Abstract
Chronic elevated nitrogen (N) deposition has altered the N status of temperate forests, with significant implications for ecosystem function. The Bear Brook Watershed in Maine (BBWM) is a whole paired watershed manipulation experiment established to study the effects of N and sulfur (S) deposition on ecosystem function. N was added bimonthly as (NH4)2SO4 to one watershed from 1989 to 2016, and research at the site has studied the evolution of ecosystem response to the treatment through time. Here, we synthesize results from 27 years of research at the site and describe the temporal trend of N availability and N mineralization at BBWM in response to chronic N deposition. Our findings suggest that there was a delayed response in soil N dynamics, since labile soil N concentrations did not show increases in the treated watershed (West Bear, WB) compared to the reference watershed (East Bear, EB) until after the first 4 years of treatment. Labile N became increasingly available in WB through time, and after 25 years of manipulations, treated soils had 10× more extractable ammonium than EB soils. The WB soils had 200× more extractable nitrate than EB soils, driven by both, high nitrate concentrations in WB and low nitrate concentrations in EB. Nitrification rates increased in WB soils and accounted for ~ 50% of net N mineralization, compared to ~ 5% in EB soils. The study provides evidence of the decadal evolution in soil function at BBWM and illustrates the importance of long-term data to capture ecosystem response to chronic disturbance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic inputs of reactive nitrogen (N) in the twentieth and twenty-first centuries have altered the global N cycle (Galloway et al. 2008; Li et al. 2016). Forests typically considered N-limited have exhibited signs of different stages of N saturation (Aber et al. 2003; Liu et al. 2011; Niu et al. 2016). Atmospheric deposition is the primary source of anthropogenic N to forest systems (Galloway et al. 2008; Schlesinger 2009). While national and global efforts to reduce anthropogenic N emissions have been effective in some locations, some forests in the USA and Europe still experience relatively high levels of N deposition, with significant biogeochemical implications (Davidson et al. 2011; Schöpp et al. 2003).
Chronic elevated N deposition has been found to alter N availability in soils, with increased N losses via leaching (Lovett and Goodale 2011; Lu et al. 2011; Templer et al. 2012a) and denitrification (Templer et al. 2012b; Morse et al. 2015). N enrichment accelerates base cation leaching and mobilizes aluminum, increasing ecosystem vulnerability to acidification (Fernandez et al. 2003; Lucas et al. 2011). Both elevated N deposition and acidification can increase tree stress and fine root mortality (Gundersen et al. 1998; Smithwick et al. 2013; Ferretti et al. 2015; Minocha et al. 2015) and can decrease soil microbial biomass and alter microbial community structure (Treseder 2008; Kopáček et al. 2013; Carrara et al. 2018). Soil organic matter (SOM) decomposition is suppressed under high N conditions, which can increase terrestrial carbon (C) stocks (Frey et al. 2014; Van Diepen et al. 2017).
Net N mineralization (NNM) and net nitrification (NN) are frequently used as indices of soil N dynamics and availability that are relevant to ecosystem function. Soil NNM and NN represent the balance between gross NNM/nitrification and immobilization and are typically measured using in situ or laboratory incubations for a short period of time (Hart et al. 1994; Verchot et al. 2001). Soil denitrification would also be included in the immobilization calculation, but denitrification rates are typically considered low in aerated, acidic soils (McClain et al. 2003). Morse et al. (2015) reported annual denitrification rates as low as 0.3 kg N ha−1 in northeastern USA forests, although in some instances they found denitrification to be up to ~ 20% of annual N inputs.
Soil C:N ratio, vegetation type, temperature, and moisture are important controls on NNM and NN rates. High soil N concentrations (low C:N ratios) stimulate production of ammonium (NH4+-N) and nitrate (NO3−-N) (Aber et al. 2003; Ross et al. 2009; Liu et al. 2017). Deciduous forest soils typically exhibit higher nitrification rates compared to coniferous soils, because of the higher quality of deciduous litter (Lovett et al. 2004; Ross et al. 2009). Microbial activity generally increases with temperature and moisture up to ideal conditions, and therefore, so do NNM and NN rates (Knoepp and Swank 2002; Gilliam et al. 2015). NNM has been used to study soil response to disturbances, such as land use changes (Goodale and Aber 2001; Burns and Murdoch 2005), frost damage (Shibata 2016; Sorensen et al. 2016), and climate change (Knoepp and Vose 2007; Contosta et al. 2011). NNM and NN rates generally increase in N-enriched systems, and these indices have been especially useful in assessing the progression of ecosystems towards N saturation (Aber et al. 1998; Gundersen et al. 1998; Gilliam et al. 2001, 2018; Lovett and Goodale 2011; Lu et al. 2011).
The Bear Brook Watershed in Maine (BBWM) is one of the few long-term whole-ecosystem manipulation experiments in the world examining the effects of chronic, elevated N deposition on temperate forest ecosystem function on a decadal time scale. Since N additions began in 1989, studies conducted at BBWM have reported increased soil N concentrations (Wang and Fernandez 1999; Jefts et al. 2004; Fernandez et al. 2010; SanClements et al. 2010); increased stream nitrate exports (Fernandez et al. 2010; Simon et al. 2010); increased foliar N (Elvir et al. 2005, 2006); increased base cation leaching (Fernandez et al. 2010); shifts in microbial community composition and enzyme activity (Wallenstein et al. 2006; Stone et al. 2012; Mineau et al. 2014; Fatemi et al. 2016; Tatariw et al. 2018); and changes in SOM composition and decomposition rates (Ohno et al. 2007; Hunt et al. 2008). The bimonthly N treatments at BBWM were discontinued in October 2016, marking the start of the “recovery” period in the treated watershed. Studies at BBWM have examined soil NNM and NN rates and labile inorganic N in response to N enrichment and acidification (Wang and Fernandez 1999; Fernandez et al. 2000; Jefts et al. 2004). In this paper, we synthesize results from past measurements of soil N dynamics at BBWM including both published results and unpublished data in order to evaluate potential multi-decadal patterns associated with experimental N enrichment.
Methods
Site description
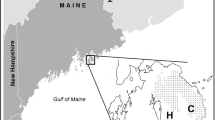
The BBWM (Fig. 1) is a long-term whole watershed acidification experiment in eastern Maine, USA (44° 52′ N, 68° 06′ W) established to study the effects of elevated N and S deposition on ecosystem processes. BBWM is comprised of two paired watersheds, the reference East Bear Brook (EB, 11.0 ha) and the manipulated West Bear Brook (WB, 10.3 ha), which was treated with bimonthly applications of ammonium sulfate [(NH4)2SO4] fertilizer from above the canopy at the rate of 28.8 kg S ha−1 year−1 and 25.2 kg N ha−1 year−1 from 1989 through 2016.
Elevation ranges from 210 to 475 m at the site. Vegetation is similar in both watersheds, with lower elevations dominated by deciduous species including Fagus grandifolia Ehrh. (American beech), Acer saccharum Marsh. (sugar maple), and Acer rubrum L. (red maple), and higher elevations dominated by coniferous species including Picea rubens Sarg. (red spruce) and Abies balsamea L. (balsam fir). The maximum stand age is ~ 70 years for the hardwoods and > 100 years for the softwoods. American beech trees show signs of beech bark disease (Elvir et al. 2010). Soils are coarse-loamy, mixed, frigid Typic, and Aquic Haplorthods (Lyman, Tunbridge, Rawsonville, Dixfield, Colonel series) (Norton et al. 1999; SanClements et al. 2010). Annual air temperature (2005–2014) at the site ranged from − 30 to + 30 °C, with a mean of 5.6 °C (Patel et al. 2018a, b). Average annual precipitation (2005–2014) was 140 cm. Maximum snow depth (2005–2014) was 50–70 cm and the snowpack generally persisted until mid–end April (Maine River Flow Advisory Commission 2018). Average annual total N deposition at the site was 3.7 kg N ha−1 year−1 and declined ~ 40% from 1990 to 2014, driven by declines in NO3−-N deposition (Fig. 2).
Time-series of total (wet + dry) deposition of ammonium (NH4+-N) and nitrate (NO3−-N) at BBWM. The x-axis is water year, defined as October 1 to September 30. Dashed lines represent linear regression, with a significant temporal trend for NO3−-N (Spearman’s ρ = − 0.8120, p < 0.0001) but not for NH4+-N (Spearman’s ρ = − 0.3532, p = 0.0768)
N-mineralization studies at BBWM
For this study, we compiled data from N-mineralization studies conducted at BBWM from 1992 to 2016. Each study included measurements of extractable inorganic N, NNM, and NN (in situ and/or laboratory incubations). We included data for soils collected during the growing season (May to September). The number of samples collected varied by study and are included in the Supplemental Tables S1–S4. The methods used in the studies are described below, and the compiled data including measures of variance can be found in Supplemental Tables S1–S4.
Sampling and processing
Soils sampled for these studies were the surface organic horizon (O horizon) that excluded loose litter at the surface but included all material to the top of the mineral soil. O horizon thicknesses were typically 2–5 cm in the deciduous stands and 5–10 cm in the coniferous stands. Soils were sampled in both vegetation types within each watershed. The morphological boundary between the surface organic horizon and subsurface mineral horizons was usually distinct and abrupt, allowing relatively consistent field identification of horizons. Soils were typically sampled in four to five plots within each compartment (EB-deciduous, EB-coniferous, WB-deciduous, and WB-coniferous). Soils were sampled using 10 × 10-cm templates or as grab samples, with grab samples collected in a manner to attempt a uniform collection of materials across the depth of the O horizon. Soils were brought back to the laboratory for processing and analysis. Soils were not pooled for analysis. Field moist soils were sieved through 6-mm screens, and gravimetric moisture was determined by drying subsamples at 65 °C for 24 h.
Inorganic N extractions
Several variations of an otherwise similar procedure were used across the various study years. Inorganic N (NH4+-N and NO3−-N) was extracted using KCl solution (1 or 2 M). Extractions were performed by shaking soil with extractant solution for either 30 or 60 min, or by leaving the soil-extractant mixture on the bench-top for 48 h. The extracts were filtered through Whatman® 42 filter paper, and NH4+-N and NO3−-N were determined colorimetrically on an autoanalyzer at the Maine Agricultural and Forest Experiment Station (MAFES) Analytical Laboratory. The extractable inorganic N was also used as the “initial” (time-zero) value for NNM and NN calculations described below.
Incubations for NNM and NN
Field incubations were performed using the method of Eno (1960). At the time of sampling, subsamples were placed in polyethylene bags and buried in the soil, with incubation periods varying from 28 to 150 days, depending on the study. At the end of the incubation, bags were retrieved, and soils were processed and extracted in the laboratory as described above. Laboratory incubations were performed according to the method of Hart et al. (1994). Sieved soils were incubated in polystyrene cups in the dark at ~ 25 °C for 14 days and were extracted post-incubation as described above. NNM (for field and laboratory incubations) was calculated as the difference between post-incubation and initial concentrations of inorganic N (NH4+-N and NO3−-N). NN was calculated as the difference between post-incubation and initial concentrations of NO3−-N. A positive value of NNM (or NN) indicates net mineralization (or nitrification); a negative value indicates immobilization of inorganic N (or NO3−-N); an NNM or NN value of zero represents no change in pre-and post-incubation concentrations of inorganic N (or NO3−-N), within instrumental detection limits of 0.18 mg kg−1 for NH4+-N and 0.06 mg kg−1 for NO3−-N.
Total C and total N
Total C (TC) and total N (TN) were determined on air-dried soils (ground to 2 mm) by dry-combustion (Sollins et al. 1999) at the MAFES Analytical Laboratory.
Statistical analysis
Testing for watershed effects
Linear mixed models (LME) were used to test for differences in TC, TN, and C:N ratio—watershed (i.e., treated vs. reference), forest type (i.e., deciduous vs. coniferous), and their interaction were fixed effects, and year was a random effect. LME was not run on NH4+-N, NO3−-N, NNM, or NN because of differences in extraction methods among study years. Instead, one-way analyses were used on these variables to test for watershed effects during each study year. None of the response variables were normally distributed, and the non-parametric Wilcoxon test was used for statistical comparisons.
Analysis of temporal trends
To account for differences in methods among studies, inorganic N, NNM, and NN absolute values were not directly compared but were analyzed as WB:EB ratios, i.e., values in the treated watershed normalized to the reference watershed. The WB:EB ratio normalizes the data for differences in methods and allows us to compare the relative change in soil properties through time. However, this technique does not allow us to assess trends in individual watersheds, which would be confounded by differences in methods over time for these time series. A Wilcoxon test run on the entire dataset indicated that forest types were different for all variables (p < 0.01); therefore, soils were separated by forest type prior to further analysis. Local regression (LOESS) (fit = linear, smoothness = 1) and the non-parametric Mann-Kendall test were used to determine long-term trends in response variables (Mann 1945; Cleveland and Devlin 1988). The Mann-Kendall test statistic (S) is a measure of the direction and the magnitude of the trend. A positive number represents increasing values through time, while a negative number represents decreasing values through time; large absolute numbers indicate a greater magnitude of change. The Mann-Kendall test can only be used on monotonic trends. When a cyclic/non-monotonic trend was detected using LOESS regression, the Mann-Kendall test was used on each monotonic segment, as described by Hipel and McLeod (1994) and Edwards et al. (2006).
Significance of statistical analyses was determined at alpha = 0.05. LOESS regression was performed using JMP® Version 13 (SAS Institute Inc., Cary, NC, 1989–2007). Mann-Kendall tests were performed using the Kendall program developed by USGS (Helsel et al. 2006).
Results
Total C and N concentrations
TC was significantly greater in coniferous than in deciduous forests (LME, p = 0.02), but did not differ significantly between watersheds (p = 0.36) (Table 1). TN did not differ between watersheds (LME, p = 0.13) or forest types (p = 0.67). C:N was greater in coniferous than in deciduous forests (LME, p < 0.01), but did not differ between watersheds (p = 0.25).
Trends in soil NH4 +-N and NO3 −-N
Deciduous soils exhibited a trend of increasing NH4+-N concentrations in WB (treated) soils relative to EB (reference) through time, evident in the trend line and the relatively high Kendall S statistic (Fig. 3a; Table 2). NH4+-N availability did not differ significantly between WB and EB watersheds in 1992, 4 years after the treatment commenced (Wilcoxon test, Z = 0.82503, p = 0.4094). WB soils had significantly higher NH4+-N concentrations than EB after 1995 (Wilcoxon test, Z = 6.87988, p < 0.0001), and the WB:EB ratios of NH4+-N concentrations were at their highest during the 2012–2016 project years, in the third decade of treatment (WB:EB ratio was ~ 10 in 2016). NO3−-N concentrations in WB relative to EB soils also increased significantly through time in deciduous soils (Fig. 3b; Table 2). EB soils had low NO3−-N concentrations, ranging from values below detection to 8.0 mg kg−1, and therefore, mathematically, even small changes in EB concentrations (denominator) resulted in the relatively large changes in the WB:EB ratios (ranging from 0 to 200) seen in Fig. 3b, compared to NH4+-N ratios (ranging from 0 to 10). Neither NH4+-N nor NO3−-N ratios showed significant temporal trends in coniferous soils. There was a significant positive correlation between NH4+-N and NO3−-N ratios for deciduous soils (Spearman’s ρ = 0.6488, p = 0.0011), but no significant correlation for coniferous soils (Spearman’s ρ = 0.0754, p = 0.7589).
Trends in NNM and NN
Neither NNM nor NN ratio showed significant temporal trends for the coniferous soils, although numerical trends from field incubations suggested increases through time for the deciduous soils (NNM: 1992–2005 and NN: 1992–2015) (Fig. 4; Table 2). For field incubations of deciduous soils, the NN ratio during the second half of the study (2004–2015) was an order of magnitude higher than during the first half (1995–2004). NN rates in EB soils were low (0.0 to 2.5 mg kg−1 day−1), which explains the relatively high WB:EB ratios of NN for deciduous soils (ranging from 0 to 725) in Fig. 4, compared to NNM ratios.
Time-series ratios for net N mineralization (NNM) from field (a) and laboratory (b) incubations, and NN from field (c) and laboratory (d) incubations, with LOESS regression lines. Data shown are expressed as rates in WB soils (N-treated) divided by rates in EB (reference) soils. Solid circles and solid lines represent deciduous soils. Open diamonds and dashed lines represent coniferous soils
Discussion
As expected, the treated watershed (WB) showed signs of N-enrichment during the 27 years of treatment, consistent with results from other long-term ecosystem manipulation experiments (Gilliam and Adams 2011; Lovett and Goodale 2011; Magill et al. 2004; Moldan and Wright 2011). Overall, WB soils exhibited higher NH4+-N and NO3−-N concentrations, and greater NN, compared to EB soils, and the magnitude of difference in N concentrations increased significantly during the course of the study. After 27 years of N additions, WB soils had 10× more NH4+-N and 200× more NO3−-N than EB soils for the deciduous stands.
Chronic N enrichment did not alter TC or TN concentrations in WB soils in most years, which suggests that addition of C or N in the soil was balanced by loss, or that spatial variability in these soils was too high to detect significant differences. In fact, the TC and TN values reported in Table 1 had coefficients of variation of ~ 20%, suggesting that the latter hypothesis was likely true. Power analysis of our data indicated that the minimum detectable change (for 95% confidence intervals with a sample size of 15) was 0.3% for TN and 7.2% for TC. These values were higher than the numerical differences in mean TN and TC concentrations between EB and WB soils (Table 1), suggesting that the high spatial variability in our soils prohibited the detection of potential statistically significant differences in this experiment. Our data provide evidence that the treatment altered labile but not total nutrient concentrations, and this was consistent with other studies conducted in the northeastern US, such as the Harvard Forest (20 years of N additions in mixed hardwood stands (Turlapati et al. 2013)) and Mt. Ascutney (14 years of N additions in red spruce stands (McNulty et al. 2005)).
Coniferous soils exhibited lower extractable NH4+-N and NO3−-N concentrations and lower NNM and NN rates than deciduous soils, consistent with the high C:N ratio and lower quality of coniferous litter (Lovett et al. 2004; Ross et al. 2009). However, it was somewhat surprising that by 2015, after 27 years of N additions, NH4+-N availability did not differ between watersheds in the coniferous stands. In 2015, although coniferous soils in WB had significantly greater NO3−-N concentrations than in EB, the concentrations were very low (0.99 ± 0.50 mg/kg in WB, 0.06 ± 0.00 mg/kg in EB). The lack of linear trends in N availability despite chronic N additions is taken as further evidence of the slow N cycling characteristic of these coniferous (spruce-fir) soils. At the Harvard Forest in MA, USA, red pine stands were found to respond more rapidly than hardwoods to elevated N deposition (Magill et al. 2000, 2004). This suggests that broad groupings of hardwoods vs. softwoods for anticipated response to N additions may be inadequate to address species differences given the results from the Harvard and BBWM experiments. In this comparison, we see very different responses between red spruce (BBWM) and red pine (Harvard). It is also important to note that the N treatments at the two sites differed in form ((NH4)2SO4 at BBWM vs. NH4NO3 at Harvard Forest) and amount of N added (25.2 kg ha−1 year−1 at BBWM vs. 50/150 kg N ha−1 year−1), and these factors may have also contributed to the rapid response in pine stands at the Harvard Forest.
Temporal trends in soil NH4 +-N and NO3 −-N
In deciduous soils, NH4+-N availability in WB increased through time, compared to EB (Fig. 3a). This was primarily attributable to declining NH4+-N concentrations in EB soils with little to no change in WB soils (Supplemental Table S1). Declining NH4+-N concentrations in EB soils could be due to (i) decreased input of N, (ii) decreased NNM, (iii) increased NN, (iv) increased plant uptake of NH4+-N, (v) increased runoff/leaching losses, and/or (vi) increased gaseous losses due to oxidation (anammox) or ammonia volatilization. Atmospheric inputs are the primary external source of N in the EB watershed and N concentrations in ambient precipitation have been declining at the site (Fatemi et al. 2012), with ~ 60% decline in wet NO3−-N deposition from 1989 to 2014 (Fig. 2). It is possible that the lower N inputs resulted in slower N cycling processes, with declines in NH4+-N availability. Our results in this paper suggest no temporal trends in NNM or NN in EB soils, contradicting hypotheses (ii) and (iii) (Supplemental Table S2). We do not have recent data on tree N uptake (hypothesis (iv)), but Elvir et al. (2005) reported no change in foliar N concentrations in EB between 1989 and 2003, suggesting that N uptake rates may have remained unchanged during this period. Conversely, at the Hubbard Brook Experimental Forest in NH, USA, Groffman et al. (2018) suggested that declining N deposition and increased primary productivity due to increasing atmospheric CO2 levels might increase plant resorption of N, further decreasing soil N availability. Hypothesis (v) is contradicted by results from Fatemi et al. (2012), who reported declining N export in EB from 1989 to 2007. Forest soils in the northeastern US generally exhibit negligible N volatilization fluxes, attributed to their low pH (Magill et al. 1997; Hong et al. 2005; Templer et al. 2012a). While we do not have data for gaseous losses of NH4+-N at BBWM, the low soil pH (pH-CaCl2 < 3.50 (Fernandez et al. 2003; SanClements et al. 2010)) in EB soils suggests that these gaseous fluxes were likely minimal. Anammox is generally low in aerated/oxic temperate forest soils (Xi et al. 2016), such as at our research site, further contradicting hypothesis (vi). Therefore, we suggest that the declining NH4+-N concentrations in EB soils may have been driven by declining atmospheric N inputs (hypothesis (i)) and perhaps also by increased plant uptake (hypothesis (iv)). Additionally, soil NH4+-N concentrations may also be influenced by the availability of other soil cations on soil exchange sites. However, this is not a likely cause of NH4+-N declines in our soils, because (a) soil Ca and Mg concentrations did not change significantly over time (SanClements et al. 2010), and (b) there is evidence of recovery from acidification in the northeastern US, with declining exchangeable aluminum concentrations (Lawrence et al. 2015)—this would likely reduce cation competition from Al+3 for exchange sites and increase percent saturation of monovalent cations like NH4+, not reduce it.
The increasing WB:EB soil extractable NO3−-N ratios (Fig. 3b) were a consequence of increasing WB NO3−-N concentrations through time. Soil NO3−-N concentrations in EB were low across all study years (Supplemental Table S1). The increased NO3−-N in WB soils compared to EB was consistent with our results showing increased NN through time, and reflected the cumulative effects of long-term, chronic N additions from the WB treatments, including the greater abundance of ammonia oxidizing gene AmoA reported by Tatariw (2016).
Temporal trends in NN and NNM
NN rates in EB soils were consistently low across the entire study period, as is common for acidic, N-limited soils (Verchot et al. 2001; Ross et al. 2009), while the treated WB soils showed significantly higher NN rates overall. Since these incubations were conducted on sieved soils in plastic bags or cups, the NN values were not influenced by root uptake or leaching losses. Furthermore, due to the aerobic nature of these soils, denitrification losses at our site were low (Venterea et al. 2004; Morse et al. 2015). The NN rates therefore reflected the difference between the microbial processes of gross nitrification and immobilization, and the increases in NN detected in WB soils were attributable to changes through time in one or both of the mechanisms: (i) N additions in WB increased the availability of NH4+-N as a substrate for nitrification and (ii) increased NH4+-N availability suppressed NO3−-N immobilization by microbes (Emmett 2007). Despite increases in NN, NNM remained largely unchanged with no difference between watersheds for most years, indicating that there was a balance between the total N (NH4+-N + NO3−-N) mineralized and immobilized. Thus, for NNM to remain unchanged as NN increased through time, net ammonification (data not presented here) decreased through time. The lack of temporal trends in relative NNM values was somewhat unexpected, because previous studies at this site have reported ~ 80% retention of the added N in WB (Kahl et al. 1993; Jefts et al. 2004), suggesting an increasing potential for NNM. However, studies have reported that gross NNM rates generally remain unaffected by chronic N enrichment (Christenson et al. 2009; Venterea et al. 2004), and that only large increases in soil TN concentrations tend to increase gross NNM rates (Booth et al. 2005). The lack of an NNM response in WB is consistent with these studies and indicates that our N treatment may not have been large enough to stimulate gross NNM.
Studies have found conflicting effects of elevated N deposition on NNM rates. Some studies have reported increased NNM in response to increased N availability (Magill et al. 2000; Aber et al. 2003; Fenn et al. 2005; McNulty et al. 2005; Perakis and Sinkhorn 2011), while others have not (Lovett and Rueth 1999; Zak et al. 2006; Adams et al. 2007; Lovett and Goodale 2011). Aber et al. (1998) predicted that increased N availability would stimulate SOM decomposition and NNM in the short term, but that the depletion of the labile SOM pool would cause declines in decomposition and NNM in the long term. Consistent with this hypothesis, a meta-analysis conducted by Nave et al. (2009) showed that chronic N additions increased NNM in the short term (1 to 4 years), but these values decreased back to reference rates after 4 years. The studies in their analysis (which included BBWM) involved long-term N additions in temperate coniferous and deciduous forests of USA and Europe and varied in terms of experimental design including the form of N (ammonium sulfate, ammonium nitrate, sodium nitrate, and ammonium chloride) and amount of N added (15–150 kg N ha−1 year−1). The BBWM treatment consisted of 25.2 kg N ha−1 year−1 as ammonium sulfate and falls within the range of conditions covered in the meta-analysis.
Despite this variable response of NNM to N enrichment in forest soils, most studies have reported increasing nitrification rates in response to greater N availability (Magill et al. 2000; Fenn et al. 2005; Lu et al. 2011; Ross et al. 2012). It is worth noting that NN accounted for ~ 5% of NNM in EB soils, but ~ 50% of NNM in WB soils. This index, termed “relative net nitrification” is typically low in N-limited soils and increases with N-enrichment (Aber et al. 2003; Fenn et al. 2005; Gilliam et al. 2018). Our data provide evidence of a shift from an ammonification-dominated system to one where nitrification contributes as much as 50% to the total inorganic N pool. In the northeastern US, the Harvard Forest in MA (Magill et al. 2000) and Mt. Ascutney in VT (McNulty et al. 2005) have reported similar NN contributions of 10–50% to NNM in N-amended soils, compared to ~ 0% in the reference soils. At the Fernow Experimental Watershed in WV, nitrification accounted for 100% of the total N mineralized in the untreated and treated stands (Gilliam et al. 2010). Given the advanced stages of N saturation in both watersheds at Fernow, NN rates were not influenced by the N addition, but instead by factors such as tree species, C:N ratio, pH, and temperature (Gilliam et al. 2018). Despite this lack of NN response, stream NO3−-N losses increased in the N-manipulated watershed, likely due to reduced biotic demand (Gilliam et al. 2018). Our findings at BBWM suggest that the reference, EB, remained N-limited for the duration of the study, and the treated watershed, WB, showed signs of N-enrichment, but did not progress to advanced N saturation like Fernow. However, as discussed above, it must also be noted that type and amount of N added differed among all these studies, as did the ambient N deposition and type of soil sampled (organic vs. mineral).
Thresholds in long-term N dynamics
Soil extractable NH4+-N and NO3−-N concentrations did not differ between watersheds in 1992, suggesting that for the first 3 years of additions, the added NH4+ was taken up by roots and microbes and/or converted to NO3−, which was subsequently taken up or lost via leaching—evidenced by elevated N concentrations in soil solution and stream in the treated watershed (Fatemi et al. 2012). Evidence of N enrichment in WB soils (increased NH4+-N and NO3−-N concentrations, increased NN rates) was evident only after 1992, suggesting that it took several years before N inputs began to exceed the rates of N uptake and loss. Results from previous studies suggested a shift in ecosystem processes around 1993, approximately 4 years after treatments began. Elvir et al. (2005) reported on foliar chemistry at BBWM from 1989 to 2003 and found that foliar N concentrations in WB stands (both deciduous and coniferous forests) increased until 1993, but remained stable (American beech, red spruce) or declined (sugar maple) post-1993. They attributed these changes to reduced root N uptake, consistent with declines seen in fine root biomass in WB. Fatemi et al. (2012) reported on soil solution chemistry at BBWM from 1989 to 2007 and found that NO3−-N concentrations in WB were higher than EB. However, they found no change in soil solution NO3− concentrations from 1989 to 1993, and then WB soil solution NO3− concentrations increased significantly after 1993. They attributed this shift to an increase in nitrification, which is consistent with our results of increased NN and soil extractable NO3−-N concentrations during this period. Threshold transitions in other soil properties were reported to have occurred in the mid-1990s in the WB watershed. Fernandez et al. (2003) reported a shift from increasing to decreasing stream Ca and Mg concentrations around 1995, reflecting a shift in the soil buffering capacity from base cations to aluminum. The increased aluminum-saturation in our soils could have induced root stress and mortality (Boyce et al. 2013; Smithwick et al. 2013), reducing root N uptake and increasing soil N availability.
Our results for BBWM are reinforced by findings from other studies that reported similar ecosystem transitions occurring years after N-additions began. At the Harvard Forest, Aber et al. (1998) reported that NN and NO3−-N leaching in deciduous stands increased significantly only after 6 years of N additions. In the NITREX experiments in Gårdsjön, Sweden, researchers used 15N to determine the source of NO3−-N in runoff. For the first 6 years of N additions, most of the NO3−-N in runoff was due to direct losses of the added NO3− that had not undergone immobilization and nitrification. During later years, NO3−-N in runoff originated from nitrification in the soil (Kjønaas and Wright 2007; Moldan and Wright 2011). In mixed-deciduous stands in New York, Lovett and Goodale (2011) reported increases in foliar N concentrations shortly after initiating N additions, but NN rates increased significantly only in the sixth year of treatment. Thus, there appear to be multiple lines of evidence for a temporal lag between the onset of N additions and observed changes in soil N dynamics, and this would be consistent with changes through time in the strength of multiple ecosystem N sinks (Ågren and Bosatta 1988; Lovett and Goodale 2011; Perakis et al. 2005).
Conclusions
Our findings at BBWM offer insights into long-term forest ecosystem function under chronic N enrichment. Labile N concentrations were found to decline in the reference watershed (EB) through time, likely related to the declining atmospheric N deposition and increasing plant N demand during this period. Compared to the reference watershed, labile N concentrations in the treated watershed (WB) increased significantly over the 27 years of our experiment. Consistent with the predicted progression of N saturation, WB demonstrated a shift from an N-limited system dominated by NH4+-N to a system where NO3−-N became increasingly available through time. Despite almost immediate increases in stream N losses and foliar N concentrations reported in the literature for this study site, results reported here suggest that chronic elevated N deposition resulted in a delayed response in soil N dynamics. The findings presented in this paper, along with previous studies at BBWM (Elvir et al. 2005; Fatemi et al. 2012), document the emergence of enhanced N availability and nitrification only after four to 5 years of watershed N additions, suggesting a threshold in the evolution of ecosystem function. The results presented here reinforce the importance of long-term ecosystem studies that are essential to improve our understanding of multi-decadal ecosystem responses to environmental change.
References
Aber, J. D., Mcdowell, W., Nadelhoffer, K. J., Magill, A. H., Berntson, G., McNulty, S. G., et al. (1998). Nitrogen saturation in temperate forest ecosystems: hypotheses revisited. BioScience, 48(11), 921–934.
Aber, J. D., Goodale, C. L., Ollinger, S. V., Smith, M.-L., Magill, A. H., Martin, M. E., et al. (2003). Is nitrogen deposition altering the nitrogen status of northeastern forests? BioScience, 53(4), 375.
Adams, M. B., Kochenderfer, J. N., & Edwards, P. J. (2007). The Fernow Watershed acidification study: Ecosystem acidification, nitrogen saturation and base cation leaching. In P. Brimblecombe, H. Hara, D. Houle, & M. Novak (Eds.), Acid rain—deposition to recovery (pp. 267–273). Dordrecht: Springer.
Ågren, G. I., & Bosatta, E. (1988). Nitrogen saturation of terrestrial ecosystems. Environmental Pollution, 54(3–4), 185–197.
Booth, M. S., Stark, J. M., & Rastetter, E. (2005). Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecological Monographs, 75(2), 139–157.
Boyce, R. L., Schaberg, P. G., Hawley, G. J., Halman, J. M., & Murakami, P. F. (2013). Effects of soil calcium and aluminum on the physiology of balsam fir and red spruce saplings in northern New England. Trees—Structure and Function, 27(6), 1657–1667.
Burns, D. A., & Murdoch, P. S. (2005). Effects of a clearcut on the net rates of nitrification and N mineralization in a northern hardwood forest, Catskill Mountains, New York, USA. Biogeochemistry, 72(1), 123–146.
Carrara, J. E., Walter, C. A., Hawkins, J. S., Peterjohn, W. T., Averill, C., & Brzostek, E. R. (2018). Interactions among plants, bacteria, and fungi reduce extracellular enzyme activities under long-term N fertilization. Global Change Biology.
Christenson, L. M., Lovett, G. M., Weathers, K. C., & Arthur, M. A. (2009). The influence of tree species, nitrogen fertilization, and soil C to N ratio on gross soil nitrogen transformations. Soil Science Society of America Journal, 73(2), 638.
Cleveland, W. S., & Devlin, S. J. (1988). Locally weighted regression: an approach to regression analysis by local fitting. Journal of the American Statistical Association, 83(403), 596–610.
Contosta, A. R., Frey, S. D., & Cooper, A. B. (2011). Seasonal dynamics of soil respiration and N mineralization in chronically warmed and fertilized soils. Ecosphere, 2(3), art36.
Davidson, E. A., David, M. B., Galloway, J. N., Goodale, C. L., Haeuber, R., Harrison, J. A., et al. (2011). Excess nitrogen in the U.S. environment: trends, risks, and solutions. Issues in Ecology, 15, 1–16.
Edwards, P. J., Williard, K. W. J., Wood, F., & Sharpe, W. E. (2006). Soil water and stream water chemical responses. In M. B. Adams, D. R. Dewalle, & J. Hom (Eds.), The Fernow Watershed acidification study (pp. 71–136). New York: Springer Berlin Heidelberg.
Elvir, J. A., Rustad, L. E., Wiersrna, G. B., Fernandez, I. J., White, A. S., & White, G. J. (2005). Eleven-year response of foliar chemistry to chronic nitrogen and sulfur additions at the Bear Brook Watershed in Maine. Canadian Journal of Forest Research, 35(6), 1402–1410.
Elvir, J. A., Wiersma, G. B., Day, M. E., Greenwood, M. S., & Fernandez, I. J. (2006). Effects of enhanced nitrogen deposition on foliar chemistry and physiological processes of forest trees at the Bear Brook Watershed in Maine. Forest Ecology and Management, 221(1–3), 207–214.
Elvir, J. A., Wiersma, G. B., Bethers, S., & Kenlan, P. (2010). Effects of chronic ammonium sulfate treatment on the forest at the Bear Brook Watershed in Maine. Environmental Monitoring and Assessment, 171(1–4), 129–147.
Emmett, B. A. (2007). Nitrogen saturation of terrestrial ecosystems: Some recent findings and their implications for our conceptual framework. Water, Air, & Soil Pollution: Focus, 7(1–3), 99–109.
Eno, C. F. (1960). Nitrate production in the field by incubating the soil in polyethylene bags. Soil Science Society of America Proceedings, 24, 277–279.
Fatemi, F. R., Fernandez, I. J., Szillery, J., Norton, S. A., & Rustad, L. E. (2012). Soil solution chemical response to two decades of experimental acidification at the bear brook watershed in Maine. Water, Air, and Soil Pollution, 223(9), 6171–6186.
Fatemi, F. R., Fernandez, I. J., Simon, K. S., & Dail, D. B. (2016). Nitrogen and phosphorus regulation of soil enzyme activities in acid forest soils. Soil Biology and Biochemistry, 98, 171–179.
Fenn, M. E., Poth, M. A., Terry, J. D., & Blubaugh, T. J. (2005). Nitrogen mineralization and nitrification in a mixed-conifer forest in southern California: controlling factors, fluxes, and nitrogen fertilization response at a high and low nitrogen deposition site. Canadian Journal of Forest Research, 35(6), 1464–1486.
Fernandez, I. J., Simmons, J. A., & Briggs, R. D. (2000). Indices of forest floor nitrogen status along a climate gradient in Maine, USA. Forest Ecology and Management, 134(1–3), 177–187.
Fernandez, I. J., Rustad, L. E., Norton, S. A., Kahl, J. S., & Cosby, B. J. (2003). Experimental acidification causes soil base-cation depletion at the Bear Brook Watershed in Maine. Soil Science Society of America Journal, 67(6), 1909–1919.
Fernandez, I. J., Adams, M. B., Sanclements, M. D., & Norton, S. A. (2010). Comparing decadal responses of whole-watershed manipulations at the Bear Brook and Fernow experiments. Environmental Monitoring and Assessment, 171(1–4), 149–161.
Ferretti, M., Calderisi, M., Marchetto, A., Waldner, P., Thimonier, A., Jonard, M., et al. (2015). Variables related to nitrogen deposition improve defoliation models for European forests. Annals of Forest Science, 72(7), 897–906.
Frey, S. D., Ollinger, S., Nadelhoffer, K. J., Bowden, R., Brzostek, E., Burton, A., et al. (2014). Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry, 121(2), 305–316.
Galloway, J. N., Townsend, A. R., Erisman, J. W., Bekunda, M., Cai, Z., Freney, J. R., et al. (2008). Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science, 320(May), 889–892.
Gilliam, F. S., & Adams, M. B. (2011). Effects of nitrogen on temporal and spatial patterns of nitrate in streams and soil solution of a central hardwood forest. International Scholarly Research Network ISRN Ecology.
Gilliam, F. S., Somerville, C. C., Lyttle, N. L., & Adams, M. B. (2001). Factors influencing spatial variability in nitrogen processing in nitrogen-saturated soils. The Scientific World Journal, 1(November), 505–513.
Gilliam, F. S., Cook, A., & Lyter, S. (2010). Effects of experimental freezing on soil nitrogen dynamics in soils from a net nitrification gradient in a nitrogen-saturated hardwood forest ecosystem. Canadian Journal of Forest Research, 40(3), 436–444.
Gilliam, F. S., Galloway, J. E., & Sarmiento, J. S. (2015). Variation with slope aspect in effects of temperature on nitrogen mineralization and nitrification in mineral soil of mixed hardwood forests. Canadian Journal of Forest Research, 45(7), 958–962.
Gilliam, F. S., Walter, C. A., Adams, M. B., & Peterjohn, W. T. (2018). Nitrogen (N) dynamics in the mineral soil of a central Appalachian hardwood forest during a quarter century of whole-watershed N additions. Ecosystems, 1–16.
Goodale, C. L., & Aber, J. D. (2001). The long-term effects of land-use history on nitrogen cycling in northern hardwood forests. Ecological Applications, 11(1), 253–267.
Groffman, P. M., Driscoll, C. T., Durán, J., Campbell, J. L., Christenson, L. M., Fahey, T. J., et al. (2018). Nitrogen oligotrophication in northern hardwood forests. Biogeochemistry.
Gundersen, P., Emmett, B. A., Kjønaas, O. J., Koopmans, C. J., & Tietema, A. (1998). Impact of nitrogen deposition on nitrogen cycling in forests: a synthesis of NITREX data. Forest Ecology and Management, 101(1–3), 37–55.
Hart, S. C., Stark, J. M., Davidson, E. A., & Firestone, M. K. (1994). Nitrogen mineralization, immobilization, and nitrification. In W. R. W, A. Scott, P. Bottomley, D. Bezdicek, S. Smith, A. Tabatabai, & A. Wollum (Eds.), Methods of soil analysis: microbiological and biochemical properties (pp. 985–1019). Madison: Soil Science Society of America, Inc..
Helsel, D. R., Mueller, D. K., & Slack, J. R. (2006). Computer program for the Kendall family of trend tests. U.S. Geological Survey Scientific Investigations Report 2005–5275, 4.
Hipel, K. W., & McLeod, A. I. (1994). Nonparametric tests for trend detection. In Time series modelling of water resources and environmental systems (pp. 853–938). Amsterdam: Elsevier Science, Ltd..
Hong, B., Swaney, D. P., Woodbury, P. B., & Weinstein, D. A. (2005). Long-term nitrate export pattern from Hubbard Brook watershed 6 driven by climatic variation. Water, Air, and Soil Pollution, 160(1–4), 293–326.
Hunt, J. F., Ohno, T., & Fernandez, I. J. (2008). Influence of foliar phosphorus and nitrogen contents on chemical properties of water extractable organic matter derived from fresh and decomposed sugar maple leaves. Soil Biology and Biochemistry, 40(7), 1931–1939.
Jefts, S., Fernandez, I. J., Rustad, L. E., & Dail, D. B. (2004). Decadal responses in soil N dynamics at the Bear Brook Watershed in Maine, USA. Forest Ecology and Management, 189(1–3), 189–205.
Kahl, J. S., Norton, S. A., Fernandez, I. J., Nadelhoffer, K. J., Driscoll, C. T., & Aber, J. D. (1993). Experimental inducement of nitrogen saturation at the watershed scale. Environmental Science and Technology, 27(3), 565–568.
Kjønaas, O. J., & Wright, R. F. (2007). Use of 15N-labelled nitrogen deposition to quantify the source of nitrogen in runoff at a coniferous-forested catchment at Gårdsjön, Sweden. Environmental Pollution, 147(3), 791–799.
Knoepp, J. D., & Swank, W. T. (2002). Using soil temperature and moisture to predict forest soil nitrogen mineralization. Biology and Fertility of Soils, 36(3), 177–182.
Knoepp, J. D., & Vose, J. M. (2007). Regulation of nitrogen mineralization and nitrification in Southern Appalachian ecosystems: separating the relative importance of biotic vs. abiotic controls. Pedobiologia, 51(2), 89–97.
Kopáček, J., Cosby, B. J., Evans, C. D., Hruška, J., Moldan, F., Oulehle, F., et al. (2013). Nitrogen, organic carbon and sulphur cycling in terrestrial ecosystems: Linking nitrogen saturation to carbon limitation of soil microbial processes. Biogeochemistry, 115(1–3), 33–51.
Lawrence, G. B., Hazlett, P. W., Fernandez, I. J., Ouimet, R., Bailey, S. W., Shortle, W. C., et al. (2015). Declining acidic deposition begins reversal of forest-soil acidification in the northeastern U.S. and eastern Canada. Environmental Science and Technology, 49(22), 13103–13111.
Li, Y., Schichtel, B. A., Walker, J. T., Schwede, D. B., Chen, X., Lehmann, C. M. B., et al. (2016). Increasing importance of deposition of reduced nitrogen in the United States. Proceedings of the National Academy of Sciences, 113(21), 5874–5879.
Liu, X., Duan, L., Mo, J., Du, E., Shen, J., Lu, X., et al. (2011). Nitrogen deposition and its ecological impact in China: an overview. Environmental Pollution, 159(10), 2251–2264.
Liu, Y., Wang, C., He, N., Wen, X., Gao, Y., Li, S., et al. (2017). A global synthesis of the rate and temperature sensitivity of soil nitrogen mineralization: latitudinal patterns and mechanisms. Global Change Biology, 23(1), 455–464.
Lovett, G. M., & Goodale, C. L. (2011). A new conceptual model of nitrogen saturation based on experimental nitrogen addition to an oak forest. Ecosystems, 14(4), 615–631.
Lovett, G. M., & Rueth, H. (1999). Soil nitrogen transformations in beech and maple stands along a nitrogen deposition gradient. Ecological Applications, 9(4), 1330–1344.
Lovett, G. M., Weathers, K. C., Arthur, M. A., & Schultz, J. C. (2004). Nitrogen cycling in a northern hardwood forest: do species matter? Biogeochemistry, 67, 289–308.
Lu, M., Yang, Y., Luo, Y., Fang, C., Zhou, X., Chen, J., et al. (2011). Responses of ecosystem nitrogen cycle to nitrogen addition: a meta-analysis. New Phytologist, 189(4), 1040–1050.
Lucas, R. W., Klaminder, J., Futter, M. N., Bishop, K. H., Egnell, G., Laudon, H., & Högberg, P. (2011). A meta-analysis of the effects of nitrogen additions on base cations: implications for plants, soils, and streams. Forest Ecology and Management, 262(2), 95–104.
Magill, A. H., Aber, J. D., Hendricks, J. J., Bowden, R. D., Jerry, M., & Steudler, P. A. (1997). Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecological Applications, 7(2), 402–415.
Magill, A. H., Aber, J. D., Berntson, G. M., McDowell, W. H., Nadelhoffer, K. J., Melillo, J. M., & Steudler, P. (2000). Long-term nitrogen additions and nitrogen saturation in two temperate forests. Ecosystems, 3(3), 238–253.
Magill, A. H., Aber, J. D., Currie, W. S., Nadelhoffer, K. J., Martin, M. E., McDowell, W. H., et al. (2004). Ecosystem response to 15 years of chronic nitrogen additions at the Harvard Forest LTER, Massachusetts, USA. Forest Ecology and Management, 196(1), 7–28.
Maine River Flow Advisory Commission. (2018). Maine Cooperative Snow Survey. Maine Emergency Management Agency, http://www.maine.gov/rfac/rfac_snow.shtml.
Mann, H. B. (1945). Nonparametric tests against trend. Econometrica, 13(3), 245.
McClain, M. E., Boyer, E. W., Dent, C. L., Gergel, S. E., Grimm, N. B., Groffman, P. M., et al. (2003). Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems, 6(4), 301–312.
McNulty, S. G., Boggs, J., Aber, J. D., Rustad, L. E., & Magill, A. H. (2005). Red spruce ecosystem level changes following 14 years of chronic N fertilization. Forest Ecology and Management, 219(2–3), 279–291.
Mineau, M. M., Fatemi, F. R., Fernandez, I. J., & Simon, K. S. (2014). Microbial enzyme activity at the watershed scale: response to chronic nitrogen deposition and acute phosphorus enrichment. Biogeochemistry, 117(1), 131–142.
Minocha, R., Turlapati, S. A., Long, S., McDowell, W. H., Minocha, S. C., & Millard, P. (2015). Long-term trends of changes in pine and oak foliar nitrogen metabolism in response to chronic nitrogen amendments at Harvard Forest, MA. Tree Physiology, 35(8), 894–909.
Moldan, F., & Wright, R. F. (2011). Nitrogen leaching and acidification during 19 years of NH4NO3 additions to a coniferous-forested catchment at Gårdsjön, Sweden (NITREX). Environmental Pollution, 159(2), 431–440.
Morse, J. L., Durán, J., Beall, F., Enanga, E. M., Creed, I. F., Fernandez, I. J., & Groffman, P. M. (2015). Soil denitrification fluxes from three northeastern North American forests across a range of nitrogen deposition. Oecologia, 177(1), 17–27.
Nave, L. E., Vance, E. D., Swanston, C. W., & Curtis, P. S. (2009). Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N-mineralization. Geoderma, 153(1–2), 231–240.
Niu, S., Classen, A. T., Dukes, J. S., Kardol, P., Liu, L., Luo, Y., et al. (2016). Global patterns and substrate-based mechanisms of the terrestrial nitrogen cycle. Ecology Letters, 19(6), 697–709.
Norton, S. A., Kahl, J. S., & Fernandez, I. J. (1999). Altered soil-soil water interactions inferred from stream water chemistry at an artificially acidified watershed at Bear Brook Watershed, Maine USA. Environmental Monitoring and Assessment.
Ohno, T., Fernandez, I. J., Hiradate, S., & Sherman, J. F. (2007). Effects of soil acidification and forest type on water soluble soil organic matter properties. Geoderma, 140(1–2), 176–187.
Patel, K. F., Nelson, S. J., Spencer, C. J., & Fernandez, I. J. (2018a). Soil temperature record for the Bear Brook Watershed in Maine. PANGAEA. https://doi.org/10.1594/PANGAEA.885860.
Patel, K. F., Nelson, S. J., Spencer, C. J., & Fernandez, I. J. (2018b). Fifteen-year record of soil temperature at the Bear Brook Watershed in Maine. Scientific Data, 5, 180153.
Perakis, S. S., & Sinkhorn, E. R. (2011). Biogeochemistry of a temperate forest nitrogen gradient. Ecology, 92(7), 1481–1491.
Perakis, S. S., Compton, J. E., & Hedin, L. O. (2005). Nitrogen retention across a gradient of 15N additions to an unpolluted temperate forest soil in Chile. Ecology, 86(1), 96–105.
Ross, D. S., Wemple, B. C., Jamison, A. E., Fredriksen, G., Shanley, J. B., Lawrence, G. B., et al. (2009). A cross-site comparison of factors influencing soil nitrification rates in northeastern USA forested watersheds. Ecosystems, 12(1), 158–178.
Ross, D. S., Shanley, J. B., Campbell, J. L., Lawrence, G. B., Bailey, S. W., Likens, G. E., et al. (2012). Spatial patterns of soil nitrification and nitrate export from forested headwaters in the northeastern United States. Journal of Geophysical Research: Biogeosciences, 117(1), 1–14.
SanClements, M. D., Fernandez, I. J., & Norton, S. A. (2010). Soil chemical and physical properties at the Bear Brook Watershed in Maine, USA. Environmental Monitoring and Assessment, 171(1–4), 111–128.
Schlesinger, W. H. (2009). On the fate of anthropogenic nitrogen. Proceedings of the National Academy of Sciences, 106(1), 203–208.
Schöpp, W., Posch, M., Mylona, S., & Johansson, M. (2003). Long-term development of acid deposition (1880-2030) in sensitive freshwater regions in Europe. Hydrology and Earth System Sciences, 7(4), 436–446.
Shibata, H. (2016). Impact of winter climate change on nitrogen biogeochemistry in forest ecosystems: a synthesis from Japanese case studies. Ecological Indicators, 65, 4–9.
Simon, K. S., Chadwick, M. A., Huryn, A. D., & Valett, H. M. (2010). Stream ecosystem response to chronic deposition of N and acid at the Bear Brook Watershed, Maine. Environmental Monitoring and Assessment, 171(1–4), 83–92.
Smithwick, E. A. H., Eissenstat, D. M., Lovett, G. M., Bowden, R. D., Rustad, L. E., & Driscoll, C. T. (2013). Root stress and nitrogen deposition: consequences and research priorities. New Phytologist, 197(3), 712–719.
Sollins, P., Glassman, C., Paul, E. A., Swanston, C. W., Lajtha, K., Heil, J. W., & Elliott, E. T. (1999). Soil carbon and nitrogen pools and fractions. In G. P. Robertson, D. C. Coleman, C. S. Bledsoe, & P. Sollins (Eds.), Standard soil methods for long-term ecological research (pp. 89–105). New York: Oxford University Press.
Sorensen, P. O., Templer, P. H., Christenson, L., Duran, J., Fahey, T., Fisk, M. C., et al. (2016). Reduced snow cover alters root-microbe interactions and decreases nitrification rates in a northern hardwood forest. Ecology, 97(12), 3359–3367.
Stone, M. M., Weiss, M. S., Goodale, C. L., Adams, M. B., Fernandez, I. J., German, D. P., & Allison, S. D. (2012). Temperature sensitivity of soil enzyme kinetics under N-fertilization in two temperate forests. Global Change Biology, 18(3), 1173–1184.
Tatariw, C. (2016). The impact of anthropogenic disturbance on soil microbial community composition and activity: implications for ecosystem function. Ph. D. dissertation. University of Maine. 158 pp.
Tatariw, C., MacRae, J. D., Fernandez, I. J., Gruselle, M.-C., Salvino, C. J., & Simon, K. S. (2018). Chronic nitrogen enrichment at the watershed scale does not enhance microbial phosphorus limitation. Ecosystems, 21(1), 178–189.
Templer, P. H., Mack, M. C., Chapin, F. S., Christenson, L. M., Compton, J. E., Crook, H. D., et al. (2012a). Sinks for nitrogen inputs in terrestrial ecosystems: a meta-analysis of 15N tracer field studies. Ecology, 93(8), 1816–1829.
Templer, P. H., Pinder, R. W., & Goodale, C. L. (2012b). Effects of nitrogen deposition on greenhouse-gas fluxes for forests and grasslands of North America. Frontiers in Ecology and the Environment, 10(10), 547–553.
Treseder, K. K. (2008). Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecology Letters, 11(10), 1111–1120.
Turlapati, S. A., Minocha, R., Bhiravarasa, P. S., Tisa, L. S., Thomas, W. K., & Minocha, S. C. (2013). Chronic N-amended soils exhibit an altered bacterial community structure in Harvard Forest, MA, USA. FEMS Microbiology Ecology, 83(2), 478–493.
Van Diepen, L. T. A., Frey, S. D., Landis, E. A., Morrison, E. W., & Pringle, A. (2017). Fungi exposed to chronic nitrogen enrichment are less able to decay leaf litter. Ecology, 98(1), 5–11.
Venterea, R. T., Groffman, P. M., Verchot, L. V., Magill, A. H., & Aber, J. D. (2004). Gross nitrogen process rates in temperate forest soils exhibiting symptoms of nitrogen saturation. Forest Ecology and Management, 196(1), 129–142.
Verchot, L. V., Holmes, Z., Mulon, L., Groffman, P. M., & Lovett, G. M. (2001). Gross vs net rates of N mineralization and nitrification as indicators of functional differences between forest types. Soil Biology and Biochemistry, 33(14), 1889–1901.
Wallenstein, M. D., McNulty, S. G., Fernandez, I. J., Boggs, J., & Schlesinger, W. H. (2006). Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. Forest Ecology and Management, 222(1–3), 459–468.
Wang, Z., & Fernandez, I. J. (1999). Soil type and forest vegetation influences on forest floor nitrogen dynamics at the Bear Brook Watershed in Maine (BBWM). Environmental Monitoring and Assessment, 55(1), 221–234.
Xi, D., Bai, R., Zhang, L., & Fang, Y. (2016). Contribution of anammox to nitrogen removal in two temperate forest soils. Applied and Environmental Microbiology, 82(15), 4602–4612.
Zak, D. R., Holmes, W. E., Tomlinson, M. J., Pregitzer, K. S., & Burton, A. J. (2006). Microbial cycling of C and N in northern hardwood forests receiving chronic atmospheric NO3 − deposition. Ecosystems, 9(2), 242–253.
Acknowledgments
We thank Jean D. MacRae, Sarah J. Nelson, Tsutomu Ohno, and Aaron Weiskittel for their input on this manuscript. We are extremely grateful to Cheryl Spencer for her assistance in the laboratory and field, and with data handling. This is a MAFES publication.
Funding
This study was supported by grants from the National Science Foundation (DEB-1119709) and the Maine Agriculture and Forest Experiment Station (MAFES).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 278 kb)
Rights and permissions
About this article
Cite this article
Patel, K.F., Fernandez, I.J. Nitrogen mineralization in O horizon soils during 27 years of nitrogen enrichment at the Bear Brook Watershed in Maine, USA. Environ Monit Assess 190, 563 (2018). https://doi.org/10.1007/s10661-018-6945-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6945-3