Abstract
The diversity of mycoflora associated with grass and sedges belonging to 24 species of eight plant families inhabiting three regions of the Hyrcanian Forest in Iran was surveyed. Fungal isolates were recovered from the roots, stems and leaves of plants, and ITS sequences of ribosomal DNA were determined. The 113 fungal isolates were categorized into the lowest taxonomic rank possible. Surprisingly, pathogen-like fungi encompassed 34% of the endophytic isolates. Colletorichum, Fusarium, and Alternaria, all genera containing important pathogenic species, were abundant. Occurrence of Fusarium was highest in root tissues, while Colletotrichum appeared more dominant in leaves and stems. Wheat seedlings were exposed to inocula of 25 of these endophytic isolates. Eleven isolates inhibited growth of the seedlings, whereas 14 isolates promoted growth compared to uninoculated controls. Further, wheat seedlings treated with isolates reported as etiological agents such as Parastagonospora nodorum and Fusarium sp., promoted growth. We report first-time isolation of Darksidea sp., a genus of root-colonizing dark septate endophytes (DSE), from herbaceous vegetation of Hyrcanian forests of Iran.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With burgeoning population growth, sustainable agricultural production requires fresh strategies to maintain and increase the rate of food production, while concurrently protecting the remaining natural environment (Tian et al., 2021). All plants live in intimate association with diverse microorganisms that affect growth in both positive and negative ways (Schirawski & Perlin, 2018). Relationships between microorganisms and plants comprise interactions ranging from obligate symbiosis to pathogenesis (Collinge et al., 2022). Endophytes – microorganisms that live asymptomatically within plants – have attracted the interest of researchers because of their roles in enhancing growth characteristics in wild systems under both ideal and stress conditions (Huang et al. 2008; Das & Varma 2009; Dastogeer et al., 2018). Isolation, identification, growth-promoting and disease-inhibiting activities of pathogen-like endophytic microbes infecting wild plant communities have been surveyed worldwide (Chand et al., 2020; Jia et al., 2020; Rosa et al., 2009; Terhonen et al., 2019; Tournay & Doty, 2022; Xiao et al., 2021; Yuan et al., 2010).
Deliberate promotion of positive interactions between domesticated plants and beneficial microorganisms isolated from wild plants inhabiting forests is a relatively new paradigm in agriculture, a strategy with considerable potential (Dudeja et al. 2012; Kleczewski et al., 2012; Ismail et al., 2021; Morales-Cedeño et al., 2021). The practical application of such endophytes as plant growth enhancers in agriculture is not yet widely realized because of questions of species-to-species compatibility, and the true nature of such interactions remain unclear for most microorganism-plant combinations (Prasad & Dagar 2014). The potential for an endophyte in one host species to become a pathogen in another is one such question that requires on-going investigation (Card et al., 2016), as is its host range (Kumar et al., 2021). Cultivated plant species belonging to families comprising wild species, such as the Poaceae, may offer sources of compatible, potentially growth-enhancing endophytes (Kumar et al., 2021), but such new interactions require considerable research before commercial release. Application of systemic fungicides to control fungal pathogens in crops may negatively affect beneficial fungal endophytes (Vasanthakumari et al., 2019), and as such, agronomic practices may require modification before specialized endophyte-based technology is commercially adopted (Watts et al., 2023). Some beneficial plant-associated microorganisms are recalcitrant in vitro, making commercial application problematic (De Silva et al., 2019; Kia et al., 2018). Commercial monocultures of crops differ widely from mixed-species natural systems, as do moisture and nutrient regimes, and responses to such factors by endophytes are largely unknown (De Silva et al., 2019). Assays are required to assess pathogenicity and distinguish latent pathogens from endophytes (Lugtenberg et al., 2016). Assigning uncharacterized endophytic microbes that belong to known pathogenic taxa to an ecological role is not possible using simple genetic identity approaches such as ITS/18S sequences (Doilom et al., 2017); instead, biological tests are required. Before commercial application of wild-plant endophytes to cropping systems, the complex plant health triangle of host–microbiota–environmental interactions must be fully investigated (Leveau, 2024). In a worst-case scenario, an apparently mutualistic microbial partner becomes pathogenic in a commercial cultivar when triggered by certain biotic and/or abiotic conditions (Jones, 2020).
Caspian Hyrcanian mixed forests occur mainly in three northern provinces of Iran, namely, Golestan, Mazandaran and Guilan, which border the Caspian Sea (Akhani & Ziegler 2002). Parts of these forested areas served as floristic refuges during the period of last ice age, and, as such, served to reseed Eurasian forests when the ice sheet receded (Tohidifar et al., 2016). The forests retain critically-important floristic biodiversity; members of the Poaceae dominate, with 246 species described (Akhani et al. 2010). The allied family Cyperaceae, commonly known as sedges, is another dominant plant family in the Hyrcanian forests, of which genus Carex is particularly diverse (Homami Totmaj et al., 2021; Naqinezhad et al., 2015). In Iran, there have been studies on endophytes colonizing wild plants as potential biocontrol agents (Alidadi et al., 2019; Hagh-Doust et al., 2017; Rostami et al., 2021; Rostami et al., 2022), but very little is known about the identity of microbial communities in the flora of the Iranian Hyrcanian forest (Kasaei et al., 2017). Similarly, little is known about their roles in the forest ecosystem in terms of its health as well as adverse effects.
Because of the prevalence of grasses and the allied sedges in the Hyrcanian forests, and the overwhelming importance of grasses such as wheat, rice and corn to human civilization (FAOSTAT, 2023), we undertook a study of the endophytic fungi living within wild grasses and sedges in the forests. The aim of this work was to deepen our understanding of how endophytes isolated from wild grasses and grass-like species may respond to colonization with a new related host species, in this case, wheat.
Materials and methods
Plant materials
During the springs and summers of 2017 and 2018, and summer-autumn period of 2019, sampling sites were: (i) an arid region (Gorgan and Bandare Gaz: Golestan province), (ii) a salty soil site influenced by desert (Shahroud: Semnan province), and (iii) a high-rainfall region (Astara: Gilan province) (Fig. 1). Forty-one randomly-chosen plant samples mainly, from the family Poaceae and its allied family Cyperaceae (the two dominant plant families in the Hyrcanian forests) as well as Asteraceae (another important floristic component of these forests), were selected for the following steps in the present study (Table 1).
Fungal endophyte isolation
Leaves, stems and roots of plant samples were cleaned under running tap water, and cut into 1–2 cm segments. Segments were surface-sterilised by immersion in 1% sodium hypochlorite for 4 min, followed by 70% ethanol for 1 min, and then rinsed in sterile distilled water three times. After removing excess water by blotting with sterile filter paper, samples were cut into 3–5 mm-long sections. These were placed in Petri dishes containing 1/10th potato dextrose agar (PDA) (Sigma-Aldrich, USA) amended with 100 mg L-l streptomycin sulfate (Azmiran, Tehran, Iran) to isolate endophytic fungi. The imprint method was employed to verify the efficacy of the surface sterilization procedure (Taufiq & Darah, 2018). Moreover, to evaluate the efficacy of surface sterilization procedure, the remnant of the final water used in the procedure was incubated in PDA and nutrient agar (NA) medium cultures to observe any fungal and bacterial growth. Hyphal tip culture was done at least three times to obtain pure cultures from each fungus that grew. Plates containing pure cultures were kept in the dark for 10 days at 25 °C and checked periodically for fungal growth.
DNA extraction and identification
Total DNA was extracted from pure cultures of fungal isolates following a modified cetyltrimethylammonium bromide (CTAB)-based protocol (Dellaporta et al. 1983). Briefly, 40 mg of fresh mycelium was harvested by scraping it from the surface of each culture with a sterile scalpel before being ground under liquid nitrogen. The ground tissue was transferred to microtubes and suspended in 700 μl CTAB extraction buffer [2% CTAB, 100 mM Tris-HCl, 1.4 M NaCl, 20 mM ethylenediaminetetraacetic acid (EDTA), 0.5 g polyvinylpyrrolidone (PVP), pH 8.0]; the microtubes were then kept at 65 °C for 30 min. Then, 2 μl of Proteinase K (Thermo Scientific, Vilnius, Lithuania) was added to the solution and the mixture was kept at 37 °C for 30 min. Next, 700 μL of chloroform:isoamyl alcohol (24:1) was added and mixed, and the microtubes were centrifuged at 20,000 xg for 10 min at 4 °C. The aqueous phase was transferred to a new sterile microtube and kept at 37 °C for 30 min, followed by adding 2 μl of RNase A (Thermo Scientific, Vilnius, Lithuania). An equal volume of chilled chloroform:isoamyl alcohol (24:1) was then added to the tube and the solution was mixed for 5 min, and subsequently centrifuged for 10 min at 20,000 xg at 4 °C. Again, the aqueous layer was transferred into a new microtube, chilled isopropanol (0.6 volume) was added and kept for 30 min at −20 °C. DNA was recovered by centrifugation at 20,000 xg for 10 min at 4 °C. The pellet was then washed with 1 mL of 75% chilled ethanol, centrifuged at 20,000 xg at 4 °C, and dried at room temperature. Total DNA was resuspended in 25 μL water and stored at −20 °C. The quality of the genomic DNA was assessed visually by electrophoresis in 0.8% agarose gel, and quantity was estimated by NanoDrop spectrophotometry (Thermo Scientific, Wilmington, DE, USA).
Molecular identification was performed using universal primer pair ITS1 (5’-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3′) to amplify internal transcribed spacer (ITS) regions of the ribosomal RNA gene (rDNA) of the fungi (Tan et al., 2018). Independent PCR products were sequenced in both directions with the amplification primers using the Sanger method. Bio-Edit software (Ver. 7.0.4.1; Hall 1999) was used to trim off the primer sequences and assemble sequences. Then, to identify the sequences that most closely matched the nucleotide sequences obtained in the current study, the basic local alignment search tool BLAST (Altschul et al. 1997), available at the National Center for Biotechnology Information (NCBI) and MycoBank engine (Robert et al., 2005) were employed to search GenBank databases for closest matches. Fungal taxonomic identification was done according to previous recommendations (Hofstetter et al. 2012; Hofstetter et al. 2019). Phylogenetic analysis of the isolates was done using reference sequences selected from NCBI GenBank and MycoBank databases, and three representative sequences of zygomycetous fungi, genus Mortierella (GenBank accessions AB542112, AB542092 and EU877758), as outgroup sequences (Douanla-Meli & Langer, 2012). Multiple sequence alignment of the sequences was done using CLUSTAL OMEGA (Madeira et al., 2019). Phylogenetic relationships were then estimated by the maximum-likelihood (ML) method (Nei & Kumar, 2000) with the “complete deletion” option of alignment gaps using MEGA software (version 10.1.1; Kumar et al., 2018). Having the lowest values for Bayesian information criterion (BIC) and Akaike information criterion, corrected (AICc) scores (Nei & Kumar, 2000), the Tamura-Nei model with a proportion of invariant sites and a gamma distribution of site-rate variants (TN93 + G + I) was selected as the best nucleotide substitution model and used to determine genetic relationships between the sequences under study. The robustness of nodes was tested with 1000 bootstrap replicates (Efron et al., 1996).
Wheat seed preparation and germination
Pure seed of autumn wheat (Triticum aestivum L.) cultivar Moghan-3, a high-yielding cultivar compatible with warm and humid climates (Anonymous, 1974–2002), was obtained from the Seed and Plant Research Improvement Institute, Karaj, Iran, and used for this study. Before breaking seed dormancy, wheat seeds were subjected to surface sterilization in 1% NaOCl for 1 min, immersed in 70% ethanol for 1 min, and serially rinsed six times in sterile distilled water (Dastogeer et al. 2017). To overcome wheat seed dormancy, a prechilling method was used as reported by the Association of Official Seed Analysts (AOSA) and the International Seed Testing Association (ISTA) (Aghilian et al., 2014). Surface-sterilized seeds were imbibed in water and stored in a refrigerator at 4 °C for 24 h to give them the required cold treatment (Aghilian et al., 2014; Nyachiro et al., 2002). Seed germination was done using a between-paper method (Aghilian et al., 2014). Seeds were placed between two layers of moist sterile paper towels and exposed to indirect sunlight. Germination of seeds was checked daily, and after the radicle was 2 cm long (Fig. 2a), seedlings were inoculated with one fungal endophyte (Fig. 2b, c).
Germination and inoculation processes of wheat seeds: (a) germinated seeds (2-cm long) ready for inoculation step; (b) non-inoculated seeds (negative control); (c) endophyte-infected wheat seeds; and (d) size of seedling on culture media 10 days post-inoculation by endophytic fungi with positive growth impact
Inoculation of wheat seedlings by fungal isolates
On the basis of databases searches results and sequence identities, twenty-five pathogen- and non-pathogen-like fungal isolates with high growth rates on culture media were selected as candidates for growth efficacy tests on wheat seedlings. Assuming that plants that survived adverse conditions have microbial communities with growth-promoting properties, the fungal isolates used in this step were from regions with relatively unfavorable environmental conditions, i.e., an arid region with low rainfall (Golestan province) and a region with salty soil (Semnan province) (Fig. 1). Pure cultures of the chosen isolates were first grown in Petri dishes containing PDA medium for about 10 days at 25 °C in the dark. A set of seeds was maintained uninoculated to use as the control group (Fig. 2c). Ten germinated wheat seedlings were then incubated next to growing margins of 10-day-old fungal colonies in PDA and placed in natural light at room temperature. Wheat seedlings grew directly on the media culture. The growth changes of the seedlings were evaluated four, seven, and ten days after the inoculation process, and records taken included the number of surviving seedlings, number of leaves and roots, length of seedlings and roots, and whole seedling length in both the control and endophyte-infected seeds at 10 days post-inoculation (dpi) (Fig. 2d). The experiment was performed using a completely random design of 10 replicates.
Statistical analysis
Documenting of data was done using Microsoft Excel (Version 2716.16.27). Statistical data analysis was done using SPSS (Version 27). To estimate effects of the fungal isolates on seedling growth traits, one-way ANOVA followed by Duncan’s multi-range average comparison test (DMRT) were performed using root number, shoot number, stem length, total root length, total leaf length, and whole seedling length, among and between the 25 fungal treatments and controls.
Results
Host diversity
Forty-one samples from 12 plant species belonging to families Poaceae, Cyperaceae and Asteraceae were surveyed. The majority of the samples collected were from poaceous species, i.e., Bromus sp., Dactylis glomerota, Eluesina indica, Microstegium vimineum, Oplismenus hirtellus, Poa nemoralis, Glyceria plicata, and Setaria viridis. Carex remota was the sole representative of the family Cyperaceae, which was collected from three sites (Table 1). Recovering 113 fungal isolates from different tissue segments, i.e., root, stem and leaf, of the selected plants demonstrated that the diversity of these fungi was different in various plant organs, and that, the leaf contained a higher diversity of endophytic fungi that were able to grow on PDA medium (Table 2).
Fungal diversity
PCR assays were performed on the DNAs of the 113 fungal isolates under study to amplify their ITS regions (Table 2). Sequences shared the highest identities (96–100%) with reference sequences available in GenBank and MycoBank databases. Based on molecular analysis of ITS sequences, eight isolates were identified to the species level, 67 isolates were classified to the genus level, 10 to the family level, one to the suborder level, 15 to the order level, and 12 isolates could not be categorized below the level of order (Table 2). Thirty-nine isolates (34%) belonged to taxa associated with pathogenesis, viz Fusarium, Alternaria, Parastagonospora, Pyrenophora and Aspergillus (Supplementary Fig. 1). These fungi were isolated from seven host species (Supplementary Fig. 2). Fourteen isolates (12%) were from apparently non-pathogenic fungal genera belonging to Annulohypoxylon, Periconia, Darksidea, Coniochaeta, Biscogniauxia, Corpinellus, Penicillium, and Trichoderma (Supplementary Fig. 3). The non-pathogen-like fungi were recovered from six plant species (Supplementary Fig. 4). The highest community composition and diversity of endophytes was obtained from leaves. Colletorichum, Fusarium, and Alternaria were the most abundant genera, with 22, 19, and 9 isolates, respectively. While, Colletotrichum was the most dominant genus in leaves and stems of host plants, the distribution of Fusarium was higher in root tissues. The sequence of isolate Se-WS31R2 perfectly matched to a representative sequence from Darksidea sp., a genus of root-colonizing dark septate endophytes (DSE) (Santos et al., 2021).
The analyses of genetic relationship and isolate distribution revealed that more than 90% of the isolates belonged to division Ascomycota, while two isolates were members of Basidiomycetes (Fig. 3). Isolates belonging to Colletotrichum and Fusarium were the two biggest clades. Isolates of Alternaria were distributed in two groups across the tree of which the smaller one was closely related to Pyrenophora and Bipolaris, whereas Curvularia strains were less related to Alternaria groups. The phylogenetic relationship and placement of remaining isolates was in agreement with BLAST search results. ITS sequences were deposited in the GenBank database of the NCBI under accessions listed in Table 2 (Accession Nos. OL314558-OL314610; OP650938-OP650997).
A maximum-likelihood (ML) phylogenetic tree constructed using ITS sequences of ribosomal DNA gene of fungal isolates recovered from some herbaceous species in the present study (marked in blue) and representative isolates from GenBank and Mycobank datasets. Numbers at each node show the percentage of supporting bootstrap samples in ML methods. Three sequences of Mortierella species were chosen as outgroups to root the tree. Scale bar defines nucleotide replacements per site. Isolates marked in red represent chosen endophytes for growth efficacy test
Impact of wild-plant endophytic fungi on wheat
Twenty-five fast-growing isolates (Table 2; Fig. 4) recovered from 13 plant samples collected from two collection regions (Fig. 5) were selected for wheat seedling inoculation. Based on Duncan’s multi-range average comparison test, it was determined that the fungi under study could be divided into six to eleven statistical groups, ranging from those with severe adverse effects on the growth and development of wheat seedlings, to super growth-promoting effects (Table 3; Fig. 6). While some isolates, e.g., Se-WS23R2, Se-WS21St1, Se-WS22St1, Go-WS1R2, Se-WS23R4 C1, Se-WS36R2, Se-WS35R2, Se-WS33R3, and Se-WS23R4 had the lowest average growth promotion effects, i.e. the highest pathogenic effects, other isolates had the highest average growth promotion effects, i.e. the greatest beneficial effects, e.g., Se-WS34L1, Go-WS3L3, Se-WS31L1, Se-WS22R1, Se-WS32R2, Se-WS22R4, Se-WS32R3, Se-WS31R3, Go-WS2L2, Se-WS24St1, Se-WS21R1, Se-WS21R3, Se-WS34L3, Se-WS31R2, and Se-WS22L1.
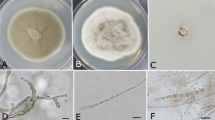
Morphological diversity of some fungal colonies used in wheat tests on potato dextrose agar (PDA) culture media: (a) Pyrenophora trichostoma (Se-WS31L1), (b) Fusarium sp. (Se-WS36R2), (c) Parastagonospora nodorum (Se-WS34L1), (d) Diaporthales sp. (Se-WS22R1), (e) Alternaria sp. (Se-WS21St1), (f) Diaporthales sp. (Se-WS22R4), (g) Diaporthales sp. (Se-WS32R4), (h) Stagonospora sp. (Se-WS32R2), (i) Phaeosphaeriaceae sp. (Se-WS22St1), (j) Pleosporales sp. (Se-WS21R1), (k) Fusarium sp. (Go-WS2L2), (l) Pleosporales sp. (Se-WS32R3), (m) Darksidea sp. (Se-WS31R2), (n) Penicillium sp. (Se-WS22L1), (o) Lasiosphaeriaceae sp. (Go-WS3L3), (p) Parastagonospora nodorum (Se-WS34L3), (q) Pleosporales sp. (Se-WS21R3)
The growth and development test indicated that pathogen-like endophytes Se-WS21St1 (Alternaria sp.) and Se-WS23R2 (Diaporthales sp.) resulted in the deaths of most seedlings, with only two and one seedlings remaining viable by 10 dpi, respectively (Fig. 6). Performance of seedlings colonized by Go-WS1R2, Se-WS22St1, Se-WS23R4, Se-WS23R4/C1, Se-WS33R3, and Se-WS36R2, belonging to Fusarium sp., Phaeosphaeriaceae sp., Diaporthales sp., Diaporthales sp., Ceratobasidiaceae sp., and Fusarium sp., respectively, was equal or weaker than control (un-inoculated) seedlings growing on the same medium. In contrast, almost all features examined, including number of leaves, leaf-root lengths, and whole seedling biomass were significantly enhanced upon the treatment of seedlings with Se-WS34L1 and Se-WS31L1 inocula identified to the level of species as Parastagonospora nodorum and Pyrenophora trichostoma, respectively. Moreover, the inoculation of seedlings with Go-WS3L3 isolate (Lasiosphaeriaceae sp.) caused remarkable increases in number of roots and stem length (Fig. 6). Colonization of seedlings by the dark septate endophyte, isolate Se-WS31R2, conferred positive effects on most plant growth features. The treatment of seedlings with the other isolates illustrated lesser enhancements in growth features and biomass. The results of the analysis are shown in detail in Fig. 7 and Supplementary Tables 1,2.
Effects of pathogen/non-pathogen-like endophyte treatments on wheat growth attributes: (a) number of root, (b) number of leaf, (c) stem length, (d) total root lengths, (e) total leaf lengths, and (f) whole seedling length. Identical lower letters indicate that there were no statistically significant differences between the groups (Duncan test, 𝑃≤0.01)
Discussion
In the current study, screening three different plant organs of some wild graminoids and grass-like herbs, belonging to 24 species and eight plant families residing in three geographically different regions of the Iranian Hyrcanian forest was done to reveal fungal community composition. Isolation of fungi from the root, stem and leaf tissues of studied plants indicated that most, if not all, plant tissues of grass and grass-like species in these mountainous forests maintain endophytic partnerships (Douanla-Meli & Langer, 2012). Previous studies have reported that roots of host plants had the highest richness and diversity of pathogen (−like) endophytes (Wang et al., 2022), however, in this study the endophytic fungi of the leaves were more diverse. Our results were in agreement with former reports and suggest that organ specificity of non-pathogen/pathogen-like endophytic fungi may differ from one host and/or ecosystem to another (Nessa et al., 2023; Sawmya et al., 2013). Considering that the sampling sites chosen all had extreme unique features, we hypothesized that fungal isolates recovered from asymptomatic plants inhabiting these regions may assist growth under these unfavorable conditions. Isolates of Annulohypoxylon cf. stygium, Periconia sp., and Trichoderma sp. were derived from these plants (Cheng et al., 2023; Harman et al., 2021; Liu et al., 2020). Isolate Se-WS31R2 was identified as a species of Darksidea, a common genus of dark septate endophytes (DSE). This is the first report of the presence of this fungus from the Hyrcanian forested massifs of Iran. They are a group with world-wide distribution and are found as root-colonising fungi. They are often found associated with extremophile plants that colonise environments with strong abiotic stress (Knapp et al., 2015), such as those sampled for the current study.
The term ‘endophyte’ refers to micro-organisms that colonize internal plant tissues without pathogenesis (Wilson, 1995). Fungal endophytes lead a wide range of lifestyles from latent pathogens or saprotrophs to mutualists (Redman et al., 2001). Sequence analysis of isolates under study showed the prevalence of fungi within genera where some species/pathotypes are serious pathogens, such as Fusarium, Alternaria, Parastagonospora, and Pyrenophora (Russo et al., 2016; Sajeena et al., 2020). The seed growth efficacy experiment revealed that some strains of these pathogen-like fungi positively affected the growth and vegetative attributes of treated wheat seedlings. The transition from the the endophytic to the pathogenic state has been reported previously in some strains of Alternaria alternata associated with specific plant species/genera (DeMers, 2022).
On the other hand, as the plant health triangle points out, coexistence of the three factors microbiota, host plant, and environment is needed for plant health. If one of these three factors is not optimal, then disease may occur (Francl, 2001; Leveau, 2024). The climate continues to change and its negative impact on crop production is inevitable. Employing wild-plant microbiota on cultivated crops in managed ecosystems to provide more optimized food products, specifically high-yielding and compatible crops, while it is a promising strategy, it is also potentially threatening. Despite the growth-promoting potential of pathogen-like endophytes in wheat, effects on other species must be tested under a range of climatic and agronomic circumstances. Kuo et al. reported that changes in environmental conditions and host plants stimulated the interactions of the model fungus Neurospora crassa which led to a shift from endophytic mode to pathogenic mode (Kuo et al., 2014). Additionally, according to the definition of ‘endophyte’, the term endophyte can be applied to latent pathogens that reside in host plants for a long period with no apparent metabolic activity. However, stimulating factors such as biotic and abiotic stresses may affect their seemingly harmless interaction with the host with the possibility of causing diseases (Hai-Tao et al., 2021; Tsers et al., 2023).
In the current study, to assess the pathogenic and non-pathogenic impacts of the isolates, seedlings were directly subjected to colonies of pathogen/non-pathogen-like fungal endophytes under laboratory conditions, while filtrates and extracts obtained from fungal endophytes were common methods to inoculate seeds/seedlings of target plants to evaluate the efficacy of non-pathogen-like fungi on non-host plants (García-Latorre et al., 2023; Jaber & Enkerli, 2016). Infecting wheat plantlets through direct exposure to endophytic fungal colonies rather than filtrate or extract approaches is a simple method of inoculation. For instance, endophytic or non-endophytic impacts of microbiota colonizing wild vegetations in various ecosystems on non-host plants can be specified in a short period of time after direct inoculation, unlike culture filtrate and extract methods whereby the endophytic/pathogenic nature of applied microbes could be discovered only after planting and growing non-host treated seeds/seedlings in a distinct period (García-Latorre et al., 2023; Jaber & Enkerli, 2016; Yan et al., 2011). We are aware that inoculation of single pure endophyte cultures to seedlings derived from surface-sterile seed does not closely mimic the situation in the field where large numbers of competing microbes are present in the soil. Field-grown plants may be co-colonized by many microorganisms belonging to several taxonomic groups up to the level of Kingdom. Introducing endophytic isolates to wheat seedling under heavily-controlled conditions is just the first step in identifying commercially valuable fungi that benefit crop production (Sela Saldinger et al., 2023). Additionally, we assumed that introducing novel isolates to wheat (or other plants) using the direct inoculation method we applied as well as soilless/hydroponic systems can make least the microbial contamination (Sela Saldinger et al., 2023). Thus, the present study was small-scale, designed to explore the concept that fungal pathogens and growth-promoting fungal endophytes living inside wild plants may differentially affect growth of a new host (Figs. 6, 7). Fungal endophyte-plant interactions are not entirely predictable and may alter under various conditions in vivo. It is possible that pathogen-like endophytes associated asymptomatically with wild plants can cause pathogenesis in cultivated plants. Therefore, matching together beneficial endophytes isolated from wild plants with cultivars growing in highly-managed agricultural systems offer future promise, but only after thorough assessment. Practically speaking, wild-plant fungal isolates will themselves undergo domestication for such application, beginning with culture of pure stable isolates and the development of efficient inoculation systems. There is likely to be a vast untapped resource of fungal (and other) endophytes suitable for this task available from wild habitats such as the Hyrcanian Forests of Iran, but their isolation and identification is the first step in their domestication. As forests are destroyed to make room for more crops, two clear risks emerge. One is that new pathogens present in wild habitats spillover to crops, causing disease. The other is that potentially priceless beneficial endophytes are lost without us ever identifying them.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article, its supplementary materials, and GenBank datasets.
References
Aghilian, S., Khajeh-Hosseini, M., & Anvarkhah, S. (2014). Evaluation of seed dormancy in forty medicinal plant species. International Journal of Agriculture and Crop Sciences, 7(10), 760.
Akhani, H., & Ziegler, H. (2002). Photosynthetic pathways and habitats of grasses in Golestan National Park (NE Iran), with an emphasis on the C4-grass dominated rock communities. Phytocoenologia, 32(3), 455–501.
Akhani, H., Djamali, M., Ghorbanalizadeh, A., & Ramezani, E. (2010). Plant biodiversity of Hyrcanian relict forests, N Iran: an overview of the flora, vegetation, palaeoecology and conservation. Pakistan Journal of Botany, 42(Special Issue), 231–258.
Alidadi, A., Kowsari, M., Javan-Nikkhah, M., Jouzani, G. R., & Rastaghi, M. E. (2019). New pathogenic and endophytic fungal species associated with Persian oak in Iran. European Journal of Plant Pathology, 155(3), 1017–1032.
Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., & Lipman, D. J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402.
Anonymous. (1974-2002). Wheat breeding annual report (bread and durum wheat). Cereal Research Department, Seed and Plant Improvement Institute.
Card, S., Johnson, L., Teasdale, S., & Caradus, J. (2016). Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiology Ecology, 92(8), 114.
Chand, K., Shah, S., Sharma, J., Paudel, M. R., & Pant, B. (2020). Isolation, characterization, and plant growth-promoting activities of endophytic fungi from a wild orchid Vanda cristata. Plant Signaling & Behavior, 15(5), 1744294.
Cheng, M. J., Wu, M. D., & Chen, J. J. (2023). Secondary metabolites from the Endophytic fungus of Annulohypoxylon stygium var. annulatum. Chemistry of Natural Compounds, 1–3.
Collinge, D. B., Jensen, B., & Jorgensen, H. J. (2022). Fungal endophytes in plants and their relationship to plant disease. Current Opinion in Microbiology, 69, 102177.
Das, A., & Varma, A. (2009). Symbiosis: the art of living. In A. Varma & A. C. Kharkwal (Eds.), Symbiotic Fungi Principles and Practice (pp. 1–28).
Dastogeer, K. M., & Wylie, S. J. (2017). Plant–Fungi Association: Role of Fungal Endophytes in Improving Plant Tolerance to Water Stress, Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer, 143–159.
Dastogeer, K. M., Li, H., Sivasithamparam, K., Jones, M. G., & Wylie, S. J. (2018). Fungal endophytes and a virus confer drought tolerance to Nicotiana benthamiana plants through modulating osmolytes, antioxidant enzymes and expression of host drought responsive genes. Environmental and Experimental Botany, 149, 95–108.
De Silva, N. I., Brooks, S., Lumyong, S., & Hyde, K. D. (2019). Use of endophytes as biocontrol agents. Fungal Biology Reviews, 33(2), 133–148.
Dellaporta, S. L., Wood, J., & Hicks, J. B. (1983). A plant DNA minipreparation: Version II. Plant Molecular Biology Reporter, 1(4), 19–21.
DeMers, M. (2022). Alternaria alternata as endophyte and pathogen. Microbiology, 168(3), 001153.
Doilom, M., Manawasinghe, I. S., Jeewon, R., Jayawardena, R. S., Tibpromma, S., Hongsanan, S., Meepol, W., Lumyong, S., Jones, E. B. G., & Hyde, K. D. (2017). Can ITS sequence data identify fungal endophytes from cultures? A case study from Rhizophora apiculata. Mycosphere, 8(10), 1869–1892.
Douanla-Meli, C., & Langer, E. (2012). Diversity and molecular phylogeny of fungal endophytes associated with Diospyros crassiflora. Mycology, 3(3), 175–187.
Dudeja, S. S., Giri, R., Saini, R., Suneja‐Madan, P., & Kothe, E. (2012). Interaction of endophytic microbes with legumes. Journal of basic microbiology, 52(3), 248–260.
Efron, B., Halloran, E., & Holmes, S. (1996). Bootstrap confidence levels for phylogenetic trees. PNAS USA, 93, 7085–7090.
Food and Agriculture Organization of the United Nations. (2023). FAOSTAT statistical database.
Francl, L. J. (2001). The disease triangle: A plant pathological paradigm revisited. The Plant Health Instructor.
García-Latorre, C., Rodrigo, S., Marin-Felix, Y., Stadler, M., & Santamaria, O. (2023). Plant-growth promoting activity of three fungal endophytes isolated from plants living in dehesas and their effect on Lolium multiflorum. Scientific Reports, 13(1), 7354.
Hagh-Doust, N., Akbarinia, M., Safaie, N., Yousefzadeh, H., & Bálint, M. (2017). Community analysis of Persian oak fungal microbiome under dust storm conditions. Fungal Ecology, 29, 1–9.
Hai-Tao, Y., Luo, S. Q., Zhan-Nan, Y., Yuan-Shuai, W., & Ding, Q. (2021). Latent pathogenic Fungi in the medicinal plant Houttuynia cordata Thunb. Are modulated by secondary metabolites and colonizing microbiota originating from soil. Polish Journal of Microbiology, 70(3), 359.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Harman, G. E., Doni, F., Khadka, R. B., & Uphoff, N. (2021). Endophytic strains of Trichoderma increase plants’ photosynthetic capability. Journal of Applied Microbiology, 130(2), 529–546.
Hofstetter, V., Buyck, B., Croll, D., Viret, O., Couloux, A., & Gindro, K. (2012). What if esca disease of grapevine were not a fungal disease? Fungal Divers, 54, 51–67.
Hofstetter, V., Buyck, B., Eyssartier, G., Schnee, S., & Gindro, K. (2019). The unbearable lightness of sequenced-based identification. Fungal Diversity, 96, 243–284.
Homami Totmaj, L., Ramezani, E., Alizadeh, K., & Behling, H. (2021). Four millennia of vegetation and environmental history above the Hyrcanian forest, northern Iran. Vegetation History and Archaeobotany, 30(5), 611–621.
Huang, W. Y., Cai, Y. Z., Hyde, K. D., Corke, H., & Sun, M. (2008). Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Diversity, 33, 61–75.
Ismail, M. A., Amin, M. A., Eid, A. M., Hassan, S. E. D., Mahgoub, H. A., Lashin, I., Abdelwahab, A. T., Azab, E., Gobouri, A. A., Elkelish, A., & Fouda, A. (2021). Comparative study between exogenously applied plant growth hormones versus metabolites of microbial endophytes as plant growth-promoting for Phaseolus vulgaris L. Cells, 10(5), 1059.
Jaber, L. R., & Enkerli, J. (2016). Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biological Control, 103, 187–195.
Jia, Q., Qu, J., Mu, H., Sun, H., & Wu, C. (2020). Foliar endophytic fungi: Diversity in species and functions in forest ecosystems. Symbiosis, 80(2), 103–132.
Jones, R. A. (2020). Disease pandemics and major epidemics arising from new encounters between indigenous viruses and introduced crops. Viruses, 12(12), 1388.
Kasaei, A., Mobini-Dehkordi, M., Mahjoubi, F., & Saffar, B. (2017). Isolation of Taxol-producing Endophytic Fungi from Iranian yew through novel molecular approach and their effects on human breast Cancer cell line. Current Microbiology, 74(6), 702–709. https://doi.org/10.1007/s00284-017-1231-0
Kia, S. H., Jurkechova, M., Glynou, K., Piepenbring, M., & Maciá-Vicente, J. G. (2018). The effects of fungal root endophytes on plant growth are stable along gradients of abiotic habitat conditions. FEMS Microbiology Ecology, 94(2), 162.
Kleczewski, N. M., Bauer, J. T., Bever, J. D., Clay, K., & Reynolds, H. L. (2012). A survey of endophytic fungi of switchgrass (Panicum virgatum) in the Midwest, and their putative roles in plant growth. Fungal Ecology, 5(5), 521–529.
Knapp, D. G., Kovács, G. M., Zajta, E., Groenewald, J. Z., & Crous, P. W. (2015). Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia-Molecular Phylogeny and Evolution of Fungi, 35(1), 87–100.
Kumar, S., Bhowmick, M. K., & Ray, P. (2021). Weeds as alternate and alternative hosts of crop pests. Indian Journal of Weed Science, 53(1), 14–29.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6), 1547.
Kuo, H. C., Hui, S., Choi, J., Asiegbu, F. O., Valkonen, J. P., & Lee, Y. H. (2014). Secret lifestyles of Neurospora crassa. Scientific Reports, 4(1), 5135.
Leveau, J. H. (2024). Re-envisioning the plant disease triangle by integration of host microbiota and a pivot in focus to health outcomes. Annual Review of Phytopathology, 62.
Liu, J., Chen, M., Chen, R., Xie, K., Chen, D., Si, S., & Dai, J. (2020). Three new compounds from endophytic fungus Periconia sp. F-31. Journal of Chinese Pharmaceutical Sciences, 29(4).
Lugtenberg, B. J. J., Caradus, J. R., & Johnson, L. J. (2016). Fungal endophytes for sustainable crop production. FEMS Microbiology Ecology, 92(12), fiw194. https://doi.org/10.1093/femsec/fiw194
Madeira, F., Park, Y. M., Lee, J., Buso, N., Gur, T., Madhusoodanan, N., Basutkar, P., Tivey, A. R., Potter, S. C., Finn, R. D., & Lopez, R. (2019). The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Research, 47(W1), W636–W641.
Morales-Cedeño, L. R., del Carmen Orozco-Mosqueda, M., Loeza-Lara, P. D., Parra-Cota, F. I., de Los Santos-Villalobos, S., & Santoyo, G. (2021). Plant growth-promoting bacterial endophytes as biocontrol agents of pre-and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiological Research, 242, 126612.
Naqinezhad, A., Zare-Maivan, H., & Gholizadeh, H. (2015). A floristic survey of the Hyrcanian forests in northern Iran, using two lowland-mountain transects. Journal of Forestry Research, 26(1), 187–199.
Nei, M., & Kumar, S. (2000). Molecular evolution and phylogenetics. Oxford University Press.
Nessa, F., Hosen, S., & Shamsi, S. (2023). Seasonal variation and diversity of endophytic fungi from different parts of Centella asiatica (L.) urban in Dhaka city, Bangeladesh. Bioresearch Communications, 9(2), 1236–1269.
Nyachiro, J. M., Clarke, F. R., DePauw, R. M., Knox, R. E., & Armstrong, K. C. (2002). Temperature effects on seed germination and expression of seed dormancy in wheat. Euphytica, 126(1), 123–127.
Prasad, M. P. & Dagar, S. (2014). Identification and characterization of Endophytic bacteria from fruits like Avacado and Black grapes. International Journal of Current Microbiology and Applied Sciences, 3(8), 937–947.
Redman, R. S., Dunigan, D. D., & Rodriguez, R. J. (2001). Fungal symbiosis from mutualism to parasitism: Who controls the outcome, host or invader? New Phytologist, 151, 705–716.
Robert, V., Stegehuis G., & Stalpers, J. (2005). The MycoBank engine and related databases. https://www.MycoBank.org/
Rosa, L. H., Vaz, A. B., Caligiorne, R. B., Campolina, S., & Rosa, C. A. (2009). Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae). Polar Biology, 32, 161–167.
Rostami, S., Hasanzadeh, N., Rajaei, S., Golnaraghi, A., & Azizinezhad, R. (2021). A study on endophytic bacteria isolated from wild legumes against Xanthomonas phaseoli. Applied Entomology and Phytopathology, 89(1), 1–16.
Rostami, S., Hasanzadeh, N., Rajaei, S., Golnaraghi, A., & Azizinezhad, R. (2022). Composition of endophytic bacterial communities of wild legumes as potential biological control agents of crop legumes diseases (case study: Kermanshah Zagros Forest). Iranian Journal of Forest and Range Protection Research, 19(2), 266–278.
Russo, M. L., Pelizza, S. A., Cabello, M. N., Stenglein, S. A., Vianna, M. F., & Scorsetti, A. C. (2016). Endophytic fungi from selected varieties of soybean (Glycine max L. Merr.) and corn (Zea mays L.) grown in an agricultural area of Argentina. Revista Argentina de Microbiología, 48(2), 154–160.
Sajeena, A., Nair, D. S., & Sreepavan, K. (2020). Non-pathogenic Fusarium oxysporum as a biocontrol agent. Indian Phytopathology, 73, 177–183.
Santos, M., Cesanelli, I., Diánez, F., Sánchez-Montesinos, B., & Moreno-Gavíra, A. (2021). Advances in the role of dark septate endophytes in the plant resistance to abiotic and biotic stresses. Journal of Fungi, 7(11), 939.
Sawmya, K., Vasudevan, T. G., & Murali, T. S. (2013). Fungal endophytes from two orchid species pointer towards organ specificity. Czech Mycology, 65(1), 89–101.
Schirawski, J., & Perlin, M. H. (2018). Plant–microbe interaction 2017—The good, the bad and the diverse. International Journal of Molecular Sciences, 19(5), 1374.
Sela Saldinger, S., Rodov, V., Kenigsbuch, D., & Bar-Tal, A. (2023). Hydroponic agriculture and microbial safety of vegetables: Promises, challenges, and solutions. Horticulturae, 9(1), 51.
Tan, X. M., Zhou, Y. Q., Zhou, X. L., Xia, X. H., Wei, Y., He, L. L., Tang, H. Z., & &Yu, L. Y. (2018). Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Scientific Reports, 8(1), 5929.
Taufiq, M. M. J., & Darah, I. (2018). Fungal endophytes isolated from the leaves of a medicinal plant, Ocimum sanctum Linn and evaluation of their antimicrobial activities. African Journal of Microbiology Research, 12(26), 616–622.
Terhonen, E., Blumenstein, K., Kovalchuk, A., & Asiegbu, F. O. (2019). Forest tree microbiomes and associated fungal endophytes: Functional roles and impact on forest health. Forests, 10(1), 42.
Tian, Z., Wang, J. W., Li, J., & Han, B. (2021). Designing future crops: Challenges and strategies for sustainable agriculture. The Plant Journal, 105(5), 1165–1178.
Tohidifar, M., Moser, M., Zehzad, B., & Ghadirian, T. (2016). Biodiversity of the Hyrcanian forests: A synthesis report. Tehran.
Tournay, R. J., & Doty, S. L. (2022). Microbial endophytes for clean-up of pollution. Good Microbes in Medicine, Food Production, Biotechnology, Bioremediation, and Agriculture, 358–371.
Tsers, I., Parfirova, O., Moruzhenkova, V., Petrova, O., Gogoleva, N., Vorob’ev, V., Gogolev, Y., & Gorshkov, V. (2023). A switch from latent to typical infection during Pectobacterium atrosepticum—Tobacco interactions: Predicted and true molecular players. International Journal of Molecular Sciences, 24(17), 13283.
Vasanthakumari, M. M., Shridhar, J., Madhura, R. J., Nandhitha, M., Kasthuri, C., Janardhana, B., Nataraja, K. N., Ravikanth, G., & Uma Shaanker, R. (2019). Role of endophytes in early seedling growth of plants: A test using systemic fungicide seed treatment. Plant Physiology Reports, 24, 86–95.
Wang, R., Zhang, Q., Ju, M., Yan, S., Zhang, Q., & Gu, P. (2022). The endophytic fungi diversity, community structure, and ecological function prediction of Sophora alopecuroides in Ningxia, China. Microorganisms, 10(11), 2099.
Watts, D., Palombo, E. A., Jaimes Castillo, A., & Zaferanloo, B. (2023). Endophytes in agriculture: Potential to improve yields and tolerances of agricultural crops. Microorganisms, 11(5), 1276.
Wilson, D. (1995). Endophyte: The evolution of a term, and clarification of its use and definition. Oikos, 73, 274.
Xiao, J. L., Sun, J. G., Pang, B., Zhou, X., Gong, Y., Jiang, L., Zhang, L., Ding, X., & Yin, J. (2021). Isolation and screening of stress-resistant endophytic fungus strains from wild and cultivated soybeans in cold region of China. Applied Microbiology and Biotechnology, 105, 755–768.
Yan, X. N., Sikora, R. A., & Zheng, J. W. (2011). Potential use of cucumber (Cucumis sativus L.) endophytic fungi as seed treatment agents against root-knot nematode Meloidogyne incognita. Journal of Zhejiang University Science B, 12, 219–225.
Yuan, Z. L., Zhang, C. L., Lin, F. C., & Kubicek, C. P. (2010). Identity, diversity, and molecular phylogeny of the endophytic mycobiota in the roots of rare wild rice (Oryza granulate) from a nature reserve in Yunnan, China. Applied and Environmental Microbiology, 76(5), 1642–1652.
Acknowledgments
The authors sincerely thank Dr. Valiollah Mozaffarian and Dr. Mostafa Assadi from the Research Institute of Forests and Rangelands for their help in identifying plant species. We also wish to thank Ms. Zohreh Bayat from the Seed and Plant Research Improvement Institute for providing wheat seeds, and Mr. Shahab Hajmansour and Mr. Younes Yazdani-Khameneh for their kind help and advice.
Funding
This work was supported by grants from the Iranian Group for the Promotion of Science (IGPS, Iran), no. 96001001, and BoomZista Institute (BZI, Canada), no. 21001001.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare that they have no competing interests.
Supplementary information
ESM 1
Supplementary Table 1 Statistical results of variables measured in wheat seedlings inoculated with pathogen/non-pathogen-like endophytesa (DOCX 39 kb)
ESM 2
Supplementarty Tables 2 Effects of pathogen/non-pathogen-like endophytes on vegetative attributes of wheat seedlings 10dpia, b. Means for groups in homogeneous subsets are displayed. Based on observed means. a Uses Harmonic Mean Sample Size = 10.000; b Alpha = 0.01. (PNG 587 kb)

ESM 3
Supplementary Fig. 1 Morphological diversity of pathogen-like fungal isolates on potato dextrose agar (PDA) culture media: (a) Parastagonospora nodorum (Se-WS34L3), (b) Fusarium sp. (Go-WS4L1), (c) Fusarium sp. (GI-WS477R1), (d) Pyrenophora trichostoma (Se-WS31L1), (e) Alternaria sp. (Se-WS24L4), (f) Fusarium sp. (Go-WS1R2), (g) Alternaria sp. (Go-WS2St1), (h) Fusarium sp. (Go-WS1L1), (i) Fusarium sp. (Go-WS11R1), (j) Alternaria sp. (Se-WS24St1), (k) Fusarium sp. (Se-WS35R2), (l) Parastagonospora nodorum (Se-WS34L2), (m) Fusarium sp. (GI-WS489R1), (n) Alternaria sp. (Se-WS21St1), (o) Aspergillus sp. (Go-WS6R1), (p) Aspergillus sp. (Go-WS3R7), (q) Alternaria chlamydosporigena (Se-WS31R4), (r) Aspergillus sp. (Go-WS6St3), (s) Parastagonospora sp. (Se-WS32R2), (t) Alternaria sp. (Se-WS24R1), (u) Alternaria sp. (GI-WS481R4), (v) Parastagonospora nodorum (Se-WS34L1), (w) Alternaria sp. (Se-WS24L1), (x) Aspergillus sp. (Go-WS6St4). (PNG 30178 kb)
ESM 4
Supplementary Fig. 2 Plant representatives from which the pathogen-like fungi were isolated: (a) Carex remota (Cyperaceae), (b) Eleusine indica (Poaceae), (c) Oplismenus hirtellus subsp. undulatifolius (Poaceae), (d) Setaria viridis (Poaceae), (e) Dactylis glomerata (Poaceae), (f) Microstegium vimineum (Poaceae), (g) Poa nemoralis (Poaceae), (h) Conyza canadensis (Asteraceae). (JPG 2022 kb)

ESM 5
Supplementary Fig. 3 Morphological diversity of non-pathogen-like fungal isolates on potato dextrose agar (PDA) culture media: (a) Coprinellus radians (GI-WS487St4), (b) Periconia sp. (GI-WS481R2), (c) Trichoderma sp. (Go-WS2R1), (d) Penicillium sp. (Se-WS22L1), (e) Trichoderma sp. (Go-WS3R5), (f) Darksidea sp. (Se-WS31R2), (g) Trichoderma sp. (Go-WS9R1), (h) Annulohypoxylon cf. stygium (Go-WS12L2). (PNG 10525 kb)
ESM 6
Supplementary Fig. 4 The non-pathogen-like fungi were recovered from six plant species: (a) Carex remota (Cyperaceae), (b) Setaria viridis (Poaceae), (c) Conyza canadensis (Asteraceae), (d) Bromus ramosus (Poaceae), (e) Oplismenus hirtellus subsp. Undulatifolius (Poaceae), (f) Dactylis glomerata (Poaceae). (JPG 1824 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yazdani-Khameneh, S., Golnaraghi, A., Wylie, S.J. et al. Prevalence and diversity of pathogen-like endophytic fungi from wild grasses and sedges of Iran’s Hyrcanian forests. Eur J Plant Pathol (2024). https://doi.org/10.1007/s10658-024-02937-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s10658-024-02937-7