Abstract
The concentrations and spatial distributions of eight heavy metals in surface sediments and sediment core samples from a shallow lake in China were investigated to evaluate the extent of the contamination and potential ecological risks. The results showed that the heavy metal concentrations were higher in the northern and southwestern lake zones than those in the other lake zones, with lower levels of As, Hg, Zn, Cu, Pb, Cr, and Ni primarily observed in the central and eastern lake regions and Cd primarily confined to areas surrounding the lake. The concentrations of the eight heavy metals in the sediment profiles tended to decrease with increasing sediment depth. The contents of Ni, Cu, Zn, Pb, and Cd in the surface sediment were approximately 1.23–18.41-fold higher than their background values (BVs), whereas the contents of Cr, As, and Hg were nearly identical to their BVs. The calculated pollution load index (PLI) suggested that the surface sediments of this lake were heavily polluted by these heavy metals and indicated that Cd was a predominant contamination factor. The comprehensive potential ecological risk index (PERI) in the surface sediments ranged from 99.2 to 2882.1, with an average of 606.1. Cd contributed 78.7 % to the PERI, and Hg contributed 8.4 %. Multivariate statistical analyses revealed that the surface sediment pollution with heavy metals mainly originated from industrial wastewater discharged by rivers located in the western and northwestern portion of the lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, thousands of contaminants, including heavy metals, organochlorine pesticides, chlorinated hydrocarbons, and polycyclic aromatic hydrocarbons, have entered the environment through natural processes, including ore-bearing rock erosion, volcanic activity, and forest fires, and anthropogenic activities, such as industrial and energy production, vehicle exhaust, waste disposal, fossil fuel combustion, and fertilizer and pesticide use (Bryan and Langston 1992; Mahbub et al. 2010; Yang et al. 2015). Heavy metal contamination has become a common problem worldwide and attracted increasing attention because of their non-biodegradable nature as well as their bioaccumulation and biomagnification through food chains, which pose a potential threat to human health (Zhang et al. 2011; Chabukdhara and Nema 2012; Yang et al. 2014). As (metalloid) and the metals Cr, Cd, Cu, Pb, Hg, Ni, and Zn are considered the most hazardous toxic heavy metals and are included in the US Environmental Protection Agency’s list of priority pollutants (Cameron 1992; Sofianska and Michailidis 2015).
Sediment is a basic component of aquatic habitats because it provides nutrients for macrophytes, zooplankton, and phytoplankton (Mil-Homens et al. 2013). Sediment also faithfully records information associated with anthropogenic activities (Heikkilä et al. 2010; Farmaki et al. 2014). Wastewater discharge and atmospheric deposition are regarded as the dominant pathways for heavy metal inputs to aquatic ecosystems (Olivares-Rieumount et al. 2005; Radakovitch et al. 2008; Sanei et al. 2010). A large proportion of heavy metals in the water column is adsorbed to and co-precipitate with suspended particles. These particles migrate to bottom sediments in calm hydrodynamic regimes, and heavy metals are concentrated in the surface sediment at levels several orders of magnitude higher than in the overlying water (Gaur et al. 2005; Liu et al. 2014). Thus, sediments are heavy metal sinks in aquatic systems (Delgado et al. 2011; Li et al. 2013). However, sediment can also become a potential source of heavy metals when they are released to overlying waters in response to disturbances associated with wind, waves, trawling, dredging, and shipping; thus, heavy metals impose an adverse effect on water quality (Jara-Marini et al. 2008). Moreover, the benthic biota can ingest sediment particles containing toxic heavy metals and accumulate the metals in their tissues, which ultimately has a detrimental impact on human health (Suresh et al. 2012; Swarnalatha et al. 2014).
The sediments in aquatic ecosystems are subject to heavy metal contamination (Chatterjee et al. 2007) and have been recognized as an important indicator that can be used to monitor contaminants in the aquatic environment (Bettinetti et al. 2003; Suresh et al. 2012). The distribution of heavy metals in sediments adjacent to populated areas could be used to investigate the anthropogenic effects of these metals on ecosystems and assess the ecological risks caused by discharged waste (Bai et al. 2011). The analysis of heavy metal concentrations and distributions in sediment can provide a better understanding of their behavior in aquatic ecosystems and plays an important role in evaluations of heavy metal contamination in sediments, thereby assisting environmental managers in supervising water quality.
In recent years, different assessment indexes of heavy metal pollution in sediments have been developed (Caeiro et al. 2005; Chabukdhara and Nema 2012; Hahladakis et al. 2013). Geochemical approaches have been successfully used to identify the sources of contaminants and indicate the effect of anthropogenic activities on sediment quality (Loska and Wiechula 2003; Chabukdhara and Nema 2012). Numerical sediment quality guidelines (SQGs) have been developed by environmental agencies in North America for aquatic ecosystems to identify critical concentrations associated with the presence or absence of biological effects of individual metals on communities (MacDonald et al. 2000; Caeiro et al. 2005; Swarnalatha et al. 2015). The potential ecological risk index (PERI), which was first proposed by Håkanson (1980), represents the sensitivity of various biological communities to toxic substances and evaluates the potential ecological risk imposed by heavy metals in sediment (Bai et al. 2011; Yi et al. 2011). The high ecological risks imposed by heavy metals are caused by their high toxic-response factors (Wang et al. 2014). The combination of these evaluation methods can effectively increase the reliability of heavy metal pollution assessments in sediments (Delgado et al. 2011).
Since the 1980s, China has experienced rapid urbanization and intensive industrialization, which has greatly accelerated increases in the gross domestic product at a rate of approximately 8 % per year. This rapid economic expansion has resulted in serious heavy metal environmental contamination, notably in lake sediments (Wang et al. 2014). Most freshwater lakes in China serve multiple functions, including flood control, irrigation, tourism, recreation, water transport, and aquaculture, and they are also important sources of drinking water for surrounding cities. Lake Changdang has been significantly affected by anthropogenic activities, such as the discharge of industrial and domestic effluents, and heavy metal contamination of the surface sediments of this lake threatens the water quality. To prevent the pollution of sediment, it is necessary to clarify the spatial and vertical distribution of contaminants and risks of heavy metal pollution in the sediments of this lake.
The main objectives of the present study were to (i) determine the spatial and vertical distributions of heavy metals in the sediments; (ii) evaluate the pollution status and contamination risks of heavy metals in the surface sediment of this lake using contamination factors, pollution load indexes, sediment quality guidelines, and potential ecological risk indexes; and (iii) explore the natural and/or anthropic sources of heavy metals using multivariate statistical techniques.
Materials and methods
Study area
Lake Changdang is located in southern Jiangsu Province to the west of the Lake Taihu Basin, one of the most industrialized and urbanized regions in China. Lake Changdang is a shallow lake with an average water depth of 1.2 m, a surface area of 85.3 km2, and a mean hydraulic retention time of 55.5 day, and it is 15.5-km long from south to north and 9.0-km wide from east to west. The primary inflow rivers include the Sudu, Xinjian, Xinhe, Dapu, Baishi, and Houdu, and the important outflow rivers surrounding the lake are the Huangli, Beigan, and Zhonggan (Fig. 1). This region belongs to a subtropical humid monsoon climate zone. The annual average rainfall is 1100.0 mm, of which approximately 60.7 % occurs from May to September. The annual mean evaporation is 1058 mm, and the perennial dominant wind directions are southeasterly and/or easterly with a multi-year average wind speed of 3.5 m/s. Wind-induced flow dominates in the lake, and the flow rate varies between 0.2 and 3.0 cm/s. A remarkable heterogeneity is noted in the spatial distribution of the sediment depth, which ranges from 0 to 110 cm. The maximum sediment depth occurs in the northeast and western lake zones, and the minimum depth occurs in the central lake region.
Sampling sites and sample collection
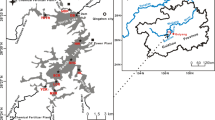
The lake monitoring sites are shown in Fig. 1. Forty-eight sites (42 for surface sediments and 6 for sediment cores) were selected based on the spatial distribution of the inflow and outflow river estuaries and the sediment depth. All of the sediment samples were collected in January 2014. The surface sediments (0–10 cm) were sampled from each site using a Van Veen grab Sampler (Eijkelamp, the Netherlands). Sediment cores were also collected by a self-made core sampler coupled with a polyvinylchloride tube, which had an inner diameter of 9.0 cm and a length of 50.0 cm. These cores were sliced at depths of 0–1, 1–2, 2–7, 7–12, 12–17, 17–22, 22–27, and 27–34 cm, and the samples were sealed in self-sealing polyethylene bags with marked labels and then placed in a cooler at 4 °C. The samples were immediately transported to the laboratory for further analysis. Subsamples were freeze dried, ground in an agate grinder, passed through 100-mesh nylon sieves, and stored at 4 °C prior to analysis.
Analysis and quality control
For the total heavy metal concentration analysis, 0.10–0.15 g of each preprocessed sediment sample was digested by an HClO4–HNO3–HF mixture in Teflon vessels. The contents of Cu, Cd, Pb, Zn, As, Cr, and Ni in the digestions were measured by inductively coupled plasma-atomic emission spectrometry (ICP-AES; Perkin-Elmer DV4300). Furthermore, atomic fluorescence spectrometry (AFS) at 253.65 nm was used to determine the Hg content. Reagent blanks were analyzed throughout the analysis to correct the analytical results. Certified reference materials (GBW07309) were obtained from the General Administration of Quality Supervision, Inspection, and Quarantine of the People’s Republic of China (NCRMAC 2007) to verify the quality of the samples. The percentage recoveries of the heavy metals measured in the sediment samples ranged from 94 to 105 %. The precision of the analytical procedures, which was expressed as the relative standard deviation, was within 10 %. All of the analyses were performed in triplicate, and the results were expressed as the mean. The total organic carbon (TOC) content was determined according to the K2Cr2O7–H2SO4 wet oxidation method described by Walkley and Black (De Vos et al. 2007), and the precision of the duplicate analysis was within 10 %. After the elimination of the organic matter in the dry subsamples with hydrogen peroxide, sediment particle size distribution was determined using a laser diffraction particle size analyzer (MS-2000, Malven Instruments Ltd., UK) at the State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences (CAS).
Sediment pollution assessment
Contamination factor
The contamination factor (CF) is calculated by the following equation:
where C heavy metal represents the concentration of each heavy metal determined in the sediment, and C background is the baseline value of each heavy metal in a specific region according to Qu et al. (2001). A value of CF < 1 indicates low contamination; 1 < CF < 3 indicates moderate contamination; 3 < CF < 6 indicates considerable contamination; and CF > 6 indicates high contamination (Håkanson 1980).
Pollution load index
The pollution load index (PLI) is defined as the nth root of the multiplied contents (CF metals), which is shown in the following equation:
where CFmetals is the contamination factor. The PLI is an assessment of the comprehensive toxicity status of heavy metals in a sediment sample and provides a simple, comparative method of assessing the level of heavy metal pollution. A PLI < 1 indicates no contamination, and a PLI > 1 represents heavy metal pollution in the sediment (Varol 2011).
Sediment quality guidelines
The heavy metal concentrations in sediments at each site were compared with the SQG values and classified according to the proposal by MacDonald et al. (2000) as follows: effect range low (ERL), effect range median (ERM), threshold effect level (TEL) and probable effect level (PEL). The ERL and TEL indicate contents at which detrimental effects on sediment dwelling fauna are infrequent, whereas the ERM and PEL represent concentrations at unfavorable effects are likely to occur (Hahladakis et al. 2013; Wang et al. 2014).
Potential ecological risk index
The PERI value can be calculated using the following formulas:
where RI is a comprehensive ecological risk index for heavy metals in each sample, \( {E}_r^i \) is the potential ecological risk index for a specific heavy metal, \( {T}_r^i \) is the toxic-response factor for a given heavy metal, \( {C}_f^i \) is the pollution factor, \( {C}_0^i \) is the content of metals in the sediments, and \( {C}_n^i \) is a background level for metals. The toxic-response factors for Hg, Cd, As, Pb, Cu, Ni, Cr and Zn were 40, 30, 10, 5, 5, 5, 2, and 1, respectively (Håkanson 1980; Liu et al. 2014). The potential ecological risks for a simple metal can be classified as follows: low risk (\( {E}_r^i \) < 40), moderate risk (40 ≤ \( {E}_r^i \) < 80), considerable risk (80 ≤ \( {E}_r^i \) < 160), high risk (160 ≤ \( {E}_r^i \) < 320), and very high risk (\( {E}_r^i \) ≥ 320). The potential ecological risk for the overall heavy metal content in the sediments of each sample is expressed as follows: low ecological risk (PERI < 150), moderate ecological risk (150 ≤ PERI < 300), considerable ecological risk (300 ≤ PERI < 600), and very high ecological risk (PERI ≥ 600) (Li et al. 2013; Wang et al. 2014).
Statistical analysis
The total heavy metal and organic matter contents in the surface sediments and sediment cores are presented as the mean ± standard deviation in this study. The normal distribution of heavy metal levels in the six sediment cores were tested using the one-sample Kolmogorov-Smirnov test (1-sample K-S), and probability values (2-tailed) ranging from 0.08 to 0.91 for As, 0.06 to 0.67 for Cd, 0.32 to 0.88 for Cr, 0.15 to 0.94 for Cu, 0.55 to 0.96 for Hg, 0.22 to 0.95 for Ni, 0.09 to 0.89 for Pb, and 0.11 to 0.74 for Zn indicated that the data follow a normal distribution. Next, the average concentrations of heavy metals at the different depths in the sediment cores at specific sampling sites were compared by performing a one-way ANOVA in conjunction with Duncan’s multiple comparison methods. Statistical analyses, including linear regression analyses, principal component/factor analyses, and cluster analyses, were conducted in the software SPSS for Windows (Version 11.5; Chicago, IL, USA). Significant differences were noted at a level of 0.05.
Results and discussion
Spatial distribution of heavy metals in the surface sediment
The spatial distribution patterns of heavy metals in the surface sediment are shown in Fig. 2 using contour maps plotted by Surfer 8.0 (Golden Software, Inc., Colorado, USA). The trends for all the heavy metals (except Cd) were similar. Higher contents of As, Hg, Zn, Cu, Pb, Cr, and Ni were mainly observed in the northern and southwestern areas of the lake, whereas lower contents were observed in the center and eastern areas of the lake. Although patches with higher concentrations of Cd were observed in the northern and southern areas, lower concentrations were primarily confined to areas around the lake. The total contents of the eight heavy metals in the surface sediment showed evident inhomogeneity (p < 0.01) associated with the location of inflow estuaries, enclosure culture regions, and sediment spatial distributions. The total contents of heavy metals in the surface sediment ranged from 60.56 to 138.61 (average 83.78 ± 15.65) mg/kg for Cr; 28.02 to 71.59 (average 44.52 ± 9.22) mg/kg for Ni; 19.13 to 101.19 (average 45.73 ± 17.91) mg/kg for Cu; 71.20 to 310.19 (average 147.56 ± 60.95) mg/kg for Zn; 4.86 to 14.19 (average 9.72 ± 2.20) mg/kg for As; 0.60 to 27.38 (average 5.24 ± 6.63) mg/kg for Cd; 19.49 to 53.25 (average 34.97 ± 7.67) mg/kg for Pb; and 0.05 to 0.26 (average 0.11 ± 0.05) mg/kg for Hg (Table 1). The coefficients of variation varied from 0.19 for Cr to 1.27 for Cd and increased in the order of Cr < Ni < Pb < As < Cu < Zn < Hg < Cd. This result indicated that the spatial distribution of these heavy metals in the surface sediment was substantially heterogeneous and may be caused by different sources (Wang et al. 2014). Additionally, the Cd contents in the surface sediment of this lake showed a larger variation relative to the distribution of the other heavy metals. Han et al. (2006) and Yang et al. (2011) stated that heavy metal contamination exhibiting low coefficients of variation is associated with natural sources, and contamination with high coefficients of variation is primarily derived from anthropogenic sources.
Average concentrations of Ni, Cu, Zn, Pb, and Cd above their corresponding background values were found at all the sites, and 58.3, 56.3, and 43.8 % of the 48 sites displayed Cr, As, and Hg contents higher than their background values, respectively (Table 1). Ni, Cu, Zn, Cd, and Pb were 1.28-, 1.42-, 1.50-, 18.41-, and 1.23-fold higher than their background values, respectively, and the Cr, As, and Hg contents were nearly identical to their background values. The extent of heavy metal enrichment above background values in the surface sediment increased in the order of Hg < As < Cr < Pb < Ni < Cu < Zn < Cd. The concentrations of heavy metals in the surface sediment also reflected their corresponding ERL/ERM and TEL/PEL values, with 54.2, 97.9, 14.6, 60.4, 25.0, 47.9, and 20.8 % of the sites exhibiting Cr, Ni, Cu, Zn, Cd, Pb, and Hg contents that exceeded their corresponding ERL; only As exhibited content in all the sites that was lower than its ERL. The Ni levels at eight sites (16.3 %), Zn at two sites (3.8 %), and Cd at four sites (8.5 %) were higher than their respective ERM values, whereas the remaining heavy metals were lower than their respective ERM values at all the sites (Table 1). The Cr, Ni, and Cd content in the surface sediment of all the sites exceeded their respective TEL values. Additionally, of the 48 sites, 75.0, 60.4, 95.8, 47.9, and 12.5 % displayed contents of Cu, Zn, As, Pb, and Hg that exceeded their corresponding TEL values, respectively. The Cr contents at 14 sites (29.2 %), Ni contents at 39 sites (81.3 %), and Cd contents at 18 sites (37.5 %) were higher than their respective PEL values, whereas the remaining other heavy metals were lower than their respective PEL values at all the sites (Table 1).
A comparison of the heavy metal concentrations in the surface sediment from the studied lake with that of other freshwater lakes in China and other countries is shown in Table 2. The Cd, Cu, Ni, and Zn contents in the surface sediments of this lake were higher than the contents in the other domestic lakes in Table 2, whereas the As and Pb contents and the Cr and Hg contents were lower than and similar to these contents, respectively. Compared with the published data on the heavy metal contents in surface sediment from other countries (Table 2), the Cd content in all the lakes (except Manchar Lake (Pakistan)) was lower than that in Lake Changdang; the Cr and Ni contents in lakes Manchar (Pakistan), Qaroun (Egypt), and Veeranam (India) were higher than that in Lake Changdang; the As and Pb contents in lakes Erie (USA), Manchar (Pakistan), and Songkhla (Thailand) were higher than that in Lake Changdang; the Cu and Zn contents in lakes Manchar (Pakistan), Songkhla (Thailand), and Veeranam (India) were higher than that in Lake Changdang. These findings indicate that the extent of select heavy metal pollution, notably Cd, in the surface sediments of Lake Changdang is serious, which may be potentially explained by the intensive discharge of industrial and domestic effluents from the rivers located along the western and northern portion of Lake Changdang.
Vertical variations in the heavy metal levels in the sediment cores
Figure 3 displays the vertical distribution of heavy metal concentrations in the sediment cores at site 4, site 9, site 17, site 25, site 27, and site 41 in Lake Changdang. The heavy metal levels in the sediment profiles ranged from 70.07 to 127.98 mg/kg for Cr, 33.62 to 68.24 mg/kg for Ni, 26.58 to 92.13 mg/kg for Cu, 80.43 to 246.53 mg/kg for Zn, 6.14 to 13.06 mg/kg for As, 0.70 to 15.78 mg/kg for Cd, 26.10 to 49.74 mg/kg for Pb, and 0.05 to 0.24 mg/kg for Hg, and the average coefficient of variation was 11.7, 15.4, 29.8, 30.0, 32.9, 74.5, 23.0, and 36.1 %, respectively. The Cd levels in the sediment profile indicated larger variations with increasing sediment depth at six sites relative to that of the other heavy metals, indicating that Cd could be influenced by anthropogenic activities. The average coefficient of variation at a specific sampling site decreased in the order site 27 > site 4 > site 17 > site 25 > site 41 > site 9, which may be explained by the location of the inflowing and outflowing estuary mouths.
Vertical distributions of heavy metals in the sediment cores from Lake Changdang. a, b, c, d, e, and f refer to site 4, site 9, site 17, site 25, site 27, and site 41, respectively. All data are expressed as the mean ± standard deviation; heavy metal concentrations marked with identical letters do not differ significantly at different sediment depths (Duncan’s test, p ≤ 0.05)
The maximum contents of 151.7 mg/kg for Cr, 80.8 mg/kg for Ni, 114.5 mg/kg for Cu, 282.0 mg/kg for Zn, 56.7 mg/kg for Pb, and 0.3 mg/kg for Hg occurred at site 41, whereas the maximum contents of 15.9 mg/kg for As and 33.7 mg/kg for Cd occurred at site 25. The minimum contents of almost all of the heavy metals (except As) in the different sediment layers were observed at site 4. As shown in Fig. 3, the distribution trends of the eight heavy metals measured in the sediment cores were similar at six sites, and their concentrations at each site increased slightly in the upper 0–2-cm layer and then decreased as the sediment depth increased, with a sharp decrease observed in the 20–34-cm layer. This distribution was also observed by Yin et al. (2011a) and Naimi and Ayoubi (2013). Heavy metals easily form complexes with organic matter, and this process may be primarily responsible for the high concentrations of heavy metals in the upper layer (McManamon et al. 2012; Huo et al. 2015). For a specific site, such as site 25, the concentrations of heavy metals in the 0–1-cm layer were lower than those in the 1–2-cm layer, indicating a likely dilution because of processes associated with sediment suspension induced by wind-driven currents, bioturbation, trawling, dredging, and shipping (Yin et al. 2011b). In this study, the contents of organic matter in the upper layers of the sediment core at site 4, site 9, site 17, site 25, site 27, and site 41 were 2.5-, 1.9-, 2.5-, 2.2-, 2.6-, and 1.3-fold higher, respectively, than that in the 20–34-cm layer, and a significant logarithmic correlation was also found between the total organic carbon contents and As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn contents, which presented R-squared values of 0.6936 (p < 0.01, n = 46), 0.2951 (p < 0.01, n = 46), 0.2804 (p < 0.01, n = 46), 0.4542 (p < 0.01, n = 46), 0.4584 (p < 0.01, n = 46), 0.4252 (p < 0.01, n = 46), 0.5506 (p < 0.01, n = 46), and 0.5224 (p < 0.01, n = 46), respectively. The contents of clay in the sediment profile decreased with increasing sediment depth at six sites; the opposite distribution trend was observed for sand levels. The silk levels in the sediment profile decreased with increasing sediment depth at site 4, site 9, site 25, and site 41, whereas the opposite trend occurred at site 17 and site 27 (Fig. 4). Heavy metals were easily adsorbed on the surface of clay minerals, and the ability of clay minerals to adsorb heavy metals has been well recognized (He et al. 2012; Li et al. 2014). A significant power function relationship was found between the clay levels and each of the As, Cd, Cr, Cu, Hg, Pb, and Zn contents, with r values of 0.4202 (p < 0.01, n = 46), 0.4040 (p < 0.01, n = 46), 0.2961 (p < 0.05, n = 46), 0.2956 (p < 0.05, n = 46), 0.3079 (p < 0.05, n = 46), 0.4215 (p < 0.01, n = 46), and 0.3045 (p < 0.05, n = 46), respectively. However, there were no significant correlations between the heavy metal contents and either sand or silk levels for six sediment cores. Therefore, organic matter and clay may play an important role in the retention of heavy metals in the surface sediment of this lake, which suggest that heavy metals are only weakly mobile in the sediment core.
Sediment contamination index
The CF values calculated for each heavy metal and PLI values calculated for the sediments of each site are presented in Table 3. The CF values ranged from 0.76 to 1.75 for Cr, 1.44 to 3.67 for Ni, 1.01 to 5.36 for Cu, 1.21 to 5.26 for Zn, 0.52 to 1.51 for As, 2.21 to 101.42 for Cd, 1.24 to 3.39 for Pb, and 0.43 to 2.32 for Hg, and the averages were 1.06 ± 0.20, 2.28 ± 0.47, 2.42 ± 0.95, 2.50 ± 1.03, 1.03 ± 0.23, 19.39 ± 24.54, 2.23 ± 0.49, 0.99 ± 0.48, and 2.14 ± 0.74, respectively. The maximum CF values for Cr, Ni, Cu, As, Pb, and Hg were observed at site 41, and the maximum CF values of Zn and Cd were observed at site 25, whereas the lowest CF values for each heavy metal were found at site 39, which was located near an estuary of the outflowing Zhonggan River. The CF values for Cr, As, and Hg were lower than three in the sediments from all the sites, suggesting a “moderate contamination” level. The CF values indicate “considerable contamination” for Ni at 3 sites (6.3 %), Cu at 9 sites (18.8 %), Zn at 12 sites (25.0 %), Pb at 3 sites (6.3 %), and Cd at 10 sites (20.8 %). Cd was detected in the sediment of 37 sites (77.1 %) at a “very high contamination” level, indicating that Cd pollution in the surface sediment of this lake was likely associated with exogenous input of domestic, municipal, and industrial wastewater discharges from inflow rivers along the northwestern and southwestern portions of this lake.
The PLI values for the sediment of each site varied between 0.98 and 3.98 and had an average value of 2.14 ± 0.74 (Table 3), representing a substantial fluctuation. The extent of heavy metal contamination increased as the PLI value increased (Suresh et al. 2012). PLI values above three were found at site 15, site 25, site 26, site 32, site 33, site 35, and site 41, and the heavy metal pollution classification suggests that the sediment exhibited significant pollution by all the eight heavy metals, which is caused by the input of metallic discharge by the inflowing river located along the northwestern portions of this lake. Except at site 48, the remaining PLI values ranged from one to three, indicating moderate contamination by these heavy metals. The variation in PLI values observed here was higher than that in Lake Vembanad in India and Lake Rawal in Pakistan, in which the PLI values of the sediments ranged from 0.49 to 3.70 (Prinju and Narayana 2006) and from 0.85 to 3.56 (Zahra et al. 2014), respectively. The range in the sediments of Lake Changdang was lower than that in the sediment of Lake Veeranam (Suresh et al. 2012); however, the average PLI values in the sediment of Lake Veeranam matched the average observed here.
Assessment of heavy metals in the surface sediment using the potential ecological risk index
The potential ecological risk of the surface sediment in the present lake was classified by the PERI values of the individual and overall heavy metals (Table 4). According to the PERI values of the individual heavy metals, Cr, Zn, Pb, Ni, As, and Cu present a low potential ecological risk in the sediment of this lake; Hg presents low and moderate potential ecological risk in the sediment of 38 and 10 sites, respectively; and Cd presents moderate, considerable, high, and serious risk levels in the sediments of 1, 9, 17, and 21 sites, respectively. Consequently, the Cd and Hg levels in the sediments might lead to higher potential ecological risks relative to that of the other metals investigated. In terms of the average PERI values of an individual heavy metal, the ecological risk level increased in the order Cr < Zn < Pb < Ni = As < Cu < Hg < Cd. The highest PERI values for a single heavy metal were observed at site 41 for Cr, Ni, Cu, As, Pb, and Hg; at site 26 for Zn; and at site 25 for Cd, whereas the lowest values were found at site 48 for almost all of the heavy metals (except Hg, for which the lowest value was at site 8).
The comprehensive PERI values calculated for the eight heavy metals in the surface sediments from the 48 sites varied between 99.2 and 2882.1 and had an average of 606.1, and the average value was higher than the threshold of the very high ecological risk level. The lowest and highest integrated PERI values were observed at site 48 and site 25, respectively. The spatial distribution of the comprehensive PERI in the surface sediment was characterized by an increase from east to west and south to north. The surface sediments with a very high potential ecological risk level were mainly located in a region 1.5 km from the estuaries of the Xinhe and Dapu rivers, whereas sediments with a low potential ecological risk level were located in the northeast and southern regions of the lake, which were dominated by outflow zones. The surface sediments with low, moderate, considerable, and very high potential ecological risk levels accounted for 6.3 % (3 sites), 33.3 % (16 sites), 35.4 % (17 sites), and 25.0 % (12 sites) of all the sites, respectively, suggesting that the surface sediment pollution caused by heavy metals was serious. Among the eight heavy metals studied, Cd contributed 78.7 % to the comprehensive PERI, Hg contributed 8.4 %, and all of the others contributed 12.9 %, indicating that Cd and Hg are the dominant factors that should be addressed when assessing the potential ecological risks in the surface sediments of this lake. Moreover, ecological risk assessments of heavy metals should consider the pollution characteristics of an individual heavy metal and cumulative contamination caused by different heavy metals in the surface sediments.
Multivariate statistical analyses
Correlation matrix
A linear regression analysis was performed to determine the relationships between different variables and explore the potential sources of different heavy metals. Pearson’s correlation coefficients for the different variables, including the eight heavy metals, TOC, PLI, and PERI, were determined using SPSS 12.0 software and are shown in Table 5. Significant linear correlations were observed among the contents of the eight heavy metals in the surface sediment in this lake. Suresh et al. (2012) stated that the significant correlation coefficients among the different heavy metals indicate mutual contaminant sources and identical environmental behaviors during transport. The strong significant correlations among the eight heavy metals investigated in this study indicate that these metals may be derived from exogenous inputs from the inflowing rivers located along the western and northwestern portions of this lake. The correlation coefficient between the Cd value and PERI value was higher than that between the other heavy metals and their PERI values, indicating the greater contribution of Cd to the comprehensive potential ecological risk in the surface sediment of this lake. However, the single trace element contributions to the PLI were nearly identical. No significant linear correlation was observed between the TOC and any of the heavy metals (except Cr and Ni) in the surface sediment, suggesting that the TOC contents may be affected by inputs from the inflowing rivers and decomposition of the aquatic vegetation and feeding culture; thus, the TOC levels may be more changeable than the heavy metal content in the surface sediment.
Principal component analysis/factor analysis
Prior to performing the principal component and factor analyses, the suitability of the experimental data was examined by the Kaiser–Meyer–Olkin (KMO) and Bartlett’s sphericity tests. According to Varol (2011), a KMO value approaching one generally indicates that the principal component and factor analyses are valuable. A probability value of less than 0.05 for the Bartlett’s sphericity test indicates a significant correlation among parameters. In the present study, the KMO value and Bartlett’s sphericity test probability value were 0.88 and 0, respectively, indicating that the results derived from the principal component and factor analyses were credible. The principal component and factor analyses were applied to the normalized experimental dataset, which included the heavy metals, TOC, PERI, and PLI values. This analysis was performed to determine the factors that influence each variable, and it employs a correlation matrix among the above variables followed by a varimax rotation. The loading coefficients, eigenvalues, and percentage of the total variance of the different parameters in the correlation matrix and varimax rotation are detailed in Table 6. Two components with eigenvalues exceeding one explained 86.0 % of the total variance. Component 1 was dominated by all the variables (0.72–0.99) except the Cd and TOC levels, and this component accounted for 73.2 % of the total variance (Table 6). Component 2 explained 12.8 % of the total variance and was not correlated with each variable. Two other components were obtained through the varimax rotation of the correlation matrix. Component 1 explained 57.7 % of the total variance and had significant loadings (0.78–0.95) for Cr, Ni, Cu, Zn, As, Pb, Hg, and the PLI (Table 6), suggesting that almost all of the heavy metals (except Cd) were important for the PLI. Component 2 was dominated by Cd and the PERI and explained 28.3 % of the total variance, indicating that the Cd content in the surface sediment significantly influenced the PERI.
Cluster analysis
A cluster analysis was also performed on the heavy metal, TOC, PLI, and PERI data to classify similar objects into groups. Figure 5 presents a dendrogram of all parameters grouped into three clusters. Cluster 1 included As, Cr, Cu, Ni, Pb, Zn, and the PLI, and it indicates that the concentrations of these heavy metals were responsible for the PLI and suggests that mutual pollution sources may have occurred for these heavy metals. Cluster 2 consisted of Cd and the PERI, and it suggests that the Cd level in the surface sediment was significantly related to the integrated PERI. In recent years, a number of industrial plants located along the inflow rivers in the north and northwestern portion of this lake and associated with metal processing, smelting, battery production, and electronic component production have been decommissioned, which has caused the discharge of a large amount of untreated industrial wastewater into the lake water. However, heavy metals have accumulated in the surface sediment and aggravated sediment pollution because of their non-biodegradable nature. Cluster 3 consisted of the TOC, which only significantly correlated with the metals Cr and Ni. As discussed above, the results obtained from the cluster analysis are consistent with those from the principal component/factor analyses and Pearson correlation analysis.
Conclusions
The concentrations of the eight heavy metals in the surface sediment of Lake Changdang were determined, and the regions with high contents of heavy metals were mainly confined to the northern and southwestern lake zones, which are strongly influenced by the inflowing rivers. The vertical distributions of As, Cd, Cr, Cu, Hg, Ni, Pb, and Zn in the sediment cores displayed sharp concentration decreases below the 20-cm layer. Eight heavy metal levels in the surface sediments were comparable to the corresponding background, SQGs, PLI, and comprehensive PERI values, showing that the sediments in this lake were contaminated by these heavy metals, particularly Cd. Additionally, over 94 % of the sites evaluated for Lake Changdang displayed at least a moderate potential ecological risk. Industrial wastewater discharge that contains metals from industrial plants associated with metal processing and treatment was the dominant pollution source, which is consistent with the results of the multivariate statistical analyses. These findings will provide useful information for the control and treatment of heavy metal pollution in surface sediments by local environmental managers and regulators.
References
Arain, M. B., Kazi, T. G., Jamli, M. K., Jalbani, N., Afridi, H. I., & Shah, A. (2008). Total dissolved and bioavailable elements in water and sediment samples and their accumulation in Oreochromis mossambicus of polluted Manchar lake. Chemosphere, 70, 1845–1856.
Bai, J., Cui, B., Chen, B., Zhang, K., Deng, W., Gao, H., & Xiao, R. (2011). Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecological Modelling, 222, 301–306.
Bettinetti, R., Giarei, C., & Provini, A. (2003). Chemical analysis and sediment toxicity bioassays to assess the contamination of the River Lambro (Northern Italy). Archives of Environmental Contamination and Toxicology, 45, 72–78.
Bryan, G. W., & Langston, W. J. (1992). Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environmental Pollution, 76, 89–131.
Caeiro, S., Costa, M. H., Ramos, T. B., Fernandes, F., Silveira, N., Coimbra, A., Medeiros, G., & Painho, M. (2005). Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecological Indicators, 5, 151–169.
Cameron, R. (1992). Guide to site and soil description for hazardous waste site characterization Vol. 1: metals. Environmental Protection Agency EPA/600/4-91/029.
Chabukdhara, M., & Nema, A. K. (2012). Assessment of heavy metal contamination in Hindon River sediments: a chemometric and geochemical approach. Chemosphere, 87, 945–953.
Chatterjee, M., Filho, E. V. S., Sarkar, S. K., Sella, S. M., Bhattacharya, A., Satpathy, K. K., Prasad, M. V. R., Chakraborty, S., & Bhattachary, B. D. (2007). Distribution and possible source of trace elements in the sediment cores of a tropical macrotidal estuary and their ecotoxicological significance. Environment International, 33, 346–356.
De Vos, B., Lettens, S., Muys, B., & Deckers, J. A. (2007). Walkley–Black analysis of forest soil organic carbon: recovery, limitations and uncertainty. Soil Use and Management, 23, 221–229.
Delgado, J., Barba-Brioso, C., Nieto, J. M., & Boski, T. (2011). Speciation and ecological risk of toxic elements in estuarine sediments affected by multiple anthropogenic contributions (Guadiana saltmarshes, SW Iberian Peninsula): I. Surficial sediments. The Science of the Total Environment, 409, 3666–3679.
El-Sayed, S. A., Moussa, E. M. M., & El-Sabagh, M. E. I. (2015). Evaluation of heavy metal content in Qaroun Lake, El-Fayoum, Egypt. Part I: bottom sediments. Journal of Radiation Research and Applied Sciences, 8(3), 276–285.
Farmaki, E. G., Thomaidis, N. S., Pasias, I. N., Baulard, C., Papaharisis, L., & Efstathiou, C. E. (2014). Environmental impact of intensive aquaculture: investigation on the accumulation of metals and nutrients in marine sediments of Greece. Science of the Total Environment, 485–486, 554–562.
Gaur, V. K., Gupta, S. K., Pandey, S., Gopal, K., & Misra, V. (2005). Distribution of heavy metals in sediment and water of river Gomti. Environmental Monitoring and Assessment, 102, 419–433.
Hahladakis, J., Smaragdaki, E., Vasilaki, G., & Gidarakos, E. (2013). Use of sediment quality guidelines and pollution indicators for the assessment of heavy metal and PAH contamination in Greek surficial sea and lake sediments. Environmental Monitoring and Assessment, 185, 2843–2853.
Håkanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water Research, 14, 975–1000.
Hallare, A. V., Kosmehl, T., Schulze, T., Hollert, H., Kohler, H. R., & Triebskorn, R. (2005). Assessing contamination levels of Laguna Lake sediments (Philippines) using a contact assay with zebrafish (Danio rerio) embryos. Science of the Total Environment, 347, 254–271.
Han, Y., Du, P., Cao, J., & Posmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Science of the Total Environment, 355, 176–186.
He, C., Bartholdy, J., & Christiansen, C. (2012). Clay mineralogy, grain size distribution and their correlations with trace metals in the salt marsh sediments of the Skallingen barrier spit, Danish Wadden Sea. Environment and Earth Sciences, 67, 759–769.
Heikkilä, M., Edwards, T. W. D., Seppä, H., & Sonninen, E. (2010). Sediment isotope tracers from Lake Saarikko, Finland, and implications for Holocene hydroclimatology. Quaternary Science Reviews, 29, 2146–2160.
Huo, S., Zhang, J., Yeager, K. M., Xi, B., Qin, Y., He, Z., & Wu, F. (2015). Mobility and sulfidization of heavy metals in sediments of a shallow eutrophic lake, Lake Taihu, China. Journal of Environmental Sciences, 31, 1–11.
Jara-Marini, M. E., Soto-Jiménez, M. F., & Páez-Osuna, F. (2008). Bulk and bioavailable heavy metals (Cd, Cu, Pb, and Zn) in surface sediments from Mazatlán Harbor (SE Gulf of California). Bulletin of Environmental Contamination and Toxicology, 80, 150–153.
Kishe, M. A., & Machiwa, J. F. (2003). Distribution of heavy metals in sediments of Mwanza Gulf of Lake Victoria, Tanzania. Environment International, 28, 619–625.
Li, F., Huang, J., Zeng, G., Yuan, X., Li, X., Liang, J., & Wang, X. (2013). Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. Journal of Geochemical Exploration, 132, 75–83.
Li, Q., Ji, H., Qin, F., Tang, L., Guo, X., & Feng, J. (2014). Sources and the distribution of heavy metals in the particle size of soil polluted by gold mining upstream of Miyun Reservoir, Beijing: implications for assessing the potential risks. Environmental Monitoring and Assessment, 186, 6605–6626.
Liu, M., Yang, Y., Yun, X., Zhang, M., Li, Q., & Wang, J. (2014). Distribution and ecological assessment of heavy metals in surface sediments of the East Lake, China. Ecotoxicology, 23, 92–101.
Loska, K., & Wiechula, D. (2003). Application of principal component analysis for the estimation of source heavy metal contamination in surface sediments from Rybnik Reservoir. Chemosphere, 51, 723–733.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
Mahbub, P., Ayoko, G. A., Goonetilleke, A., Egodawatta, P., & Kokot, S. (2010). Impacts of traffic and rainfall characteristics on heavy metals build-up and wash-off from urban roads. Environmental Science & Technology, 44, 8904–8910.
McManamon, C., Burke, A. M., Holmes, J. D., & Morris, M. A. (2012). Amine-functionalised SBA-15 of tailored pore size for heavy metal adsorption. Journal of Colloid and Interface Science, 369, 330–337.
Mil-Homens, M., Costa, A. M., Fonseca, S., Trancoso, M. A., Lopes, C., Serrano, R., & Sousa, R. (2013). Characterization of heavy-metal contamination in surface sediments of the Minho River estuary by way of factor analysis. Archives of Environmental Contamination and Toxicology, 64, 617–631.
Naimi, S., & Ayoubi, S. (2013). Vertical and horizontal distribution of magnetic susceptibility and metal contents in an industrial district of central Iran. Journal of Applied Geophysics, 96, 55–67.
Olivares-Rieumount, S., Rosa, D. D. L., Lima, L., Graham, D. W., D’Alessandro, K., Borroto, J., Martínez, F., & Sánchez, J. (2005). Assessment of heavy metal levels in Almendares River sediments—Havana City, Cuba. Water Research, 39, 3945–3953.
Opfer, S. E., Farver, J. R., Miner, J. G., & Krieger, K. (2011). Heavy metals in sediments and uptake by burrowing may flies in western Lake Erie basin. Journal of Great Lakes Research, 37, 1–8.
Pradit, S., Wattayakorn, G., Angsupanich, S., Baeyens, W., & Leermakers, M. (2010). Distribution of trace elements in sediments and biota of Songkhla lake, Southern Thailand. Water, Air, & Soil Pollution, 206, 155–174.
Prinju, C. P., & Narayana, A. C. (2006). Spatial and temporal variability of trace element contents in tropical lagoon, southwest coast of India: environmental implications. Journal of Coastal Research, 39, 1053–1057.
Qu, W., Dickman, M., & Wang, S. (2001). Multivariate analysis of heavy metal and nutrient concentrations in sediments of Taihu Lake, China. Hydrobiologia, 450, 83–89.
Radakovitch, O., Roussiez, V., Ollivier, P., Ludwig, W., Grenz, C., & Probst, J. (2008). Input of particulate heavy metals from rivers and associated sedimentary deposits on the Gulf of Lion continental shelf. Estuarine, Coastal and Shelf Science, 77, 285–295.
Sanei, H., Outridge, P. M., Goodarzi, F., Wang, F., Armstrong, D., Warren, K., & Fishback, L. (2010). Wet deposition mercury fluxes in the Canadian sub-Arctic and southern Alberta, measured using an automated precipitation collector adapted to cold regions. Atmospheric Environment, 44, 1672–1681.
Sofianska, E., & Michailidis, K. (2015). Chemical assessment and fractionation of some heavy metals and arsenic in agricultural soils of the mining affected Drama plain, Macedonia, northern Greece. Environmental Monitoring and Assessment, 187, 101.
Suresh, G., Sutharsan, P., Ramasamy, V., & Venkatachalapathy, R. (2012). Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicology and Environmental Safety, 84, 117–124.
Swarnalatha, K., Letha, J., & Ayoob, S. (2014). Effect of seasonal variations on the surface sediment heavy metal enrichment of a lake in South India. Environmental Monitoring and Assessment, 186, 4153–4168.
Swarnalatha, K., Letha, J., Ayoob, S., & Nair, A. G. (2015). Risk assessment of heavy metal contamination in sediments of a tropical lake. Environmental Monitoring and Assessment, 187, 322.
Varol, M. (2011). Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. Journal of Hazardous Materials, 195, 355–364.
Wang, L., Yang, L., Kong, L., Li, S., Zhu, J., & Wang, Y. (2014). Spatial distribution, source identification and pollution assessment of metal content in the surface sediments of Nansi Lake, China. Journal of Geochemical Exploration, 140, 87–95.
Yang, Z., Lu, W., Long, Y., Bao, X., & Yang, Q. (2011). Assessment of heavy metals contamination in urban topsoil from Changchun City, China. Journal of Geochemical Exploration, 108, 27–38.
Yang, J., Chen, L., Liu, L., Shi, W., & Meng, X. (2014). Comprehensive risk assessment of heavy metals in lake sediment from public parks in Shanghai. Ecotoxicology and Environmental Safety, 102, 129–135.
Yang, X., Duan, J., Wang, L., Li, W., Guan, J., Beecham, S., & Mulcahy, D. (2015). Heavy metal pollution and health risk assessment in the Wei River in China. Environmental Monitoring and Assessment, 187, 111.
Yi, Y., Yang, Z., & Zhang, S. (2011). Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environmental Pollution, 159, 2575–2585.
Yin, H., Deng, J., Shao, S., Gao, F., Gao, J., & Fan, C. (2011a). Distribution characteristics and toxicity assessment of heavy metals in the sediments of Lake Chaohu, China. Environmental Monitoring and Assessment, 179, 431–442.
Yin, H., Gao, Y., & Fan, C. (2011b). Distribution, sources and ecological risk assessment of heavy metals in surface sediments from Lake Taihu, China. Environmental Research Letters, 6, 044012.
Zahra, A., Hashmi, M. Z., Malik, R. N., & Ahmed, Z. (2014). Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah—feeding tributary of the Rawal Lake Reservoir, Pakistan. Science of the Total Environment, 470–471, 925–933.
Zhang, Z., Rengel, Z., Meney, K., Pantelic, L., & Tomanovic, R. (2011). Polynuclear aromatic hydrocarbons (PAHs) mediate cadmium toxicity to an emergent wetland species. Journal of Hazardous Materials, 189, 119–126.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (No. 41271213, 41230853), the China National Fund for Water Pollution Control and Management Project (No. 2014ZX07101-011) and the Key Research Program of the Chinese Academy of Sciences (Grant No. KZZD-EW-10). The authors express their thanks to Chen Yun and Xu Weiwei for their assistance with obtaining samples of the surface sediment and sediment cores. The authors are grateful to Zhang Hongmei, Gao Ming, and Zhu Yuxin for their assistance with the total organic carbon and total contents of heavy metals analyses. We are also grateful to the anonymous reviewers for providing constructive comments that helped improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, J., Wang, Y., Liu, X. et al. Spatial distribution and risk assessment of heavy metals and As pollution in the sediments of a shallow lake. Environ Monit Assess 188, 296 (2016). https://doi.org/10.1007/s10661-016-5301-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5301-8