Abstract
Trees in cutaway peatland are growing in difficult conditions. Fertilization with nutrient-rich wood ash helps improve growth conditions. Photosynthesis and carbohydrate concentration along leaf anatomy were studied on plots treated with 10 and 5 t ha−1 wood ash (WA10 and WA5) and on untreated (Control) plot to explain the physiological background of the differences in tree growth. The leaves from WA10 had the largest leaf area, total thickness, the thickest mesophyll and also significantly larger average values of all anatomical parameters of the shoots. The photosynthetic assimilation was significantly higher on treated plots at 200 and 400 ppm CO2 levels. In leaves on the treated plots, the sucrose concentration was lower while that of starch was higher than in trees on untreated soil. The differences in the maximum photosynthesis were relatively small. At unit ground, the leaf area provided for a wood ash-treated tree an efficient surface for CO2 assimilation, light interception and some starch storage during the growing period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stress factors are described as extreme environmental conditions that have an effect on functional changes in plants to an extent that stress of the organism develops, resulting in inhibited growth, reduced bioproduction, physiological acclimatization and adaptation of species or some combination of these changes.
Throughout their lives, woody plants are subjected to multiple abiotic and biotic stresses of varying intensity and duration that, by influencing physiological processes, modify their growth including the anatomical structure of the canopy (Pallardy 2008; Lukjanova and Mandre 2009). In any case, stress and disturbances are limiting biomass increment by causing shortages of light, water and mineral nutrients; changes in metabolism and photosynthetic activity and partial or total destruction of plants (Thomas and Packham 2007).
The conditions prevailing on cutaway peatlands are unsuitable for the development of new vegetation: the microclimate is characterized by late and early frosts and extreme surface temperatures in summer; the water regime is unfavourable, and the surface is eroded away by wind and water. Therefore, natural revegetation of these areas is complicated and may take several decades (Kaunisto and Aro 1996; Campbell et al. 2002; Groeneveld and Rochefort 2002; Lavoie et al. 2005; Hytönen and Saarsalmi 2009; Hytönen and Aro 2012).
Since cutaway peat is very poor in mineral nutrients, deficiencies of phosphorus and potassium are considered to be the most limiting factors to plant growth (Paavilainen and Päivänen 1995). Peat is acidic and has low availability of macro- and micronutrients such as N, P, K and Cu that are limiting the growth of trees (Renou and Farrell 2005). There are still uncertainties in the responses of trees to increased substrate pH after ash treatment, added nutrients, understory vegetation development, dynamics of peat decomposition, soil water accumulation, aeration, etc. Practical interest in growing forest on abandoned peatlands is considerable because cutaway peatlands could be a significant source of atmospheric CO2 due to the decomposition of peat. Fertilization with wood ash increases soil respiration in drained peatlands (Moilanen et al. 2012). There are many studies on the stimulation of photosynthetic rate under elevated CO2 (Norby et al. 1999; Gielen and Ceulemans 2001), which enhances the growth and productivity of trees (Ceulemans and Mousseau 1994; Rey and Jarvis 1997). Afforestation of former peatlands is known to reduce the CO2 emission (Black and Gallagher 2010).
The use of wood ash affects soil acidity and the availability of many nutrients to the trees, activating soil microbial activity and increasing mineralization of nitrogen and phosphorus availability (Hytönen 2005). Wood ash is rich in compounds of K, Ca and Mg and microelements, including B, which are essential nutrients for forest trees. Wood ash often raises the potassium concentration in soil and plants. Due to the easy release of K from wood ash and its active uptake by trees, K concentration in spruce needles and birch leaves is markedly elevated shortly after the wood ash application in different soils (Mandre et al. 2004; Kikamägi et al. 2013) and also B may increase due to its availability from wood ash (Jacobson 2003).
Leaf internal structure depends on the specific ecological conditions, particularly water, nutrients and light availability. Wood ash affects the morphology and anatomy of Scots pine needles; the needles of pine growing on the treated plots have mostly larger average values of the anatomical parameters compared to the untreated plot (Lukjanova and Mandre 2010). Potassium in leaves is required to regulate the stomata and the water balance in a tree (Benlloch-González et al. 2008). Birch is an isohydric plant (it uses osmotic adjustment in leaves), and the concentration of soluble carbohydrates may rise in response to an osmotic stress in birch leaves. Sucrose levels can be larger in leaves that are actively exporting sucrose. In stress conditions, hydrolysis of sucrose to glucose and fructose can increase (Einig et al. 1997).
A low K concentration in leaves augments the concentrations of glucose and fructose concomitant with the increase in acid invertase activity in leaves since possibly K and soluble sugars are interchangeable for the osmotic function (Huber 1984; Itoh et al. 1997).
Carbohydrates commonly accumulate as a response of plants to nutrient shortages. Nitrogen deficiency increases starch concentration because of reduced leaf growth and reduced channelling of carbon to the synthesis of amino acids or proteins for the growth and storage, while the maintaining of photosynthesis has higher priority (Wallenda et al. 1996).
Studies on mineral soil show that in Norway spruce, the fructose and glucose concentration in needles is reduced whereas the sucrose concentration is unaffected in response to wood ash (Klõšeiko et al. 2006). This suggests an influence of K, since accumulation of soluble, in particular reducing carbohydrates in K-deficient plants, in leaves is the most consistent observation (Amtmann et al. 2008).
To explain the physiological background of the differences in tree growth between wood ash-fertilized and unfertilized trees, photosynthesis and carbohydrate concentration along leaf anatomy were studied. Specifically, the aim of the present study with silver birch (Betula pendula Roth) was to estimate the effect of wood ash on leaf size, anatomy of leaf and shoot, photosynthesis, soil nutrients (N, P, K, Ca, Mg) and carbohydrate concentrations in trees on a cutaway peatland.
Materials and methods
Study area
The experimental plots are located in a cutaway peat-milling area (58° 22’ N, 26° 26’ E) 1 km north-west of the settlement of Ulila in Tartu County (South Estonia). The area of the abandoned field at Ulila is 236.64 ha, of which old milling fields make up 49.9 ha (Ramst et al. 2006). The peat harvesting in Ulila was completed in about 2003.
The average thickness of the Ulila cutaway peatland of the poorly decomposed peat layer is 0.9-m thick, which has the average decomposition rate of 10 % and ash content 2.6 % (Ramst et al. 2006). The well-decomposed peat layer is 2.4-m thick, which has the average decomposition rate of 26 % and ash content 3.8 %. The remaining peat thickness reaches in some places to 3.3 m. The pHKCl in the top 10-cm layer is acidic (pH = 2.3–3.0) (Kikamägi et al. 2013). The Ulila cutaway peatland, located in the southern part of the country, is the average nutrient-rich peatland in Estonia, but Ca and Mg content is less, 10 and 2 times, respectively, than in peatlands of north-east Estonia.
The plants growing on the area include Eriophorum spp., whose coverage is 5–10 %, and Calluna vulgaris, Ledum palustre, Andromeda polifolia, Vaccinium vitis-idaea and Empetrum nigrum, which grow by ditches; in lower places, patches of Eriophorum angustifolium are found. The moss layer is mainly made up of Polytrichum strictum. In ditches, Carex rostrata and Comarum palustre are growing. The total vegetation coverage is 14 %.
According to the data from a meteorological station (the closest to the study area was the Tartu Tõravere station), the mean annual temperature was 6 °C in 2009, 5.1 °C in 2010, 6.9 °C in 2011 and 5.3 °C in 2012. The amount of precipitation was 805 mm in 2009, 838 mm in 2010, 511.3 mm in 2011 and 771.5 mm in 2012 (www.emhi.ee, accessed 02.04.2014).
In June 2009, the following three plots were established:
-
1.
Untreated plot (Control)
-
2.
Wood ash 5 t ha−1 (WA5)
-
3.
Wood ash 10 t ha−1 (WA10)
The wood ash generated as production waste originated from the local boiler station at Elva. Concentrations of plant macronutrients in the wood ash (mg kg−1) are presented in (Kikamägi et al. 2013). Ash was applied before planting within 1 m × 1 m around the seedling location. One-year-old silver birches (B. pendula Roth.) were hand-planted at the beginning of June 2009 at a 1.2 m × 2 m grid. The total sample of each plot included 50 trees. The survival and growth of the planted trees has been described previously (Kikamägi and Ots 2010; Kikamägi et al. 2013).
Measurements and analysis methods
Sampling
The leaves (n = 36–42) from five trees were collected in July 2012 for the determination of leaf characteristics. For anatomical analyses, five leaves and three current-year shoots from each of the three model trees felled for the determination of biomass and biomass production (Kikamägi et al. 2013) were collected in September 2012.
To measure the leaf carbohydrate concentration, leaves without petioles were collected between 1200 and 1300 hours, at the end of June and at the beginning of August 2011. In each treatment area, five leaves of middle age classes from each randomly selected eight trees were put in aluminium foil in liquid nitrogen. Because of the poor growth and survival of trees on the untreated soil, too few leaves were left on the control trees in August. Therefore, for the control samples, leaves from several trees and from the natural birch regeneration around the control plot were pooled in August.
To determine the concentrations of nutrients in the growth substrate, peat samples (n = 3) were taken from random quadrates at depths of 0–10, 11–20, 21–30, 31–40 and 41–60 cm in September 2012.
Leaf characteristics
The collected leaves were herbarized. The areas of single leaves (S) were measured using the package WinFolia (Regent Instruments Inc.). All measured leaf blades with the petiole were weighed to 0.0001 g. Specific leaf area (SLA, m2 kg−1) and foliar assimilation efficiency (FOE, g g−1 year−1) were calculated. FOE of a tree was calculated by dividing current annual production by leaf mass (Uri et al. 2009).
Leaf and shoot anatomy analysis
The leaves and shoots of birch were pre-fixed with 3 % glutaraldehyde in 0.1 mol L−1 phosphate buffer, pH 7.3 and fixed in 1 % solution of OsO4 (Bozzola and Russell 1992; Ruzin 1999). Then, tissues were dehydrated with ethanol and xylol (Fluka, USA) and embedded in paraffin. The cross-sections of leaves (5 μm) and shoots (20 μm) were cut with a microtome (CUT 4060 RE, USA) and mounted on glass. The cross-sections were viewed in the bright field at ×100 and ×50 magnification and photographed with a Leica DM3000 LED microscope (Germany).
Anatomical characteristics of the leaves and shoots were analysed. Leaf thickness and mesophyll (palisade and spongy) and epidermis (upper and lower) tissue thickness were measured on the cross-sections in micrometres. The area of the pith and the thickness of other tissues of the shoots were also measured on cross-sections in micrometres and then recalculated in millimetres.
Gas exchange measurements
The measurements were carried out in June and August 2013. Gas exchange measurements were made using a CIRAS-2 portable gas exchange system (PP Systems, UK) with a 2.5-cm2 broad leaf insert installed in a cuvette PLC6. Temperature in the cuvette was controlled at 25 °C, the humidity of the air entering the cuvette at 10 mb and the light level was at 1500 μmol m−2 s−1 of photosynthetically active radiation from light-emitting diodes. To determine how the wood ash influences the photosynthesis of birches and how the photosynthesis act in different CO2 environments, gas exchange was measured at an ambient CO2 concentration (Ca) of 400, and at 200 and 800 ppm CO2 surrounding the leaf. Maximum photosynthesis (Amax), internal CO2 (Ci) and stomatal conductance (Gs) were determined. As no statistical differences between June and August data were found, the data are shown together.
Carbohydrate analysis
To stop changes in the concentration of carbohydrates, enzymes in leaves were deactivated by boiling in 96 % ethanol for 3 min. After boiling, the leaves were dried at 60 °C for 48 h. Samples were further ground with a ball mill MM200 (Retsch, Haan, Germany). Carbohydrates were extracted from 50 to 60 mg of needle powder in 4 mL of 5 mmol L-1 HEPES buffer (pH 6.8) at 50 °C for 1 h with mixing after every 15 min on a vortex mixer. The insoluble residue was separated with centrifugation at 1900g for 10 min. Extracts were purified by adding 600 μL of 80 mg mL−1 polyamide suspension to 600 μL of the extract and keeping for 20 min with shaking at 5 min intervals. Suspensions were centrifuged at 9500g for 10 min, and the obtained clear solutions were used for carbohydrate determination.
Glucose, fructose and sucrose were determined enzymatically (Steen and Larsson 1986). Starch was analysed from insoluble residue with thermostable α-amylase (162 U mL−1; A-4551; Sigma-Aldrich, Steinheim, Germany) and amyloglucosidase (235 U mL−1; from Aspergillus niger; Merck, Darmstadt, Germany) in line with the AACC Method 76.13. Starch was gelatinized with dimethyl sulfoxide. Carbohydrates were expressed in hexose units, milligrammes per gramme d.m.
Fructose concentration was small and about 30 % of the glucose concentration. Spectrometer readings for fructose were below the recommended lower limit of 0.1 absorbance units. Therefore, the sum of glucose and fructose concentrations was used.
To estimate the mass of the solid residue lost from leaf samples during the fixation, the residue of the ethanol boiled with leaves was gathered separately for different plots. Larger debris was removed from the ethanol residue by passing the solutions through a coarse filter. The ethanol solutions were evaporated and dried at 60 °C. The mass of the solid residue lost from leaf samples during the fixation varied from 31 to 36 % of the total leaf dry mass in different treatments.
Chemical analyses
The pHKCl of the peat and the concentrations of N, P, K, Ca and Mg in the peat were determined in the Laboratory of Plant Biochemistry of the Estonian University of Life Sciences. The peat samples were analyzed for their extractable concentration of P, K Ca and Mg and for total concentration of N. The methods of chemical analyses are described in (Kikamägi et al. 2014).
Statistical analyses
Averages of analyses and standard errors (average ± SE) for leaf characteristics, anatomy and mineral nutrients were calculated. The Shapiro–Wilk test was used to check the normality of the distribution. Levene’s test was used to check the equality of variances. ANOVA (Tukey’s test and Tukey’s Unequal N test) were used for the multiple comparisons of the averages. The Statistica 10.0 software was used, and the significance level of α = 0.05 was accepted in all cases.
To analyze differences between treatment plots in the carbohydrates on the two dates, a multivariate analysis of variance (MANOVA) with type III sum of squares was used. Tree was used as an experimental unit. Treatment and date were independent factors. Treatment was a repeated factor, and it was accounted for in MANOVA by including the measurements on different dates as dependent variables. MANOVA test results were interpreted by Wilks’ statistic. Interaction with time was tested by applying ANOVA to the difference in the tree between dates. Contrast between the two wood ash doses was tested by using the sum of carbohydrate concentrations on different sampling dates and applying ANOVA with post hoc contrast 1, −1 and 0 for the WA10, WA5 and control treatments, respectively. Systat 10.0 for Windows (Systat Software GmbH, Erkrath) software was used for the carbohydrates statistical testing. Log-transformation was used to reduce differences in variances and to better meet the assumption of normal distribution. Exceptional data points were determined by univariate tests, and these data were excluded from the MANOVA. Effects were considered as significant when the p value was less than 0.05. Two-dimensional error degrees of freedom were (4, 32) for glucose + fructose and (4, 40) for sucrose and starch.
Results
Leaf characteristics
Wood ash treatment increased the areas of leaves significantly (p < 0.05). The areas of leaves were five times greater on ash-treated plots than on the control (Table 1). SLA was greatest on the WA5 plot and smallest on the control but there were no significant differences (p > 0.05) between the treated plots and the control plot (Table 1). Contrary to SLA, FOE was greatest on the control plot and smallest on the WA5 plot, but no significant differences between the treated plots and the control plot were found either.
Anatomy of leaves and shoots
Analysis of the histological preparations of leaves showed that the leaves from WA10 had the largest total thickness (p˂0.00013) and the thickest mesophyll (p˂0.00023). The leaves from the WA5 plot had the thickest upper epidermis (p˂0.00017) and the thinnest lower epidermis (p˂0.0047) (Fig. 1). The average values of the parameters measured are presented in Table 2. However, in general, the partitioning of the tissues in the leaf was similar: palisade mesophyll made up 29 % and epidermis 16 % (upper epidermis 11–12 % and lower epidermis 4–5 %) in the leaves from all plots.
Slight differences were found in the partitioning of spongy mesophyll and upper epidermis in the leaves from the WA5 plot compared to the other plots. The proportion of spongy mesophyll of the total leaf thickness was the smallest in the leaves from WA5, where it made up 52 % of the thickness of the leaf. At the same time, the proportion of the upper epidermis in these leaves was above 1 % and of the lower epidermis below 1 % compared to leaves from other plots.
The average values of all anatomical parameters of the shoots were significantly larger (p˂0.05) in the trees from WA10 than in trees from the other plots (Table 3, Fig. 2). But then, the partitioning of the tissues, except the xylem, was largest in the shoots from the control plot. In the shoots from WA10, the xylem made up 55 % of the thickness of the shoot and the xylem was 10 times thicker than the phloem. In the shoots from the control plot, the proportion of the phloem was 12 % and the phloem was only 3 times thinner than the xylem.
Gas exchange measurements
The maximum photosynthesis of silver birches on WA5 and WA10 was very similar at all CO2 levels; however, there were significant differences between the treated plots and the control plot (Fig. 3). The maximum photosynthesis was significantly higher than the control on treated plots at 200 and 400 ppm CO2 levels (p < 0.05), but no significant differences were found at the level of 800 ppm (p > 0.05). Maximum photosynthesis was increasing with raising CO2 levels. There were no significant differences in internal CO2 between different treatments; however, it was different in each plot at each CO2 level. Stomatal conductance was the highest in the leaves of trees growing on WA5, and it differed significantly from the control. Stomatal conductance was similar at each CO2 level in all plots (Fig. 3).
Carbohydrates
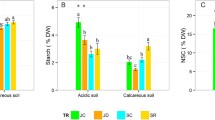
No effect of the wood ash treatments on the concentration of fructose + glucose was found (p = 0.37 and p = 0.196 for the treatment and treatment × date effects in MANOVA) (Fig. 4). Differences in the sucrose concentration between treatments varied depending on the date (p = 0.002 for the treatment × date interaction). Earlier, in June, the sucrose concentrations in the wood ash treatments were lower than the control (about 60 % of the control). During the sampling period, the sucrose concentration increased in the wood ash-treated trees, while there was little change in this soluble carbohydrate in the control trees. However, in the course of the seasonal increase, the sucrose concentration did not exceed the control in the wood ash treatments. On average, the sucrose concentration was lower in wood ash-treated trees in comparison with trees on the untreated peat (p < 0.001) and different doses of wood ash resulted in different sucrose concentrations (p = 0.022). The smallest sucrose concentration was measured in the largest treatment WA10 on both dates. The differences between the two wood ash-treated plots were 9 and 18 % at the end of June and at the beginning of August, respectively.
The starch concentration was 230 to 280 % larger in the wood ash treatments than the control (p < 0.001) (Fig. 4). The interactive terms in MANOVA with date and contrast between the wood ash doses were not significant for starch (p = 0.141 and p = 0.169).
Soil nutrients
Wood ash treatment has the greatest effect on P and K concentrations in cutaway peatlands. After 4 years, the P and K concentrations in the upper layer of soil were significantly higher in treated plots than in the control while the concentrations of N, Ca and Mg showed no substantial differences between treatments (Fig. 5). There were no leaching signs of N, P, Ca and Mg, but the K concentration in the lower layers was significantly higher in the treated plots than in the control plot (p < 0.05) (Fig. 5). Large differences were observed between P (1.7–2.5 times at depths of 0–10 cm) and K (1.7–3.6 times at depths of 11–20 cm and 1.2–3.4 times in deeper layers) concentrations on ash-treated plots and the control (Fig. 5). The average K concentrations were greater in plots where larger amounts of ash had been applied (Fig. 5). Four years after treatment, ash had no liming effect any more: the pH was below 3, and the soil was very strongly acidic.
Discussion
According to several studies, fertilization with wood ash increases the productivity of birches in cutaway peatlands (Huotari et al. 2009; Hytönen and Aro 2012; Kikamägi et al. 2013). Photosynthesis is the basis of tree growth and biomass production. Improved nutrient availability may enhance leaf photosynthesis and/or increase leaf area index, both resulting in greater canopy photosynthesis and, thus, in larger productivity (Merilo et al. 2005).
In our experiment, fertilization with wood ash in a cutaway peatland increased the leaf area of silver birches but it did not affect SLA. An increase of the photosynthetically active leaf area is a typical response to an elevated level of nutrients for many tree species (Maier et al. 2008; Kleczewski et al. 2012). Our anatomical study showed that on WA10, i.e. the plot treated with the greatest amount of wood ash, the leaves were thicker and, accordingly, had a thicker mesophyll than on the other plots. We can assume that the thicker palisade mesophyll promotes the photosynthetic rate in the leaves from WA10. The palisade cells are located on the upper side of the leaf and are directly responsible for photosynthesis. The spongy mesophyll, which is located on the lower side of the leaf and is for most part responsible for the interchange of gases and the transport of CO2 to the chloroplasts, was also thicker on leaves on the WA10 plot. Thus, we can conclude that the foliar biomass of the birch on the WA10 plot grew best due to more intensive photosynthesis and gas exchange in the thicker layer of the mesophyll. The average leaf thickness of different 2-year-old silver birch clones is 194 μm and in August when affected by low ozone exposure 181 μm, the palisade thickness is, respectively, 72 and 66 μm and spongy thickness, respectively, 90 and 87 μm (Pääkkönen et al. 1995). In the current study, the leaf thickness and palisade mesophyll thickness were much smaller, which indicates that the plants were growing in stress conditions.
Fertilizing cutaway peatlands with wood ash helps raise the concentrations of P, K, Ca and Mg in soil and in plants (Hytönen 1995; Hytönen and Aro 2012; Kikamägi et al. 2013). These nutrients are playing important roles in plant growth and photosynthesis in one or another way (Hopkins and Hüner 2009).
Our study revealed an increase of the tissue thickness not only in leaves but also in shoots due to the use of wood ash. One of the major components of woody plant is xylem, which provides mechanical support, forms the wood and conducts water and nutrients from the roots to all other parts of the plant. According to Sellin et al. (2012), larger shoots have a larger transport tissue area and afford higher water flux. Based on our results, we can also state that the largest xylem in the shoots from WA10 allow transporting the largest amount of required soluble minerals to leaves. Good nutritional status is critical for an optimum wood production but also increases resistance against stress from biotic and abiotic factors (Renou-Wilson and Farrell 2007). The current study showed that the effect of fertilization is short. The concentrations of P, K, Ca and Mg fell drastically during a year compared to published soil chemistry data from 2011 (Kikamägi et al. 2013), although the concentrations were still significantly higher in the wood ash-treated plots than the control. Hytönen and Aro (2012) found that most of the applied K probably leaches out to groundwater and surface waters in ditches or leaches to deeper peat layers. The same was observed also in the current study: the K concentration in the 41–60-cm layer on the WA10 plot was over 3 times higher compared to the control. As no signs of leaching were observed for other elements, it can be assumed that trees are using the nutrients for growth and additional fertilizing is essential.
The CO2 flux to a stoma is influenced by light, temperature, ambient CO2 concentration, photosynthetic capacity of the mesophyll cells, size of stomatal opening, leaf structure and diffusion coefficients in different parts of the system (Aalto and Juurola 2002). The regulation of leaf stomatal conductance is a key phenomenon in plants as it is vital for both prevention of desiccation and CO2 acquisition (Dodd 2003). The decrease in the photosynthetic rate under stressful conditions is normally attributed to a suppression in the mesophyll conductance and the stomata closure at moderate and severe stress (Chaves et al. 2009; Ashraf and Harris 2013). Mesophyll structure may influence photosynthetic capacity by affecting the diffusion of CO2 in the leaf (Terashima et al. 2001). Kenzo et al. (2004) found a strong correlation between the photosynthetic rate at light saturation and leaf mesophyll structures, including the palisade cell layer thickness. The maximum photosynthetic rate occurs near the upper cuticle (about 14–74 μm from the surface) due to efficient diffusion and rapidly decreasing irradiance. The rate is almost constant in the palisade and uppermost spongy mesophyll cells, decreasing towards the lower cuticle, which can be seen from increasing concentration in spongy mesophyll cells (Aalto and Juurola 2002). Doubled ambient CO2 concentration increases the total flux by 60 %. The saturation point for the photosynthetic photon flux density in silver birch is around 900 μmol m−2 s−1; exceeding this point causes no increase in photosynthesis (Wang et al. 1995). Fertilizing enhances photosynthesis, which increases carbohydrate availability for the growing organs (Maier et al. 2008).
Concentrations of carbohydrates in this study were generally in the range found in previous studies with birch leaves (Rey and Jarvis 1998; Riipi et al. 2002; Riikonen et al. 2003). Relatively high levels of galactose, possibly as a defence sugar during larvae feeding, are found in birch leaves. Starch responds easily to variations in growing conditions and accumulates at high CO2 concentrations in the air (Rey and Jarvis 1998). There were differences in the dynamics of some carbohydrates. In our study, sucrose increased in wood ash-treated trees. Nevertheless, the average sucrose concentration in the control trees was the largest, which linked well with the largest proportion of the phloem of the shoots of these trees. Phloem is the tissue that carries organic nutrients, in particular sucroses and hexoses, to all non-photosynthetic parts of the plant where needed. We can assume that in the trees from the untreated plot, the reallocation of photosynthates is more intensive compared to the treated plots. The same conclusion was reached by Puijalon et al. (2008), who argue that plants that grow under nutrient-poor conditions have low resource loss. Differently from our findings, starch has been found to increase during summer (Rey and Jarvis 1998; Riikonen et al. 2003). Carbohydrates as the product of CO2 assimilation are substrates for growth and biosynthesis. Also, soluble carbohydrates are involved in osmotic adjustment and participate in complex signalling networks (Koch 2004). Sucrose is transported from sinks to sources in conifers. Starch is a storage carbohydrate to be used in stress conditions or when photosynthesis is slow. Wood ash could affect the carbohydrate metabolism in needles in various ways. Metabolic impairment due to nutrient deficiencies, ion or heavy metal toxicity may increase carbohydrate concentration when the photosynthesis is affected less than carbohydrate consumption for respiration, biosynthesis or export from needles.
A reduction in concentrations of soluble carbohydrates fructose and glucose of conifer needles seems to be quite a common effect of wood ash on mineral soils a few years after the treatment (Klõšeiko et al. 2006). However, no reduction in fructose and glucose was found in birch leaves on the cutaway peatland in the present study. Potassium deficiency is known to increase severalfold the concentration of soluble carbohydrates, including sucrose in plant leaves (Armengaud et al. 2009). Potassium deficiency seems to reduce the synthesis of sucrose and therefore its availability for export from leaves in addition to the decreased phloem flow by reduced xylem-to-phloem transfer of K, which is important in the transport processes between sources and sinks (Hölttä et al. 2009). Therefore, the lower concentration of sucrose in wood ash treatments was possibly associated with the better availability of K in trees in the current experiment. The K concentration of birch leaves was increased in the ash-treated area (0.87–0.94 %) compared to the control (0.59 %) also in the Ulila study area (Kikamägi et al. 2013), although K was still deficient (optimum 1.5–3.1 %) according Ingestad’s (1962) scale.
Starch as a storage compound may easily respond to changes in the carbon balance, which is affected by nutrient availability. Nutrient deficiencies affect the net carbon gain and the carbon partitioning between plant organs and different compounds. Starch concentration in plant leaves is increased after induction of deficiencies in many different nutrients such as N, P and Mg but not K (Hermans et al. 2006). In nutrient-deficient plants, starch may accumulate because the translocation of carbohydrates to growing organs is difficult or the sink activity is reduced and photosynthesis is less affected. However, in birch seedlings, Mg deficiency at a steady growth rate does not increase the concentration of starch in leaves because in birch the main constraint associated with a shortage of Mg could be a reduced net carbon gain (Ericsson and Kähr 1995). In the present study, the control plants grew extremely poorly, their establishment and survival were low in the permanently nutrient limited environment and no excess of starch accumulation was observed. Therefore, in the wood ash-treated plots, where trees were well growing and with healthy leaves and a dense foliage, the higher concentration of starch was possibly a result of a higher net photosynthesis rate. Wood ash-treated trees with improved nutrition may fill up the starch storage pools quickly as well as mobilize more efficiently the carbohydrate reserves during stress events. Control trees with a sparse foliage may struggle with a stress from excess light while not able to use the light for photosynthesis. Carbohydrate requirements in roots or for growth could be high while nutrient deficiencies impair the production of carbohydrates during a stressful photosynthesis. Distribution of carbohydrates from leaves to other organs and availability of carbohydrates in stems and roots for both growth and storage was possibly also low in birches on the untreated peatland substrate.
In the present study, an improved nutrient availability in wood ash-treated trees enhanced leaf maximum photosynthesis and increased leaf area. The sparse and poor foliage in the control plot and the healthy looking foliage with the common leaf size in the wood ash-treated plots suggested a larger leaf area index in the wood ash-treated plots. There were no differences in SLA and FOE between plots. Relatively larger effects were found in the growth of trees than in the maximum photosynthesis. Thus, the larger productivity in wood ash-treated plots was explainable mainly by enhanced canopy photosynthesis from the larger foliage area providing an efficient surface for CO2 assimilation, light interception and starch storage.
Conclusions
Trees growing in cutaway peatlands are affected by different stresses. Fertilization with wood ash helps improve growth conditions and increases the density of the canopy. The characteristics, including the anatomical parameters, of leaves of birches were much larger in the treated plots than in the control. Photosynthesis, which is the basis of tree growth and biomass production, was higher in wood ash-treated plots. The total concentration of soluble carbohydrate fructose and glucose was much less than the concentration of sucrose. In fructose and glucose, no differences between plots were found. The higher concentration of sucrose in the control plot was possibly caused by a low concentration of solubilized mineral compounds, which required a replacement for their osmotic effects in leaves or effects of nutrient deficiencies on the sucrose export from leaves. The leaf area of the treated trees was an efficient surface for CO2 assimilation, light interception and some starch storage during the growing period. Wood ash raises the nutrient content in soil but the effect is not very long-lasting and re-fertilization is necessary.
References
Aalto, T., & Juurola, E. (2002). A three-dimensional model of CO2 transport in airspaces and mesophyll cells of a silver birch leaf. Plant, Cell and Environment, 25, 1399–1409.
Amtmann, A., Troufflard, S., & Armengaud, P. (2008). The effect of potassium nutrition on pest and disease resistance in plants. Physiologia Plantarum, 133(4), 682–691.
Armengaud, P., Sulpice, R., Miller, A. J., Stitt, M., Amtmann, A., & Gibon, Y. (2009). Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots. Plant Physiology, 150(2), 772–785.
Ashraf, M., & Harris, P. J. C. (2013). Photosynthesis under stressful environments. Photosynthetica, 51(2), 163–190.
Benlloch-González, M., Arquero, O., Fournier, J. M., Barranco, D., & Benlloch, M. (2008). K+ starvation inhibits water-stress-induced stomatal closure. Journal of Plant Physiology, 165, 623–630.
Black, K., & Gallagher, G. (2010). The greenhouse gas balance of peatland forest. Environ. 11, c/o COFORD, Department of Agriculture, Fisheries and Food.
Bozzola, J.J., & Russell, L.D. (1992). Electron microscopy. Jones & Bartlett Learning, LLC.
Campbell, D. R., Lavoie, C., & Rochefort, L. (2002). Wind erosion and surface stability in abandoned milled peatlands. Canadian Journal of Soil Science, 82, 85–95.
Ceulemans, R., & Mousseau, M. (1994). Effects of elevated atmospheric CO2 on woody plants. New Phytologist, 27, 425–446.
Chaves, M. M., Flexas, J., & Pinheiro, C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of Botany, 103, 551–560.
Dodd, I. C. (2003). Hormonal interactions and stomatal responses. Journal of Plant Growth Regulation, 22, 32–46.
Einig, W., Lauxmann, U., Hauch, B., Hampp, R., Landolt, W., Maurer, S., & Matyssek, R. (1997). Ozone-induced accumulation of carbohydrates changes enzyme activities of carbohydrate metabolism in birch leaves. New Phytologist, 137, 673–680.
Ericsson, T., & Kähr, M. (1995). Growth and nutrition of birch seedlings at varied relative addition rates of magnesium. Tree Physiology, 15, 85–93.
Gielen, B., & Ceulemans, R. (2001). The likely impact of rising atmospheric CO2 on natural and managed Populus: a literature review. Environmental Pollution, 115, 335–358.
Groeneveld, E., & Rochefort, L. (2002). Nursing plants in peatland restoration: on their potential use to alleviate frost heaving problems. Suoseura, 53(3-4), 73–85.
Hermans, C., Hammond, J. P., White, P. J., & Verbruggen, N. (2006). How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science, 11(12), 610–617.
Hölttä, T., Mencuccini, M., & Nikinmaa, E. (2009). Linking phloem function to structure: analysis with a coupled xylem-phloem transport model. Journal of Theoretical Biology, 259(2), 325–337.
Hopkins, W. G., & Hüner, N. P. A. (2009). Introduction to plant physiology. Hoboken: Wiley.
Huber, S. C. (1984). Biochemical basis for effects of K-deficiency on assimilate export rate and accumulation of soluble sugars in soybean leaves. Plant Physiology, 76(2), 424–430.
Huotari, N., Tillman-Sutela, E., & Kubin, E. (2009). Ground vegetation exceeds tree seedlings in early biomass production and carbon stock on an ash-fertilized cut-away peatland. Biomass and Bioenergy, 33, 1108–1115.
Hytönen, J. (1995). Effect of fertilizer treatment on the biomass production and nutrient uptake of short-rotation willow on cutaway peatlands. Silva Fennica, 29, 21–40.
Hytönen, J. (2005). Effects of liming on the growth of birch and willow on cut-away peat substrates in greenhouse. Baltic Forestry, 11(2), 68–74.
Hytönen, J., & Aro, L. (2012). Biomass and nutrition of naturally regenerated and coppiced birch on cutaway peatland during 37 years. Silva Fennica, 46, 377–394.
Hytönen, J., & Saarsalmi, A. (2009). Long-term biomass production and nutrient uptake of birch, alder and willow plantations on cut-away peatland. Biomass and Bioenergy, 33, 1197–1211.
Ingestad, T. (1962). Macroelement nutrition in pine, spruce and birch seedlings in nutrient solutions. Medd statens skogsforskningsinst, 51, 1–150.
Itoh, R., Yamagishi, J., & Ishii, R. (1997). Effects of potassium deficiency on leaf growth, related water relations and accumulation of solutes in leaves of soybean plants. Japanese Journal of Crop Science, 66(4), 691–697.
Jacobson, S. (2003). Addition of stabilized wood ashes to Swedish coniferous stands on mineral soils—effects on stem growth and needle nutrient concentrations. Silva Fennica, 37(4), 437–450.
Kaunisto, S., & Aro, L. (1996). Forestry use of cutaway peatlands. In H. Vasander (Ed.), Peatlands in Finland (pp. 130–134). Helsinki: Finnish Peatland Society.
Kenzo, T., Ichie, T., Yoneda, R., Kitahashi, Y., Watanabe, Y., Ninomiya, I., & Koike, T. (2004). Interspecific variation of photosynthesis and leaf characteristics in canopy trees of five species of Dipterocarpaceae in a tropical rain forest. Tree Physiology, 24, 1187–1192.
Kikamägi, K., & Ots, K. (2010). Stimulating the growth of trees with ashes of various biofuels (wood, peat) on a cutaway peatland. Forestry Studies, 52, 60–71.
Kikamägi, K., Ots, K., & Kuznetsova, T. (2013). Effect of wood ash on the biomass production and nutrient status of young silver birch (Betula pendula Roth.) trees on cutaway peatlands in Estonia. Ecological Engineering, 58, 17–25.
Kikamägi, K., Ots, K., Kuznetsova, T., & Pototski, A. (2014). The growth and nutrients status of conifers on ash-treated cutaway peatland. Trees, 28(1), 53–64.
Kleczewski, N. M., Herms, D. A., & Bonello, P. (2012). Nutrient and water availability alter belowground patterns of biomass allocation, carbon partitioning, and ectomycorrhizal abundance in Betula nigra. Trees, 26, 525–533.
Klõšeiko, J., Mandre, M., & Korsjukov, R. (2006). Needle carbohydrate concentrations in Norway spruce as affected by wood ash application to soil. Proceedings of the Estonian Academy of Sciences: Biology Ecology, 55(2), 123–136.
Koch, K. (2004). Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology, 7(3), 235–246.
Lavoie, C., Saint-Louis, A., & Lachance, D. (2005). Vegetation dynamics on an abandoned vacuum-mined peatland: 5 years of monitoring. Wetlands Ecology and Management, 13, 621–633.
Lukjanova, A., & Mandre, M. (2009). The effect of wood ash fertilisation on the anatomy and localisation of lignin in Scots pine (Pinus sylvestris L.) needles. Baltic Forestry, 15(2), 177–185.
Lukjanova, A., & Mandre, M. (2010). Effects of alkalization of the environment on the anatomy of Scots pine (Pinus sylvestris) needles. Water, Air, and Soil Pollution, 206(1–4), 13–22.
Maier, C. A., Palmroth, S., & Ward, E. (2008). Short-term effects of fertilization on photosynthesis and leaf morphology of field-grown loblolly pine following long-term exposure to elevated CO2 concentration. Tree Physiology, 28, 597–606.
Mandre, M., Korsjukov, R., & Ots, K. (2004). Effect of wood ash application on the biomass distribution and physiological state of Norway spruce seedlings on sandy soils. Plant and Soil, 265(1–2), 301–314.
Merilo, E., Heinsoo, K., & Kull, O. (2005). Leaf photosynthetic properties in willow (Salix viminalis and Salix dasyclados) plantation in response to fertilization. European Journal of Forest Research, 125(2), 93–100.
Moilanen, M., Hytönen, J., & Leppälä, M. (2012). Application of wood ash accelerates soil respiration and tree growth on drained peatland. European Journal of Soil Science, 63, 467–475.
Norby, R. J., Wullschleger, S. D., Gunderson, C. A., Johnson, D. W., & Ceulemans, R. (1999). Tree responses to rising CO2 in field experiments: implications for the future forest. Plant, Cell and Environment, 22, 683–714.
Pääkkönen, E., Holopainen, T., & Kärenlampi, L. (1995). Ageing-related anatomical and ultrastructural changes in leaves of birch (Betula pendula Roth.) clones as affected by low ozone exposure. Annals of Botany, 75, 285–294.
Paavilainen, E., & Päivänen, J. (1995). Peatland forestry: ecology and principles (Ecological studies 111). Berlin: Springer.
Pallardy, S. G. (2008). Physiology of woody plants (3rd ed.). Amsterdam: Academic.
Puijalon, S., Piola, F., & Bornette, G. (2008). Abiotic stresses increase plant regeneration ability. Evolutionary Ecology, 22, 493–506.
Ramst, R., Orru, M., Salo, V., Halliste, L. (2006). Revision of Estonian cutaway peatlands. The second stage: Ida-Viru, Lääne-Viru, Jõgeva, Järva ja Tartu County. Geological survey of Estonia, Tallinn (in Estonian).
Renou, F., & Farrell, E. P. (2005). Reclaiming peatlands for forestry: the Irish experience. In J. A. Stanturf & P. A. Madsen (Eds.), Restoration of boreal and temperate forests (pp. 541–557). Boca Raton: CRC Press.
Renou-Wilson, F., & Farrell, E. P. (2007). The use of foliar and soil information for optimising the nutrition of Sitka spruce and Norway spruce on cutaway peatlands. Silva Fennica, 41, 409–424.
Rey, A., & Jarvis, P. G. (1997). Growth response of young birch trees (Betula pendula Roth.) after four and half years of CO2 exposure. Annals of Botany, 80, 809–816.
Rey, A., & Jarvis, P. G. (1998). Long-term photosynthetic acclimation to increased atmospheric CO2 concentration in young birch (Betula pendula) trees. Tree Physiology, 18, 441–450.
Riikonen, J., Oksanen, E., Peltonen, P., Holopainen, T., & Vapaavuori, E. (2003). Seasonal variation of physiological characteristics of two silver birch (Betula pendula) clones in the field. Canadian Journal of Forest Research, 33, 2164–2176.
Riipi, M., Ossipov, V., Lempa, K., Haukioja, E., Koricheva, J., Ossipova, S., & Pihlaja, K. (2002). Seasonal changes in birch leaf chemistry: are there trade-offs between leaf growth and accumulation of phenolics? Oecologia, 30, 380–390.
Ruzin, E.S. (1999). Plant microtechnique and microscopy. Oxford University Press, 322 p.
Sellin, A., Õunapuu, E., Kaurilind, E., & Alber, M. (2012). Size-dependent variability of leaf and shoot hydraulic conductance in silver birch. Trees, 26(3), 821–831.
Steen, E., & Larsson, K. (1986). Carbohydrates in roots and rhizomes of perennial grasses. New Phytologist, 104(3), 339–346.
Terashima, I., Miyazawa, S.-I., & Hanba, T. Y. (2001). Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. Journal of Plant Research, 114, 93–105.
Thomas, P. A., & Packham, J. R. (2007). Ecology of woodlands and forests: description dynamics and diversity. Cambridge: Cambrige University Press.
Uri, V., Lõhmus, K., Kiviste, A., & Aosaar, J. (2009). The dynamics of biomass production in relation to foliar and root traits in a grey alder (Alnus incana (L.) Moench) plantation on abandoned agricultural land. Forestry, 82(1), 61–74.
Wallenda, T., Schaeffer, C., Einig, W., Wingler, A., Hampp, R., Seith, B., George, E., & Marschner, H. (1996). Effects of varied soil nitrogen supply on Norway spruce (Picea abies [L.] Karst.). II. Carbon metabolism in needles and mycorrhizal roots. Plant and Soil, 186(2), 361–369.
Wang, T., Tigerstedt, P. M. A., & Viherä-Aarnio, A. (1995). Photosynthesis and canopy characteristics in genetically defined families of silver birch (Betula pendula). Tree Physiology, 15, 665–671.
Acknowledgments
This work was supported by the institutional research funding IUT21-4 (B21004) of the Estonian Ministry of Education and Research, by the Environmental Investment Centre (Projects Nos 29 and 3708) and by the State Forest Management Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguraijuja, K., Klõšeiko, J., Ots, K. et al. Effect of wood ash on leaf and shoot anatomy, photosynthesis and carbohydrate concentrations in birch on a cutaway peatland. Environ Monit Assess 187, 444 (2015). https://doi.org/10.1007/s10661-015-4681-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4681-5