Abstract
Key message
Use of wood ash or a mixture of wood and oil shale ashes increases the concentrations of P and K in the assimilation organs of conifers and stimulates tree growth.
Abstract
The effect of fertilization with wood ash (10 and 15 t ha−1) and a mixture of wood ash (10 t ha−1) and oil shale ash (8 t ha−1) on the growth (height, root collar diameter, biomass, biomass production) and nutrient concentrations in subsoil and needles of young Pinus sylvestris and Picea abies plants on the Puhatu (Northeast Estonia) cutaway peatland in the first 2 years were studied. After the second growing year differences in the average height growth of P. abies and P. sylvestris were statistically significantly higher on ash-treated plots than on the control plots (p < 0.05), being respectively 1.4–1.6 and 1.5–1.7 times greater than height growth of the control trees. The best results on root collar diameter were observed on mixture ash treatments: the root collars were 1.9 (P. abies) and 2.2 (P. sylvestris) times larger than of the control trees. The biomass of the two conifer species and the biomass production of P. sylvestris in 2012 was the greatest on the mixture ash treatments. Five months after fertilization with ashes the concentrations of P, K, Ca and Mg were higher on the treated plots than on the control plot. Although the concentrations of P and K in P. sylvestris needles rose after the treatment with ash, seedlings suffered from P and K deficiency. The concentrations of P and K in P. abies needles were on optimum. The P/N and the K/N ratios in needles were also improved compared to control trees needles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estonia is one of the European regions with large wetland areas. Peatlands in Estonia cover 1,009,100 ha or 22.3 % of the territory (Paal and Leibak 2011). Estonia has a long tradition of peat extraction. Peat was already milled since 19th century for heating and litter (Orru 2008). To date, 9,371 ha of peatland in Estonia has already damaged by peat milling (Ramst and Orru 2009). The revision of Geological Survey of Estonia has made recommendations of re-use in all 81 of Estonian abandoned peat production areas: afforestation 22, re-use for milling 26, restoration back to mire 15, cultivating of berries (blueberry, cranberry) 8, bring into order 7 and waste management 1 area (Ramst et al. 2006). Today, these recommendations are not implemented, and large mined areas are standing abandoned without vegetation. A small area is used in scientific purposes as experimental areas to find options to afforestation, bog restoration and growing biomass.
After peat extraction the land is bare of plants and therefore the residual peat is practically free from seeds (Curran and MacNaeidhe 1984) and colonisation may take several years due to microclimatic and substrate conditions of the cutaway peatlands (Salonen and Laaksonen 1994; Tuittila et al. 2000). Peat harvesting interrupts the carbon-storage to turn peatlands a source of carbon emissions (Tuittila et al. 1999). Afforestation is expected to be a main after-use of cutaway peatlands in Finland (Huotari et al. 2009), Sweden (Hånell et al. 1996) and Ireland (Renou et al. 2006). Forests on abandoned cutaway peatlands can be established to produce quality timber, pulp and fibre biomass (Hall and House 1994) and restore CO2 binding and accumulation of carbon in trees (Huotari et al. 2009). It is important to revegetate these sites to reduce CO2 emission and restore biological diversity.
Cutaway peatland areas are characterised by their variable peat thickness, low pH, high nitrogen concentrations and low phosphorus and potassium concentrations (Hytönen 1996). As remining peat layer is considerably poor in nutrient, it needs to be enriched with nutrient prior to afforestation actions. Ash has long been used to improve soil fertility. In Finland the use of ash as a forest fertilizer has increased considerably, and currently ash is spread annually to nearly 10,000 ha of land, mostly in drained peatlands (Moilanen et al. 2013). Today in Estonia the amount of wood ash produced as energy generation waste is about 20,000 tonnes a year (Pärn et al. 2010). In 1986 more than half of the oil shale ashes was used, but only 3–6 % used in the last decade. In deposition areas 280 million tonnes of oil shale ash has already accumulated and every year 4–5 million tonnes is added (Kuusik et al. 2012), thus it is necessary to find re-use options to ashes to mitigate the waste problem.
Recycling the ashes, which contains the nutrients, now mostly disposed of as waste, could be an alternative for improving the nutritional status in cutaway peatlands. Ashes are a good fertilizer to acidic peatsoils because they act as a liming agent, increasing the soil pH. Wood ash is rich in phosphorus and potassium (Silfverberg 1996), which are the most growth-limiting mineral nutrients in cutaway peatlands (Paavilainen and Päivänen 1995). Oil shale ash generally contains only small amounts of phosphorus and some micronutrients like boron, copper, manganese, zinc etc., but riches in calcium, magnesium, potassium and sulphur (Kärblane 1996). As oil shale ash is relatively poor in P, a mixture of it with P-rich wood ash could be an alternative to encourage the growth of trees in cutaway peatlands. Bare oil shale ash with amount 20–25 t ha−1 did not show positive effect to trees growth, fertilized trees were even smaller than control trees, also frost damage was increased (Seemen et al. 2000). The positive effect of wood ash to trees in cutaway peatlands has been noticed in several studies (Hytönen and Saarsalmi 2009; Huotari et al. 2009; Kikamägi and Ots 2010; Hytönen and Aro 2012; Kikamägi et al. 2013 etc.).
Norway spruce (Picea abies (L.) Karst.) is the main commercial species recommended on this site type in Ireland (Renou and Farrell 2005). Besides P. abies the most common tree species planted on former agricultural lands and cutaway peatlands in Finland have been P. sylvestris and Betula pendula (Aro et al. 1997; Hynönen and Hytönen 1998). In Estonia, Norway spruce is the most productive tree species in fertile drained peatsoils (Valk 1992) and Scots pine is a common tree species, which can grow in very extreme conditions. Therefore, seedlings of P. abies and P. sylvestris were used in our pilot study to investigate the effect of wood ash and a mixture of wood ash and oil shale ash as fertilizers on tree growth on cutaway peatlands.
In particular, the aim of the study was to investigate the effect of fertilization with different amounts of wood ash (10 and 15 t ha−1) and a mixture of wood ash (10 t ha−1) and oil shale ash (8 t ha−1) on the growth (height, root collar diameter, biomass, biomass production), survival, morphology of needles (length of needles, dry mass of 100 needles and density of needles on the branches) and chemical properties of soil and needles of young P. abies and P. sylvestris plants on the Puhatu (Northeast Estonia) cutaway peatland in the first 2 years after fertilization.
Materials and methods
Study site and treatments
The experimental area of Puhatu is located on a cutaway peat-milling area in Ida-Viru County (Northeast Estonia) (59°19′N, 27°35′E). The area of the abandoned field at Puhatu is 1359.80 ha (Ramst et al. 2006). The average thickness of the well-decomposed peat is 0.9 m (Ramst et al. 2006). The area is covered with Phragmites peat. The number of trees on the plots is small. The coverage of Eriophorum vaginatum is on average 10 %; in lower places Phragmites australis, Eriophorum angustifolium, Carex spp. and Equisetum fluviatile are growing. Ditches are half filled with peat and have rich growth of Eriophorum vaginatum, E. angustifolium, Typha latifolia, Carex pseudocyperus, Juncus effuses and J. bufonius. Therefore, some areas have no functioning drainage systems and are in places seasonally flooded. The amount of precipitation was 704.2 mm in 2011 and 758.7 mm in 2012; the average temperature was 6.1 °C in 2011 and 4.5 °C in 2012 (data from the Estonian Meteorological and Hydrological Institute).
In April 2011 the following treatments were established: (1) wood ash 15 t ha−1 (WA15); (2) wood ash 10 t ha−1 (WA10); (3) wood ash 10 t ha−1 + oil shale ash 8 t ha−1 (WA + OSA) and (4) unfertilized (control). Ash was applied just before planting using a square system (1 m2 area around the plants). The ash application amount per hectare, based on the dry weight of ash, was calculated. The amount of ash applied in a square was 0.5–1.8 kg ash per tree. The wood ash generated as production waste originated from Sonda boilerhouse and is bottom ash. The oil shale ash originated from energy company Eesti Energia Narva Elektrijaamad and is cyclone ash. Two-year-old seedlings of Scots pine (Pinus sylvestris L.) and three-year-old seedlings of Norway spruce (Picea abies (L.) Karst.) were hand-planted with planting density 1.2 × 2 m in 9 × 14.5 m plots. The total sample of each treatment included 50 trees.
Measurements and analysis methods
Sampling
The height of all living seedlings was measured after planting (Ho) and at the end of the growing season (October) in 2011 (H2011) and in 2012 (H2012). Survival (%) of planted trees was calculated as the ratio of the number of survived trees to the total number of trees ×100. The root collar diameters were measured in September 2012.
The model trees (n = 3–5) for the determination of biomass and biomass production of the two-year-old stands were felled in September 2012 (one average tree from each row or every second row).
Biomass and biomass production
The model trees were divided into leaves, branches, stem and roots. Stem and branches were selected by year: current year and older (age >1 year). After drying at 70 °C until constant weight the dry mass of all fractions was determined.
Average current annual production (kg year−1) of a model tree consists of the annual increment of leaves, shoots and stem. The current annual production of the leaves, current-year branches and stem is equal to their biomass. The production of older branches and stem (age >1 year) was estimated by dividing branch and stem mass by their age. Root systems were washed with tap water until soilfree, dried at 70 °C and weighed to find their biomass.
Morphometrical measurements
The length (cm) of current-year needles (n = 90–150) were measured with the software WinSeedle (Regents Instruments Inc.). The dry mass (g) of 100 needles (n = 28–30) was weighed. For dry mass determination the needles were stored in a thermostat at 70 °C until they reached a constant mass. The density of P. sylvestris and P. abies needles on the branches (number of needles on a branch divided by the length of a branch, No. cm−1) was calculated.
Chemical analyses
To determine the concentrations of nutrients in the growth substrate, peat samples (n = 3) were taken from random quadrates at depths of 0–10 cm from each treatment and from the unfertilized control plots in September 2011 and at depths of 0–10, 11–20, 21–30, 31–40 and 41–50 cm in September 2012. The needles samples (n = 3) were taken from each treatment and from the unfertilized plots in September 2012.
The pHKCl of peat and the concentrations of N, P, K, Ca and Mg in the peat and plant material were determined in the Laboratory of Plant Biochemistry of the Estonian University of Life Sciences. The peat samples were analysed for their extractable concentration of P (ammonium lactate; by FiaStar5000 (Flow Injection Analyser), K (ammonium lactate; Flame Photometric Method), Ca (ammonium acetate; Flame Photometric Method), Mg (ammonium acetate; by FiaStar5000 (Flow Injection Analyser)) and for total concentration of N (Copper Catalyst Kjeldahl Method). The samples of current-year needles were converted to Kjeldahl digest. N concentration was analysed by Kjeldahl’s copper catalyst method, P by the formation of molybdate blue from orthophosphate in the digest by Fiastar 5000, Ca with o-cresolphthalein complexone in the presence of 8-hydroxyquinoline to mask the influence of Mg by Fiastar 5000, and Mg by means of titan yellow dye by Fiastar 5000, K by flame photometry.
The chemical analyses for assessing the concentrations of heavy metals and macroelements in wood and oil shale ash were made in the Laboratory of the Estonian Environmental Research Centre. Homogeneous material of ashes was subjected to chemical analyses after mineralisation with HNO3 in a microwave digester Mars 5 (USA). The concentration of K was determined by flame emission spectrometry, Ca and Mg by flame atomic absorption spectrometry and S, B, Cd, Cr, Ni. Pb, Fe, Zn, Cu were determined with an inductively coupled plasma optical emission spectroscopy. The pH of ashes was determined potentiometrically in water solution. To measure N the method of Kjeldahl was used and P was determined by ammonium molybdate spectrometric method. The chemical composition of the wood ash and oil shale ash is presented in Table 1.
Statistical analyses
Averages of analyses and standard errors (average ± SE) for morphological parameters and mineral nutrients were calculated. The Shapiro–Wilk test was used to check the normality of the distribution. Levene’s test was used to check the equality of variances. ANOVA (Tukey’s test and Tukey’s Unequal N test) were used for the multiple comparisons of the averages. Pearson correlation coefficients (r) between leaf and soil nutrients were calculated. The Statistica 10.0 software was used, and the significance level of α = 0.05 was accepted in all cases.
Results
Nutrient concentrations in peat
As wood ash and oil shale ash are alkaline (Table 1), they act as liming agents, neutralising the acidic peatsoils. After 5 months the soil pHKCl in the treated plots was around 7, while in the control plots the pHKCl was 4 in both P. abies and P. sylvestris stands in 2011 (Table 2). The pHKCl was decreased on treated plots on the second year, but was still higher than on control in depth 0–10 cm (Table 2).
Five months after fertilization the P concentration in P. abies and P. sylvestris stands was 169 and 57 (WA10), 245 and 59 (WA15) and 7 and 19 (WA + OSA) times higher than control, respectively (Table 2). The P concentration in wood ash-treated plots differed significantly (p < 0.001) from control and WA + OSA treated plots. The P concentration was 43 (WA15), 26 (WA10), 13 (WA + OSA) times higher from control in the second year in depth 0–10 cm. Also the K concentration was substantially higher in treated plots, but it differed significantly (p < 0.05) from control only on WA15 treatment in 2011 (Table 2). In the second year the K concentration was noticeably lower than in 2011 but compared with control plot the concentration of K in 2012 was significantly higher on WA15 and WA + OSA. The Ca and Mg concentrations in fertilized plots were significantly higher (p < 0.05) from control in 2011, but no significant differences was observed between Ca concentration on different plots in 2012 (Table 2). The concentration of Mg was significantly higher on WA15 and WA + OSA from control in depth 0–10 cm in 2012. As ashes are poor in N, treatments did not increase its concentration, quite the contrary, the N concentrations were lower on fertilized plots compared with control plots. The concentration of N on the WA15 and WA + OSA plots of P. abies and WA15, WA10 and WA + OSA plots of P. sylvestris were significantly lower than that in control plots (p < 0.05) in 2011 (Table 2). On the second year, the concentration of N was a little increased on treated plots at depth 0–10 cm, but compared to control, no signifincant differences were found (Table 2).
The P and K concentratios were noticeably decreased in the directions of deeper layer, the Ca and Mg concentrations on the contrary were increased (Table 2). The concentrations of K and Mg were significantly higher on WA + OSA plot compared to control on layers 11–20, 21–30, 31–40 and 41–50 cm (p < 0.05). The concentration of P was significantly higher on WA10 plot compared to control on layers 21–30 and 31–40 cm layers (Table 2).
Tree growth parameters and survival
In the first year after planting and treating with ashes the average height of P. abies and P. sylvestris was almost equal (p > 0.05) on all plots (Fig. 1). In the second year the average height of P. abies and P. sylvestris was already significantly higher (p < 0.05) on ash-treated plots than those on control, being, respectively 1.4 and 1.7 times (WA10), 1.6 and 1.8 times (WA15) and 1.4 and 1.5 times (WA + OSA) greater (Fig. 1). While WA15 was the most effective on average height, the WA + OSA treatment gave the best results on root collar diameter, which was 1.9 (P. abies) and 2.2 (P. sylvestris) times larger than in control trees (Fig. 2). Root collar diameter differed significantly (p < 0.05) from the control plots in all treatments.
Height (average ± SE) of P. abies (A) and P. sylvestris (B) on the ash-fertilized plots and on the unfertilized plot (Control). Different letters indicate a statistically significant difference between treatments within tree species (small letters indicates to P. abies and capital letters to P. sylvestris) in the Tukey test p < 0.05
Root collar diameter (average ± SE) of P. abies and P. sylvestris on the ash-fertilized plots and on the unfertilized plot (Control). Different letters indicate a statistically significant difference between treatments within tree species (small letters indicates to P. abies and capital letters to P. sylvestris) in the Tukey test p < 0.05
The largest biomass of P. abies and P. sylvestris was resulted on the WA + OSA treatment, as well as the biomass production of P. sylvestris in 2012 (Table 3). The biomass production of P. abies was the greatest on the WA15 treatment. The biomass and biomass production of P. abies and P. sylvestris on WA + OSA and WA15 treatment differed significantly from control (p < 0.05), while the two treatments did not differed from each other (p > 0.05) (Table 3).
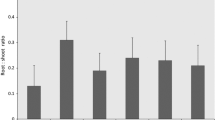
Biomass allocation varied among species and treatments (Fig. 3). At the end of the second year after treatment the largest part of the biomass of P. abies control trees was allocated to the roots, followed by needles, stem and branches. On the wood ash-treated plots the order of biomass distribution was as follows: needles, roots, stem and branches, and on the WA + OSA plot roots, needles, branches and stem. In the case of P. sylvestris the stem made up the largest part of the biomass, followed by roots, needles and branches on the control plot, but on the ash-treated plots the needles made up the largest part of the biomass, followed by stem, roots and branches.
There was no mortality of P. abies on the WA15 and WA + OSA treatments after the first and the second growing season, on the WA10 treatment the mortality was only 4 %. On the control plot the mortality was 6 % after the first growing season and 8 % after the second growing season. The survival of P. sylvestris on the WA15 and WA + OSA treatments and the control plot was over 90 %, but in the WA10 treatment the survival fell to 52 % after the first growing season. After the second growing season the survival of P. sylvestris was in all plots the same as after the first year, except for the control plot, where the survival fell by 2 %, but was still over 90 % (Table 3). Besides, frost damages were noticed on the control plot.
Needles characteristics
Ash fertilization increased significantly (p < 0.05) the length of needles and dry mass of 100 needles of both P. abies and P. sylvestris seedlings (Fig. 4). The needles of P. abies was 2.7 (WA15), 3.3 (WA10), and 3.5 (WA + OSA) times longer and 2.4 (WA15), 3.2 (WA10) and 4.0 (WA + OSA) times heavier than those in the control plot (Fig. 4). The needles of P. sylvestris was 2.4 (WA15 and WA10) and 3.2 (WA + OSA) times longer and 3.8 (WA10), 4.9 (WA15) and 7.3 (WA + OSA) times heavier than of those in the control plot (Fig. 4). The density of needles on the branch of P. abies and P. sylvestris seedlings was significantly smaller (p < 0.001) on the ash-treated plots than on the control plots (Fig. 4).
Needle length (L, cm), density of needles on branches (DNS, No. cm −1) and dry mass of 100 needles (DM of 100 needles, g) (average ± SE) of P. abies and P. sylvestris. Different letters indicate a statistically significant difference between treatments within tree species (small letters indicates to P. abies and capital letters to P. sylvestris) in the Tukey test p < 0.05
Concentration of chemical elements in needles
The concentration of N in P. abies needles on all plots were average out optimal and did not differ significantly from each other (p > 0.05) (Fig 5). The concentration of N in P. sylvestris needles was significantly lower on ash-treated plots than on the control (Fig. 6). The concentrations of P in P. abies and P. sylvestris needles on ash-treated plots were 2–2.6 and 1.5–1.7 times higher than on the control plots, respectively; the differences were statistically significant (p < 0.01), except on P. abies WA10 plot in which no significant differences were found (Figs. 5 and 6). The concentration of K was almost the same on all plots (p > 0.05), and the highest for P. sylvestris needles was on WA + OSA treatment and for P. abies needles was on WA10 treatment (Figs. 5 and 6). The concentration of Ca in P. abies and P. sylvestris needles did not differ between ash-treated plots and control (p > 0.05) (Figs. 5 and 6). The concentration of Mg in P. abies needles on ash-treated plots did not differ from control either (p > 0.05) (Figs. 5 and 6). The concentration of Mg in P. sylvestris needles on WA10 was 1.4 times significantly higher (p < 0.05) from control.
Concentration of mineral elements (average ± SD) in needles of P. abies on the Puhatu cutaway peatlands. From above continuous line to dashed line indicates the optimum level and below to dashed line the range of moderate deficiency suggested by Ingestad (1962). The letters a, b and c indicate a statistically significant difference between treatments in the Tukey test p < 0.05
Concentration of mineral elements (average ± SD) in needles of P. sylvestris on the Puhatu cutaway peatlands. From above continuous line to dashed line indicates the optimum level and below to dashed line the range of moderate deficiency suggested by Ingestad (1962). The letters a, b and c indicate a statistically significant difference between treatments in the Tukey test p < 0.05
Foliar N concentrations of P. abies were positively correlated to the soil nitrogen content (r = 0.72, p = 0.009) and foliar P concentration positively correlated to the soil phosphorus content (r = 0.82, p = 0.001) of layer 0–10. There was also relation between foliar and soil P and Ca content of P. sylvestris (r = 0.87, p = 0.000; r = 0.62, p = 0.033, respectively). There was no relationships between foliar and soil K content of P. abies and P. sylvestris.
It is also important to show nutritional balance in needles. The P/N, K/N, Ca/N and Mg/N ratios on ash-treated plots were improved compared to control. However, considering the optimum ratios suggested by Ingestad (1987), the P/N and K/N ratios were below optimum (Table 4).
Discussion
It is difficult to estimate the nutritional suitability of cutaway peatlands for growing trees because chemical properties are often quite complex (Renou-Wilson and Farrell 2007). Also the chemical composition of ashes can vary a lot. Therefore, it is necessary to experiment with different amounts of ashes to find out the most favourable amounts of ashes to trees.
The imbalance of nutrients or their occurrence in unfavourable ratios can have a negative impact on the timber quality (Kaunisto and Aro 1996). The benefits of ash fertilization in terms of optimising tree growth are maximal on peatland soils rich in nitrogen (Silfverberg and Moilanen 2000). However, a high N concentration in soil and/or acidity may eventually induce P deficiency in plants (Clarholm 1998) and P will become a growth-limiting factor in ecosystems (Koerselman and Meuleman 1996). Trees need N and P in the ratio of 100/10–13 (Hånell et al. 1996; Kaunisto and Aro 1996; Aro 2000). In our study the N/P ratio of peat was improved by wood ash fertilization which was 100/>18. As wood and oil shale ashes are alkaline, they act as liming agents neutralising the acidic peat soils. Liming and fertilization might increase long-term biomass production of trees due to increases in the resistance of trees to extreme temperatures, drought, pathogens and pests (Raid 1986). Peat with a higher Ca content (i.e. higher pH) or ash application may allow for faster microbial activity to decompose peat and release organically bound N (Renou-Wilson and Farrell 2007). Shortage of P and K is considered to be growth limiting in peat soils (Hytönen 2003). High concentrations of P and K in ash are of great importance, improving preconditions for the uptake of N and thus for successful forestation of cutaway peatlands (Hytönen 1995; Hytönen and Saarsalmi 2009). Five months after fertilization with ashes the concentrations of P and K, and also Ca and Mg in peat were higher on the treated plots than on the control plot. After year the concentrations of P, K, Ca and Mg were significantly lower that year before. Phosphorus in peat soil has a low sorption capacity (Nieminen and Jarva 1996). P leaching signs could be noticed only in WA10 plot (the P content in lower layers was significantly higher than in control). In addition, K and Mg content in WA + OSA plot was higher in lower layers than in control plot. Other leaching signs were not observed.
Good nutritional status is critical for an optimum wood production but also increases resistance against stress from both biotic and abiotic agents (Renou-Wilson and Farrell 2007). Tree average height after the first growing year did not differ between ash-treated and control plots, which may be due to stress after planting, as the plants adapt with the new habitat and growth conditions. Besides, Norway spruce grows slowly for the first year after planting on cutaway peatlands (Renou and Farrell 2007). After the second growing year the differences of tree average height and root collar diameter between the ash-treated and control plots were significant. According to Seemen et al. (2000) application of 20–25 t ha−1 oil shale ash to a 4-year-old spruces showed no effect on the height growth of trees: 4 years after the fertilization the height of treated trees was 77 cm and control trees 80 cm. The height increment and root collar diameter of trees observed in a study performed in Ireland, with different stock types of Norway spruce in a cutaway peatland (Renou-Wilson and Farrell 2008), after two growing seasons, are comparable with our control tree parameters, which shows the necessity to fertilize such sites to assure normal growth of trees. Numerous studies show a positive effect of fertilization with ashes on tree growth (Huotari et al. 2008; Sikström et al. 2010; Hytönen and Aro 2012; etc.), which depends on ashes as well as fertilizer amount, site type, tree species, etc. Most studies are focused on growing deciduous trees (birches, willows) on cutaway peatlands or fertilizing older conifer stands. Comparable data on early development of stands in cutaway peatlands are scare. The growth and biomass estimation in chronosequences give additional data about stand development and with this data it is possible to complement the growth dynamics of the cutaway peatland forests.
Silfverberg (1996) observed that an ash dose of less than 2000 kg ha−1 usually results in small or negligible increases in tree growth. Promising results in managing site nutrition have been achieved by wood ash fertilization, where applied amounts have been 4000–5000 kg ha−1 (Aro 2008). Early development of seedlings should be ensured by spot fertilization with a low application rate of potassium and phosphorus (P and K 3–5 g/seedlings), using of larger amount of fertilizer will increase the mortality of seedlings. In peatland fertilization, wood ash is currently recommended only for fertile sites (Moilanen et al. 2013). Puhatu cutaway peatland, the area of our experiments with wood ash and oil shale ash, is one the most nutrient-rich (especially N and Ca) peatlands in Estonia. We achieved the best results in height increment with the wood ash dose of 15 t ha−1. The best results in root collar diameter were observed in the treatment with wood ash 10 t ha−1 mixed with oil shale ash 8 t ha−1, although the difference between WA15 and WA + OSA was not statistically significant. Cutaway peatland forests could have an important productive potential for timber and biomass, as well as for providing a valuable amenity for recreation and a contribution to regional biodiversity (Renou and Farrell 2005). Because of the high nitrogen availability in the soil, the planting density for P. sylvestris should be 3000–5000 seedlings/ha to ensure good quality timber (Aro 2008). In our study 3831 pine and spruce seedlings per hectare were planted.
P. sylvestris has been the main subject of study in the afforestation of cut-away peatlands in Finland (Hytönen 2003, 2005; Moilanen et al. 2013). P. abies is very sensitive to early summer frosts and needs shelter trees on cut-away peatlands located at lower elevation than their surroundings (Aro 2008). Therefore, P. abies has not been used in afforestation in Finland, but it may be possible to grow spruces as the second tree generation after birch or pine. On the other hand, spruce is the safest choice in field afforestation: it can be planted on peat fields as long as one avoids sites low in fertility and sites with an especially high risk of early summer frost damages (Hytönen 2008). Spruce has only a few slightly serious agents of damage, and it tolerates competition from weeds better than other tree species (Hytönen 2008). P. abies appeared to be the more productive species: a tree grew on average 65.5 g biomass on the WA15 plot in the second growing year after fertilization, that is 251 kg ha−1 year−1. The production of two-year-old stand of P. sylvestris was 95.9 kg ha−1 year−1 on the WA + OSA plot. According to Kuznetsova et al. (2011), the aboveground biomass production in reclaimed oil shale mining areas on two-year-old P. sylvestris stand was 2.04 kg ha−1 year−1. The ashes increased the biomasses and biomass production of conifers in peatland, better results were observed when wood ash was mixed with oil shale ash. Hytönen and Kaunisto (1999) found a very good response to wood ash application in pine stands, and the response lasted longer compared to PK-fertilization. Ferilization with wood ash and PK-fertilizer increased the growth of P. sylvestris in peatland, particularly the number of trees and the higher growth rate of individual trees increased, compared with the control (Sikström et al. 2010). Increased needle length, what is good indicator of changes in the growth environment, can be considered as primary signs of a positive effect of ash fertilization. The needles dry mass and length increased significantly on the ash-treated plots compared with control trees. Drainage, cultivation and fertilization are practices often required for the satisfactorily establishment of trees on these sites (Renou and Farrell 2005). The high mortality of P. sylvestris on the WA10 plot in the first growing season may be due to stress after planting, or the plants may have suffered excessive moisture, which was caused by no functioning drainage systems.
An important indicator of tree productivity is needle mass, which is affected by photosynthesis, metabolism and growth processes (Kuznetsova and Mandre 2006). The nutrient content of the needles may cause differences in the productivity of the needles (Ågren 1996). The fertilization with ashes increased conifers’ needles parameters. The average length of needles of P. sylvestris was 5.3–6.9 cm on the ash-treated plots, which is about the same as their average length in Estonia (Laas 2004), while the length of needles on control trees was only 2.2 cm. The needle length of P. abies on the ash-treated plots (1.3–1.6 cm) was also in the range of their average length in Estonia (Laas 2004). The higher density of needles on branches in poorer areas is due to adaptation to adverse environmental conditions, which retard the branches length growth, while trees try to maintain their assimilation surface (Ots 2000). The density of needles on branches was higher on control plots than on ash-fertilized plots.
The ash application increased also the nutrition content in needles. The results of experiments in Finland showed also that higher doses of wood ashes significantly increase P, K and Ca concentrations in P. sylvestris needles (Moilanen et al. 2013). Analysis carried out on 1–4-year-old seedlings after 5-year of application with hardened wood ash (3 t ha−1) showed an increase of P, K and Ca in current and 1-year-old needles in Norway spruce stands (Arvidsson and Lundkvist 2002). Foliar P content of P. abies and P. sylvestris was strongly correlated with soil P content, which shows that P is important element to tree growth in cutaway peatlands. In ash-treated plants, the P content, as well as K and N content in P. abies needles was optimal. To the contrary the N, P and K content in P. sylvestris needles was under optimum. As the P. abies is more nutrient demanding than P. sylvestris (Miller 1995) and physiological condition (greater hight, diameter, needles length and mass etc.) of P. sylvestris is good in fertilized plots, it can be say that P and K content in needles was adequate to P. sylvestris in cutaway peatland. The reduced N concentrations in P. sylvestris needles in the treated plots may be a dilution effect due to excellent growth previous years. Similar results with P. sylvestris have been observed in other studies in cutaway peatlands and drained mires (Silfverberg and Moilanen 2008; Sikström et al. 2010; Kaunisto 1987). Interaction of different mineral elements in plant tissues and their balance are of great importance for tree growth and survival under stress conditions (Ingestad and Ågren 1988; Marschner 2002; Portsmuth et al. 2005). Plants growing in natural conditions can regulate nutrient concentrations and, therefore, sustain quite a constant ratio between N and other nutrients (Ingestad 1979; Linder 1995). The ratios of P/N and K/N in the current-year needles of P. abies and P. sylvestris were improved on the ash-treated plots but stayed under the optimum suggested by Ingestad (1962). The optimum N and P relationship means that plant growth is regulated in the same way by both these elements. Güsewell (2004) supported the use of N/P ratio to predict nutrient limitation and proposed a broader range of ratios, with N/P ratio <10 for N limitation and >20 for P limitation. Koerselman and Meuleman (1996) suggested that foliar N/P values >16 are critical for P limitation in wetlands. Analyses of the N/P ratio indicated that the growth-limiting factor on the control plot was P as the N/P ratio was 32 (P. sylvestris) and 25 (P. abies). In Finland Moilanen et al. (2013) found in needles of Scots pine the N/P ratio of 10 (dose of wood ash 5 Mg ha−1) and 22 (dose of wood ash 15 Mg ha−1). In our experiments the N/P ratio of P. sylvestris needles on WA10 and WA15 was 11 and on WA + OSA was 14, which shows that after fertilization P was not any longer a growth-limiting factor to trees on ash-treated plots in the cutaway peatland. If P is limiting, increased N deposition does not stimulate plant growth but rather reduces it (Gotelli and Ellison 2002). The deficiency of P seems to have a more negative impact than N deficiency on long-term plant performance, for example through increased mortality.
Conclusions
Afforestation is a most promising and sustainable recovery option for cutaway peatlands as it creates renewable energy sources and restores the biological diversity and aesthetic value of these areas. Use of ashes (wood ash, oil shale ash) as fertilizers is beneficial in restoring the fertility of peat and thereby stimulating the tree growth. In the plots treated with wood ash or a mixture of wood and oil shale ashes a considerable increase in the concentrations of P and K, which are growth-limiting elements in cutaway peatlands, was observed in the peat substrate but also in the assimilation organs of trees. Fertilization with nutrient-rich ashes notably increased tree growth (biomass, height, root collar diameter). The best results were registered on the WA15 and WA + OSA treatments. As the tree growth parameters between these two treatments did not differ significantly, it can be said that both ashes were good fertilizers to conifers on the cutaway peatland. P. abies was the more productive species on the Puhatu cutaway peatland. However, because of the short experimental time the effect of wood and oil shale ashes on the growth of conifers on cutaway peatlands needs further investigations.
References
Ågren GI (1996) Nitrogen productivity or photosynthesis minus respiration to calculate plant growth. Oikos 76:529–535
Aro L (2000) Afforestation of cutaway peatlands in Finland. In: Åman P (ed) Re-use of peat production areas. EU’s Northern Periphery Programme project, Proc 1st Int Semin, Oulu, Finland, pp 43–45
Aro L (2008) Cut-away peatlands in forestry. In: Korhonen R, Korpela L, Sarkkola S (eds) Finland. Research and sustainable utilisation of mires and peat Maahenki, Finnish Peatland Society, Helsinki, pp 207–211
Aro L, Kaunisto S, Saarinen M (1997) Afforestation of Peat Cutaway Areas. Project Report in 1986–1995. Research Papers 634. Finnish Forest Research Institute
Arvidsson H, Lundkvist H (2002) Needle chemistry in young Norway spruce stands after application of crushed wood ash. Plant Soil 238:159–174
Clarholm M (1998) Wood ash to counteract potential phosphorus and potassium limitations in a Norway spruce forest subjected to air pollution. Scand J For Res 2:67–75
Curran PL, MacNaeidhe FS (1984) The weed problem of cultivated bog. Ir J Agric Res 23:59–70
Gotelli NJ, Ellison AM (2002) Nitrogen deposition and extinction risk in the northern pitcher plant, Sarracenia purpurea. Ecology 83:2758–2765
Güsewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Hall DO, House JI (1994) Trees and biomass energy: carbon storage and/or fossil fuel substitution? Biomass Bioenergy 6:11–30
Hånell B, Svensson J, Magnusson T (1996) Reclamation of peat winning fields—a literature review with special reference to the establishment of forest stands. Reports in Forest Ecology and Forest Soils 70. Swedish University of Agricultural Sciences, Uppsala
Huotari N, Tillman-Sutela E, Pasanen J, Kubin E (2008) Ash-fertilization improves germination and early establishment of birch (Betula pubescens Ehrh.) seedlings on a cut-away peatland. For Ecol Manag 255:2870–2875
Huotari N, Tillman-Sutela E, Kubin E (2009) Ground vegetation exceeds tree seedlings in early biomass production and carbon stock on an ash-fertilized cut-away peatland. Biomass Bioenergy 33:1108–1115
Hynönen T, Hytönen J (1998) Afforestation of Agricultutal Lands. Metsälehti Kustannus, Saarijärvi
Hytönen J (1995) Effect of fertilizer treatment on the biomass production and nutrient uptake of short-rotation willow on cut-away peatlands. Silva Fenn 29:21–40
Hytönen J (1996) Biomass Production and Nutrition of Short-rotation Plantations. Research Papers 586. Finnish Forest Research Institute
Hytönen J (2003) Effects of wood, peat and coal ash fertilization on Scots pine foliar nutrient concentrations and growth on afforested former agricultural peat soils. Silva Fenn 37:219–239
Hytönen J (2005) Effects of liming on the growth of birch and willow on cut-away peat substrates in greenhouse. Balt For 11(2):68–74
Hytönen J (2008) Afforestation of peatland fields. In: Korhonen R, Korpela L, Sarkkola S (eds) Finland. Research and sustainable utilisation of mires and peat Maahenki, Finnish Peatland Society, Helsinki, pp 212–216
Hytönen J, Aro L (2012) Biomass and nutrition of naturally regenerated and coppiced birch on cutaway peatland during 37 years. Silva Fenn 46:377–394
Hytönen J, Kaunisto S (1999) Effect of fertilisation on the biomass production of coppiced mixed birch and willow stands. Biomass Bioenergy 17:455–469
Hytönen J, Saarsalmi A (2009) Long-term biomass production and nutrient uptake of birch, alder and willow plantations on cut-away peatland. Biomass Bioenergy 33:1197–1211
Ingestad T (1962) Macroelement nutrition in pine, spruce and birch seedlings in nutrient solutions. Medd statens skogsforskningsinst 51:1–150
Ingestad T (1979) Mineral nutrient requirements of Pinus silvestris and Picea abies seedlings. Physiol Plant 45:373–380
Ingestad T, Ågren GI (1988) Nutrient uptake and allocation at steady-state nutrition. Physiol Plant 72:450–459
Kärblane H (1996) The Handbook of Nutrition and Fertilization of Plants. Ministry of Agryculture, Tallinn
Kaunisto S (1987) Effect of refertilization on the development and foliar nutrient contents of young Scots pine stands on drained mires of different nitrogen status. Communicationes Instituti Forestalis Fenniae, Finlande, p 140
Kaunisto S, Aro L (1996) Forestry use of cutaway peatlands. In: Vasander H (ed) Peatlands in Finland. Finnish Peatland Society, Helsinki, pp 130–134
Kikamägi K, Ots K (2010) Stimulating the growth of trees with ashes of various biofuels (wood, peat) on a cutaway peatland. Forest Stud 52:60–71
Kikamägi K, Ots K, Kuznetsova T (2013) Effect of wood ash on the biomass production and nutrient status of young silver birch (Betula pendula Roth) trees on cutaway peatlands in Estonia. Ecol Eng 58:17–25
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Kuusik R, Meriste T, Pototski A (2012) Several new projects have been launched to expand the use of oil shale ash. Keskkonnatehnika 3:8–9
Kuznetsova T, Mandre M (2006) Chemical and morphological indication of the state of lodgepole pine and Scots pine in restored oil shale opencast mining areas in Estonia. Oil Shale 23:366–384
Kuznetsova T, Lukjanova A, Mandre M, Lõhmus K (2011) Aboveground biomass and nutrient accumulation dynamics in young black alder, silver birch and Scots pine plantations on reclaimed oil shale mining areas in Estonia. For Ecol Manag 262:56–64
Laas E (2004) Conifers. Atlex, Tartu
Linder S (1995) Foliar analysis for detecting and correcting nutrient imbalances in Norway spruce. Ecol Bull (Copenhagen) 44:178–190
Marschner H (2002) Mineral nutrition of higher plants, 3rd edn. Academic Press, London
Miller HG (1995) The influence of stand development on nutrient demand, growth and allocation. Plant Soil 168–169:225–232
Moilanen M, Saarsalmi A, Kukkola M, Issakainen J (2013) Effects of stabilized wood ash on nutrient status and growth of Scots pine—comparison between uplands and peatlands. For Ecol Manage 295:136–144
Nieminen M, Jarva M (1996) Phosphorus adsorption by peat from drained mires in southern Finland. Scand J For Res 11:321–326
Orru M (2008) A survey of the history of research on mires and peat (from the 19th century to 1966). In: Vingisaar P, Kivisilla J (eds) Half a century of peat Research. Geological Survey of Estonia, Tallinn, pp 6–12
Ots K (2000) Impact of air pollution on morphological parameters of conifers in an area of oil shale mining and processing. For Stud 33:143–157
Paal J, Leibak E (2011) Estonian mires: inventory of habitats. Regio, Tartu
Paavilainen E, Päivänen J (1995) Peatland Forestry. Ecology and Principles. Ecological Studies 111, Springer-Verlag, Berlin
Pärn H, Mandre M, Ots K, Klõšeiko J, Lukjanova A, Kuznetsova T (2010) Use of biofuel ashes in forestry. For Stud 52:40–50
Portsmuth A, Niinemets U, Truus L, Pensa M (2005) Biomass allocation and growth rates in Pinus sylvestris are interactively modified by nitrogen and phosphorus availabilities and by tree size and age. Can J For Res 35:2346–2359
Raid L (1986) Fertilization of cultivated forests. Mets Puit Paber 5:7–10
Ramst R, Orru M (2009) Revegetation of cutaway peatlands in Estonia. Estonian Combustible Natural Resources and Wastes 1–2:6–7
Ramst R, Orru M, Salo V, Halliste L (2006) Revision of Estonian abandoned peat production areas. The second phase. Ida-Viru, Lääne-Viru, Jõgeva, Järva ja Tartu Counties. Geological Survey of Estonia, Department of Geology and Mineral Resources, Tallinn
Renou F, Farrell EP (2005) Reclaiming peatlands for forestry: the Irish experience. In: Stanturf JA, Madsen PA (eds) Restoration of boreal and temperate forests. CRC Press, Boca Raton, pp 541–557
Renou F, Egan T, Wilson D (2006) Tomorrow’s landscapes: studies in the after-uses of industrial cutaway peatlands in Ireland. Suo 57:97–107
Renou-Wilson F, Farrell EP (2007) The use of foliar and soil information for optimising the nutrition of Sitka spruce and Norway spruce on cutaway peatlands. Silva Fenn 41:409–424
Renou-Wilson F, Farrell EP (2008) The use of foliage and soil information for managing the nutrition of Sitka and Norway spruce on cutaway peatlands. Silva Fenn 41:409–424
Salonen V, Laaksonen M (1994) Effects of fertilization, liming, watering and tillage on plant colonization of bare peat surfaces. Ann Bot Fenn 31:29–36
Seemen H, Pikk J, Valk U (2000) The Rae bog—a unique patch of experimental forestry. Akadeemilise Metsaseltsi Toimetised 12. Töid Eesti metsanduse ajaloost 3:95–112
Sikström U, Almqvist C, Jansson G (2010) Growth of Pinus sylvestris after the application of wood ash or P and K fertilizer to a peatland in southern Sweden. Silva Fenn 44:411–425
Silfverberg K (1996) Nutrient status and development of tree stands and vegetation on ash-fertilized drained peatlands in Finland. PhD Thesis, University of Helsinki
Silfverberg K, Moilanen M (2000) Environmental consequences of recycling wood ash to forests. Extended abstracts from the SNS workshop at Grimsö, Sweden, 22–25 May 2000. In Högbom L, Nohrstedt H-Ö (eds) SkogForsk Report. Uppsala, pp 25–28
Silfverberg K, Moilanen M (2008) Long-term nutrient status of PK fertilized Scots pine stands on drained peatlands in North-Central Finland. Suo 59:71–88
Tuittila E-S, Komulainen VM, Vasander H, Laine J (1999) Restored cut-away peatland as a sink for atmospheric CO2. Oecologia 120:563–574
Tuittila E-S, Rita H, Vasander H, Laine J (2000) Vegetation patterns around Eriophorum vaginatum L. tussocks in a cut-away peatland in southern Finland. Can J Bot 78:47–58
Valk U (1992) The results of forestation of peatlands. Eesti Mets 4–5:13–16
Acknowledgments
This work was funded by the Environmental Investment Centre (Projects Nos 29 and 3708), the State Forest Management Centre and Eesti Energia Narva Power Plants AS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Koike.
Rights and permissions
About this article
Cite this article
Kikamägi, K., Ots, K., Kuznetsova, T. et al. The growth and nutrients status of conifers on ash-treated cutaway peatland. Trees 28, 53–64 (2014). https://doi.org/10.1007/s00468-013-0929-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-013-0929-2