Abstract
Polyaromatic hydrocarbons (PAHs) utilizing bacteria were isolated from soils of seven sites of Mathura refinery, India. Twenty-six bacterial strains with different morphotypes were isolated. These strains were acclimatized to utilize a mixture of four polycyclic aromatic hydrocarbons, i.e., anthracene, fluorene, phenanthrene, and pyrene, each at 50 mg/L concentration as sole carbon source. Out of total isolates, 15 potent isolates were subjected to 16S rDNA sequencing and identified as a member of diverse genera, i.e., Bacillus, Acinetobacter, Stenotrophomonas, Alcaligenes, Lysinibacillus, Brevibacterium, Serratia, and Streptomyces. Consortium of four promising isolates (Acinetobacter, Brevibacterium, Serratia, and Streptomyces) were also investigated for bioremediation of PAH mixture. This consortium was proved to be efficient PAH degrader resulting in 40–70 % degradation of PAH within 7 days. Results of this study indicated that these genera may play an active role in bioremediation of PAHs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyaromatic hydrocarbons (PAHs) represent a large group of environmentally persistent, organic pollutants which are ubiquitous in aquatic and terrestrial ecosystems (Shiaris 1989). These are formed due to incomplete combustion of organic substances such as coal, oil, gas, wood, garbage, and from petrochemical/oil refining industries (Nam et al. 2003). These PAHs gain entry into the soil and gets accumulated in zones rich in organic matter, where they are likely to be retained for many years due to their persistence and hydrophobicity (Krauss et al. 2000). However, the threat of percolation of recalcitrant polyaromatics from the spent oily sludge into the ground water looms large and environmental management of the recalcitrant polyaromatic hydrocarbons becomes more significant because of their carcinogenicity and mutagenicity.
PAHs concentrations in Asian countries have shown an increasing trend in the last decade due to increase in fossil fuel combustion and indiscriminate dumping of petroleum refinery effluents. Factors like hydrophobicity, aqueous solubility, and polarity have a large influence on the bioavailability of pollutants. Contaminated soils often contain a separate non-aqueous phase liquid (NAPL) that may be present as droplets or films on soil surfaces. Many pollutants, especially those that are hydrophobic, are virtually insoluble in water and remain adsorbed in the NAPL (Pandey and Jain 2002). PAHs have been found to persist in the NAPL due to their low water solubility and high octanol-water partition coefficients. Therefore, for biodegradation to occur, bacteria must have access to the target compounds, either by dissolution of the target compounds in the aqueous phase or by adhesion of the bacteria directly to the NAPL-water interface. However, the intracellular localization of the PAH degrading enzymes implies that the PAHs have to be solubilized and must enter into the cytoplasm before they can be metabolized (Herrenkohl et al. 2001; Schluep et al. 2001; Wick et al. 2001)
In India, a study on PAHs and PCBs contamination level in soil sample around Mathura refinery indicated that the contamination of PAHs ranged between 6.76 and 13.44 μg g−1 including fluoranthene, chrysene, benzo (b) fluoranthene in the samples collected throughout the year from Mathura-Agra highway (Sharma and Rawat 2013). Nowadays, there is a general awareness about the detrimental effects of PAH products and sub-products that are being released and dispose into the environment as many PAHs and their epoxides are highly toxic, mutagenic, and/or carcinogenic to microorganisms as well as to higher forms of life including humans (Rengarajan et al 2015). Natural habitats usually exhibit highly diversified microbial communities which exhibit high taxonomic and functional diversity (Lindstrom et al 1999). Contaminated sites may harbor a number of microorganisms capable of degrading and utilizing polyaromatic hydrocarbons which can be exploited for amelioration of such sites (Baboshin et al. 2008; Kang et al. 2003). Potential isolates from PAH-polluted sites have been found to be more active than those originating from uncontaminated sites; as these bacteria are adapted to the contaminated environments. The metabolic cooperation of several microorganisms may result in enhanced PAH utilization, since metabolic intermediates produced by one group of microorganisms may serve as substrates for the growth of others.
Several reports are available wherein biodegradation of PAHs was achieved by bacteria (Arulazahan and Vasudevan 2011; Hamamura et al 2013). Soil spiked with high molecular weight PAHs benzoanthracene, benzopyrene was bioremediated effectively achieved by using Sphingobium indicum, Sphingobium japonicum, and Stenotrophomonas maltophilia bioaugmented with different co-substrates (Gurjeet et al 2014).
Bioremediation by soil bacteria has been investigated extensively during the last few decades as an alternative, and less expensive, strategy for the destruction of PAH contaminants. A number of strains such as S. maltophilia strain VUN 10003, Mycobacterium sp., Gardona sp., Rhodococcus sp., Pseudomonas aeruginosa, Burkholderia cepacia, Flavobacterium sp., and Cycloclasticus sp. have been found to utilize benz[a]anthracene, chrysene, fluoranthene, and pyrene as a source of carbon and energy. However, most of the information about metabolic pathways, enzymes, and genes has been restricted to low molecular weight (LMW) PAHs. Much less information is available on the metabolism of high molecular weight (HMW) PAHs, albeit research in the past decade on the bacterial degradation of four-ring PAHs such as fluoranthene, pyrene, benz[a]anthracene, etc. has advanced significantly. Most of these bacteria involved in bioremediation are gram-positive, suggesting that these organisms play a more important role than gram-negative bacteria in the environmental degradation of HMW PAHs (Juhasz et al. 2000; Kanaly and Harayama 2000; Watanabe 2001). Studying the structure and dynamics of microbial communities, capable of degrading different pollutants in contaminated ecosystems, is relevant in microbial ecology for the development of bioremediation strategies (Zhang et al. 2010). With this view, the present study was undertaken with the soil collected from the vicinity of an Indian oil refinery to isolate promising bacteria for bioremediation of PAHs
Materials and method
Chemical sample collection and media
Polyaromatic hydrocarbon (PAHs) anthracene (97 % purity), fluorene (95 %), phenanthrene (97 %), and pyrene (96 %) were purchased from Merck Germany. Other chemicals used in this study were purchased from HiMedia India. Soil samples were collected from sites of Mathura refinery India situated 27° 30′ N 77° 41′ E 27.5° N 77.68° E. For the purpose of soil sample collection, Mathura refinery premises was divided into seven parts based on the locations of different petroleum processing waste outlets and the flanking garden. Samples were collected with the help of an auger from 0 to 15 cm of top soil from seven different points within the same area. A total of 35 soil samples were collected (5 from each 7 locations), i.e., Mathura agricultural field (site 1, village, Dhana Teja 1 km from refinery), Mathura refinery agricultural field (site 2, range 100 m), sewage sample Mathura refinery (site 3), which was accumulated outside the outlet pipe discharging refinery waste, bitumen unit Mathura refinery (site 4), kerosene unit Mathura refinery (site 5), aviation turbine fuel refining unit (site 6), and sulphur unit (site 7, sulphur depositions in soil through chimney of refinery). Five samples from each location were pooled together, air dried, sieved through 200 mesh sieve, stored in polybags, and kept in refrigerator till further analysis.
Enrichment, isolation, and identification of PAH utilizing bacterial strain
Soil sample (10 g) from each site was taken into pre sterilized flask containing 100 mL Bushnell and Haas 1941 of medium spiked with PAH mixture 50 mg L−1 comprising of Bushnell and Haas medium (B&H medium) and minimal medium comprised of NH4NO3 (1 g), MgSO4.7H2O (0.2 g), CaCl2.2H2O (0.02 g), and KH2PO4 (1 g), K2HPO4 (1 g), FeCl3 (0.001 g), and agar (20 g) per liter of the medium (pH 7.0–7.2) was used for isolation of PAH utilizing bacteria. Medium supplemented with PAH mixture at 50 mg L−1 each was achieved after evaporating PAH solution in acetone in pre sterilized flask followed by addition of B&H medium. Flasks were kept for 15 days in dark at 30 °C in Kuhner incubator shaker at 150 rpm. Medium (1 mL) was transferred to 99 mL of fresh BH medium containing 50 mg L−1 PAH mixture and incubated under same conditions. This process was repeated three times. A portion of 100 μL of the suspension was plated onto solid minimal PAH medium plate containing 50 mg L−1 PAH mixture as a sole source of carbon and incubated for 7 days at 30 °C. Different morphotypes were selected and purified further. Nutrient agar medium for bacteria and GYMA for actinobacteria were used for further sub culturing. Screening of the isolates for their PAH utilization efficiency was done by observing their growth potential on PAH containing minimal medium plates. The presence or absence of growth was recorded after 24 h of incubation at 30 ± 1 °C.
Chemotaxis assay
Chemotaxis response of various isolates for PAH was determined by drop assay method. Bacterial cells in logarithmic phase of growth were harvested from 40 mL nutrient broth and resuspended in 12 mL of chemotaxis buffer (100 mM potassium buffer pH 7, 20 mM EDTA to and optical density 0.7 approximately at 600 nm). This suspension (0.1 mL) was spread plated on Nutrient agar/GYMA plates. A test attractant, i.e., anthracene or fluorene, was added to the center of petri dish. Formation of concentric ring of turbidity near center of petri dish was recorded as positive chemotactic assay. Succinate was used as chemo attractant in positive control.
Biosurfactant production
The amount of crude biosurfactants produced by PAH cultures in the culture medium was estimated by turbidimetric method. Fifty milliliters of sterilized minimal medium with glucose (2 % w/v) as carbon source was taken in 250 mL of Erlenmeyer flask. Flasks were inoculated with PAH utilizing bacteria and incubated at 30 °C, 180 rpm for 48 h. Supernatant was obtained after centrifugation of broth at 10,000 rpm for 30 min. HCl (6 N) was added to cell-free supernatant till pH 2 was attained. Further supernatant was allowed to stand for 30 min at 4 °C. The mixture was vortexed to get homogeneous solution. Absorbance was recorded at 600 nm and concentration of biosurfactant was calculated by using method of Mukherjee et al. (2009).
Analysis of 16S rDNA sequences
For 16S rDNA sequence analysis of the bacterial strains, their genomic DNA were isolated following the method of Charles and Nester (1993) followed by 16S RNA gene amplification using primers, pA forward, (5′–CACGGATCCAGAGTTTGATTCTGGCTCAG–3′) and pH reverse, (5′–GTGCTGCAGGGTTACCTTGTTACGACT–3′) for bacteria (Edwards et al. 1989, Xcleris, Ahmedabad India). The purified PCR product was sequenced using ABI PRISM™ 3130xl Genetic analyzer (Applied Biosystems, CA, USA). All the sequence were determined and aligned with BLAST on the NCBI site website (http://www.ncbi.nlm.nih.gov). Phylogenetic tree were constructed with MEGA 4 software (version) and the neighbor joining (NJ) method with pairwise alignment and bootstrap 1000 replications.
PAH degradation assay
In order to confirm the extent of degradation, four isolates were chosen on the basis of their growth at higher concentration (100 mg L−1) of PAHs. The four strains PL-2, PL-44, PL-68, and PAH-13 were sub-cultured at 30 °C on PAH medium. Degradation of four PAHs (fluorene, phenanthrene, anthracene, and pyrene) was studied as mixture, with 50 mg L−1 of each PAH compound, under controlled conditions in BH medium incubated with chosen strains. The medium was inoculated with cell suspension (OD600 = 0.6) followed by incubation in shaker at 30 °C and 150 rpm for 7 days. After incubation, the samples were used to quantify the residual PAHs. For the extraction of residual PAHs, the samples were extracted with ethyl acetate and the resulting extract after drying with sodium sulphate was quantified by HPLC. HPLC analysis was carried out using a Hewlett Packard HPLC instrument (series 1100) equipped with degasser, quaternary pump, photo diode-array detector connected with rheodyne injection system (20 μL loop), and a computer (model vectra). The stationary phase consisted of PAH column LiChroCART 250-4LiChrospher PAH. Chromatogram was recorded in a Windows’ NT based HP chemstation program. Acetonitrile: water (75:25) at 0.5 mL min−1 flow rate was used as mobile phase. HPLC analysis was performed at 254 nm wavelength suitable for each compound by checking the absorption maxima in isoplot option using photodiode array. Twenty microliters of sample and standard PAH compounds were injected into HPLC under standardized conditions. Each run was repeated thrice and the detector response was measured in terms of peak areas. Four PAHs, i.e., fluorene, phenanthrene, anthracene, and pyrene under the described conditions of HPLC appeared at retention times 6.98, 8.08, 8.98, and 13.0 min, respectively. The data was analyzed and results of PAH dissipation were expressed as percent degradation of each PAH compound. Recovery studies of all the PAHs (fluorene, phenanthrene, anthracene, and pyrene) were calculated at five different concentrations. Recoveries were found in the range of 90–97 % except for fluorene which was 77–78 %. The standard curve was found to be linear and limit of detection of PAHs were 0.05, 0.02, 0.001, and 0.1 μg g−1 for fluorene, phenanthrene, anthracene, and pyrene, respectively.
Results and discussion
A total of 66 bacterial strains were isolated from 7 soil samples. Out of them, 26 potent bacteria were selected for biochemical characterization on the basis of growth on high concentration of individual PAH (fluorene, phenanthrene, anthracene, and pyrene) as well as the mixture of 4 PAHs. The Bacillus sp. together accounted for 33 % of the total number of bacteria. Streptomyces, Serratia, Aeromonas, Burkholderia, and Aerobacter each accounted for smaller proportion about 4 % while other strain accounted for 7 % and more as shown in Fig. 1.
Classification of PAH degrading bacteria in refinery samples
Distribution of 26 isolates according to the site is presented in Table 1. Two Bacillus strains, one Lysinibacillus, Serratia, Pseudomonas and one Aeromonas were isolated from Mathura field soil (site 1). A total of five bacterial strains belonging to different genera were isolated from site 2 (Mathura refinery field soil). Nine isolates were isolated from the sewage sample of refinery, i.e., site 3 (Sewage Mathura refinery) which included strains belonging to genera Acinetobacter and Bacillus, Brevibacterium, Burkholderia, and Sphingomonas. Streptomyces and Bacillus strain were isolated from the Bitumen unit refinery (site 4); Bacillus and Stenotrophomonas strain were isolated from kerosene unit of refinery site 5. From site 6 (aviation turbine fuel site) and site 7 (sulphur site), one-one strain of Bacillus was isolated.
Taxonomic identification of the selected strains
Fifteen potent bacterial strains were subjected to 16S rDNA sequencing and were identified as Acinetobacter sp., Brevibacterium sp., Alcaligenes sp., Stenotrophomonas sp., Lysinibacillus sp., Bacillus sp., Serratia sp., and Streptomyces sp. (Chaudhary et al. 2011). Homology analysis of 16S rRNA provides specific data that could be used to determine both close and very distant relationships (Bull et al. 1992). BLAST homology search for 16S rRNA gene sequences of the isolates indicated 98–99 % similarity with their closest relative. Analysis of 16S rDNA gene sequence of four experimental strains indicates that they belong to genus Streptomyces, Serratia, Acinetobacter, and Brevibacterium and submitted to NCBI with accession nos. GQ904711, HQ536226, HQ536214, and HQ536221 (Fig. 2).
Chemotaxis assay
Out of 15, 4 strain were selected for PAH degradation as they showed maximum growth on mixture of PAH. Affinity of PL68 was maximum toward three-ring and four-ring PAH. But we failed to observe the affinity of potent isolates PAH-13, PL-2, and PL-44 on petriplate as the selected strains were nonmotile.
Biosurfactant production
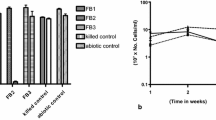
Biosurfactant secreted by bacteria are more effective than chemical surfactants in enhancing the solubility and biodegradation of petroleum hydrocarbons including PAH (Cybulski et al. 2003; Wong et al. 2004). Production of biosurfactant is related to the utilization of available hydrophobic substrates by microbes from their natural habitat, presumably by increasing the surface area of substrates and increasing their apparent solubility. However, there are only a few reports of microorganisms producing surface-active compounds while growing on crude petroleum-oil hydrocarbons (Chhatre et al. 1996). In the present study, PAH bacteria were found to be effective biosurfactant producers; the amount ranging between 0.22 and 1.69 g L−1 on minimal medium, providing the advantage of a continuous supply of natural, non-toxic, and biodegradable surfactants by bacteria for solubilizing the hydrophobic hydrocarbons prior to biodegradation.
Degradation of PAH
Four bacterial strain were selected on the basis of their growth at higher concentration of PAH, Acinetobacter calcoaceticus (PL-2) and Brevibacterium (PL-44) from refinery sewage, Serratia (PL-68) from agricultural field, Strepomyces rochei PAH-13 from bitumen unit of Mathura refinery. When consortium of these four cultures were evaluated for degradation of PAH mixture by HPLC analysis, the degradation rate of fluorene, anthracene, and phenanthrene was higher as compared to that of pyrene (four-ring PAH). PAH dissipation levels ranged between 40 and 70 % in minimal media after 7-day incubation with highest depletion recorded in the case of fluorene (70 %). Phenanthrene and anthracene degradation was 55 and 53 %, and only 40 % of pyrene degradation was achieved by combination of all four bacterial strains (Fig. 3). The present results showed that the consortium of the four microbes could degrade PAH but individual microbes were less efficient, except S. rochei PAH 13 which could degrade PAH efficiently in 15 days in submerged medium supplemented with yeast extract (0.1 %) as indicated in our earlier studies (Chaudhary et al. 2011). However, the percent degradation (28–92 %) was less as observed at 100 ppm PAH concentration after 15 days as compared to the present study where the consortia resulting in faster PAH degradation within 7 days. Several bacterial strains have been reported to possess the metabolic routes required for the degradation of recalcitrant compounds. Species of Pseudomonas, Mycobacterium, Haemophilus, Rhodococcus, Paenibacillus, and Ralstonia are some of the most extensively studied bacteria for their bioremediation capability (Farhadian et al. 2008; Haritash and Kaushik 2009; de Carvalho et al. 2005). These strains are able to degrade petroleum hydrocarbons and aromatic hydrocarbons such as benzene, toluene, ethyl benzene, and xylene (Pseudomonas, Rhodococcus, and Ralstonia), as well as polyaromatic hydrocarbons such as naphthalene (Pseudomonas), phenanthrene (Pseudomonas and Haemophilus), anthracene (Rhodococcus), pyrene (Haemophilus and Mycobacterium) and the highly carcinogenic benzo[a]pyrene (Rhodococcus and Mycobacterium).
HPLC chromatogram showing residual PAHs [F fluorene (6.7 min), Ph phenanthrene (7.9 min), A anthracene (9.4 min), Py pyrene (13.0 min)] at 7 days after incubation; C-Residual PAHs from control flask (PAHs at 50 μg g−1 in medium with no microbial inoculation); final dilution before HPLC 2 mL; T-Residual PAHs from treated flask (PAHs at 50 μg g−1 in medium with microbial inoculation); final dilution before HPLC 5 mL
PAHs are environmental pollutants which are widely distributed in the world. Pollutants introduced into soil exert a negative influence on the microbiota. PAHs exhibit toxic properties which clearly inhibit development and metabolic activity of microorganism in most cases (Kanaly and Harayama 2000). In some cases, the stimulatory activity of substance originating from crude oil had also been observed (Boopathy 2000) which result from gradual adaptation of microorganisms to pollutants and utilization of xenobiotics as sole source of carbon and energy. Similarly in our study, the isolated strains from Mathura region have potential to grow on higher concentration of PAH. A major factor in PAH degradation is the lack of bioavailability of the PAH due to poor solubility in water, leading to accumulation. These isolated strain used in the study are smart enough to tackle the PAH uptake/degradation problem either by direct contact, e.g., attaching themselves to these compounds or indirectly by production of biosurfactant which make PAHs bioavailable. In general, biosurfactant-enhanced bioremediation technology is still at its early phase. There is growing interest in the use of biosurfactant for environmental applications, as synthetic surfactants are generally considered to be more toxic and require higher concentrations than biosurfactants. In the past two decades, a number of bacteria have been identified as “PAH degraders,” including the common genera of Burkholderia (Kim et al. 2003), Rhodococcus (Di Gennaro et al. 2001), Polaromonas (Pumphrey and Madsen 2007), Bacillus (Annweiler et al. 2000), Alcaligenes, and Stenotrophomonas (Boonchan et al. 1998). Thus, the combination of S. rochei (GQ904711), A. calcoaceticus (HQ536214), Brevibacterium sp. (HQ536221), and Serratia (HQ536226) could be employed in the degradation of PAHs contamination as consortium through biostimulation and bioaugmentation approach. Taxonomic analysis indicated that gamma-proteobacteria and actinobacteria were the most abundant PAH degrading bacterial lineages in PAH-polluted soils. The findings revealed differences in bacterial community structures within the refinery site and confirm their potential to degrade higher levels of PAHs. These results call for more intensive study to monitor microbial communities at petroleum waste polluted areas through modern diagnostic tools.
Conclusion
This study is based on the morphological, physiological, and phylogenetic profiling of PAH utilizing bacteria in the soil of Mathura refinery which may be playing important role in natural bioremediation of polyaromatics. A significant representation of gamma-proteobacteria and actinobacteria is a novel finding and should be taken into consideration while developing bioremediation strategies. Manipulation of such microbial diversity in contaminated soils may provide safe, economical, effective “green,” and economically viable technology for bioremediation of PAH-contaminated soils. Bacterial-PAH association formed in the vicinity of refinery soils showed their preference for PAH. The role of PAH degrading genes may be explored in further study to decipher their role in metabolism of low molecular and high molecular weight PAHs. Similarly, bioprospecting of potent microbes has led to consortium development which may be tested in other PAH-contaminated sites to efficiently biodegrade/bioremediate the contaminated sites in India.
References
Annweiler, E., Materna, A., Safinowski, M., Kappler, A., Richnow, H. H., Michaelis, W., & Meckenstock, R. U. (2000). Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture. Applied Environmental Microbiology, 66, 5329–5333.
Arulazahan, P., & Vasudevan, N. (2011). Role of nutrients in the utilization of polycyclic aromatic hydrocarbons by halotolerant bacterial strain. Journal of Environmental Science, 23, 282–287.
Baboshin, M., Akimov, V., Baskunov, B., Born, T. L., Khan, S. U., & Golovleva, L. (2008). Conversion of polycyclic aromatic hydrocarbons by Sphingomonas sp. VKM B-2434. Biodegradation, 19, 567–576.
Boonchan, S., Britz, M. L., & Stanley, G. A. (1998). Surfactant- enhanced biodegradation of high molecular weight polycyclic aromatic hydrocarbons by Stenotrophomonas maltophilia. Biotechnology Bioengineering, 59, 482–494.
Boopathy, R. (2000). Factors limiting bioremediation technologies. Bioresource Technology, 74, 63–67.
Bull, A. T., Goodfellow, M., & Slater, J. H. (1992). Biodiversity as a source of innovation in biotechnology. Annual Reviews of Microbiology, 46, 219–252.
Bushnell, L. D., & Haas, H. F. (1941). The utilization of certain hydrocarbons by microorganisms. Journal of Bacteriology, 41, 653–673.
Charles, T. C., & Nester, E. W. (1993). A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. Journal of Bacteriology, 175, 6614–6625.
Chaudhary, P., Sharma, R., Singh, S. B., Chaudhary, P., Sharma, R., Singh, S. B., & Lata. (2011). Bioremediation of PAH by Streptomyces sp. Bulletin of Environmental Contamination and Toxicology, 86, 268–271.
Chaudhary, P., Singh, S. B., Chaudhry, S., & Lata. (2012). Impact of PAH on biological health parameters of soils of an Indian refinery and adjoining agricultural area-A case study. Environmental Assessment and Monitoring, 184(2), 1145–1156.
Chhatre, S., Purohit, H., Shanker, R., & Khanna, P. (1996). Bacterial consortia for crude oil spill remediation. Water Science and Technology, 34, 187–193.
Cybulski, Z., Dziurla, E., Kaczorek, E., & Olszanowski, A. (2003). The influence of emulsifiers on hydrocarbon biodegradation by Pseudomondacea and Bacillaea strains. Spill Science Technology Bulletin, 8, 503–507.
De Carvalho, C. C. C. R., Parreno-Marchante, B., Neumann, G., da Fonseca, M. M. R., & Heipieper, H. J. (2005). Adaptation of Rhodococcus erythropolis DCL14 to growth on n-alkanes, alcohols and terpenes. Applied Microbiology Biotechnology, 67, 383–388. doi:10.1007/s00253-004-1750-z.
Di Gennaro, P., Rescalli, E., Galli, E., Sello, G., & Bestetti, G. (2001). Characterization of Rhodococcus opacus R7, a strain able to degrade naphthalene and o-xylene isolated from a polycyclic aromatic hydrocarbon-contaminated soil. Research Microbiology, 152(7), 641–651. doi:10.1016/S0923-2508(01)01243-8.
Edwards, U., Rogall, T., Blocker, H., Emde, M., & Böttger, E. C. (1989). Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acid Research, 19(19), 7843–7853.
Farhadian, M., Vachelard, C., Duchez, D., & Larroche, C. (2008). In situ bioremediation of monoaromatic pollutants in groundwater: a review. Bioresource Technology, 99, 5296–5308. doi:10.1016/j.biortech.2007.10.025.
Gurjeet, P., Kothiyal, N. C., & Kumar, V. (2014). Bioremediation of some polycyclic aromatic hydrocarbons (PAH) from soil using Sphingobium indicum, Sphingobium japonicum and Stenotrophomonas maltophilia bacterial strains under aerobic conditions. Journal of Environmental Research and Development, 8, 396–405.
Hamamura, N., Ward, D. M., & Inskeep, W. P. (2013). Effects of petroleum mixture types on soil bacterial population dynamics associated with the biodegradation of hydrocarbons in soil environments. FEMS Microbiology Ecology, 85, 168–178.
Haritash, A. K., & Kaushik, C. P. (2009). Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. Journal of Hazardous Material, 169, 1–15.
Herrenkohl, M. J., Lunz, J. D., Sheets, R. G., & Wakeman, J. S. (2001). Environmental impacts of PAH and oil release as a NAPL or as contaminated pore water from the construction of a 90-cm in situ isolation cap. Environmental Science Technology, 35, 4927–4932.
Juhasz, A. L., Stanley, G. A., & Britz, M. L. (2000). Microbial degradation and detoxification of high molecular weight polycyclic aromatic hydrocarbons by Stenotrophomonas maltophilia strain VUN 10,003. Letters in Applied Microbiology, 30, 396–401.
Kanaly, R. A., & Harayama, S. (2000). Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. Journal of Bacteriology, 182, 2059–2067.
Kang, H., Hwang, S. Y., Kim, Y. M., Kim, E., Kim, Y. S., & Kim, S. K. (2003). Degradation of phenanthrene and naphthalene by a Burkholderia species strain. Canadian Journal of Microbiology, 49, 39–144.
Kim, Y. H., Engesser, K. H., & Cerniglia, C. E. (2003). Two polycyclic aromatic hydrocarbon o-quinone reductases from a pyrene-degrading Mycobacterium. Archives of Biochemistry Biophysics, 416, 209–217.
Krauss, M., Wilcse, W., & Zech, W. (2000). Availability of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) to earthworms in urban soils. Environmental Science Technology, 34, 4335–4340.
Lindstrom, J. E., Barry, R. P., & Braddock, J. F. (1999). Long-term effect on microbial communities after a sub artic oil spill. Soil Biology and Biochemistry, 31, 1677–1689.
Mukherjee, S., Das, P., & Sen, R. (2009). Rapid quantification of a microbial surfactant by a simple turbidometric method. Journal of Microbiology Methods, 76, 38–42.
Nam, J. J., Song, B. H., Eom, K. C., Lee, S. H., & Smith, A. (2003). Distribution of polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in South Korea. Chemosphere, 50, 1281–1289.
Pandey, G., & Jain, R. K. (2002). Bacterial chemotaxis toward environmental pollutants: role in bioremediation. Applied Environmental Microbiology, 68, 5789–5795.
Pumphrey, G. M., & Madsen, E. L. (2007). Naphthalene metabolism and growth inhibition by naphthalene in Polaromonas naphthalenivorans strain CJ2. Microbiology, 153, 3730–3738. doi:10.1099/mic.0.2007/010728-0.
Rengarajan, T., Rajendran, P., Nandakumar, N., Lokeshkumar, B., Rajendran, P., & Nishigaki, I. (2015). Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pacific Journal of Tropical Biomedicine, 5, 182–189.
Schluep, M., Imboden, D. M., Galli, R., & Zeyer, J. (2001). Mechanisms affecting the dissolution of non aqueous phase liquids into the aqueous phase in slow stirring batch system. Environmental Toxicology Chemistry, 20, 459–466.
Sharma, M., & Rawat, M. K. (2013). Study on polycyclic aromatic hydrocarbons and poly chlorinated biphenyls yearly based concentration in waste oil-sludge at Mathura-Agra Region. Journal of Current Chemical & Pharmaceutical Sciences, 3(1), 16–22.
Shiaris, M. P. (1989). Seasonal biotransformation of naphthalene, phenanthrene and benzo (a) pyrene in superficial estuarine sediments. Applied Environmental Microbiology, 55, 1391–1399.
Watanabe, K. (2001). Microorganisms relevant to bioremediation. Current Opinions in Biotechnology, 12, 237–241.
Wick, L. Y., Colangelo, T., & Harms, H. (2001). Kinetics of mass transfer-limited bacterial growth on solid PAHs. Environmental Science Technology, 35, 354–361.
Wong, J. W. C., Fang, M., Zhao, Z., & Xing, B. (2004). Effect of surfactants on solubilization and degradation of phenanthrene under thermophilic conditions. Journal of Environmental Quality, 33, 2015–2025.
Zhang, W., Wang, H., Zhang, R., Yu, X. Z., Qian, P. Y., & Wong, M. H. (2010). Bacterial communities in PAH contaminated soils at an electronic-waste processing center in China. Ecotoxicology, 19, 96–104.
Acknowledgments
The financial assistance provided by the Indian council of Agricultural Research, New Delhi through AMAAS network project is gratefully acknowledged. The authors are also grateful to the division of Microbiology and Agricultural chemicals for providing the necessary facilities to undertake this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Chaudhary, P., Sahay, H., Sharma, R. et al. Identification and analysis of polyaromatic hydrocarbons (PAHs)—biodegrading bacterial strains from refinery soil of India. Environ Monit Assess 187, 391 (2015). https://doi.org/10.1007/s10661-015-4617-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4617-0