Abstract
The aim of the work was to evaluate the ecotoxicity of reclaimed agroindustrial wastewaters used for irrigation through ecotoxicological bioassays and biomarkers. The ecotoxicological monitoring was addressed on both treated wastewaters and irrigated soils. Wastewater biomonitoring was performed by the acute test with Daphnia magna, the Microtox® test, and a new in vitro patented method. Soil quality monitoring was performed by the acute and chronic tests with the earthworm Eisenia fetida and biomarker analysis, such as lysosomal membrane stability, general stress biomarker of chemical pollution, and metallothionein, specific biomarker of exposure to heavy metals. Overall the integrated ecotoxicological analysis excluded the presence of ecotoxicity both in the reclaimed waters resulting from tertiary treatment and in the irrigated soils. In particular, the analysis of metallothionein allowed to exclude the accumulation of bioavailable heavy metals in the soil. This study suggests the suitability of ecotoxicological methods for the biomonitoring of water and soil during the reclaimed wastewaters reuse for irrigation, contributing to improving the process of agricultural re-use of wastewater in terms of assessment of the toxicological safety of the waters for the environment, for traders and consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agricultural food production involves intensive use of water and soil, and at the same time it produces a considerable impact on their quality and availability (Krishnan 2009). To date agricultural activities consume about 50–60 % of the available water, including withdrawal from surface waters, natural or artificial reservoirs and groundwater. In addition, the activities of agricultural food production are often sources of pollution to the waters themselves and to the soil. The misuse of pesticides and fertilizers, the mismanagement of manure and agribusiness wastewater can contribute to pollution of ground and surface water and to the accumulation of pollutants in the soil.
The protection of water and soil in the agri-food cycle involves the need to save these resources and at the same time to realize an efficient and effective monitoring of their status in relationship to the impacts.
The need to save water in the agricultural activities has contributed to the awareness of the importance of the reuse of water for irrigation. The use of treated wastewater in agriculture is one of the priorities expressed by the legislation concerning the management and protection of water resources in several countries, including Italy (Law 36/94, Decree 152/06 and DMN 185/2003 Directive EC/2000/60). In particular, a great importance has been attributed to the reuse concept in the agri-food cycle. For example, food processing industries produce large quantities of wastewater that, if refined with tertiary treatment, could be an important source of water, converting a “refuse” in “resource” and contributing to the preservation of water resources in the environment.
However, the reuse of wastewater raises concern about treated wastewater quality assessment. In fact, the quality of reclaimed water should be well established prior to its reuse, in order to prevent potential long-term pollution hazard for the environment and potential risks for human health. Chemical analysis cannot meet alone the requirement of an effective and efficient monitoring. In fact, the chemical classes of compounds to be monitored in wastewaters and in the irrigated soils are not always known, the high cost of chemical analysis makes very expensive the determination of all the possible pollutants that can be present in water and soil, and the analytical determination of all the single pollutants does not provide information about the bioavailability of the pollutants themselves and the toxicity for living organisms resulting from the additives and/or synergistic effects that more contaminants simultaneously present can exert. Therefore, there is a great interest in the use of sensitive analytical methods that can be simple to use, inexpensive and potentially transportable in situ.
The use of effect-based techniques such as ecotoxicological bioassays and biomarkers is receiving a growing interest in the monitoring and assessment of wastewater treatment. Ecotoxicity bioassays are used worldwide to help assess environmental media quality, because they can integrate the various complex effects of contaminants on the test organism (Costa et al. 2008). However, because of the varying sensitivity of indicator organisms to harmful factors, ecotoxicological tests are carried out with the simultaneous use of several assays (Manzo et al. 2008). On the other hand, molecular and cellular biomarkers on exposed organism can be helpful for gaining insight regarding the mechanisms causing observed effects of chemicals and may, in some cases, provide useful indicators of exposure (Forbes et al. 2006; Schettino et al 2012). The strength of the use of bioassays and biomarkers is that these endpoints can provide reliable indications of the degree of exposure and of the resulting effects on the test organism. In this regard, the use of bioassay/biomarkers offers early warning information to be used to improve the processes of environmental risk assessment (Martínez-Gómez et al. 2010).
The present study was aimed at the evaluation of the ecotoxicity of treated reclaimed agroindustrial wastewaters utilized for irrigation by the integrated use of ecotoxicological bioassays and biomarkers on both treated wastewaters and irrigated soils. This study wants to lay the groundwork for the development of ecotoxicological protocols useful for improving the assessment of treated agroindustrial wastewater quality and suitable to detect the occurrence of any signs of pollution in the reclaimed wastewaters and in the irrigated soils. The treated reclaimed agroindustrial wastewaters analyzed in the present study came from the treatment plant of an agricultural and food manufacturing company provided with a secondary and a membrane filtration tertiary treatment. Membrane filtration in combination with other physicochemical processes is receiving a recent growing interest in the treatment of agroindustrial wastewaters (Zagklis and Paraskeva 2014).
The acute test with Daphnia magna and the Microtox® test (ISPRA 2013) were chosen for treated wastewater monitoring. In parallel, the applicability of a new in vitro patented method (Patent n. WO 2009/135537) for the toxicity assessment of reclaimed wastewaters was tested. The method is based on the in vitro inhibition measurement of the enzyme carbonic anhydrase by multiple contaminants present in aqueous environmental samples. The inhibition of the enzyme is proportional to the toxicity of the sample. Soil quality monitoring was performed by the acute and chronic tests (OECD 1984b, 2004) with the earthworm Eisenia fetida. Earthworms are particularly useful in the soil biomonitoring. They are essential for soil fertility, as they contribute greatly to the recycling of organic materials and its transformation into humic compounds and nutrients easily absorbed by plants. Due to their close interaction with the soil, earthworms are very sensitive to the presence of pollutants in the soil. Therefore, they can be called as “sentinel organisms” of soil chemical contamination (Calisi et al. 2009, 2011a, b, 2013, 2014). The study on the earthworms was deepened by the analysis of molecular and cellular biomarkers, such as lysosomal membrane stability, general stress biomarker of chemical pollution, and metallothionein, specific marker of exposure to heavy metals, in specimens exposed for 30 days to the soil irrigated with the agroindustrial treated wastewaters.
2 Methods
2.1 Experimental design
The experimental activity was carried out in an agricultural area in the Foggia district (Stornarella: 41°15′N, 15°44′E; altitude, 154 m a.s.l.) of the Apulian region in Southern Italy, on a site belonging to an agricultural and food manufacturing company, which produces and processes vegetables. The experimental area is characterized by a Mediterranean climate, with warm to hot, dry summers and mild to cool, wet winters (mean annual rainfall of 590 mm). The treated wastewaters used in this study were taken from the treatment plant that purifies all of the wastewater produced by the company during industrial processing of vegetables. The incoming wastewaters undergo a preliminary treatment through a 6-mm sieve screen, to separate out the coarse organic waste. The effluent water then goes into an equalization tank, for the secondary biological treatment. At the end of this phase, the wastewater is clarified in a secondary settler, and the sludge is separated out (Gatta et al 2015). The plant is also provided with a membrane-based tertiary treatment, preceded by a quartzite prefilter that removes any suspended matter from the secondary sedimentation tank.
The experiment of irrigation reuse of reclaimed agroindustrial wastewaters was carried out in the period April to August 2013 on an agricultural soil cultivated with tomato (Solanum lycopersicum L.; formerly Lycopersicon esculentum Mill.) and located near to the company wastewater treatment plant. The main characteristics of the soil layer of the experimental site (0–60 cm) were: sand, 40.1 %, loam 32.5 %, clay 27.4 %, organic matter 1.6 %, pH 7.9 (Gatta et al. 2015). The agricultural soil was divided in plots (15 m × 30 m) exposed to three different experimental irrigation treatments during the growing season (April–August): irrigation with groundwater, irrigation with secondary and tertiary treated agroindustrial wastewater, respectively. Each irrigation treatment was replicated on three plots. The ground water was from a water source that is commonly applied for crop irrigation in the experimental area.
Three water samplings (groundwater, secondary and tertiary treated waters) were performed at monthly intervals throughout the tomato irrigation period. The samples were collected in sterile 500-ml glass bottles, and immediately transported to the laboratory in refrigerated bags for the analysis.
The soil samples were collected from the plots (one soil sample from each plot) from a depth of 10–50 cm before starting the experimentation (time 0) and at the end of the tomato growing season, respectively. In parallel, OECD artificial soil (OECD 1984a) was utilized as standard sample. The soils were air-dried, passed through a 5-mm sieve, and kept at 4–5 °C until used in bioassays (within 1 month) according to Colacevich et al. (2011). Just before the starting of the soil toxicity tests with E. fetida, the soil moisture content was adjusted to 45 % of the water holding capacity with deionized water.
2.2 Ecotoxicological bioassays on treated agroindustrial wastewater samples
The ecotoxicological bioassays used for water quality monitoring include the acute test with D. magna and the Microtox® test (ISPRA 2013). The cladoceran D. magna is one of the most popular test organisms used for environmental pollution biomonitoring. It represents primary consumers in freshwater aquatic ecosystems providing important source of food for fish. Its wide use is also due to its high sensitivity to toxicants, its parthenogenetic reproduction and its easy culture. In the acute test with D. magna the effects exerted by an environmental sample on the swimming capability of Daphnia following 24–48 h exposure was assessed as a measure of the toxicity of the sample according to OECD (1984a).
Microtox® test is an acute toxicity test using the marine luminescent bacterium Vibrio fischeri, which emits light as a result of normal metabolic processes (Jennings et al. 2001). A reduction in the bacteria bioluminescence during exposure to pollutants is taken as a measure of toxicity. The test responds to a very broad range of toxicants and different classes of chemical agents, including metals, pesticides, fungicides, chlorinated solvents, industrial chemicals and other similar materials. The bioassays on wastewater samples were performed according to the standardized protocol Comparison Protocol according to Azur Environmental (1994). All the measurements were performed by using the M500 luminometer equipped with the appropriate cells incubator at 15 °C for acute toxicity and with the 4 °C cell incubator for the bacterial reagent.
The patented in vitro toxicity method (Patent n. WO 2009/135537) is based on the in vitro inhibition measurement of the enzyme carbonic anhydrase (Lionetto et al. 2005, 2012b) by multiple contaminants present in aqueous environmental samples. It measures the toxicity of a sample in a sensitive, rapid, and cost-effective way and it was applied for the first time to the monitoring of treated wastewaters in this work. It displays sensitivity (expressed as percentage inhibition of the enzymatic activity) to the main classes of chemical contaminants relevant for water contamination. Moreover, it showed sensitivity to the synergic effects that combined pollutants in mixture can have on biological systems.
2.3 Earthworm acute and chronic toxicity test
The E. fetida acute toxicity test was performed according to OECD (1984b). The test lasted 14 days and the number of surviving E. fetida was recorded at the end of the 2-week exposure. The test was carried out in triplicated for each soil sample. The E. fetida chronic toxicity test was performed according to OECD (2004) and lasted 8 weeks. During the first 4 weeks adult growth and mortality were recorded. On day 28 living adult worms were removed from the test medium, counted, and weighed. Then, the soil samples excluding adult worms but including cocoons were incubated for another 4 weeks under the same test conditions. At the end of the second 4 weeks the number of the juvenile earthworms was counted. The test was carried out in triplicated for each soil.
2.4 Biomarker analysis in Eisenia fetida: metallothionein tissutal concentration and lysosomal membrane stability
Metallothioneins are low-molecular weight cysteine-rich metal-binding proteins that are involved in regulation of metabolism of trace metals and protection against heavy metal toxicity and oxidative stress (Costello et al. 2004; Amiard et al. 2006) in a wide range of phylogenetic groups (Viarengo et al. 1999; Lionetto et al. 2001), including earthworms (Kammenga et al. 2000; Lionetto et al. 2012a). Metallothionein tissue concentration was determined in E. fetida by the spectrophotometric method previously described (Viarengo et al. 1997; Gastaldi et al. 2007) in the whole organism. Briefly, tissues were homogenized in three volumes of 0.5 M sucrose, 20 mM Tris–HCl buffer, pH 8.6, added with 0.006 mM leupeptin, 0.5 mM phenylmethylsulphonylfluoride as antiproteolytic agents, and 0.01 % β-mercaptoethanol as a reducing agent. Then, the homogenate was treated to obtain a partially purified metallothionein fraction by ethanol/chloroform precipitation. Metallothionein concentration in the samples was quantified by spectrophotometric determination of the sulphydryl residues using the Ellman’s reagent (5,5′-dithiobis-2-nitrobenzoic acid) using reduced glutathione (GSH) as a standard. Data were expressed as µg MT/g of wet weight.
Lysosomal membrane stability is routinely used as an early indicator of the adverse effects of chemical contaminants (both organic and inorganic) across a wide range of animals including earthworms (Svendsen et al. 1996, 2004; Scott-Fordsmand et al. 1998). Lysosomal membrane stability was analyzed in coelomocytes by the neutral red retention assay (NRRA) (Lowe et al. 1992, 1995). The retention of the cationic probe neutral red within the lysosomal compartment over time was determined as a measure of damage to the lysosomal membrane (Svendsen et al. 2004). The NRRA method used in the present study has been described previously by Weeks and Svendsen (1996). Briefly, 40 µl of coelomic fluid (diluted 1:1 as above) were dispensed on a poly-l-lysine coated slide and incubated in a humid chamber (16 °C) for 30 min. 40 µl of neutral red solution (995 μl of saline solution and 5 μl of neutral red stock solution obtained by dissolving 20 mg of neutral red powder in 1 ml dimethylsulfoxide) were added and the slides were left in a humid chamber (16 °C) for 15 min. Then, a cover slide was applied and the slide was ready to be observed under the microscope. The slides were observed every 15 min for the first hour and every 30 min for the next 2 h thereafter. The time necessary for 50 % of the cell lysosomes to leak neutral red into the cytosol was determined.
2.5 Statistical analysis
Data were analyzed by one-way ANOVA and Newman–Keuls post hoc test. The homogeneity of variance was tested by Cochran’s test. Data are expressed as mean ± SEM.
3 Results and discussion
Table 1 shows the results obtained with the D. magna test (mortality at 24 h) and the Microtox test (bioluminescence inhibition at 15 min) on wastewaters resulting from secondary and tertiary treatment. The results have been compared with data obtained from ground water. The results are expressed as the percentage of the effect exerted by the water sample as such. The interpretation of data is referred to the Report RTI CTN_AIM 4/2001 of ANPA which is inspired by the sample toxicity classification system reported by several authors (Põllumaa et al. 2004; Ricco et al. 2004): a percentage of effect within 20 % is considered an indicator of absence of toxicity, a percentage of effect between 20 and 50 % is considered an indicator of low toxicity, over 50 % indicator of toxicity. Table 1 shows that the secondary wastewater samples overcome the threshold of 20 % in two of the three samplings, suggesting that the secondary treatment is not sufficient to completely remove the ecotoxicity risk associated with wastewaters. However, the samples become negative following the tertiary treatment. All the tertiary samples were below the threshold of 20 %, suggesting the efficiency of tertiary treatment in the removal of bioavailable toxic chemical that could be responsible for ecotoxicity of wastewaters.
Table 2 shows the results obtained with the in vitro AC test on the water samples analyzed with Daphnia test and Microtox test. The in vitro bioassay showed a high degree of concordance with the D. magna toxicity test and Microtox test, as revealed by the comparison of data obtained with the three tests on the same water samples (Tables 1, 2). More research is still needed to evaluate the sensitivity of the in vitro method to physicochemical variables of environmental samples other than pollutants that could affect the enzymatic measurement. However, the obtained results already suggest the potential applicability of the method to the screening of treated wastewaters reclaimed for irrigation as complement to the ecotoxicological analysis of reclaimed wastewaters performed by in vivo bioassays. Being based on the use of a free enzyme in the reaction mixture, the AC test allows to overcome a limit of the in vivo bioassays represented by the presence of allelopathic substances for the test species in the environmental sample.
In parallel to the analysis of reclaimed wastewaters by the Daphnia test, the Microtox test and the AC test, the study has included also the analysis of the toxicity of soils, experimentally irrigated with treated wastewaters, by using earthworms (E. fetida) as bioindicator organisms.
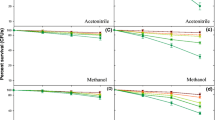
The results obtained by acute and chronic toxicity test with E. fetida are reported in Fig. 1a, b. As shown in Fig. 1, none of the soil analyzed showed positivity to the acute test or the chronic test, excluding the presence of ecotoxicity in the soil irrigated with the treated wastewaters. The analysis of the soil was further deepened by the biomarker analysis in earthworms exposed for 30 days to the test soil samples. It is known that the effects of contaminants at lower levels of biological organization (e.g., biochemical, cellular, physiological) in general occur more rapidly than those at higher levels (e.g., ecological effects) and therefore may provide a more sensitive early warning of toxicological effects within populations (Clements 2000).
Coelomocytes lysosomal membrane stability and tissutal metallothionein concentration were chosen as general and specific biomarker, respectively. Lysosomal membrane stability represents one of the most used cellular biomarker of chemical exposure and associated biological effects (Svendsen et al. 2004). Alterations of the lysosomal system provide an early response to pollutant exposure in a wide variety of animals including earthworms. Several studies have been demonstrated that lysosomal membrane destabilization is a useful predictor of adverse effect on lifecycle parameters such as survival, reproduction, and growth (Sanchez-Hernandez 2006). In the present study the lysosomal membrane stability was determined on coelomic fluid cells by the NRRA method. In earthworms coelomocytes are involved in the internal defence system of the animal (Engelmann et al. 2004). Thanks to the important role played by coelomocytes in the animal physiology, any impairment of their functioning can alter the health of the entire organism.
Metallothioneins are low-molecular weight cysteine-rich metal-binding proteins responsible for the homeostasis of essential metals such as Cu and Zn and at the same time the detoxification of non-essential metals such as Ag, Cd and Hg (Costello et al. 2004; Amiard et al. 2006). They play also an important role as free radical scavengers in the cell. Metallothioneins contain 25–30 % cysteine, but few aromatic or histidine residues. Induction of this proteins by metal exposure has been detected in a wide variety of organisms including earthworms (Stürzenbaum et al. 2001; Demuynck et al. 2006; Ndayibagira et al. 2007; Brulle et al. 2007; Calisi et al. 2009; 2011a, b, 2013, 2014) both in laboratory and field exposure conditions. The choice of this specific biomarker arises from the fact that heavy metals are among the most diffusive chemicals occurring in soil and they can often be present in wastewaters, resulting from different anthropogenic sources. In contrast to harmful organic compounds, heavy metals do not decompose and do not disappear from soil even if their release to the environment can be restricted. Therefore, the effects of heavy metal contamination on soil organisms and decomposition processes persist for many years. Figure 2a shows the results obtained by the analysis of the coelomocyte lysosomal membrane stability, a general biomarker of effect. The statistical analysis by one-way ANOVA and Newman–Keuls post test did not found any significant differences between the lysosomal membrane stability measured in animals exposed to soils irrigated with treated agroindustrial wastewaters and animals exposed to control soils. Therefore, it is possible to exclude the presence of a stress syndrome in the earthworm exposed to soil irrigated with agroindustrial treated wastewaters coming from the processing of agricultural products.
Figure 2b shows the results obtained by the analysis of metallothionein, specific biomarker of exposure to heavy metals. MT has been linked to heavy metal (copper, zinc, mercury and cadmium) detoxification in a wide range of phylogenetic orders, including earthworms. It is known that earthworms share a high tolerance to heavy metal exposure also thanks to the fundamental contribution of these metal-binding proteins (Stürzenbaum et al. 1998). The statistical analysis by one-way ANOVA and Newman–Keuls post test did not find any statistical differences between the soil irrigated with treated agroindustrial wastewaters and the control soils. Therefore, the irrigation of soil with treated agroindustrial wastewaters seems to be not associated with the accumulation of heavy metals in soil and soil organisms.
4 Conclusions
In this study, the applicability of ecotoxicological bioassays and biomarkers to the monitoring of treated wastewaters, produced during the transformation of agricultural products and addressed to irrigation, has been successfully assessed in the framework of the project PON IN.TE.R.R.A. The developed protocol included two standardized ecotoxicological bioassays, D. magna toxicity test and Microdot test, and a patented toxicity test based on the in vitro inhibition of the activity of the enzyme carbonic anhydrase. For a more complete evaluation of the safe use of the reclaimed wastewaters for irrigation, the developed protocol included also the ecotoxicological analysis of the soils irrigated with the reclaimed wastewaters. The ecotoxicological investigation of the soil was performed by the integrated analysis of acute and chronic toxicity test on the earthworm E. fetida and the biomarker analysis on tissue and body fluids of the bioindicator organism. Overall the integrated ecotoxicological study excluded the presence of ecotoxicity both in reclaimed waters resulting from tertiary treatment and in the irrigated soils. Moreover, the analysis of metallothionein, specific biomarkers of exposure to zinc, copper, cadmium and mercury, in the tissues of earthworms exposed to the irrigated soil for 30 days, allowed to exclude the accumulation of bioavailable heavy metals in the soil.
This study suggests the possibility of large-scale use of ecotoxicological methods for the biomonitoring of water and soil in the reclaimed wastewaters reuse for irrigation. The application of ecotoxicological bioassays can contribute to improve the process of re-use of wastewater for irrigation in terms of assessment of the toxicological safety of the waters for the environment, for traders and consumers, and, therefore, can contribute to the safeguard of water resources.
References
Amiard JC, Amiard-Triquet C, Barka S, Pellerin J, Rainbow PS (2006) Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat Toxicol 76:160–202. doi:10.1016/j.aquatox.2005.08.015
Azur Environmental (1994) Microtox® M500 manual (A toxicity testing handbook). Carlsbad, CA, USA
Brulle F, Mitta G, Leroux R, Lemière S, Leprêtre A, Vandenbulcke F (2007) The strong induction of metallothionein gene following cadmium exposure transiently affects the expression of many genes in Eisenia fetida: a trade-off mechanism? Comp Biochem Physiol Part C 144:334–341. doi:10.1016/j.cbpc.2006.10.007
Calisi A, Lionetto MG, Schettino T (2009) Pollutant-induced alterations of granulocyte morphology in the earthworm Eisenia foetida. Ecotoxicol Environ Safe 72:1369–1377. doi:10.1016/j.ecoenv.2009.03.010
Calisi A, Lionetto MG, Schettino T (2011a) Biomarker response in the earthworm Lumbricus terrestris exposed to chemical pollutants. Sci Total Environ 409:4456–4464. doi:10.1016/j.scitotenv.2011.06.058
Calisi A, Lionetto MG, Sanchez-Hernandez JC, Schettino T (2011b) Effect of heavy metal exposure on blood haemoglobin concentration and methemoglobin percentage in Lumbricus terrestris. Ecotoxicology 20:847–854. doi:10.1007/s10646-011-0641-1
Calisi A, Zaccarelli N, Lionetto MG, Schettino T (2013) Integrated biomarker analysis in the earthworm Lumbricus terrestris: application to the monitoring of soil heavy metal pollution. Chemosphere 90:2637–2644. doi:10.1016/j.chemosphere.2012.11.040
Calisi A, Lionetto MG, De Lorenzis E, Leomanni A, Schettino T (2014) Metallothionein induction in the coelomic fluid of the earthworm Lumbricus terrestris following heavy metal exposure: a short report. Biomed Res Int. doi:10.1155/2014/109386
Clements WH (2000) Integrating effects of contaminants across levels of biological organization: an overview. J Aquat Ecosyst Stress Recover 7:113–116. doi:10.1023/A:1009927612391
Colacevich A, Sierra MJ, Borghini F, Millán R, Sanchez-Hernandez JQ (2011) Oxidative stress in earthworms short- and long-term exposed to highly Hg-contaminated soils. J Hazard Mater 194:135–143
Costa CR, Olivi P, Botta CMR, Espindola ELG (2008) Toxicity in aquatic environments: discussion and evaluation methods. Quim Nova 31:1820–1830. doi:10.1590/S0100-40422008000700038
Costello LC, Guan Z, Franklin RB, Feng P (2004) Metallothionein can function as a chaperone for zinc uptake transport into prostate and liver mitochondria. J Inorg Biochem 98:664–666. doi:10.1016/j.jinorgbio.2004.02.005
Demuynck S, Grumiaux F, Mottier V, Schikorski D, Lemière S, Leprêtre A (2006) Metallothionein response following cadmium exposure in the oligochaete Eisenia fetida. Comp Biochem Physiol Part C 144:34–46. doi:10.1016/j.cbpc.2006.05.004
Engelmann P, Molnar L, Palinkas L, Cooper EL, Nemeth P (2004) Earthworm leukocyte populations specifically harbor lysosomal enzymes that may respond to bacterial challenge. Cell Tissue Res 316:391–401. doi:10.1007/s00441-004-0874-x
Forbes VE, Palmqvist A, Bach L (2006) The use and misuse of biomarkers in ecotoxicology. Environ Toxicol Chem 25:272–280. doi:10.1897/05-257R.1
Gastaldi L, Ranzato E, Capri F, Hankard P, Peres G, Canesi L, Viarengo A, Pons G (2007) Application of a biomarker battery for the evaluation of the sublethal effects of pollutants in the earthworm Eisenia andrei. Comp Biochem Physiol C146:398–405. doi:10.1016/j.cbpc.2007.04.014
Gatta G, Libutti A, Gagliardi A, Beneduce L, Brusetti L, Borruso L, Disciglio G, Tarantino E (2015) Treated agro-industrial wastewater irrigation of tomato crop: effects on qualitative/quantitative characteristics of production and microbiological properties of the soil. Agric Water Manag 149:33–43
ISPRA (2013) Batterie di saggi ecotossicologici per sedimenti e acque interne. Manuali e Linee Guida, 88/2013
Jennings VLK, Rayner-Brandes MH, Bird DJ (2001) Assessing chemical toxicity with the bioluminescent photobacterium (Vibrio fischeri): a comparison of three commercial systems. Water Res 35:3448–3456. doi:10.1016/S0043-1354(01)00067-7
Kammenga JE, Dallinger R, Donker MH, Köhler HR, Simonsen V, Triebskorn R, Weeks JM (2000) Biomarkers in terrestrial invertebrates for ecotoxicological soil risk assessment. Rev Environ Contam Toxicol 164:93–147
Krishnan P (2009) Environmental impact of food production and consumption. In: Ting KC, Fleisher DH, Rodriguez LF (eds) Systems analysis and modeling in food and agriculture, UNESCO-EOLSS, pp 56-68
Lionetto MG, Giordano ME, Caricato R, Pascariello MF, Marinosci L, Schettino T (2001) Biomonitoring of heavy metal contamination along the Salento coast (Italy) by metallothionein evaluation in Mytilus galloprovincialis and Mullus barbatus. Aquat Conserv 11:305–310. doi:10.1002/aqc.458
Lionetto MG, Caricato R, Erroi E, Giordano ME, Schettino T (2005) Carbonic anhydrase-based environmental bioassay. Int J Environ Anal Chem 85:895–903. doi:10.1080/03067310500154320
Lionetto MG, Calisi A, Schettino T (2012a) Earthworm biomarkers as tools for soil pollution assessment. In: Hernandez-Soriano MC (ed) Soil health and land use management, ISBN 978-953-307-614-0. InTech-Open Access Publisher in Science, Rijeka (Croatia), pp. 305–332
Lionetto MG, Caricato R, Giordano ME, Erroi E, Schettino T (2012b) Carbonic anhydrase as pollution biomarker: an ancient enzyme with a new use. Int J Environ Res Public Health 9:3965–3977. doi:10.3390/ijerph9113965
Lowe D, Moore MN, Evans BM (1992) Contaminant impact on interactions of molecular probes with lysosomes in living hepatocytes from dab Limanda limanda. Mar Ecol Progr Ser 91:135–140. doi:10.3354/meps091135
Lowe DM, Soverchia C, Moore MM (1995) Lysosomal membrane responses in the blood and digestive cells of mussels experimentally exposed to fluoranthene. Aquat Toxicol 33:105–112. doi:10.1016/0166-445X(95)00015-V
Manzo S, De Nicola F, De Luca Picione F et al (2008) Assessment of the effects of soil PAH accumulation by a battery of ecotoxicological tests. Chemosphere 71:1937–1944
Martínez-Gómez C, Vethaak AD, Hylland K, Burgeot T, Köhler A, Lyons BP, Thain J, Gubbins MJ, Davies IM (2010) A guide to toxicity assessment and monitoring effects at lower levels of biological organization following marine oil spills in European waters. J Mar Sci 67:1105–1118
Ndayibagira A, Sunahara GI, Robidoux PY (2007) Rapid isocratic HPLC quantification of metallothionein-like proteins as biomarkers for cadmium exposure in the earthworm Eisenia andrei. Soil Biol Biochem 39:194–201. doi:10.1016/j.soilbio.2006.07.008
OECD (1984a) Daphnia sp., Acute immobilisation test and reproduction Test. nr. 202. OECD, Paris, France
OECD (1984b) Earthworms, acute toxicity tests. OECD, guideline for testing chemicals nr. 207. OECD, Paris, France
OECD (2004). Earthworms reproduction tests. Guideline for testing chemicals. no 222. OECD, Paris, France
Põllumaa L, Kahru A, Manusadzianas L (2004) Biotest- and chemistry based hazard assessment of soils, sediments and solid wastes. J Soils Sediments 4:267–275
Ricco G, Tomei MC, Ramadori R et al (2004) Toxicity assessment of common xenobiotic compounds on municipal activated sludge: comparison between respirometry and Microtox. Water Res 38:2103–2110
Sanchez-Hernandez JC (2006) Earthworm biomarkers in ecological risk assessment. Rev Environ Contam Toxicol 188:85–126. doi:10.1007/978-0-387-32964-2_3
Schettino T, Caricato R, Calisi A, Giordano ME, Lionetto MG (2012) Biomarker approach in marine monitoring and assessment: new insights and perspectives. Open Environ Sci 6:20–27
Scott-Fordsmand JJ, Weeks JM, Hopkin SP (1998) Toxicity of nickel to the earthworm and the applicability of the neutral red retention assay. Ecotoxicology 7:291–295
Stürzenbaum SR, Kille P, Morgan AJ (1998) The identification, cloning and characterization of earthworm metallothionein. FEBS Lett 431:437–442. doi:10.1016/S0014-5793(98)00809-6
Stürzenbaum SR, Winters C, Galay M, Morgan AJ, Kille P (2001) Metal ion trafficking in earthworms. Identification of a cadmium-specific metallothionein. J Biol Chem 276:34013–34018. doi:10.1074/jbc.M103605200
Suter GW (2006) Ecological risk assessment, 3rd edn. Lewis Publishers, Boca Raton, pp 355–356
Svendsen C, Meharg AA, Freestone P, Weeks JM (1996) Use of an earthworm lysosomal biomarker for the ecological assessment of pollution from an industrial plastics fire. Appl Soil Ecol 3:99–107. doi:10.1016/0929-1393(95)00085-2
Svendsen C, Spurgeon DJ, Hankard PK, Weeks JM (2004) A review of lysosomal membrane stability measured by neutral red retention: is it a workable earthworm biomarker? Ecotoxicol Environ Saf 57:20–29. doi:10.1016/j.ecoenv.2003.08.009
Viarengo A, Ponzano E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar Environ Res 44:69–84. doi:10.1016/s0141-1136(96)00103-1
Viarengo A, Burlando B, Dondero F, Marro A, Fabbri R (1999) Metallothionein as a tool in biomonitoring programmes. Biomarkers 4:455–466
Weeks JM, Svendsen C (1996) Neutral red retention by lysosomes from earthworm (Lumbricus rubellus) coelomocytes: a simple biomarker of exposure to soil copper. Environ Toxicol Chem 1996(15):1801–1805. doi:10.1897/1551-5028(1996)015<1801:NRRBLF>2.3.CO;2
WO 2009/135537. Metodo per la valutazione enzimatica della tossicità di matrici acquose ambientali. Authors: Schettino T., Lionetto M.G., Erroi E. Concessed on 13/04/2011
Zagklis DP, Paraskeva CA (2014) Membrane filtration of agro-industrial wastewaters and isolation of organic compounds with high added values. Water Sci Technol 69:202–207
Acknowledgments
The authors wish to thank the Italian Ministry of University and Research (MIUR) for its financial support under the Project In.T.e.R.R.A. (Contract No. 01 01480) cofounded within the Italian Program PON/Ricerca e Competitività 2007–2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
This contribution is the written, peer-reviewed version of a paper presented at the XV Giornata Mondiale dell’Acqua “Grado di inquinamento naturale di acque e suoli in Italia”, held at the Accademia Nazionale dei Lincei on March 20, 2015.
Rights and permissions
About this article
Cite this article
Lionetto, M.G., Caricato, R., Calisi, A. et al. Biomonitoring of water and soil quality: a case study of ecotoxicological methodology application to the assessment of reclaimed agroindustrial wastewaters used for irrigation. Rend. Fis. Acc. Lincei 27, 105–112 (2016). https://doi.org/10.1007/s12210-015-0486-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-015-0486-2