Abstract
Rhodococcus erythropolis DCL14 has the ability to convert the terpene (−)-carveol to the valuable flavour compound (−)-carvone when growing on a wide range of carbon sources. To study the effect of carbon and energy sources such as alkanes, alkanols and terpenes on the biotechnological process, the cellular adaptation at the level of fatty acid composition of the membrane phospholipids and the (−)-carvone production were examined. All tested carbon sources caused a dose-dependent increase in the degree of saturation of the fatty acids. The exception was observed with short-chain alcohols such as methanol and ethanol, to which the cells adapted with a concentration-dependent decrease in the saturation degree of the membrane phospholipids. This influence of the different carbon sources on the rigidity of the cell membrane also had an impact on the (−)-carvone productivity of the strain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The physico-chemical properties of cellular membranes are affected by solvents such as hydrocarbons and terpenes and especially by aromatic compounds. According to Sikkema et al. (1995), the main target of organic solvent toxicity in bacteria is in fact the cell membrane. The compounds partition preferentially to membranes, causing an increase in membrane fluidity and leading to non-specific permeabilization (Heipieper et al. 1992). Incorporation of a solute in a bilayer perturbs lipid–lipid and lipid–water interactions and consequently affects membrane functionality. n-Alkanes, n-alkanols and terpenes destabilize the bilayer structure of microbial membranes (Weber and de Bont 1996). However, micro-organisms are able to adapt to the presence of toxic organic solvents, including aliphatic alcohols, using a whole cascade of adaptive mechanisms (for reviews, see Isken and de Bont 1998; Isken and Heipieper 2002; Sikkema et al. 1995). Biological functions depend on the ability of micro-organisms to adapt their membranes in order to survive under the destructive effects of solvents. Adaptation mainly consists of changes in membrane lipid composition, with some changes in protein, sterol, hopanoid and carotenoid content (Heipieper et al. 1994; Weber and de Bont 1996). Among the adaptive mechanisms, those leading to a modification of the membrane to keep it in the same condition of fluidity have been described most intensively. Changes in the fatty acid composition of membrane lipids appear to be the major response of bacteria against membrane-active substances. Thereby, bacteria maintain a constant membrane fluidity by changing the membrane fatty acid’s degree of saturation. This mechanism is called “homeoviscous adaptation” (Sinensky 1974).
In the present work, we studied the effect of alkanes, alkanols and terpenes, used as sole carbon and energy sources, on the fatty acid composition of the membrane of Rhodococcus erythropolis DCL14. This strain has been successfully used in our laboratory in the bioconversion of several terpenes in organic–aqueous systems (de Carvalho et al. 2000; de Carvalho and da Fonseca 2002a). During different studies, it was found that R. erythropolis DCL14 cells are solvent-tolerant and are able to consume a large number of alkanes and alkanols, a feature that can be exploited for bioremediation (de Carvalho and da Fonseca, in press).
Materials and methods
Strain
R. erythropolis DCL14 was provided by the Division of Industrial Microbiology, Wageningen Agricultural University, The Netherlands.
Growth
Cells were grown in cylindrical 100-ml flasks, closed with rubber bungs, containing 20 ml of minimal salts medium (Wiegant and de Bont 1980) and incubated at 28°C and 150 rpm. The organic compounds used as sole carbon and energy sources were present at an initial concentration of 0.125, 0.25, 0.5, 1.0, 2.0, 5.0, 10.0 or 20.0% (v/v). The extent of growth was monitored by measurement of the optical density at 600 nm (OD600), by dry weight and by microscopy and image analysis techniques. Assays were carried out at least in duplicate.
Carbon source consumption rate
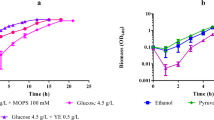
Cells were grown at 28°C and 200 rpm in at least 15 test tubes (10 ml), closed with rubber bungs wrapped in tin foil, containing 2 ml of minimal salts medium and defined amounts of each carbon source. During growth, at least five samples were taken at different times. At each sampling time, the whole contents of three test tubes were collected: one for biomass measurement and two for determination of carbon source consumption. The contents of these latter two tubes were extracted with 0.5 ml of ethyl acetate and, after phase separation, the ethyl acetate layer was analysed by gas chromatography (GC).
GC analysis
Samples were analysed by GC on a Hewlett Packard 5890 GC with a flame ionization detector (FID), connected to a HP3394 integrator. The capillary column was SGE HT5 (25 m long, 0.22 mm i.d., 0.33 mm o.d.). The oven temperature was 120°C and the injector 200°C. The detector was at 250°C.
Chemicals
The organic solvents used as carbon source were ethanol (99.8%), butanol (>99.5%), propanol (>99.5%), n-dodecanol, cyclohexane (>99.5%), toluene (>99.5%; all from Merck), n-octane (>99%; from Merck-Schuchardt), methanol (>99.8%), pentanol (>99%), n-hexane (>99%), iso-octane (>99.5%; all from Riedel-de Haën), n-hexadecane (99%; from Sigma), cyclohexanol (99%), n-hexanol (98%), n-decane (>99%), n-dodecane (>99%; all from Aldrich), pentane (99%; from Fluka) and n-nonane (99%; from Acros). The terpenes used as carbon sources were (R)-(+)-limonene (97%), (+)-limonene oxide (97%), (−)-carveol (97%), (R)-(−)-carvone (98%), α-pinene (98%) and α-pinene oxide (97%), all purchased from Aldrich Chemicals. Cis-(−)-carveol (>98%; de Carvalho et al. 2002) was diastereomerically purified from (−)-carveol using R. erythropolis DCL14 cells as described by de Carvalho and da Fonseca (2002b).
Cell hydrophobicity test
The cell hydrophobicity test was carried out according to the microbial adhesion to hydrocarbon test described by Rosenberg et al. (1980), as follows. Various volumes of n-hexadecane were added, in test tubes, to 1.2 ml of washed cells (collected at the beginning of the stationary phase) suspended in phosphate buffer (pH 7). Following 10 min of pre-incubation at 30°C, the mixtures were agitated at full speed for 120 s on a vortex (REAX 2000; Heidolph, Germany). After 15 min, the organic phase was removed and the OD600 of the aqueous phase was measured. Cell hydrophobicity refers to the percentage of cells that migrate towards the organic phase.
Changes in fatty acid composition
Cells from 20-ml suspensions (about 3×1010 cells) of R. erythropolis DCL14, grown in the presence of different carbon sources and concentrations, were centrifuged and washed with phosphate buffer (50 mM, pH 7.0). The lipids were extracted with chloroform/methanol/water, as described by Bligh and Dyer (1959). Fatty acid methyl esters (FAME) were prepared by 15 min of incubation at 95°C in boron trifluoride/methanol, using the method of Morrison and Smith (1964). FAME were extracted with hexane and analysed using a quadruple GC-MS System (HP6890, HP5973; Hewlett & Packard, Palo Alto, USA) equipped with a split/splitless injector. A CP-Sil 88 capillary column (Chrompack, Middelburg, The Netherlands; 30 m long, 0.32 mm i.d., 0.25 μm film) was used for the separation of FAME. GC conditions were: injector temperature held at 250°C, split flow 1:10, helium carrier gas. The temperature program was: 80°C for 1 min isotherm, rising by 15°C/min to 140°C, then 4°C/min to 280°C. The MS conditions were: EI ionization mode, ionization energy 70 eV. The peak areas of the carboxylic acids in total ion chromatograms were used to determine their relative amounts. The fatty acids were identified by GC-MS with co-injection of authentic reference compounds obtained from Supelco (Bellefonte, USA). Replicate determinations gave a coefficient of variation of 5%. Saturation of membrane fatty acids was defined as the ratio of two saturated fatty acids (16:0, 18:0) to two unsaturated fatty acids (16:1cis7, 18:1cis9, 18:1cis11) present in this bacterium (Tsitko et al. 1999, Gutierrez et al. 1999).
Error analysis
The error associated with the GC quantification of samples was ±6%. The errors were calculated based on the standard deviation and sample mean of seven repeated injections and are quoted for a confidence interval of 95%. Biomass concentration measurements (OD600) had an associated error of ±8%, based on the standard deviation and sample mean of eight repeated samples, quoted for a confidence interval of 95%. The error associated with the image analysis (used to assess biomass growth) was ±7%, based on the standard deviation and sample mean of 12 repeated images taken from the same sample, quoted for a confidence interval of 95%.
Results
Influence of different carbon sources on cellular membrane composition
R. erythropolis cells were grown on different alcohols, hydrocarbons and terpenes, as sole carbon and energy sources. In all experiments, the composition of the membrane was determined with cells collected during the exponential growth phase, since a strong dependency of the fatty acid pattern on growth phase was observed (data not shown).
The strain was able to grow on alcohols ranging from C1 to C12 as sole carbon and energy sources. For all investigated alcohols, a dose-dependent decrease in the growth rate could be observed (Table 1). This decrease always showed a logarithmic-shaped curve. Using two coefficients (A, B), the growth rate can thus be expressed by:
The higher the modulus of A in Eq. 1, the more abrupt is the decrease in growth rate with the initial concentration of the carbon source, i.e., the more toxic is the carbon source. In the present work, the value of coefficient A decreased towards zero with the increasing number of carbon atoms in the carbon source, between C1 and C4 (Table 2). However, its value was constant and around −0.003 for alcohols with a number of carbons higher than C4. This shows that short-chain alcohols may produce a higher impact on growing cells of R. erythropolis than the other tested alcohols. Still, at low concentrations, the observed dose-dependent decrease in growth rate in the presence of short-chain carbon sources was lower than that observed with longer-chain alcohols. Nevertheless, cells were able to grow on all methanol and ethanol concentrations in the experimental range. The highest solvent concentration in Table 1, presenting the corresponding growth rate, is the maximum concentration which allowed growth to occur without any kind of adaptation.
A dose-dependent increase in the degree of saturation of cells grown on propanol and cyclohexanol was observed (Table 3). However, the degree of saturation of the cellular membrane lipids of cells grown on methanol, ethanol and butanol decreased slightly with the increase in carbon source concentration. R. erythropolis DCL14 was able to grow on a concentration of up to 5% n-dodecanol, but the membrane composition of cells grown on concentrations higher than 0.25% could not be determined, due to interference by this compound in the determination method.
The growth rates observed with odd-numbered hydrocarbons were much lower than those measured with even-numbered carbon sources (Fig. 1). In general, the growth rate observed for cells grown in the presence of an odd-numbered hydrocarbon was at least ten-fold lower than that obtained with the next even-numbered carbon source. For the even-numbered carbon sources, the growth rates increased with the number of carbons in the carbon source (Fig. 1). Almost no variation was observed, for the same carbon source, between an initial concentration of 0.125% and 0.25%, except for toluene, n-octane and iso-octane. In the case of n-octane and iso-octane, the growth rates for an initial concentration of 0.25% were limited to 24.0% and 46.6% of those observed for a concentration of 0.125%. This indicates that a concentration of 0.25% of each of these three carbon sources might be toxic to R. erythropolis cells.
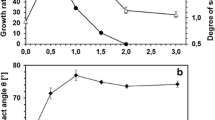
n-Pentane, cyclohexane and limonene caused a dose-dependent increase in the degree of saturation of the membrane phospholipid fatty acids (Table 4). The same effect was observed with the other hydrocarbons and terpenes tested (data not shown). In the case of cyclohexane and limonene, the increase was smooth (slope around 16%), whilst in the case of n-pentane a high increase of the degree of saturation was observed for concentrations higher than 0.25%.
R. erythropolis cells have been used in our laboratory for the biotransformation of terpenes, which were found to be toxic to these cells at relatively low concentrations, causing a loss of viability (de Carvalho and da Fonseca 2002a). Nevertheless, these cells are apparently able to respond to their presence, increasing the degree of saturation of the fatty acids in the bacterial cellular membrane. This could explain part of the successful adaptation strategy which allowed the high production of (−)-carvone in an air-driven column reactor after adapting the cells to the presence of (−)-carveol and (−)-carvone (de Carvalho and da Fonseca 2002b). Carvone accumulation up to 259 mM was achieved, thus overcoming the product toxicity problems previously encountered for concentrations higher than 50 mM.
Effect of carbon source on carvone production
The carbon source influenced both the degree of saturation of the fatty acids of the membrane and the growth rate of R. erythropolis cells, as shown in the previous paragraph. The differences in the degree of saturation of the membrane phospholipids led to differences in the hydrophobic behaviour of cells grown on the several carbon sources tested. The composition of the cell membrane thus influenced the uptake rate of hydrophobic/hydrophilic compounds. Nevertheless, the specific consumption rate of each of the carbon sources varied, not only as a result of differences in the uptake rate due to the composition of the cell membrane, but also due to specificities in the metabolism of these compounds.
As mentioned previously, R. erythropolis DCL14 has been used in our laboratory as a biocatalyst in various reaction systems. Amongst these, the bioconversion of (−)-carveol to (−)-carvone was studied in considerable detail. Since this reaction is co-factor-dependent, the viability of the cells should be maintained throughout the biotransformation course (de Carvalho and da Fonseca 2002b). Reaction conditions are therefore of paramount importance to achieve high carvone production and productivity (de Carvalho and da Fonseca 2002a).
A simple model (Eq. 2) was developed to interpret and predict the influence of: (1) cell hydrophobicity (Hydroph), (2) carbon source consumption rate (CSCR), (3) degree of saturation of the membrane fatty acids (DS), (4) growth rate (μ), (5) log P of the carbon source (log P) and (6) the number of carbon atoms in the carbon source (NC) on the carvone production rate. The model is as follows:
where A, B, C, D, E, F and G are model parameters. In Fig. 2, the predicted values of carvone production rate were plotted against the experimental results. The values assumed by the model parameters are listed in Table 5. A correlation of 0.99, 1.0 and 0.92 was obtained for the experiments carried out with, respectively, hydrocarbons, alkanols and terpenes as sole carbon and energy sources. The lower correlation obtained with cells grown on terpenes must be the result of an improvement in the metabolic route of (−)-carveol observed in the presence of cell growth on terpenes. In fact, not only the bacterial membrane is affected by the presence of an organic solvent and in particular by the presence of terpenes. The enzymes involved in their metabolism may also be activated (de Carvalho and da Fonseca 2004). In this case, CSCR and μ are probably not sufficient to predict the biocatalytic performance of whole cells. To improve the model, an extra term, reflecting the effect of the carbon source on the expression of carveol dehydrogenase, should be included on the right-hand side of Eq. 2.
Carvone production rates predicted by Eq. 2 versus experimental values
Discussion
Due to their hydrophobicity, alkanes, terpenes and alkanols tend to accumulate in membranes where they align with the acyl chains of phospholipids. The accumulation of n-alkanes, which can be visualized as a wedge between the acyl chains, destabilizes the bilayer structure and promotes the formation of a reversed hexagonal lipid phase (Weber and de Bont 1996). Incorporation of C6–C20 n-alkanes is known to promote the formation of non-lamellar structures of this kind in many phospholipids and glycolipids (Seddom 1990).
An increase in the saturation degree of the membrane fatty acids is the major mechanism by which the cytoplasmic membrane of a bacterial cell adapts to toxic organic solvents (Heipieper et al. 1994; Heipieper and de Bont 1994). It was recently shown that R. erythropolis uses the so-called aerobic biosynthesis to create unsaturated fatty acids with a cis-double bond located at position nine using a NAD(P)H-desaturase (Keweloh and Heipieper 1996; Hartig et al. 1999).
Differences in the degree of saturation of the membrane of R. erythropolis reflect differences in adaptive ability and cell composition. The only mechanism bacteria have to modify the fatty acid composition of membrane lipids is de novo synthesis, which is strictly related to cell growth. If growth is completely inhibited, fatty acid biosynthesis and the faculty of modifying the degree of saturation are also blocked.
An adaptation on the level of fatty acid composition of the membrane phospholipids was observed in R. erythropolis DCL14 cells: all tested compounds caused a dose-dependent increase in the degree of saturation of the membrane phospholipid fatty acids (Tables 3, 4). The only exceptions were short-chain alcohols, such as ethanol, that caused a concentration-dependent decrease in the membrane degree of saturation.
These differences in R. erythropolis cellular response are in agreement with those formerly published for other bacteria. It has been observed that bacteria like Escherichia coli and Pseudomonas putida react to the presence of long-chain alcohols by increasing their degree of saturation (Ingram 1976), while in the presence of short-chain alcohols, e.g. ethanol, the degree of saturation decreases (Ingram 1976; Heipieper and de Bont 1994). Very recently, Kabelitz et al. (2003) could present strong evidence that this difference in the adaptive response towards alcohols is related to the physico-chemical properties of short-chain alcohols, which can only penetrate slightly into the hydrophobic centre of the phospholipid bilayer. Therefore, short-chain alcohols only cause a swelling effect on the hydrophilic headgroups (Weber and de Bont 1996). To counteract this effect, the insertion of unsaturated fatty acids seems to be a better reaction against those short-chain alcohols. In contrast, long-chain alcohols penetrate deep into the membrane, behaving like aromatic solvents (for example) and thus causing an increase in the degree of saturation (Ingram 1976; Weber and de Bont 1996).
A clear correlation between the carbon source and the mycolic acid profiles of R. erythropolis E1 was found by Sokolovská et al. (2003). When cells grew on linear alkanes with an even number of carbon atoms, there was a lack of odd-numbered carbon chains. Mycolic acids with both even- and odd-numbered carbon chains were observed when the cells grew on compounds with an odd number of carbon atoms, branched alkanes or mixtures of compounds. These results and the available literature led the authors to conclude that there are two different pathways for the synthesis of mycolic acids in this strain: one by the addition of acetyl coenzyme A and the other by the addition of propionyl coenzyme A. According to Alvarez (2003), the strain R. erythropolis 17 produced fatty acids with a chain length associated with the chain length of the growth substrate during cultivation on n-alkanes. Other fatty acids derived from the β-oxidation pathway were also produced. Strain 17 showed a more efficient mechanism for the production of the intermediate, propionyl-CoA, a precursor of fatty acids containing an odd number of carbon atoms. In our case, the results show that R. erythropolis DCL14 cells have a less efficient mechanism for the production of the precursor propionyl-CoA than other strains in the Rhodococcus genus and therefore for the production of fatty acids with an odd number of carbon atoms.
The large number of enzymes which allow R. erythropolis DCL14 cells to carry out an enormous set of bioconversions and degradations, combined with their ability to modulate the fatty acids of the cellular membrane, fully justify the potential applications of this bacterium in biotechnology.
References
Alvarez HM (2003) Relationship between β-oxidation and the hydrocarbon-degrading profile and actinomycete bacteria. Int Biodeterior Biodegrad 52:35–42
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Carvalho CCCR de, Fonseca MMR da (2002a) Maintenance of cell viability in the biotransformation of (–)-carveol with whole cells of Rhodococcus erythropolis. J Mol Catal B Enzym 19/20:389–398
Carvalho CCCR de, Fonseca MMR da (2002b) Influence of reactor configuration on the production of carvone from carveol by whole cells of Rhodococcus erythropolis DCL14. J Mol Catal B Enzym 19/20:377–387
Carvalho CCCR de, Fonseca MMR da (2004) Principal component analysis applied to bacterial cell behaviour in the presence of organic solvents. Biocatal Biotransform 23:1–12
Carvalho CCCR de, Fonseca MMR da (in press) Degradation of hydrocarbon and alcohols at different temperatures and salinities by Rhodococcus erythropolis DCL14. FEMS Microbiol Ecol
Carvalho CCCR de, Keulen F van, Fonseca MMR da (2000) Production and recovery of limonene-1,2-diol and simultaneous resolution of a diastereomeric mixture of limonene-1,2-epoxide with whole cells of Rhodococcus erythropolis DCL14. Biocatal Biotransform 18:223–235
Carvalho CCCR de, Keulen F van, Fonseca MMR da (2002) Modelling the bio-kinetic resolution of diastereomers present in unequal initial amounts. Tetrahedron Asymmetry 13:1637–1643
Chen Q, Janssen DB, Witholt B (1995a) Growth on octane alters the membrane lipid fatty acids of Pseudomonas oleovorans due to the induction of alkB and synthesis of octanol. J Bacteriol 177:6894–6901
Chen Q, Nijenhuis A, Preusting H, Dolfing J, Janssen DB, Witholt B (1995b) Effects of octane on the fatty acid composition and transition temperature of Pseudomonas oleovorans membrane lipids during growth in two liquid phase continuous cultures. Enzyme Microb Technol 17:647–652
Chen Q, Janssen DB, Witholt B (1996) Physiological changes and alk gene instability in Pseudomonas oleovorans during induction and expression of alk genes. J Bacteriol 178:5508–5512
Gutierrez JA, Nichols P, Couperwhite I (1999) Changes in whole cell-derived fatty acids induced by benzene and occurrence of the unusual 16:1 omega 6c in Rhodococcus sp 33. FEMS Microbiol Lett 176:213–218
Hartig C, Loffhagen N, Babel W (1999) Glucose stimulates a decrease of the fatty acid saturation degree in Acinetobacter calcoaceticus. Arch Microbiol 171:166–172
Heipieper HJ, Bont JAM de (1994) Adaptation of Pseudomonas putida S1 to ethanol and toluene at the level of fatty acid composition of membranes. Appl Environ Microbiol 60:4440–4444
Heipieper HJ, Diefenbach R, Keweloh H (1992) A possible mechanism of the protection of the phenol degrading strain Pseudomonas putida P8 from the toxicity of the substrate: the conversion of cis- into trans- unsaturated fatty acids. Appl Environ Microbiol 58:1847–1852
Heipieper HJ, Weber FJ, Sikkema J, Keweloh H, Bont JAM de (1994) Mechanisms behind resistance of whole cells to toxic organic solvents. Trends Biotechnol 12:409–415
Ingram LO (1976) Adaptation of membrane lipids to alcohols. J Bacteriol 125:670–678
Isken S, Bont JAM de (1998) Bacteria tolerant to organic solvents. Extremophiles 2:229–238
Isken S, Heipieper HJ (2002) Toxicity of organic solvents to microoganisms. In: Bitton G (ed) Encyclopedia of environmental microbiology, vol 6. Wiley, New York, pp 3147–3155
Kabelitz N, Santos PM, Heipieper HJ (2003) Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol Lett 220:223–227
Keweloh H, Heipieper HJ (1996) Trans-unsaturated fatty acids in bacteria. Lipids 31:129–137
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J Lipid Res 5:107–118
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9:29–33
Seddom JM (1990) Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta 1031:1–69
Sikkema J, Bont JAM de, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222
Sinensky M (1974) Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA 71:522–525
Sokolovská I, Rozenberg R, Riez C, Rouxhet PG, Agathos SN, Wattiau P (2003) Carbon source-induced modifications in the mycolic acid content and cell wall permeability of Rhodococcus erythropolis E1. Appl Environ Microbiol 69:7019–7027
Tsitko IV, Zaitsev GM, Lobanok AG, Salkinoja-Salonen MS (1999) Effect of aromatic compounds on cellular fatty acid composition of Rhodococcus opacus. Appl Environ Microbiol 65:853–855
Weber FJ, Bont JAM de (1996) Adaptation mechanisms of microorganisms to the toxic effects of organic solvent on membranes. Biochim Biophys Acta 1286:225–245
Wiegant WM, Bont JAM de (1980) A new route for ethylene glycol metabolism in Mycobacterium E44. J Gen Microbiol 120:325–331
Acknowledgement
This study was supported by a post-doctoral grant (SFRH/BPD/14426/2003) awarded to C.C.C.R.C. by the Fundação para a Ciência e a Tecnologia, Portugal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Carvalho, C.C.C.R., Parreño-Marchante, B., Neumann, G. et al. Adaptation of Rhodococcus erythropolis DCL14 to growth on n-alkanes, alcohols and terpenes. Appl Microbiol Biotechnol 67, 383–388 (2005). https://doi.org/10.1007/s00253-004-1750-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1750-z