like the Fucino Plain and the Pescara Valley, the frequency of samples with high concentrations of iron and manganese is certainly higher (Fig. 5).
The statistical comparison between the presence of iron and manganese in soils and in groundwater indicates that the degree of contamination of the groundwater is not dependant exclusively on the absolute concentration of the two metals within the soil (Fig. 6).
Box plot showing the presence of iron and manganese in soils and groundwater within the study area (see Fig. 1 for the location of the aquifers)
Closer examination of the iron concentration in soils within the Vomano and Vibrata valleys indicates that these tend to be above the average, while the concentrations of the metal in the groundwater of the respective aquifers are amongst the lowest. The reasons behind these trends are to be found in the redox conditions of the aquifers and the consequent redox processes that arise from these conditions. The Vomano and Vibrata aquifers are in fact the ones with the highest number of locations with oxic conditions (68 and 91 %, respectively). Therefore, the reducing conditions needed to mobilise the iron present in the soils does not arise and, consequently, the groundwater cannot be enriched with this metal from soil leaching.
The opposite is true for the Saline River where the prevailing of reducing conditions (81 % of the monitoring locations) occurs, which can then mobilise the iron in the soils, allowing the iron migration towards the groundwater. The relationships described for iron are also applicable to manganese (Fig. 6).
The selected locations selected for assessing the seasonal variability of iron and manganese concentrations show differences between the minimum and maximum groundwater levels of 0.2 to 2.1 m. Comparison of the concentrations relative to minimum groundwater levels and the concentrations relative to maximum groundwater levels indicates that there is a significant seasonal variability at 14 of the 33 selected monitoring locations, while the remaining monitoring points do not show a significant variability of concentration because of their limited piezometric fluctuation.
The seasonal variability of concentration is connected to the rise of groundwater levels (Fig. 7) and is observed for both iron and manganese. There is no monitoring point where the increase in concentrations is observed for only one of the metals. Multi-seasonal analytical results over the period 2010 and 2011 are available for monitoring locations SL3, SL34 and TG16. The difference in concentrations between minimum and maximum groundwater levels is confirmed for both years at these monitoring locations.
In summary, rising groundwater levels during the wet season are responsible for increases in iron and manganese concentrations at many of the assessed monitoring locations. This is probably due to a higher degree of leaching from soils and the consequent mobilisation of oxides and hydroxides of iron and manganese that are present in the soil. Once mobilised, the solid phases of the two metals can be solubilised due to the reducing conditions of the groundwater which cause the concentrations of iron and manganese to increase.
Possible anthropogenic influences
Hydrocarbons and pesticides were selected to assess the anthropogenic contribution to iron and manganese contamination of groundwater, since they can be representative of human activities such as agriculture and fuel storage or distribution. The comparison between the presence of hydrocarbons and pesticides with land use (EEA 2006) confirms that the polluted monitoring points are located in agricultural or anthropic areas (Fig. 8). The intramontane and the Adriatic alluvial aquifers, mainly affected by agricultural and anthropic activities, are the most polluted areas.
The presence of hydrocarbons within groundwater is relatively limited in the study area. In fact, their presence is detected in only 7 % of the samples, and in less than 1 % of the samples, the concentrations exceed the legislative limits. The possible correlation with iron and manganese contamination was investigated by considering all the samples in which concentration of THC exceeded the limit of detection (Fig. 9). The reducing conditions chart indicates that higher concentrations of iron and manganese often coincide with higher concentrations of hydrocarbons. In the observed samples, iron concentrations remain below the CLC when total hydrocarbon concentrations are below 200 μg/l, while manganese concentrations quite often exceed the CLC. This behaviour probably depends on the reducing condition that moderate hydrocarbon concentrations could determinate. It is reasonable to assume that the degradation of moderate quantities of hydrocarbon cannot provide the amount of electrons necessary to reduce iron. Thus, in these cases, iron is not mobilised and its concentration in groundwater remains low.
High concentrations of iron and manganese occur at lower concentrations of hydrocarbons (THC < 100 μg/l in the analysed samples), and this is probably connected to the rise of anoxic conditions that are independent from the presence of hydrocarbons (e.g. presence of peat or long residence time).
With oxidising conditions, the concentration of THC is always observed to be low except for one sample (TR16).
Ultimately, although hydrocarbons are not a direct source of Fe and Mn, their presence is indicative of anthropogenic influences that favour the reducing conditions for the release of these two metals, providing electron donors from the oxidation of the organic matter. Therefore, it can be inferred that Fe and Mn contamination is related to anthropogenic influence for those samples that show high concentrations of THC.
Similar observations can be made with the presence of pesticides. In fact, pesticides are observed at only 4 % of the monitoring locations. Total pesticides (TPs) within samples are above the legislative limits at only 2 % of the monitoring locations.
The charts (Fig. 10) show that Fe and Mn concentrations exceed their respective CLC at some of the locations where pesticides were detected. However, the Fe content is not related to the concentration of pesticides. In fact, iron is constantly below the detection limit for those points on the left of the chart corresponding to the highest concentrations of pesticides. Even for manganese (Fig. 10), clear correlation with pesticides cannot be identified; in fact, manganese exceeds CLCs at both low and high concentrations of pesticides. Although iron and manganese are not present in the molecules of the analysis pesticides, these are believed to be representative of the agricultural impact. For this reason, since there is no correlation between iron and manganese and the presence of pesticides, it can be assumed that agricultural activities do not significantly affect the content of the two metals in groundwater.
Summarising, the assessment of anthropic impact on iron and manganese contamination of groundwater suggests that there is no connection with the use of pesticides and fertilisers and, more generally, with agriculture. On the contrary, anthropic influence related with hydrocarbons pollution is evident. This is due to the redox conditions that arise following the presence of hydrocarbons, although they are not a direct source of Fe and Mn.
Conclusions
A conceptual model that explains the hydrogeochemical processes that govern iron and manganese concentrations in groundwater was derived from the processing and assessment of the data used for this work.
Both the hydrogeochemical and the statistical analyses highlight a general independence of iron and manganese contaminations from the principal ions and, more generally, from the groundwater chemistry. On the contrary, a clear inverse correlation with nitrate and ORP suggests the main role of redox processes.
The spatial distribution of the two metals indicates that the alluvial aquifers, both intramontane and Adriatic, are the most sensitive to Fe and Mn contamination, although their distribution is irregular (patchy).
Also, the redox zonation confirms that reduction of iron and manganese takes place almost only in the alluvial aquifers. These processes are regulated by local conditions that are long residence time, presence of peat or organic-rich fine sediments and anthropic pollution, which are able to mobilise these two.
The concentration of iron and manganese identified within soil indicates that the latter is a concrete source of the two metals. However, also in this case, the local conditions regulate Fe and Mn mobility in groundwater after soil leaching.
The presence of point contamination connected to direct anthropogenic sources of Fe and Mn contamination (industrial discharges, leachate from landfills, etc.) will clearly require site-specific investigations that will need to assess the redox conditions within the aquifer. The evolution over time of a contamination, and therefore its persistence, will depend on the redox conditions within the aquifer or induced by the contamination itself.
Summing up, research results suggest that the elevated iron and manganese concentration not necessarily depend on anthropic pollution, but rather on the local conditions that favour redox processes. When Fe and/or Mn CLC exceedances are detected, it will be necessary to investigate aquifer’s hydrodynamic (i.e. residence time) and the presence of natural (e.g. peat) and/or anthropic (e.g. oil spills) organic matter that, all together, determinate the reducing condition needed to mobilise iron and manganese.
Thus, two cases can occur: the first one, in which high concentrations of Fe and Mn in groundwater, with CLC exceedances, are not related to anthropic pollution but rather to natural occurring iron and manganese (i.e. in soils) mobilised due to redox processes occurrence; and the second one, related to Fe and Mn releases caused by anthropic pollution. In this case, the presence of iron and manganese, clearly originated from anthropic pollution, can give CLC exceedances, if the redox condition, needed for these two metals mobilisation, will occur in groundwater. However, if the redox processes do not arise, there would not be necessarily CLC exceedances of Fe and Mn in groundwater, even though the anthropic pollution.
The dealt issues allow to assess the contribution of anthropic impact, in order to properly address water management policies and, in particular, whether to consider a site polluted or to derivate Fe and Mn natural background levels suitable for the different aquifers.
References
Alloway BJ (2013) Heavy metals in soils. Trace metals and metalloids in soil and their bioavailability. Environmental Pollution 22. doi:10.1007/978-94-007-4470-7
APAT, IRSA-CNR (2003) Metodi analitici per le acque [Analytical methods for water]. Manuali e Linee Guida, 29/2003. Available via dialog: http://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/metodi-analitici-per-le-acque
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. A.A. Balkema Publishers, Laiden
Atekwana EA, Atekwana E, Legall FD, Krishnamurthy RV (2005) Biodegradation and mineral weathering controls on bulk electrical conductivity in a shallow hydrocarbon contaminated aquifer. J Contam Hydrol 80(3):149–167. doi:10.1016/j.jconhyd.2005.06.009
Belkhiri L, Narany TS (2015) Using Multivariate Statistical Analysis, Geostatistical Techniques and Structural Equation Modeling to Identify Spatial Variability of Groundwater Quality. Water Resour Manag 29(6):2073–2089
Berbenni P, Pollice A, Canziani R, Stabile L, Nobili F (2000) Removal of iron e manganese from hydrocarbon-contaminated groundwaters. Bioresour Technol 74(2):109–114. doi:10.1016/S0960-8524(00)00003-1
Berner RA (1981) A new geochemical classification of sedimentary environments. J Sediment Res 51(2)
Boni C, Bono P, Capelli G (1986) Schema idrogeologico dell’Italia centrale [Hydrogeological scheme of central Italy]. Mem Soc Geol Ital 35:991–1012
Botes PJ (2004) Investigation of mobility trace elements in river sediments using ICP-OES. University of Pretoria Edition
Botes PJ, Van Staden JF (2007) Investigation of trace element mobility in river sediments using ICP-OES. Water SA 31(2):183–192
Bowen HJM (1979) Environmental Chemistry of the Elements, vol 333. Adacemic Press, London
Bradl H (2005) Heavy metals in the environment: origin, interaction e remediation (Vol. 6). Academic Press
Briz-Kishore BH, Murali G (1989) Factor analysis for revealing hydrochemical characteristics of a watershed. Environ Geol 19(1):3–9. doi:10.1007/BF01740571
Burri E, Petitta M (2004) Agricultural changes affecting water availability: from abundance to scarcity (Fucino Plain, central Italy). Irrig Drain 53(3):287–299. doi:10.1002/ird.119
Caschetto M, Barbieri M, Galassi DMP, Mastrorillo L, Rusi S, Stoch F, Di Cioccio A, Petitta M (2014) Human alteration of groundwater–surface water interactions (Sagittario River, Central Italy): implication for flow regime, contaminant fate e invertebrate response. Environ Earth Sci 71(4):1791–1807. doi:10.1007/s12665-013-2584-8
Cattel RB (1966) The screen test for the number of factors. Multivar Behav Res 1:245–276. doi:10.1207/s15327906mbr0102_10
Celico P (1983) Idrogeologia dell’Italia centro meridionale [Hydrogeology of central-southern Italy]. Quaderni della Cassa per il Mezzogiorno 4/2
Chen J, Gu B, Royer RA, Burgos WD (2003) The roles of natural organic matter in chemical and microbial reduction of ferric iron. Sci Total Environ 307(1):167–178
Cornell RM, Schwertmann U (2006) The iron oxides: structure, properties, reactions, occurrences and uses. John Wiley & Sons
Desiderio G, Rusi S (2004) Idrogeologia e idrogeochimica delle acque mineralizzate dell’avanfossa abruzzese molisana [Hydrogeology e hydrochemistry of the mineralized waters of the Abruzzo e Molise foredeep (Central Italy)]. Boll Soc Geol Ital 123(3):373–389
Desiderio G, Nanni T, Rusi S (1999) Gli acquiferi delle pianure alluvionali centro adriatiche [The aquifers of the central Adriatic alluvial plains]. Quaderni di Geologia Applicata 2:21–30
Desiderio G, Nanni T, Rusi S (2000) La pianura alluvionale del fiume Pescara (Abruzzo): idrogeologia e vulnerabilità dell’acquifero [The alluvial plain of Pescara river (Abruzzo): hydrogeology and aquifer vulnerability]. Mem Soc Geol Ital 56:197–211
Desiderio G, Nanni T, Rusi S (2002) Idrogeologia e qualità delle acque degli acquiferi della conca intramontana di Sulmona (Abruzzo). Atti I convegno AIGA, 315-342
Desiderio G, Nanni T, Rusi S (2003) La pianura del fiume Vomano (Abruzzo): idrogeologia, antropizzazione e suoi effetti sul depauperamento della falda [The Vomano river plain (Abruzzo-central Italy): hydrogeology, anthropic evolution and its effects on the depletion of the unconfined aquifer]. Boll Soc Geol Ital 122(3):421–434
Desiderio G, Ferracuti L, Rusi S (2007) Structural-Stratigraphic of Middle Adriatic Alluvial Plains e its Control on Quantitative e Qualitative Groundwater Circulation. Mem Descr Carta Geol d’It 76:147–162
Desiderio G, Rusi S, Tatangelo F (2010) Caratterizzazione idrogeochimica delle acque sotterranee abruzzesi e relative anomalie [Hydrogeochemical characterization of Abruzzo groundwaters and relative anomalies]. Ital J Geosci 129(2):207–222. doi:10.3301/IJG.2010.05
Desiderio G, D’arcevia CFV, Nanni T, Rusi S (2012) Hydrogeological mapping of the highly anthropogenically influenced Peligna Valley intramontane basin (Central Italy). J Maps 8(2):165–168. doi:10.1080/17445647.2012.680778
Dragon K (2006) Application of factor analysis to study contamination of a semi-confined acquifer (Wielkopolska buried valley acquifer, Poland). J Hydrol 331:272–279. doi:10.1016/j.jhydrol.2006.05.032
EEA - European Environment Agency (2006) Corine Land Cover. http://www.eea.europa.eu/publications/COR0-landcover
EU - European Union (1998) Direttiva 98/83/CE del Consiglio del 3 novembre 1998 concernente la qualità delle acque destinate al consumo umano. http://eur-lex.europa.eu/legal-content/IT/ALL/?uri = CELEX:31998L0083
EU – European Union (2000) Direttiva 2000/60/CE, Direttiva 2000/60/CE del Parlamento europeo e del Consiglio, del 23 ottobre 2000, che istituisce un quadro per l’azione comunitaria in materia di acque. http://eur-lex.europa.eu/legal-content/IT/ALL/?uri = CELEX:32000L0060
EU - European Union (2006) Direttiva 2006/118/CE, pubblicata sulla GU dell’UE il 27.12. 06, recante nuove misure sulla protezione delle acque sotterranee ad integrazione della direttiva quadro 2000/60/CE. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri = OJ:L:2006:372:0019:0031:IT:PDF
Ferraz HB, Bertolucci PHF, Pereira JS, Lima JGC, Andrade LAF (1988) Chronic exposure to the fungicide maneb may produce symptoms e signs of CNS manganese intoxication. Neurology 38(4):550–553. doi:10.1212/WNL.38.4.550
Fiorillo F, Petitta M, Preziosi E, Rusi S, Esposito L, Tallini M (2015) Long-term trend and fluctuations of karst spring discharge in a Mediterranean area (central-southern Italy). Environ Earth Sci 74:153–172. doi:10.1007/s12665-014-3946-6
Ford RG, Wilkin RT, Puls RW (2007) Monitored natural attenuation of inorganic contaminants in ground water volume 1 - technical basis for assessment. National Risk Management Research Laboratory Office of Research and Development. US Environmental Protection Agency, Cincinnati
Furi W, Razack M, Abiye TA, Kebede S, Legesse D (2012) Hydrochemical characterization of complex volcanic aquifer in a continental rifted zone: the Middle Awash basin. Etiopia Hydrogeol J 20:385–400. doi:10.1007/s10040-011-0807-1
Giblin AE (2009) Iron and manganese. in Chief, Encyclopedia of Inland Waters: Elsevier Press, pp 35-44
Gilmour C, Riedel G (2009) Biogeochemistry of Trace Metals e Metalloids. in Chief, Encyclopedia of Inland Waters: Elsevier Press, pp 7-15. doi:10.1016/B978-012370626-3.00095-8
Grazuleviciene R, Nadisauskiene R, Buinauskiene J, Grazulevicius T (2009) Effects of elevated levels of manganese and iron in drinking water on birth outcomes. Pol J Environ Stud 18(5):819–825
Holliger C, Zehnder AJ (1996) Anaerobic biodegradation of hydrocarbons. Curr Opin Biotechnol 7(3):326–330. doi:10.1016/S0958-1669(96)80039-5
Homoncik SC, MacDonald AM, Heal KV, Dochartaigh BÉÓ, Ngwenya BT (2010) Manganese concentrations in Scottish groundwater. Sci Total Environ 408(12):2467–2473
Howe PD, Malcolm HM, Dobson S (2004) Manganese and its compounds: environmental aspects. Concise international chemical assessment document
Huang B, Li Z, Chen Z, Chen G, Zhang C, Huang J, Nie X, Xiong W, Zeng G (2015) Study and health risk assessment of the occurrence of iron and manganese in groundwater at the terminal of the Xiangjiang River. Environ Sci Pollut Res 1-10, in press
IR - Italian Republic (2006) Decreto Legislativo 3 Aprile 2006, n. 152.“ Norme in materia ambientale”. Gazzetta Ufficiale Repubblica Italiana n. 88 del 14/06/2006. http://www.camera.it/parlam/leggi/deleghe/06152dl.htm
IR - Italian Republic (2009) Decreto Legislativo 16 Marzo 2009, n. 30. Attuazione della direttiva 2006/118/CE, relativa alla protezione delle acque sotterranee dall’inquinamento e dal deterioramento. Gazzetta Ufficiale Repubblica Italiana n. n.79 del 4-4-2009. http://www.camera.it/parlam/leggi/deleghe/09030dl.htm
Jurgens BC, McMahon PB, Chapelle FH, Eberts SM (2009) An Excel workbook for identifying redox processes in ground water. U. S. Geological Survey Open-File Report 2009-1004; USGS: Reston, VA
Kaiser HF (1958) The varimax criterion for analytic rotation in factor analysis. Psychometrka 23:187–200. doi:10.1007/BF02289233
Kim DM, Yun ST, Kwon MJ, Mayer B, Kim KH (2014) Assessing redox zones e seawater intrusion in a coastal aquifer in South Korea using hydrogeological, chemical e isotopic approaches. Chem Geol 390:119–134. doi:10.1016/j.chemgeo.2014.10.024
Langmuir D, Hall P, Drever J (1997) Environmental Geochemistry. Prentice Hall, New Jersey
Lindsay WLM (1991) Iron oxide solubilization by organic matter e its effect on iron availability. In Iron nutrition and interactions in plants. Springer Netherlands, pp 29-36
Lindsay WLM, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, e copper. Soil Sci Soc Am J 42(3):421–428. doi:10.2136/sssaj1978.03615995004200030009x
McLean and Bledsoe (1992) McLean JE, Bledsoe BE (1992) Behavior of Metals in Soils. US EPA Ground Water Issue
McMahon PB, Chapelle FH (2008) Redox processes e water quality of selected principal aquifer systems. Ground Water 46(2):259–271. doi:10.1111/j.1745-6584.2007.00385.x
Mikac N, Cosovic B, Ahel M, Andreis S, Toncic Z (1998) Assessment of groundwater contamination in the vicinity of a municipal solid waste landfill (Zagreb, Croatia). Water Sci Technol 37(8):37–44. doi:10.1016/S0273-1223(98)00233-9
Molinari A, Ayora C, Marcaccio M, Guadagnini L, Sanchez-Vila X, Guadagnini A (2014) Geochemical modeling of arsenic release from a deep natural solid matrix under alternated redox conditions. Environ Sci Pollut Res 21(3):1628–1637
Nanni T, Rusi S (2003) Idrogeologia del massiccio carbonatico della montagna della Majella (Appennino centrale) [Hydrogeology of the «montagna della majella» carbonate massif (Central Apennines-Italy)]. Boll Soc Geol Ital 122(2):173–202
Nanni T, Vivalda P (1999) Le acque salate dell’Avanfossa marchigiana; origine, chimismo e caratteri strutturali delle zone di emergenza [The salt waters of the Marche foredeep: origin, chemistry and structural characters of the emergency zones]. Boll Soc Geol Ital 118(1):191–215
O’Day PA, Vlassopoulos D, Root R, Rivera N (2004) The influence of sulfur and iron on dissolved arsenic concentrations in the shallow subsurface under changing redox conditions. Proc Natl Acad Sci U S A 101(38):13703–13708
Palmucci W, Rusi S (2013) Origin and distribution of Iron, Manganese and Boron in the Abruzzo region groundwaters. Hydrogeochemical survey on the Saline sample area. Rend Online Soc Geol Ital 22:222–224
Palmucci W, Rusi S (2014) Boron-rich groundwater in Central Eastern Italy: a hydrogeochemical e statistical approach to define origin e distribution. Environ Earth Sci 72(12):5139–5157. doi:10.1007/s12665-014-3384-5
Palmucci W, Rusi S, Tatangelo F (2016) Ring maps applied to hydrogeological and environmental studies in alluvial aquifers, central Italy. J Maps 12(1):33–44. doi:10.1080/17445647.2014.977973
Pezzetta E, Lutman A, Martinuzzi I, Viola C, Bernardis G, Fuccaro V (2011) Iron concentrations in selected groundwater samples from the lower Friulian Plain, northeast Italy: importance of salinity. Environ Earth Sci 62(2):377–391. doi:10.1007/s12665-010-0533-3
Postma D, Appelo CAJ (2000) Reduction of Mn-oxides by ferrous iron in a flow system: column experiment e reactive transport modeling. Geochim Cosmochim Acta 64(7):1237–1247. doi:10.1016/S0016-7037(99)00356-7
Ritter L, Solomon K, Sibley P, Hall K, Keen P, Mattu G, Linton B (2002) Sources, pathways, e relative risks of contaminants in surface water e groundwater: a perspective prepared for the Walkerton inquiry. J Toxicol Environ Health A 65(1):1–142. doi:10.1080/152873902753338572
Root RA, Vlassopoulos D, Rivera NA, Rafferty MT, Andrews C, O’Day PA (2009) Speciation and natural attenuation of arsenic and iron in a tidally influenced shallow aquifer. Geochim Cosmochim Acta 73(19):5528–5553. doi:10.1016/j.gca.2009.06.025
Rotiroti M, Sacchi E, Fumagalli L, Bonomi T (2014) Origin of Arsenic in Groundwater from the Multilayer Aquifer in Cremona (Northern Italy). Environ Sci Technol 48(10):5395–5403. doi:10.1021/es405805v
Roychoudhury AN, Merrett GL (2006) Redox pathways in a petroleum contaminated shallow sandy aquifer: iron e sulfate reductions. Sci Total Environ 366(1):262–274. doi:10.1016/j.scitotenv.2005.10.024
Ruijten MWMM, Sall HJA, Verberk MM, Smink M (1994) Effect of chronic mixed pesticide exposure on peripheral e autonomic nerve function. Arch Environ Health 49(3):188–195
Schwab AP, Lindsay WL (1983) The effect of redox on the solubility e availability of manganese in a calcareous soil. Soil Sci Soc Am J 47(2):217–220. doi:10.2136/sssaj1983.03615995004700020008x
Shacklette HT, Boerngen JG (1984) Element concentrations in soils e other surficial materials of the conterminous United States; Professional Paper 1270; U.S. Geological Survey, United States Printing Office: Washington, DC, 1984; p 103
Tucillo ME, Cozzarelli IM, Herman JH (1999) Iron reduction in the sediments of a hydrocarbon-contaminated aquifer. Appl Geochem 14(5):655–667. doi:10.1016/S0883-2927(98)00089-4
Upadhyaya D, Survaiya MD, Basha S, Mandal SK, Thorat RB, Haldar S, Goel S, Dave H, Baxi K, Trivedi RH, Mody KH (2014) Occurrence and distribution of selected heavy metals and boron in groundwater of the Gulf of Khambhat region, Gujarat, India. Environ Sci Pollut Res 21(5):3880–3890
Vance D (1994) Iron: the environmental impact of a universal element. Natl Environ J 4(3):24–25
Voudouris K, Lambrakis N, Papatheodorou G, Daskalaki P (1997) An application of factor analysis for the study of the hydrogeological conditions in Plio-Pleistocene aquifers of NW Achaia (NW Peloponnesus, Greece). Math Geol 29(4):43–59. doi:10.1007/BF02769619
Waber UE, Lienert C, Von Gunten HR (1990) Colloid-related infiltration of trace metals from a river to shallow groundwater. J Contam Hydrol 6(3):251–265. doi:10.1016/0169-7722(90)90020-H
Wang L, Meng XX, Xu HE (2006) Analysis of causes of superstandard Fe and Mn content in source water of catchment areas in Jiamusi City. Environ Sci Manag 1:053
WHO - World Health Organization (2003) Iron in drinking water. WHO Press, Geneva. http://www.who.int/water_sanitation_health/dwq/chemicals/iron.pdf
WHO - World Health Organization (2006) Guidelines for drinking-water quality. http://www.who.int/water_sanitation_health/dwq/gdwq0506.pdf
WHO - World Health Organization (2011a) Guidelines for drinking-water quality. http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/
WHO - World Health Organization (2011b) Manganese in drinking water. WHO Press, http://www.who.int/water_sanitation_health/dwq/chemicals/manganese.pdf
Yadav IC, Devi NL, Singh S (2015) Reductive dissolution of iron-oxyhydroxides directsgroundwater arsenic mobilization in the upstream of Ganges River basin, Nepal. J Geochem Explor 148:150–160
Acknowledgments
The authors wish to thank the Regione Abruzzo “Servizio Qualità delle Acque” and the Agenzia Regionale per la Tutela dell’Ambiente (ARTA) – “Progetto Inquinamento Diffuso” for making the groundwater analytical results available. The authors also wish to thank the Agenzia Regionale per i Servizi di Sviluppo Agricolo in Abruzzo (ARSSA) for making the soil analytical results available for this study. The authors are also grateful to reviewers for their comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Palmucci, W., Rusi, S. & Di Curzio, D. Mobilisation processes responsible for iron and manganese contamination of groundwater in Central Adriatic Italy. Environ Sci Pollut Res 23, 11790–11805 (2016). https://doi.org/10.1007/s11356-016-6371-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6371-4