Abstract
Environmental effects due to continuous accumulation of hazardous materials like heavy metals in the surface sediments of lake systems can stress fragile ecosystems. Elucidating the mechanisms influencing the concentration and distribution of heavy metals becomes vital in formulating lake management strategies to preserve the quality of the water environment. Studying of the effect of seasonal variations on surface sediments will help in understanding the different factors and sources contributing and diluting these persistent pollutants. In this study, heavy metal pollution in a tropical shallow lake (Akkulam-Veli) in South India was investigated by monitoring the seasonal variations of heavy metals and major elements in surface sediments. The metallic pollutants (Cr, Ni, Co, Cu, Zn, Pb, Fe, and Mn) and major elements (Si, Ti, Al, Ca, Mg, Na, K, and P (measured as oxides) in the surface sediments of this lake were monitored during four consecutive seasons. The results were subjected to correlation analysis and principal component analysis to study the interrelationships of different parameters as well to determine the possible origin of pollutants. Although metal concentrations were found to be unaffected by seasonal variations, the factors contributing to occurrence of these heavy metals were found to be affected by seasonal fluctuations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activities and related interventions disrupt the natural balance of lake ecosystems that have evolved over long periods of time, resulting in degradation of natural water environments (Kabata-Pendias and Pendias 2001; Salati and Moore 2010). Pollution of lake systems by heavy metals becomes alarming because of their toxic impacts (Feng et al. 2007; Yang et al. 2009). The trace metals in surface sediments are mainly distributed over minerals, organic carbon, iron, and manganese oxides and hydroxides, which are susceptible to redox changes. These redox processes driven by organic carbon degradation and numerous biogeochemical transformations influence the chronic distribution of trace metals between solid and liquid phases and beneath the sediment–water interface (Casas et al. 2002). Variations of these biogeological factors may lead to seasonal release of heavy metals in sediments. Once they desorb and become available to the benthic organisms, the risks associated with bioaccumulation of heavy metals may persist due to the long biological half-life. These metal pollutants require frequent monitoring in rivers and estuaries (Swarnalatha et al. 2013a, b). In addition, deposition of metals in sediments occurs through interaction between sediment and water, wherein metal contents of sediment and water depend on variation of metal concentrations in sediments (Dilip and Subramonian 1998; Nelson et al. 2004; Miguel et al. 2008; Harikumar and Nasir 2010; Wenzhong et al. 2010) and their speciations (Orkun et al. 2010; Yang et al. 2009). Only limited research (Akpan et al. 2002; Orkun et al. 2010) has explored the effect of seasonal variation in the metal enrichment process. Because heavy metal enrichment of surface sediments is an offshoot of geogenic and anthropogenic activities, monitoring programs covering different seasons depict spatial and temporal variations in water chemistry and may offer representative data for more reliable estimations and be useful in assessing the environmental status of any lake system. This could be a difficult task, however, if the actual source of each metal is not readily discernible. We used principal component analysis (PCA) and hierarchical clustering analysis (HCA) to identify the possible commonalities of distribution, association, and concentrations of different metal enrichments.
The present study investigates the temporal and spatial distribution of heavy metal concentration in the surface sediments of a tropical eutrophic urban lake, Akkulam-Veli Lake (AV Lake), located in the southwest of the Indian peninsula. A review of published literature indicates that very few studies (Swarnalatha et al. 2013a, b) have been reported on the heavy metal burden of this lake, but previous studies postulated that the spatial and temporal variability in the processes affecting metal enrichments may have an impact on the sediment quality (Corine et al. 2005). Lack of previous data and speciation studies of heavy metals in AV Lake can hence be successfully overcome by application of multivariate analysis to define possible sources of contamination. In this study, sediment quality in four consecutive seasons was monitored to analyze the effect of seasonal fluctuations. Heavy metals such as Cr, Ni, Co, Zn, and Pb and major elements such as Si, Ti, Al, Mn, Fe, Ca, Mg, Na, K, and P (measured as oxides) in the surface sediments were analyzed. These results were subjected to correlation studies, PCA, and HCA with the major and trace elements to identify the possible sources of contamination.
Materials and methods
Study area
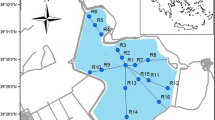
AV lake is situated about 5 km northwest of Thiruvananthapuram city (Fig. 1) in southern India (8° 25′ N–8° 35′ N, 76° 50′ E–76° 58′ E). Thiruvananthapuram is the first city along the path of the southwest monsoons and receives its first showers in early June. The city also receives rain from the receding northeast monsoons by October, before the dry season arrives in December. The area experiences a tropical humid climate (relative humidity 76.2 %) with an average air temperature of 27.5 °C and an annual average rainfall of 1,728.5 mm (Table 1). The warm, humid climate with high precipitation promotes strong physical and chemical processes and high rates of erosion and sediment deposition.
The lake has a length of 3.2 km, an average depth less than 4 m, and an area of around 1 km2, and it is surrounded by laterite hillocks. The lake is partially bisected by a lengthwise bund formation (Sheela et al. 2011). The western side (around 1.25 km long, 100 m wide) forms Veli Lake, and the northeastern part forms Akkulam Lake. For most of the year, these lakes remain separated from the sea due to a sand barrier (around 150 m long and 30 m wide), which remains open for a period of 10–14 days, depending on the influx of land drainage into the lake. Two natural channels (Kulathur and Kannamoola streams) drain into this lake. These channels have been converted into major sources of pollutants significantly contributing to the heavy metal burden of the lake (Swarnalatha et al. 2013). In addition to the huge sewage load, the lake receives surface runoff, industrial wastes, hospital wastes, tourism by-products, and wastes from various developmental activities. Because the tidal phenomena are weak, insufficient, and infrequent, complete flushing of wastewater from the lake is insufficient. As a result, the lake is in a eutrophic condition, leading to prolific growth of water hyacinth (Eichornia crassipes), a notorious aquatic weed.
Sampling and analysis
Sampling
Thiruvananthapuram has a climate that borders a tropical savanna climate and a tropical monsoon and as a result does not experience distinct seasons. The mean temperature is 34 °C, and the mean minimum is 21 °C. The annual maximum rainfall is in June, July, and August (Table 1). To study the influence of seasonal variations in the heavy metal concentrations of surface sediments of AV Lake, samples were selected in four consecutive seasons (S1, S2, S3, and S4) from January 2011 to January 2012. The post-monsoon data obtained in January 2011 (S1) is from a transition period after the monsoon showers, which was followed by a dry summer season from February to May. The pre-monsoon samples were hence collected in the dry season in May 2011 (S2), just before the onset of monsoon. The showers extended for the full rainy period up to August, and wet season samples were collected in September 2011 (S3), soon after the showers. S4 samples were again taken during post-monsoon in January 2012. The 15 sampling points (Fig. 1) were distributed in the lake to represent major pollution sources. Sampling stations 1 to 7 were on the Akkulam side of the lake and 8 to 15 on Veli side. Because the lake was partially blocked with E. crassipes, a highly invasive floating macrophyte, all 15 sampling points were not accessible at once. The upper sediments (0–15-cm depth) of the lake were taken using Van Veen grab samplers. At each station, three sediment samples were collected and mixed on site, to get representative sampling. Samples were then placed in polyethylene bags and transported to the laboratory (UNEP 1985). The collected samples were air dried, sealed in clean polythene bags, and stored in a refrigerator for characterization studies (Singh et al. 2005).
Sample preparation and analysis
Deionized water was used throughout to prepare reagents and for digestion. All glassware and polyethylene bottles were washed with metal free soap, rinsed thoroughly, and soaked overnight in 50 % nitric acid solution to prevent metal contamination. All chemicals and standard solutions used were analytical grade. The pH and oxidation reduction potential (ORP) of sediments were recorded at site. Salinity, carbonates, and sulphate content of sediments were determined per standard methods (APHA 2005). The cation exchange capacity (CEC) was determined by leaching sediment samples with ammonium acetate (Chapman 1965). Organic content of sediments was determined using the wet oxidation method by oxidizing a known quantity of chromic acid and titrating with standard ferrous ammonium sulphate solution using a diphenylamine indicator (El Wakeel and Riley 1957). Particle size distribution was determined by pipette analysis (Buchnan and Kain 1974). The dried sediment samples were further ignited at 950 °C for 1 h in a muffle furnace, and the residue was ground into microscopic particles (0.5 μm > d < 20 μm) using an agate mortar. The finely powdered sample was sprinkled over boric acid, and pressed pellets were prepared for element determination. The sample was then analyzed for total heavy metals (Cr, Ni, Cu, Zn, Pb, Co, Fe, and Mn) and major elements (Si, Ti, Al, Ca, Mg, Na, K, and P, measured as oxides) using X-ray fluorescence (XRF) spectrophotometer (Osan et al. 2002; Solecki and Chibowski 2009; CESS 2009). The XRF equipment includes a Bruker-model S4 Pioneer sequential wavelength-dispersive X-ray spectrometer with sample preparation units (CESS 2009; Swarnalatha et al. 2013a, b).
Guideline values
The threshold effect concentration (TEC) and the probable effect concentration (PEC) for sediment levels were reported by MacDonald et al. (2000). Because no previous studies were conducted on the sediment of AV Lake, no background concentration data was available; hence, the background concentrations of heavy metals in average continental shale and upper continental crust were taken from Turekian and Wedepohl (1961) and Wedepohl (1995) and used in this study for comparison. Because not all sampling stations were accessible throughout the year (due to infestation of water hyacinth), pollutant concentrations corresponding to each sampling station may not yield reliable analysis. Average values of metal concentrations were therefore considered in this study.
Descriptive analysis and multivariate analysis
The analysis of descriptive data generated maximum, minimum, median, mean, standard deviation, skewness, and variance values. Correlation, PCA, and HCA studies were used to define geogenic and anthropogenic origin and to identify possible non-point sources of contamination. Statistical analysis was carried out using SPSS 17 statistical package. Correlation analysis was performed on monsoon data (S3) between heavy metals and the physicochemical characteristics of sediments, including pH, ORP, CEC, texture, salinity, carbonates, and sulphates. PCA was conducted for metal concentrations and major elements during the four seasons (with loadings greater than 0.6 considered significant), using varimax rotation. This method can reduce the complexity of data by projecting onto a small number of variables called principal components (Ikem and Adisa 2011). HCA was also done to study the interactions of heavy metal concentrations with sediments on the four sets of data using Wards method and squared Euclidean distance as a measure of the proximity between samples.
Results and discussion
Characteristics of sediments
The physicochemical characteristics of sediments in AV Lake (Table 2) show that the adsorptive capacities of metal on sediment particles, soil, and suspended solids increase with pH. Heavy metals have maximum adsorption on sediments at its characteristic pH value (Yang 2003). The sediment of the study area was found to be nearly neutral, with an average pH of 7.39, favoring adsorption of heavy metals on sediments. The average specific gravity of 2.17 suggests that clay and silt readily accumulate at low turbulent conditions. The relevant textural and other properties of sediments (Fig. 2) show that the organic content of sediments varied from 0.6 to 3.2 %, suggesting uneven discharge of sewage from feeding canals and plant debris into the lake. Sand was found to be dominant toward the seaward side, and its content (13.6–70.3 %) was found to decrease in an opposite direction with an increased quantity of silt and clay. The carbonate content varied from 3.9 to 5.8 %.
Heavy metal analysis
The seasonal variations of heavy metal, major element concentrations, and their summary statistics (Table 3) show higher pollutant concentrations on the Akkulam side. The pollutant concentrations at all seasons were maximum near the confluence point of Kannammoola stream (near station 1) and TS canal (between stations 8 and 9). The buried barrier at the end of the Akkulam part of the lake restricts the flow and creates stagnant conditions in Akkulam Lake, resulting in accumulation of pollutants in this region (Station 7). Thus, station 7 was found to be the most polluted part of the lake on the Akkulam side, even though the point sources of discharge are far apart. The minimum concentration values toward the sea may be due to the occasional influx of sea water. Due to lack of data from all stations at all seasons, average values of metal concentrations were used to evaluate the effect of seasonal variations (Table 3).
No significant variations in metal concentrations were observed between S3 (monsoon) and S2 (pre-monsoon) sediments, indicating that seasonal changes were not sufficient to dilute the heavy metal concentrations of surface sediments in AV Lake. Similar results were obtained by Orkun et al. (2004), possibly because metal pollutants reach the lake continuously during all seasons. During monsoon, the lake was receiving wastewater from numerous sources (even from the remote point of the catchment) as runoff, as well as the urban wastes through the canals, and hence no dilution of pollutants was observed. This also indicates the constant and continuous influence of the main polluting sources (i.e., the two canals) discharging urban sewage into the lake. We observed that Cr, Ni, and Zn were available in significant concentrations in the lake sediments, with the maximum concentration found in S4 (post-monsoon) sediments.
The excessive growth of water hyacinth observed during this period may be ascribed to the increased nutrient availability due to upwelling during wet season as well as other favorable physicochemical factors. Prolific growth of aquatic plants may lead to increased production of organic carbon, followed by chelation of dissolved metals and their settlement, a situation that may lead to high heavy metal enrichment in sediments. The sediments showed distinctly higher concentrations of Fe2O3, P2O5, and TiO2. High concentrations of Fe2O3 during dry season may be ascribed to continuous discharge of municipal sewage. Fe in sediments may co-precipitate other metals and, on dissolution, may release them to the water phase under changing environmental conditions (Akpan et al. 2002). Phosphorous (P) appears in various chemical forms, and mobilities, and is adsorbed onto iron and aluminum (hydr) oxides. During sediment re-suspension events, P minerals (i.e., apatite, vivianite, and strengite) may also contribute to the suspended solids in the water column (Koelmans 1998). Heavy minerals such as ilmenite and rutile are important Ti-bearing minerals found on the west coast of South India. The moderately higher concentration of TiO2 throughout the study area in comparison with the world average could be attributed to the heavy minerals present in this region.
The maximum metal concentrations were observed in S4 sediments. Co, Cu, and Zn had slightly increased concentrations, whereas Cr had a slight decrease in concentration from S1 to S3, although there was no significant change in maximum values of metal concentrations. The average concentrations of Cr and Ni were found to exceed TEC, PEC, and upper continental crust (UCC) and average shale values, which could adversely affect aquatic life. To a lesser extent, Co, Cu, and Pb present in surface sediments were found in elevated concentrations compared to shale values. All metal concentrations were found to exceed average shale values (Turekian and Wedepohl 1961) and UCC values (Wedepohl 1995), indicating an enrichment in sediments. Zn had average values similar to TEC, although lower than shale values. The heavy metals adsorbed onto the sediments may be remobilized back to water phases, exposing aquatic biota to risks of intoxication and bioaccumulation. The significantly higher concentrations of metals and major elements during the wet season could be ascribed to anthropogenic input through surface run-off loaded with municipal wastes and catchment erosion (Handong and Neil 2005).
Statistical analyses
Correlation studies
Correlation analysis (Table 4) showed that metal concentrations of S1 have poor correlation among metals compared to other seasons. The significant relations (p < 0.01) found between metals were of Cr and Co (0.66), Ni and Pb (0.92), and Zn and Cu (0.81). Fe/Mn oxides were also correlated with metals such as Cr and Fe2O3 (0.64), Co and Fe2O3 (0.78), Cu and MnO (0.61), and Zn and MnO (0.77). SiO2, TiO2, Na2O, CaO, and MgO were found to have only insignificant correlations with all metals except Pb and Ni. The high positive relation of Ni and Pb with Al2O3 (0.68, 0.89, respectively) and insignificant relations with Fe and Mn (0.04, 0.13; 0.29, 0.17, respectively) indicate the lithogenic source of these metals in S1.
The interrelationship among metals significantly increased in the pre-monsoon season S2. Thus, Cr was correlated with Co (0.83), Ni (0.88), Cu (0.64), and Pb (0.78); Pb was related with Ni (0.96), Cu (0.92), and Zn (0.73); and Zn was found to be correlated with Cu (0.86), as reported in many previous studies (Casas et al. 2002; Dilip and Subramonian 1998; Yang et al. 2009). The metals exhibited strong association with major elements such as MgO, K2O, and MnO. We found that metals were more associated to Fe2O3 and MnO than Al2O3, implying close associations of metal concentrations with Fe/Mn oxides. The negative relationship with Na2O was indicative of dilution of pollutants. Metals showed a positive relation with Al2O3 and negative relations with SiO2, suggesting affinity of metal ions to the finer fractions of sediments (Dilip and Subramonian 1998). The relatively higher correlation between Cu and Zn, their lesser correlation with the lithophile elements Fe and Mn, and higher concentration with P2O5 suggest their source as the organic input from the sewage inputs through the canals. Higher correlation of Ni with Al2O3, Fe2O3, and MnO suggests lithogenic origin of the metal.
Regarding the correlations in wet season, S3 values were similar to those in S2. High correlations were observed for Cr and Ni (0.96), Cr and Pb (0.95), Ni and Pb (0.98), and Cu and Zn (0.94). Of the 15 correlations among metals, 14 had r > 0.70, suggesting a common origin or similar geochemical behavior (Korfali and Davies 2001) of metals during this season. All metals showed closer relations with Al2O3 (r > 0.66), MnO (r > 0.74), Fe2O3 (r > 0.69), MgO (r > 0.78), K2O (r > 0.5), and P2O5 (r > 0.72), possibly ascribed to the adsorptive properties of heavy metals with organic matter and clay fractions. The high correlation between Fe2O3 and MnO (0.95) (Table 5) suggests the presence of Fe/Mn compounds (Dilip and Subramonian. 1998; Fatma et al. 2009). Fe and Mn are dynamic participants in the redox cycle ( Zhang et al. 2009) and have a high affinity with most trace metals (Fatma et al. 2009). Diagenetic processes involving Mn and Fe and redox conditions control the behavior of metals in the sediments (Pradit et al. 2009). In general, the positive relations between metals and major elements suggest lithogenic as well as anthropogenic contributions in this season; negative relationships of SiO2 and TiO2 with metals suggest their diluting effect on metallic pollutants. Quartz can be considered a diluting phase for trace elements, which may lead to lower concentration as quartz content (often found in coarser grain size particles) increases.
Metals with significant relations in S1 such as Zn and Cu, Pb and Ni, Co and Fe2O3, Cu and Mn, and Zn and Mn also had consistent relationships (0.93, 0.65, 0.76, 0.66, and 0.70, respectively) in S4. The significant relations observed in S4 such as Cr and Ni (0.96), Co and Cu (0.76), Co and Al2O3 (0.77), Co and K2O (0.89), Ni and Al2O3 (−0.64), Ni and Fe2O3 (−0.61), and Ni and Mn (−0.6) were not found in S1. The high correlation values of Co with Fe, Al, and Mn indicate that Co originated from geogenic sources. These similarities and variations in relations suggest the complexity of pollutants as well as fresh unauthorized sources entering the lake irrespective of season, which may be linked to the rapid urbanization in this area. CaO and Na2O had insignificant relations with metals in all seasons. Ca and Na were relatively more soluble and have low affinity for particles, and therefore their concentration in sediments is less likely to be affected by processes such as exchange and adsorption.
Based on the correlation matrices, inorganic scavengers (Fe/Mn oxides followed by Al) are the dominant factors controlling trace metal distribution in AV Lake sediments in the post-monsoon and monsoon seasons, whereas both inorganic and organic (TOC) factors play a key role in the monsoon season. The relations between different metals and major elements were found to be stronger in S2 and S3 than post-monsoon periods. The number of positive relations between metals and major elements having r > 0.6 for the seasons S1, S2, S3, and S4, respectively, is Cr (2,8,10,1), Co (2,6,10,5), Ni (2,11,10,2), Cu (2,6,10,8), Zn (2,4,11,7), and Pb (2,7,10, 2). The strong association among metals, and among metals and major elements, suggests the complex nature of the system.
Correlation analysis of heavy metal concentrations with the physico − chemical parameters of sediments using S3 data (Table 5) shows that all metals were found to have significant relations (>0.7) with TOC, which is consistent with previous studies (Fatma et al. 2009; Nelson et al. 2004). The high relations among Cr, Co, Ni, Cu, Zn, Pb, Fe, Mn, and TOC (>0.73) reflect the complex nature of organic matter (Muthuraj and Jayaprakash 2008). Silty texture was found to have a stronger correlation (0.36 − 0.57) with all metals than clayey texture (0.02 − 0.42). pH (−0.04 − 0.25) and ORP (−0.28 − 0.04) had no significant relations with heavy metals, except moderate relations with Cu and Zn in this study. We found that CEC had moderately good correlation with heavy metals (0.14–0.54), especially Ni (0.54), Cu (0.53), and Pb (0.47). As surface area increases with reduction in grain size, CEC increases significantly, and hence trace metals which are mainly cations may be increasingly adsorbed. For carbonate (0.16–0.53) and sulphate (0.13–0.47) concentrations, moderate relations with metal concentrations were observed. Based on the correlation matrices, inorganic scavengers (Fe/Mn oxides followed by Al) were found to be dominant factors controlling trace metal distribution of AV Lake sediments in the post-monsoon and monsoon seasons whereas both inorganic and organic (TOC) factors seemed to play key roles in monsoon season.
PCA analysis
After varimax rotation, three principal components were retained in all four seasons for ease of comparison, which could explain 96.7, 83.7, 85.7, and 78.6 % of the total variance respectively. PCA analysis on the heavy metal concentrations was conducted for the four seasons (Table 6). In S1, the first component explained 40.84 % of the total variance and grouped Cr, Co, Fe2O3, MnO, and K2O with high positive loadings ranging from 0.63 to 0.98, suggesting that Cr and Co may be associated with Fe and Mn oxides/hydroxides. The second component explained 33.8 % of the total variance and grouped Ni and Pb along with Al2O3 with high loadings (>0.85), indicating their lithogenic sources, a finding consistent with earlier correlation studies. Cu and Zn were found to be associated with TOC in the third component, which explained 22.1 % of the variance, and the relation between Cu and Zn was found to be consistent with correlation studies, as stated earlier. Figure 3 shows a pictorial representation of metal loading in the first two principal components. Thus, association of Cr with Co and Fe2O3 and Ni with Pb and Al2O3 is clearly seen.
S2 sediments showed a similar trend to that of S1; Cr, Co, Fe2O3, MnO, and K2O with high positive loadings were placed in the first component, explaining 35.7 % of total variance in S2 sediments. The second component explained that 29.04 % of the total variance had metals Ni and Pb (>0.6) with parameters Al2O3 and Fe2O3 exhibiting high positive loadings (>0.6), reconfirming its relation with fine-grained sediments. The third component explained 19 % of the total variance and grouped Cu, Zn, and P2O5, representing the influence of organic fraction. Links to organic fractions may be ascribed to the present eutrophied condition (Sheela et al. 2011) of the lake and organic inputs (from dumping of sewage and other wastes into the lake through the two canals). TiO2 was found to be closely related to SiO2, demonstrating association with sandy texture, which was further confirmed by the negative correlation between TiO2 and Al2O3.
S3 exhibited strong relations of metals and major elements with Cr, Co, Ni, Cu, Zn, Pb, Fe, Mn, Al2O3, CaO, MgO, and P2O5 (placed in the first component) accounting for 58.6 % of total variance. The observed high loadings of Fe and Mn were found to be consistent with correlation results (Table 4) also. Association of these factors in the first component suggests that the contributions may not be limited to anthropogenic sources because natural sources such as weathering of exposed hills and erosion or runoff from catchment area also contribute. Thus, it could be observed that natural (land based) as well as anthropogenic sources/factors were instrumental in the metal enrichment of surface sediments. Further, the geochemical (Al2O3) and inorganic (Fe2O3) phases were instrumental in metal binding. In the post-monsoon sediments during S4 (Table 4), high negative loadings of Cr, Ni, and Pb (in the first component) were indicative of same source. These metals were not found related with any major elements in this season, unlike other seasons; thus, the observed high concentrations of Cr and Ni in S4 sediments were due to new or fresh waste sources into the lake. The low loading of SiO2 (0.59) may be associated with Cr and Ni released from weathering process or due to proximity to sea. Cu, Zn, Fe, and Mn were associated with organic fractions. High organic loading was found in more than one factor, indicating multiple sources of pollutants.
Cluster analysis
HCA was done on the four sets of data separately, and the results are shown in Figs. 4, 5, 6, and 7, respectively. Two main clusters with significant linkage distance and independent nature can be observed in the dendrogram (Fig. 4) from the S1 data. The first cluster housing parameters of lithogenic nature alone indicate that the metal contamination of sediments in this season is of purely anthropogenic origin. The association of Cr, Co, Fe2O3, MnO; Ni, Pb, Al2O3; Cu, Zn, TOC; and SiO2, TiO2 in the different sub-clusters are consistent with the results obtained from PCA studies. Similar results are seen in the dendrograms (Figs. 5, 6, and 7) obtained for the remaining data also.
We observed that correlation studies, PCA, and CA suggest consistent associations of Cr–Ni, Cr–Fe, Co–Fe, Ni–Al, Pb–Al, Cu–TOC, and Zn–TOC, which may be indicating possible identical sources of metal contamination. The synergy of various major elements may positively influence the occurrence of heavy metals, consistent with correlation and factor analysis (Table 7). Although there was no significant variation in the concentration of heavy metals with change of seasons, the predominant factors controlling occurrence of heavy metals changed from S1 to S4, indicating influence of multiple sources (anthropogenic, geological, or background lithogenic factors). The influx of heavy metals is a common consequence of rapid urbanization and associated land clearing, road and building construction, and increased road traffic (Ashley and Napier 2005). The lack of a clear relation between concentrations of heavy metals with seasons may be attributed to the changing rainfall pattern observed for the past decade. The rainfall data (Table 1) shows the inconsistency in the intensity of showers in the different months of a year, with rainfall occurring in varied intensities in all months of the year, leading to a prolonged wet period and, thus providing little seasonal demarcations. This unpredictable behavior may be attributed to the potential effect of global climate change scenario.
Co, Fe, Mn, and K were found to be closely related in all seasons (Table 7). The association of Cr and Co in oxy/hydroxides of Fe and Mn was well established. Si and Ti were found to be negatively related with Co, Cu, Zn, and Pb in all seasons. Ni and Pb relations seemed unaffected by seasons, as shown in correlation studies. The close association of Ni with Al2O3 could be linked to its natural origin, as cited by Weiguo et al. (2009). The close relation of Cu and Zn with Fe2O3, MnO, TOC, and P2O5 indicates the close relation of these metals with the organic inputs reaching the lake.
Conclusion
The surface sediments of AV Lake were found to be loaded with heavy metals such as Cr, Zn, Cu, Ni, and Pb, and major elements with high concentrations of Cr, Ni, and Zn. Average concentrations of Cr and Ni were found to be higher than TEC and PEC values indicating possible adverse impacts on water and the aquatic environment. The effect of seasonal variations was found to be insignificant in the heavy metal enrichment process. However, statistical analyses revealed that contributing factors of metal enrichments vary with season. Multivariate analysis established relations of Cr and Co with Fe/Mn; Ni with Al; and Zn, Cu, Pb with P. The influence of these predominating factors in different seasons favoring the presence of heavy metals highlights the complex nature of the system. This complexity may be due to the combined influence of factors such as continuous discharge of sewage wastes, runoff from urban drains, and addition of coarser sediments from catchment reaching the lake in multiple proportions. The proximity to heavily populated urban settlement combined with indiscriminate disposal of industrial effluents may be aggravating the problem. The unique features of the lake—shallow depth, proximity to sea, stagnant conditions, and lack of sufficient dilution—may also be enhancing the accumulation of pollutants. The unpredictable seasonal variations in heavy metal concentrations may be linked to the complex behavior of climate change phenomena. Due to irregular rainfall patterns, the study of seasonal variations in heavy metal concentrations is difficult, suggesting the need for a regular monitoring program for the restoration and rejuvenation of the lake environment.
References
Akpan, E. R., Ekpe, U. J., & Ibok, U. J. (2002). Heavy metal trends in the Calabar River, Nigeria. Environmental Geology, 42, 47–51.
APHA. (2005). Standard methods for the examination of water and waste water (21st ed.). Washington: American Public Health Association.
Ashley, P. M., & Napier, M. E. (2005). Heavy-metal loadings related to urban contamination in the Kooloonbung Creek catchment, Port Macquarie, New South Wales. Australian Journal for Earth Sciences, 52, 843–862.
Buchnan, & Kain. (1974). Methods for the study of Marine Benthos. In N. A. Holme & A. D. Mclntyre (Eds.), IBP handbook,16th edition (pp. 30–58). Oxford: Blackwell Scientific Publication.
Casas, J. M., Rosas, H., Sole, M., & Lao, C. (2002). Heavy metals and metalloids in sediments from the Llobregat basin, Spain. Environmental Geology, 44, 325–332.
CESS. Centre for Earth Science Studies, Trivandrum. (2009). Preparation of samples for XRF studies, www.cess.res.in, accessed 12 Jan 2011.
Chapman, H.D. (1965). Cation-exchange capacity. In: C. A. Black (ed.), Methods of soil analysis—chemical and microbiological properties. Agronomy. Vol. 9, pp. 891–901
Corine, V. G., Marloes, L., Jan, J., & Albert, A. K. (2005). Temporal variation of trace metal geochemistry in floodplain lake sediment subject to dynamic hydrological conditions. Environmental Pollution, 137, 281–294.
Dilip, K. D., & Subramonian, V. (1998). Distribution and fractionation of heavy metals in the surface sediments of the Ganges-Brahmaputra-Meghna river system in the Bengal basin. Environmental Geology, 36, 93–101.
El Wakeel, S. K., & Riley, J. P. (1957). The determination of organic carbon in marine sediments. Journal du Conseil International pour l'Exploration de la Mer, 22, 180–183.
Fatma, Ç., Münir, Z., Lugal, G., Osman, B. D., & Özlem, F. (2009). An assessment of metal pollution in surface sediments of Seyhan dam by using enrichment factor, geoaccumulation index and statistical analyses. Environmental Monitoring and Assessment, 152, 309–317.
Feng, Z., Huaicheng, G., & Lei, L. (2007). Quantitative identification and source apportionment of anthropogenic heavy metals in marine sediment of Hong Kong. Environmental Geology, 53, 295–305.
Handong, Y., & Neil, R. (2005). Trace metal pollution records in some UK lake sediments, their history, influence factors and regional differences. Environment International, 31, 63–75.
Harikumar, P. S., & Nasir, U. P. (2010). Ecotoxicological impact assessment of heavy metals in core sediments of a tropical estuary. Ecotoxicology and Environmental Safety, 73, 1742–1747.
Ikem, A., & Adisa, S. (2011). Runoff effect on eutrophic lake water quality and heavy metal distribution in recent littoral sediment. Chemosphere, 82, 259–267.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soil and plants. Boca Raton: CRC.
Koelmans, A. A. (1998). Geochemistry of suspended and settling solids in two freshwater lakes. Hydrobiologia, 364, 15–29.
Korfali, S. I., & Davies, B. E. (2001). A comparison of metals in sediments and water in the river Nahr-Ibrahim, Lebanon. Environmental Geochemistry and Health, 25, 41–50.
Mac Donald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus based sediment quality guidelines for fresh water ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
Miguel, A., Huerta, D., Francisco, D., Martin, A., Jose, A., Segovia, Z., Zaul, G. E., Hector, L., Zarate, Arturo, S. V., & Salvador, G. B. (2008). Diagnosis of trace metal contamination in sediments: the example of Ensenada and El Sauzal, two harbors in Baja California, Mexico. Marine Environmental Research, 66, 45–358.
Muthuraj, S., & Jayaprakash, M. (2008). Distribution and enrichment of trace metals in marine sediments of Bay of Bengal, off Ennore, south east of India. Environmental Geology, 56, 207–217.
Nelson, B., Yu-Wei, C., John, M., Gunnb, & Sushil, S. D. (2004). Sediment trace metal profiles in lakes of Killarney Park, Canada: from regional to continental influence. Environmental Pollution, 130, 239–248.
Orkun, I. D., Galip, S., Demet, G. K., Turan, Y., & Cagatayhan, B. E. (2010). Speciation and implications of heavy metal content in surface sediments of Akayatan Lagoon-Turkey. Desalination, 260, 199–210.
Osan, J., Kurunazi, Torok, S., & Van Grieken, R. (2002). X-ray analysis of river bank sediment of the Tisza (Hungary): identification of particles from a mine pollution event. Spectrochimica Acta Part B, 57, 413–422.
Pradit, S., Wattayakorn, G., Angsupanich, S., Baeyens, W., & Leermakers, M. (2009). Distribution of trace elements in sediments and biota of Songkhla lake, Southern Thailand. Water Air Soil Pollution. doi:10.1007/s11270-009-0093-x.
Salati, S., & Moore, F. (2010). Assessment of heavy metal concentration in the Khoshk River water and sediment, Shiraz, Southwest Iran. Environmental Monitoring and Assessment, 164, 677–689.
Sheela, A. M., Letha, J., & Joseph, S. (2011). Environmental status of a tropical lake system. Environmental Monitoring and Assessment, 180, 427–449.
Singh, K. P., Mohan, D., Vinod, K. S., & Malik, A. (2005). Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges, India. Journal of Hydrology, 312, 14–27.
Solecki, J., & Chibowski, S. (2009). Examination of trace amounts of some heavy metals in bottom sediments of selected lakes of South Eastern Poland. Polish Journal of Environmental Studies, 9, 203–208.
Swarnalatha, K., Letha, J., & Ayoob, S. (2013a). An investigation into the heavy metal burden of Akkulam–Veli Lake in south India. Environmental Earth Sciences, 68(3), 795–806.
Swarnalatha, K., Letha, J., Ayoob, S., & Sheela, A. M. (2013b). Identification of silicon as an appropriate normaliser for estimating the heavy metals enrichment of an urban lake system. Journal of Environmental Management, 129, 54–61.
Turekian, K. K., & Wedopohl, K. H. (1961). Distribution of the elements in some major units of the earth’s crust. Geological Society of America, 72, 175–192.
UNEP. (1985). Reference methods for marine pollution studies’, United Nations Environment Program Regional seas, pp. 31–39.
Wedepohl, H. (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta, 59, 1217–1239.
Wenzhong, T., Baoqing, S., Hong, Z., & Zhanpo, M. (2010). Heavy metal sources and associated risk in response to agricultural intensification in the estuarine sediments of Chaohu Lake Valley East China. Journal of Hazardous Materials, 176, 945–951.
Yang, X. (2003). World meteorological organization Operational hydrology report no. 47, Manual on sediment management and measurement, WMO No. 948.
Yang, Z., Ying, W., Zhenyao, S., Junfeng, N., & Zhenwu, T. (2009). Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. Journal of Hazardous Materials, 166, 1186–1194.
Zhang, W., Feng, H., Chang, J., Jianuo, Xie, H., & Yu, L. (2009). Heavy metal contamination in surface sediments of Yangtze river intertidal zone: an assessment from different indexes. Environmental Pollution, 157, 1533–1543.
Acknowledgments
Authors thank the Centre for Earth Science Studies (CESS), Thiruvananthapuram, for extending laboratory facilities. The financial assistance from the Kerala State Council for Science, Technology, and Environment (KSCSTE), Government of Kerala, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Swarnalatha, K., Letha, J. & Ayoob, S. Effect of seasonal variations on the surface sediment heavy metal enrichment of a lake in South India. Environ Monit Assess 186, 4153–4168 (2014). https://doi.org/10.1007/s10661-014-3687-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3687-8