Abstract
The limited postharvest life of fresh horticulture products can be ascribed to various factors among which diseases and disorders play an important role, leading to product and economic losses. Bull’s eye rot caused by Neofabraea perennans is one of the postharvest fungal diseases of apple fruits. We studied the efficacy of combined short-term hypobaric treatment and chitosan coating on fruit quality, disease incidence and consumer acceptability of apple fruits, after artificially inoculating the apples with N. perennans. Fruit were treated with hypobaric pressure (50 kPa for 4 h) and/or coated with 0.5%, 1%, and 2% chitosan, and a combination of both. All fruits were then stored for 120 days at 4 ± 1℃ and 85 ± 5% RH followed by sensory evaluation at simulated retail conditions for 15 d at 20 ± 3℃ and 65 ± 5% RH. Results showed that hypobaric treatment and chitosan coating, either alone or in combination, significantly affected enzyme activity, development of bull’s eye rot, physicochemical quality, and acceptability of apple fruit during storage. Among all the treatments, the best results were obtained by hypobaric treatment combined with 2% chitosan coating, which significantly maintained firmness (71.01 N), TSS (13.0 obrix), TA (0.34%), ethylene production rate (0.78 µmol kg−1 h−1), and respiration rate (0.40 mmol kg−1 h−1), increased the activity of Chitinase, Glucanase and Phenylalanine ammonia lyase (80, 133.67, and 156.7 U g−1 FW respectively), and prevented rot development (0%) until day 120. Moreover, the combined treatment had comparatively better overall acceptability (8.40) during its shelf life compared with control and individual treatments. However, further investigation is needed to evaluate the commercial feasibility of the study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apple (Malus x domestica Borkh) is highly popular worldwide due to its delicious taste, high nutritional value, and ease of storage (Ren et al., 2021). Globally, in terms of cultivation and consumption, apple is ranked third after banana and watermelon (Vasylieva & Harvey, 2021).
The quality and acceptability of the apple fruit are crucial factors that influence consumer decisions when purchasing fruit. Quality can be defined as a combination of the attributes of the fruit together with consumer perception, while the overall feedback on these attributes is referred to as acceptability. Apple tends to deteriorate and lose its quality during storage, leading to significant variability in consumer acceptance. During harvesting, losses in apple can exceed 8%, while post-harvest losses range from 25 to 28% (Rahman et al., 2022). These losses occur due to various reasons, among which disease attacks are of particular concern. Apple fruit is susceptible to attack by a variety of fungal pathogens, however Royal Gala is commonly infected by Bull's Eye rot caused by Neofabraea spp., which are particularly devastating during long-term cold storage. Economic losses caused by N. perennans in apple fruit result from reduced marketable yield, lower consumer demand, increased production costs, and potential damage to the reputation and market access of apple producers. Infected apples lead to financial impacts due to reduced sales, postharvest losses, and additional expenses for disease management. The severity of the rot can reach up to 60%, leading to quality and economic losses of apple fruit (González et al., 2020).
Apple fruit can be evaluated based on various physiochemical properties. Its appearance, including color, size, shape, and gloss, is the first aspect that is checked, followed by firmness, texture, taste, density, total soluble solids content, and acidity. Through these chemical and physical properties, consumers can recognize the nutritional value of the fruit. Fruits that are lightweight, colorless, too firm, or shriveled are generally not preferred by consumers (Ahmad et al., 2021).
In recent years, various treatments have been studied to enhance the quality and consumer acceptability of apple fruit during storage. These treatments include heat treatments, irradiation, edible coatings, antimicrobial and anti-browning agents, nitric oxide (NO), sulfur dioxide, ozone, ethylene, 1-Methylcyclopropene (1-MCP), pressure treatment, controlled atmosphere (CA) storage, and modified atmosphere packaging (MAP) (Mahajan et al., 2014). One effective compound for maintaining the quality of climacteric fruits like apples is chitosan, which is an edible coating. Chitosan is a long-chain polymer of 2-acetamido-D-glucose and 2- amino-D-glucose units crosslinked by β-1,4 glycosidic bonds. It is obtained from chitin, a biopolymer present inside the cell walls of fungi, exoskeletons of crustaceans (shrimp shells and crab), and other biological organisms (de Oliveira et al., 2020). Chitosan has anti-microbial properties and is non-toxic to humans (Ali et al., 2011). Application of chitosan on fruits can enhance their appearance (color and gloss), texture, TSS, acidity, reduce the respiration rate and ethylene production, elevate antioxidant activity, and activate some defense enzymes like chitinase leading to improved defense against foreign pathogens (Shah & Hashmi, 2020).
A chitosan coating has many advantages in maintaining the quality of fruits and vegetables but also has limitations due to its individual application, including insufficient microbial inhibition, and the inability to remove O2 and CO2 already present inside the fruit (Jianglian & Shaoying, 2013). To address these issues and achieve more satisfactory results, the chitosan coating is often used in combination with other treatments. Some of these approaches include the use of organic compounds (such as essential oils, organic acids, wax, and ethanol), inorganic compounds (such as metal ions), modified atmosphere packaging, heat treatment, gas fumigation, and biological control agents (Jianglian & Shaoying, 2013).
However, in recent years, hypobaric treatment has attracted considerable interest among researchers (Rahman et al., 2022). According to recent studies, hypobaric treatment can enhance the outward diffusion of CO2, O2, ethylene and some other toxic gases from tissues of the fruit by increasing the pressure gradient between the fruit and the external environment, which can maintain their quality during postharvest storage (Huan et al., 2021). Previous research has shown that hypobaric treatment is effective in reducing fruit softening, maintaining color, and minimizing weight loss in peach, pear, and jujube fruits (Wang et al., 2015). Hypobaric treatment has also been found effective in maintaining TSS, firmness, total polyphenol content, and weight loss in kiwi fruit and improving antifungal properties of strawberries, sweet cherries, and table grapes (Hashmi et al., 2013a; Huan et al., 2021; Romanazzi et al., 2008). Studies of Li et al. (2019) suggested that hypobaric treatment can maintain the nutritional value of fruit by preventing reductions in the concentrations of raw pectin, organic acid, and vitamin C, as well as by limiting the increase in anthocyanin content during storage. However, Ahmad et al. (2023) and Rahman et al. (2022) have suggested that combining hypobaric treatment with other postharvest techniques can lead to more desirable and significant results in pear and apple, respectively.
In this study, we have investigated the effect of hypobaric treatment and chitosan coating in controlling fungal rot caused by N. perennans, and maintaining the quality of apple fruit during cold storage, and shelf life.
Materials and methods
Plant materials
Apples cv. Royal Gala were harvested from an orchard block located in Baluchistan, Pakistan and transported to the postharvest laboratory of the Department of Food Science and Technology at The University of Agriculture Peshawar—Pakistan. On arrival in the lab, apples were washed with distilled water, air dried and, before applying treatments, apples were artificially infected with N. perennans as described below.
Isolation of N. perennans
During the first year of the study, N. perennans spores were isolated from decayed apple fruit, using a method reported by Aguilar et al. (2018). A small piece of symptomatic tissue was removed from the margin of lesion with a sterile blade, before being placed on plates of Potato Dextrose Agar (PDA). The plates were then incubated at 20°C for approximately 10 days. The tissue containing N. perennans was grown into a colony on the PDA plates to obtain pure culture.
Artificial inoculation
A suspension containing spores of N. perennans (110 spores/mL) was prepared. The fruits were surface sterilized for 2 min using 1% sodium hypochlorite (NaOCl) solution. The fruits were washed using sterile distilled water and dried at room temperature. A wound (2 mm depth and 1 mm width) was made on two opposite sides at the central equatorial region of the apple using a sterile needle. The wounded area was inoculated through an atomizer using the above-mentioned suspension, Tween 20 (0.05%) was used as surfactant to facilitate penetration as carried out by Abdel-Rahman et al. (2021). After inoculation, the apples were packed in perforated plastic boxes and placed in a hypobaric chamber followed by chitosan coating as described below.
Treatment of the Apples
Apple fruits were divided into four different groups. The first group served as the control and received no treatment. The second group was subjected to individual chitosan concentrations of 0.5%, 1%, or 2% (designated as CT0.5, CT1, and CT2 respectively) through a 2–3 s dipping process in chitosan solutions. The fruits in the third group were treated with 50 kPa hypobaric pressure for 4 h, while the fourth group received a combined treatment in which fruits were first exposed to 50 kPa hypobaric pressure for 4 h, followed by dipping in chitosan solutions (CTH0.5, CTH1, CTH2). Each group had three replicates with each replicate group having 15 apple fruits.
Hypobaric treatment
Hypobaric treatment (50 kPa for 4 h) was applied as described by Rahman et al. (2022) with slight modification. Fruits were placed in perforated plastic boxes and placed inside the hypobaric chamber then a partial vacuum was generated. After 4 h, output valves were opened to normalize the pressure, and fruits were removed for coating.
Chitosan coating
Chitosan solutions with 0.5%, 1% and 2% concentrations were prepared by mixing 0.5 g, 1 g, and 2 g (w/v) chitosan powder (200 viscosities, degree of deacetylation 95%, Sigma Aldrich, USA) in 1% lactic acid solution under continuous agitation with a magnetic stirrer for 1 h (Shah et al., 2021). Apple fruits were dipped into the solutions, air dried, placed in their designated perforated plastic boxes and stored at 4 ± 1 °C and 85 ± 5% RH for 120 days. The stored apples were analyzed for their physicochemical quality on day 1, 30, 60, 90, and 120.
Physicochemical analysis
Fruit firmness
The firmness was analyzed with a Texture Analyzer Lutron FR-5120 Penetrometer (Hashmi et al., 2013a). The data was noted after pressing an 11 mm probe on two sides of the fruit. The average was calculated and expressed in Newtons (N).
Fruit Weight Loss
The electronic scale G&G JJ2000 (error ± 0.01 g) was used for weight loss analysis following the method of Hashmi et al. (2016). The initial weight of all samples was measured on the initial day and then repeated at every interval (30, 60, 90, 120 d). Weight loss was expressed as a percentage by the following formula:
Ethylene production rate
The production rate of ethylene was measured using a portable ethylene analyzer, specifically the F-900 model, CID Bio-Science, Inc. WA, USA. The method of Rahman et al. (2022) was adopted with slight modifications. To conduct the test, GC mode was selected on the analyzer display, and a 10 ml gas sample was withdrawn for analysis. Three fruit samples per replicate were placed inside a sealed jar, allowing gas to accumulate. After one minute, a gas sample was withdrawn using a 10-cc syringe through a septum and injected into the ethylene analyzer to measure the initial gas concentration. After 60 min, another gas sample was withdrawn using the same procedure mentioned above. The concentration of ethylene was calculated and expressed in μmol kg‒1 h‒1.
Respiration rate
The respiration rate was measured using an F-900 portable ethylene analyzer employing the method of Ahmad et al. (2023) and measuring the CO2 rate. The monitor mode was used for measuring CO2 rate. Fruit (3) were put inside an airtight jar that was connected to the F-900 via a specified pipe. Initial data was noted soon after putting the fruit inside the jar and final readings were noted after 60 min. The respiration rate was calculated and expressed in mmol kg‒1 h‒1.
Fruit Peel color
The fruit peel color was determined using a PCE-CSM2 colorimeter as described by Kulcu (2018). The L⃰, a⃰, b⃰ mode was utilized. The colorimeter was calibrated with white and black tiles. The data was recorded from both sides of the fruit. Initial L⃰, a⃰, b⃰ values were recorded on the initial day for all fruit and later on at each 30-d interval for 120 d. Results were expressed as hue angle (H◦) (tan‒1 b/a).
Total soluble solids
A refractometer was used for to measure total soluble solids (TSS) as described by Rahman et al. (2022). The juice was extracted from three fruits per replicate and a drop was placed on the prism of the refractometer. The analysis was carried out at 20°. The results were expressed in ‘°Brix’.
Titratable acidity
The acidity was calculated using the method of Rahman et al. (2022) following the standardization of NaOH and sample titration. The results were expressed in percentage of acidity.
Defense-related enzymes activities
Chitinase, β-1,3-glucanase, phenylalanine ammonia lyase (PAL), and polyphenol oxidase (PPO) were assayed as per the method specified by Huang et al. (2021). Initially, 0.5 g of frozen fruit tissue was homogenized 5.0 ml of phosphate buffered saline (100 mmol/L) that contained 1% (w/v) polyvinylpyrrolidone for PAL and PPO, and 50 mmol/L of sodium acetate for Chitinase and Glucanase. At 4°C, centrifugation of the homogenate was carried out for 15 min at 12000x. The supernatant was then used for the determination of enzyme activities and expressed as U g−1 FW.
Disease incidence
The disease incidence (DI) was calculated following the method of Abdel-Rahman et al. (2021) using the following formula.
Overall acceptability
A nine-point hedonic scale was used for evaluating the overall acceptability in terms of appearance, color, and aroma. The procedure of Ahmad et al. (2022) was followed with slight modifications. The taste of the apples was not included for overall acceptability evaluation as the fruits were infected with N. perennans. Sensory analysis was carried out after 120 days of cold storage. Overall acceptability was assessed for 15 days with the following intervals of 3, 6, 9, 12, and 15 days at 20 ± 3℃ and 65 ± 5% RH. A trained panel of 12 judges was asked to evaluate the apples using the hedonic scale.
Statistical analysis
The data were tested for normality and homogeneity of variance and were subjected to 2-way analyses of variance (ANOVA) using Statistix 10 analytical software (2105 Miller Landing Rd Tallahassee, FL 32312). The means were differentiated via LSD test at P ≤ 0.05. The experiments were conducted in triplicate, due to similar results only one data set is presented in this paper.
Results and discussion
Fruit firmness
The firmness of the apple fruit decreased in all treatments during storage. However, a chitosan coating and hypobaric treatment, either applied alone or in combination, significantly (P ≤ 0.05) retained the firmness compared to the control. The synergetic effect of the hypobaric treatment (50 kPa – 4 h) and chitosan coating (1–2%) was more prominent in maintaining the firmness of apple fruit compared to the control (Fig. 1). Compared to the control (in which firmness decreased from 78.82 N to 54.04 N), in apples treated with 50 kPa with 2% chitosan, firmness decreased from 79.01 N to 71.01 N after 120 days of storage. These results are in line with the findings of Rahman et al. (2022), who observed that hypobaric treatment resulted in maintaining the firmness of Gala apples. Furthermore, Huan et al. (2021) reported that short-term hypobaric treatment had a significant effect on the firmness of ‘Bruno’ kiwi fruit. Ripening causes breakdown of cell walls leading to reduced mechanical strength which ultimately reduces firmness (Hashmi et al., 2013a). Hypobaric treatment delays the ripening by removing heat, ethylene, water molecules, some toxic components from the fruits and activating defense enzymes (Hashmi et al., 2016). Chitosan, on the other hand, had a significant effect on the firmness retention of apple alone or in combination as compared to control. Shao et al. (2012) reported the beneficial effect of chitosan on the firmness of apple fruit. Chitosan has also been reported to be effective against firmness loss in guava, papaya, kiwi, peach, tomato, and Japanese pear and the effectiveness of chitosan increased with increasing concentration (Shiekh et al., 2013). The possible mechanism of chitosan in maintaining the firmness is that it covers the cuticles and lenticels leading to reduce ripening by decreasing respiration, transpiration, infection and retaining other senescence related activities (Shiekh et al., 2013). In this study, the combined effect of 2% chitosan and hypobaric treatment (CTH2) maintained the highest firmness throughout the storage followed by the individual treatment of 2% chitosan (CT2) which clearly showed that the hypobaric treatment enhanced the effect of chitosan in apple fruit.
Firmness of 'Royal Gala' apple with hypobaric treatment (H: 50 kPa, 4 h), three concentrations of chitosan coating (CT: 0, 5, 1 and 2%) and combination of hypobaric and chitosan (CTH: 0.5, 1, and 2%) during postharvest storage (4 ± 1 °C, 85 ± 5% HR). Data are means of three replicates. Different letters indicate that the treatment is significant (LSD, P ≤ 0.05). Error bars represent Standard Deviation
Fruit weight loss
Fruit lost weight in all treatments during storage. However, the hypobaric treatment and chitosan coating either alone or in combination significantly (P ≤ 0.05) reduced the weight loss compared to the control (Fig. 2). In particular, the weight loss was 0.5–3 times lower in the combined treatments. In CTH2 treated apples weight loss reached 1.33% on day 120 compared to control fruit where it reached 5.49%. An increase in weight loss during storage could be ascribed to an elevated respiration rate; as the fruits ripen dehydration increases due to excessive loss of internal moisture (Kabir et al., 2020). Huan et al. (2021) recently reported that short-term hypobaric pressure (30 min) retains weight loss in kiwi fruit throughout storage. This might be because hypobaric treatment reduced respiration rate and, as a result, no or less water is produced and lost to the surrounding environment. Chitosan on the other hand had a significant effect in retaining weight loss in apples and the effect of chitosan increased with increasing concentration. Chitosan treatment forms a protective cover around the fruits and act as a good barrier against water loss (Misir et al., 2014). A reduction in weight loss of apple fruit through coating applications was reported by Shao et al. (2012) and Khalifa et al. (2016). Furthermore, chitosan has been found to be effective against weight loss in banana, grape, papaya, mango, and strawberry (Shiekh et al., 2013). In the combined treatments of hypobaric treatment and chitosan treatment, CTH2 resulted in the lowest weight loss throughout the storage followed by CT2 which clearly indicates that the combination of chitosan with hypobaric pressure treatment is a promising approach to combat weight loss in apple fruit.
Weight loss of 'Royal Gala' apple with hypobaric treatment (H: 50 kPa, 4 h), three concentration of chitosan coating (CT: 0, 5, 1 and 2%) and combination of hypobaric and chitosan (CTH: 0.5, 1, and 2%) during postharvest storage (4 ± 1 °C, 85 ± 5% HR). Data are means of three replicates. Different letters indicate that the treatment is significant (LSD, P ≤ 0.05). Error bars represent Standard Deviation
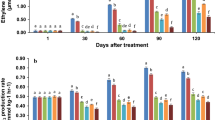
Ethylene production rate
All treatments showed a continuous rise in ethylene production. However, in this study, hypobaric treatment and chitosan coating (either applied individually or in combination) significantly (P ≤ 0.05) reduced the ethylene production compared to the control samples (Fig. 3). All concentrations of chitosan coating delayed the ethylene peak until 120 days of storage. In contrast, ethylene production in both the control and hypobaric treated (H) samples increased more rapidly when compared with other treatments and reached their climacteric peaks on day 90. However, the climacteric peak of hypobaric treatment was lower than that of control. The CTH2 treatment had a great effect on the reduction of ethylene production compared to all other treatments (Fig. 3). In the control, ethylene production rate increased from 0.07 µmol kg−1 h−1 on day 1 to 2.30 µmol kg−1 h−1 on day 90 and then decreased to 2.09 µmol kg−1 h−1 on day 120. By contrast, in CTH2 treated apples the ethylene production rate increased from 0.07 to 0.78 µmol kg−1 h−1 on day 120.
Ethylene production of 'Royal Gala' apple with hypobaric treatment (H: 50 kPa, 4 h), three concentration of chitosan coating (CT: 0, 5, 1 and 2%) and combination of hypobaric and chitosan (CTH: 0.5, 1, and 2%) during postharvest storage (4 ± 1 °C, 85 ± 5% HR). Data are means of three replicates. Different letters indicate that the treatment is significant (LSD, P ≤ 0.05). Error bars represent Standard Deviation
In fruits and vegetables, ethylene regulated ripening is the main cause of spoilage and therefore its effective management is necessary to reduce post-harvest losses (Wei et al., 2021). The mechanism by which hypobaric treatment controls ethylene production is based on its slowing down the fruit metabolism and therefore removal of heat and toxic compounds (Huan et al., 2021). Hashmi et al. (2013a) has reported that short-term hypobaric treatments (50 kPa) reduced respiration in strawberry fruit. Both ethylene production and respiration rate are linked and directly proportional to each other (Çalhan et al., 2014). Chitosan modified the internal environment of the fruit by increasing CO2 concentration and reducing O2 which suppress ethylene production and respiration rate. As a result, no or little changes occur in TSS, TA, skin color and firmness (Dong et al., 2004). Previously, Shao et al. (2012) reported that 1% chitosan reduces ethylene production in apple fruit. Dong et al. (2004) has also reported that a nano-chitosan coating (0.2% and 0.5%) had a significant effect on ethylene production in apple. Chitosan also retained the ethylene production in other fruits such as papaya, pear, tomato, and strawberry (Ali et al., 2011; Khalifa et al., 2016). However, combining hypobaric with chitosan in our study has more effectively controlled ethylene production as compared to individual treatments (Fig. 3).
Respiration rate
The respiration rate increased continuously in the control and hypobaric treatment alone (H) from day 1 to day 120 (Table 1). Both these treatments reached their climacteric peaks on day 90. However, at the climacteric peak the respiration rate of the hypobaric treated apple was lower (0.73 mmol kg−1 h−1) than that of the control (0.80 mmol kg−1 h−1). Chitosan treatments either alone or in combination with hypobaric pressure reduced the respiration rate more effectively. After 30 days, chitosan treatments showed a continuous surge in respiration but interestingly the respiration rate of chitosan treated fruit did not reach the climacteric peaks during storage (Table 1). Among all treatments, CTH2 maintained the lowest respiration rate (0.40 mmol kg−1 h−1) followed by CT2 (0.43 mmol kg−1 h−1). This could be due to the earlier treatment of hypobaric pressure which removed ethylene, CO2, and O2. After hypobaric treatment the fruits were directly coated with chitosan which acts as a barrier for gases. Moreover, the reduction in metabolism (respiration rate, ethylene biosynthesis) with the reduction in O2 and increase in CO2 may be attributed to the chitosan coating and/or the hypobaric treatment. Hence, it was not possible for O2 (a key component in respiration) to penetrate inside the fruit, leading to reduced respiration rate and fruit metabolism (Karagöz & Demirdöven, 2019).
Fruits and vegetables respire even after harvest which leads to increased susceptibility to decay. By controlling the respiration rate the quality of the fruits can be maintained, as there is reduced degradation of complex carbohydrates and organic acids (Shao et al., 2012). The respiration rate is commonly assessed from CO2 production. The effect of the chitosan coating and hypobaric treatments was significant (P ≤ 0.05) on the respiration rate of apple fruit compared to the control, either applied alone or in combination. In previous research Hashmi et al. (2016) found that short-term hypobaric treatment (50 kPa -4 h) effectively reduced the respiration rate in strawberry fruit. Additionally, Huan et al. (2021) also stated that 10–50 kPa maintained the quality of fruit including lowering the respiration rate. Karagöz and Demirdöven (2019) also demonstrated that a chitosan coating effectively reduced the respiration rate of both fresh-cut and whole apples. Other fruits such as tomato, litchi, strawberry, papaya, and Japanese pear have shown reduced respiration ratesa when treated with a chitosan coating (Shiekh et al., 2013). The combination of chitosan and hypobaric treatment in our study could have developed a synergistic effect resulting in controlling the respiration rate of apple fruit (Table 1).
Fruit peel color
The hue angle (h°) of all samples decreased throughout the storage. In particular, the hue angle of the hypobaric and control fruit decreased considerably from 84.19 on day 1 to 58.40 on day 120. Compared to the control fruit, treated fruit significantly maintained higher hue, particularly CTH2 which maintained a higher hue angle of 77 on day 120 (Fig. 4). Three pigments are involved in imparting red color to the apple fruit: anthocyanins, chlorophyll, and Xanthophyll which are red, green, and yellow, respectively (Zucoloto et al., 2017). At harvest time, for most of the fruit, the color changed from green (chlorophyll) to red (anthocyanins). Both hypobaric treatment and chitosan coating treatments significantly (P ≤ 0.05) inhibited the degradation of chlorophyll and the formation of anthocyanins (Kou et al., 2016). In this study, we found that the hypobaric treatment had a significant effect on the color change of the apple fruit compared to the control samples. Low pressure suppressed ethylene production (Fig. 3) and respiration rate (Table 1) of apple fruit leading to retention of the red color (Chen et al., 2013). Chitosan treated fruits were less ripe and the color was significantly different compared to the uncoated fruit, and the extent of the difference was much greater at higher chitosan concentrations (Shiekh et al., 2013). A chitosan coating reduces respiration and ethylene production, which leads to delayed fruit senescence and ripening. This delayed ripening helps in the retention of the color of apple fruit (Ali et al., 2011). Previously, Shao et al. (2012) had observed that a 1% chitosan coating significantly maintained the color of ‘Gala’ apples for 56 days at 0℃. Moreover, the chitosan coating delayed the activity of enzymes that cause browning in apple fruit that is evident from inhibited enzymatic activity as reported in this study (Karagöz & Demirdöven, 2019). A chitosan coating has also been found effective in delaying color changes in other fruits like papaya, strawberry, Japanese pear, peach, and kiwi fruit (Shiekh et al., 2013).
Skin color of 'Royal Gala' apple with hypobaric treatment (H: 50 kPa, 4 h), three concentration of chitosan coating (CT: 0, 5, 1 and 2%) and combination of hypobaric and chitosan (CTH: 0.5, 1, and 2%) during postharvest storage (4 ± 1 °C, 85 ± 5% HR). Data are means of three replicates. Different letters indicate that the treatment is significant (LSD, P ≤ 0.05). Error bars represent Standard Deviation
Total soluble solids
The amount of total soluble solids (TSS) in the apple fruit increased continuously in all treatments during storage (Fig. 5). A chitosan coating at 0.5% and 1% delayed the increase in TSS and similarly, when chitosan was combined with hypobaric treatments (CTH1 and CTH2), it significantly (P ≤ 0.05) slowed the increase in TSS content (13.30 and 13.0 obrix respectively) as compared to the control (13.89 obrix). At the highest concentration (2%), chitosan alone reduced TSS to 13.20 obrix however, combining this concentration with the hypobaric treatment further reduced the TSS to 13.0 obrix. A hypobaric treatment reduces the ripening process, leading to reduction in TSS (Hashmi et al., 2013a; Huan et al., 2021). The effect of a chitosan coating in modifying the internal environment and reducing ethylene production in fresh produce is well known (Çalhan et al., 2014; Dong et al., 2004); lower ethylene means lower hydrolysis of carbohydrates into sugars due to the reduced utilization of metabolites which ultimately reduced the TSS content (Ali et al., 2011). Like our results, Shao et al. (2012) has also reported that a 1% chitosan coating did not have a significant effect on Gala apples, whereas Khalifa et al. (2016) has stated that 2% chitosan coating maintained a lower TSS level in apple fruit.
TSS of 'Royal Gala' apple with hypobaric treatment (H: 50 kPa, 4 h), three concentration of chitosan coating (CT: 0, 5, 1 and 2%) and combination of hypobaric and chitosan (CTH: 0.5, 1, and 2%) during postharvest storage (4 ± 1 °C, 85 ± 5% HR). Data are means of three replicates. Different letters indicate that the treatment is significant (LSD, P ≤ 0.05). Error bars represent Standard Deviation
Titratable acidity
Throughout the storage period, there was a decrease in titratable acidity in all treatments, and this can be attributed to the fact that acid serves as the primary respiratory substrate after carbohydrates, as indicated by Chen et al. (2013). In comparison to the control, the hypobaric treatments and chitosan coatings, either alone or in combination, were able to slow down the decrease in acidity of apple fruit during the evaluations, as shown in Fig. 6. In the control, TA level decreased from 0.44% on day 1 to 0.24% on day 120. Among the treated fruits, CTH2 maintained a significantly (P ≤ 0.05) higher TA level of 0.34% on day 120. Huan et al. (2021) also observed a similar effect on the titratable acidity in kiwi fruit after treatment with short-term hypobaric pressure. Higher concentrations of the chitosan coating resulted in a small decrease in titratable acidity. Shao et al. (2012) reported that acidity of 'Gala' apples was maintained with 1–2% chitosan, while Khalifa et al. (2016) the acidity of 'Anna' apples was maintained with 2% chitosan. Both chitosan and hypobaric pressure may have reduced fruit metabolism, slowed down the ripening process thus resulting in lower acid content, and therefore TA in apple fruit was maintained, as proposed by Shiekh et al. (2013).
Titratable acidity of 'Royal Gala' apple with hypobaric treatment (H: 50 kPa, 4 h), three concentration of chitosan coating (CT: 0, 5, 1 and 2%) and combination of hypobaric and chitosan (CTH: 0.5, 1, and 2%) during postharvest storage (4 ± 1 °C, 85 ± 5% HR). Data are means of three replicates. Different letters indicate that the treatment is significant (LSD, P ≤ 0.05). Error bars represent Standard Deviation
Enzymes activities
To protect fruits from various pathogens, defense mechanisms are activated through the action of enzymes in response to different abiotic and biotic stresses (Hashmi et al., 2013b). To assess the effect of the hypobaric treatment and chitosan coating on the reduction of microbial decay in apple fruits, the contribution of key enzymes involved in fruit defense was evaluated (Shah et al., 2021).
Chitinase activity
At harvest, chitinase activity in the fruits ranged from 26.67 to 43.67 U g−1 FW, which significantly increased during storage at each subsequent period of 30, 60, 90, and 120 days (Fig. 7a). The highest chitinase activity values were observed in the control and hypobaric treated apples on day 60 at 43.33 and 47.0 U g−1 FW, respectively. However, the activity subsequently declined in both treatments until day 120 with 32.13 and 40.33 U g−1 FW, respectively. The highest chitinase activity of 80.00 U g−1 FW was observed in apples treated with 2% chitosan and hypobaric treatment on day 120. During cold storage, a combination of hypobaric treatment with 0.5% and 1% chitosan significantly (P ≤ 0.05) increased chitinase activity in apples, but the maximum chitinase activity was observed in fruits treated with 50 kPa hypobaric followed by 2% chitosan.
Defence-related enzymes activity (a) Chitinase (b) Glucanase (b) PAL and (d) PPO in 'Royal Gala' apple with hypobaric treatment (H: 50 kPa, 4 h), three concentration of chitosan coating (CT: 0, 5, 1 and 2%) and combination of hypobaric and chitosan (CTH: 0.5, 1, and 2%) during postharvest storage (4 ± 1 °C, 85 ± 5% HR). Data are means of three replicates. Different letters indicate that the treatment is significant (LSD, P ≤ 0.05). Error bars represent Standard Deviation
β-1,3-glucanase activity
The activation of enzymes that enhance the defense system of fruits against biotic and abiotic stresses is a protective mechanism against various pathogens (Hashmi et al., 2013b). To assess the contribution of the hypobaric treatment and chitosan coating in reducing microbial decay of apple fruit, the activities of key defense enzymes were evaluated (Shah et al., 2021). β-1,3-glucanase activity exhibited a steady increase during the storage of apple fruit, with an initial range of 48.33 to 68.33 U g−1 FW (Fig. 7b). On day 60, the peel glucanase activity in the control fruit peaked at 73.33 U g−1 FW but declined to 55.0 U g-1 FW on day 120. However, a combination of 50 kPa hypobaric with 2% and 1% chitosan significantly increased glucanase activity (133.67 and 122.33 U g−1 FW, respectively) until day 120, indicating the potential of this treatment in enhancing the defense system of the fruit.
Phenylalanine Ammonia Lyase (PAL) activity
In contrast to the chitinase and glucanase activities, there was a noticeable difference in the PAL activity between the control apples and those treated with other methods, with initial levels ranging from 71.67 to 97.67 U g−1 FW on day 1 (Fig. 7c). The control fruit showed a peak in PAL activity (98.30 U g−1 FW) on day 90, which was different to the peak seen in chitinase and glucanase activities on day 60. In apples treated with hypobaric and a 2% chitosan coating, PAL activity increased sharply from day 1 to day 30, and then gradually increased until day 120. Like the chitinase and glucanase activities, there was a significant increase in the PAL activity in fruit treated with hypobaric treatment and a 2% chitosan coating.
Polyphenol Oxidase (PPO) activity
On day 30, the PPO activity of the control fruit increased to 23.33 U g-1 FW, then decreased to 16.67 U g-1 FW on day 60 and again increased to 41.66 and 52.31 U g-1 FW on day 90 and 120, respectively (Fig. 7d). Treated fruit, on the other hand, exhibited significantly lower PPO activity during the cold storage as compared to the control fruit. The hypobaric and 2% chitosan treatment significantly (P ≤ 0.05) reduced the PPO activity of the apples until day 120.
Fruits have a coordinated defense system that protects them from pathogen attack, and key enzymes like chitinase, β-1,3-glucanase, phenylalanine ammonia-lyase, and polyphenol oxidase are essential for fruit defense and are often used to assess induced resistance (Liu et al., 2019). β-1,3-glucan and chitin are major components of fungal cell walls, and β-1,3-glucanase and chitinase can break down these components, making them important enzymes for fruit defense (Thakker et al., 2013). By analyzing the increase in their activities and the reduced incidence of disease in treated samples, it was confirmed that these enzymes destroyed the cell wall of Neofabraea perennans, resulting in increased shelf life and quality of apple fruit. Previous studies have reported that the activities of these enzymes can increase resistance in tomatoes against B. cinerea, and that they have a synergistic effect on their effectiveness against pathogen attack (Thakker et al., 2013).
Numerous studies have reported on the role of PAL in defending fruits, and inhibiting PAL has been found to decrease disease resistance in fruits, as noted in research by Chen et al. (2006) and Shah et al. (2021). PAL is a critical component of the phenylpropanoid pathway, which leads to the biosynthesis of various compounds such as salicylic acid and lignin, which enhance plant resistance against invading pathogens (Zhang et al., 2016). Similarly, PPO aids in the oxidation of specific phenolic compounds into antimicrobial agents, thereby protecting fruits (Huang et al., 2021). Previously, short-term hypobaric treatment has been demonstrated to activate defense-related enzymes in sweet cherries and table grapes, strawberries, and kiwifruit, according to studies by Romanazzi et al. (2001), Hashmi et al. (2013b), and Huan et al. (2021), respectively. Furthermore, hypobaric treatment has been reported to improve the shelf life of apple and pear fruit, as reported in research by Ahmad et al. (2023) and Rahman et al. (2022). Similarly, chitosan coating, either alone or in combination with other edible coating agents, has been shown to increase the disease resistance of mangos, according to research by Shah et al. (2021). It can be inferred that the combined application of hypobaric treatment and chitosan coating has a synergistic effect on the activation of defense-related enzymes in apple fruit.
Disease incidence
The efficacy of hypobaric and chitosan treatments in managing bull's eye rot in apples was influenced by the nature and concentrations of the treatments. To understand the mechanism of the combined effect of chitosan and hypobaric treatment, disease incidence was monitored in inoculated fruit. Infected apples have greyish to dark brown mycelia dark brown pigment which confirm the growth of N. perennans. The control fruit showed wide zones of bull's eye rot on the inoculated sites, which rapidly spread and reached 96.7% on 120 d. However, individual treatments of hypobaric and different concentrations of chitosan coating significantly (P ≤ 0.05) inhibited rot development. In treated apples, no rot was observed until day 30, but on day 60, 5% rot was detected in 0.5% chitosan-coated fruit, which increased with storage time (Fig. 8). However, in the apple group treated with the combination of 50 kPa hypobaric and 2% chitosan no rot development was observed during the entire storage period of 120 days. It was noted that with increase in storage life as the fruits become ripe an increase in disease incidence occured. Prevention of rot development in treated apples might be due to an increase in the activities of defense-related enzymes (Fig. 7), which inhibited the growth of N. perennans throughout the 120 days of cold storage.
Disease incidence in artificially inoculated 'Royal Gala' apple with hypobaric treatment (H: 50 kPa, 4 h), three concentration of chitosan coating (CT: 0, 5, 1 and 2%) and combination of hypobaric and chitosan (CTH: 0.5, 1, and 2%) during postharvest storage (4 ± 1 °C, 85 ± 5% HR). Data is mean of three replicates. Different letters indicate that the treatment is significant (LSD, P ≤ 0.05). Error bars represent Standard Deviation
Overall acceptability
A consumer’s decision to buy fruit is based on its overall acceptability. The major factors that consumers check in the market are appearance, color, aroma, and in some cases taste as well. All these attributes lead to overall acceptability of the fruit by consumers. In this study, the overall acceptability was assessed at 20 ± 3℃ and 65 ± 5 RH over the 15 days after 120 days of storage at 3 days interval. In all treatments, the overall acceptability of the apple fruit decreased with increasing time after storage (Fig. 9). Both the hypobaric treatments and chitosan coating (alone or in combination) had a significant (P ≤ 0.05) effect in controlling the degradation of sensory characteristics of apple fruit. The highest value for overall acceptability was observed in CTH2 followed by CT2 (8.40 and 8.10, respectively) at day 15 while the lowest value was observed in the control treatment followed by H treated apple (5.53 and 5.72, respectively) on day 9, after which the severity of rot increased and no further sensory evaluation was carried out. Fruit treated with higher concentrations (i.e., 1% and 2%) of chitosan, either alone or in combination, were not fully matured at the first 3 days, however, at the same interval, the control, H, CT0.5 and CTH0.5 were at their peak. The control and individual hypobaric treated fruit were discarded on day 12, therefore they were only evaluated until day 9, whereas CT0.5 and CTH0.5 treated apples were discarded on day 15. The overall acceptability of CTH2 and CT2 were at their peak on day 15 and probably could remain acceptable even after 15 days. Our results suggest that a combination of a chitosan coating and hypobaric treatment can manipulate the storage of apple fruit as per the requirement of suppliers. This means that if the fruit is needed to be on shelves for 12 days after storage, then CT0.5 and CTH0.5 treatment could be applied, whereas if supply is needed for more than15 days then CTH2 and CT2 treatments should be used. The effect of the hypobaric treatment and chitosan coating on the sensory attributes have also been studied in other fruits. Huan et al. (2021) recently reported that 10–50 kPa hypobaric pressure maintained the overall quality of ‘Bruno’ kiwifruit; Shao et al. (2012) reported that 1% chitosan coating significantly improved the consumer acceptability of apple fruit. Good sensory attributes in apple fruits were also reported by Karagöz and Demirdöven (2019). Similarly, Ahmad et al. (2023) demonstrated that enzymes responsible for the fermented flavor were controlled by 1-MCP + hypobaric treatment in pear, resulting in higher consumer acceptability. Together, it can be asserted that a hypobaric treatment in combination with a chitosan coating can be used as an effective postharvest treatment to maintain the sensory properties of apple fruit.
Overall acceptability of 'Royal Gala' apple with hypobaric treatment (H: 50 kPa, 4 h), three concentration of chitosan coating (CT: 0, 5, 1 and 2%) and combination of hypobaric and chitosan (CTH: 0.5, 1 and 2%) after cold storage of 120 days (20 ± 3 °C, 65 ± 5% HR). Data are means of three replicates. Different letters indicate that the treatment is significant (LSD, P ≤ 0.05). Error bars represent Standard Deviation
Conclusion
In conclusion, the findings of this study suggest that a hypobaric treatment and chitosan coating can serve as effective methods to preserve the quality and prolong the shelf life of apples. Additionally, by applying these treatments in combination, the incidence of bull's eye rot can be prevented. The results indicate that a combination of 1% chitosan and 50 kPa pressure yields nearly identical outcomes as using 2% chitosan. Furthermore, growers have the option of employing 1% chitosan with 50 kPa pressure to obtain ripe apples for a week, or 2% chitosan with 50 kPa pressure for marketable apples for 2 weeks after long-term cold storage. Despite the effectiveness demonstrated in this postharvest treatment approach, further research is necessary to ascertain its economic viability for large-scale commercial implementation.
Data Availability
Data will be available upon request.
Abbreviations
- 1-MCP:
-
1-Methylcyclopropene
- CA:
-
Controlled Atmosphere
- CO2 :
-
Carbon dioxide
- Day:
-
d
- Degree Brix:
-
OBrix
- Hour:
-
h
- Hue angle:
-
Ho
- Hypobaric:
-
h
- Kilo Pascal:
-
kPa
- MAP:
-
Modified Atmosphere Packaging
- mm:
-
millimeter
- mmol:
-
millimole
- µmol:
-
micromole
- NaOH:
-
Sodium Hydroxide
- O2 :
-
Oxygen
- Relative humidity:
-
RH
- W/V:
-
Weight/Volume
References
Abdel-Rahman, F. A., Monir, G. A., Hassan, M. S., Ahmed, Y., Refaat, M. H., Ismail, I. A., & El-Garhy, H. A. (2021). Exogenously applied chitosan and chitosan nanoparticles improved apple fruit resistance to blue mold, upregulated defense-related genes expression, and maintained fruit quality. Horticulturae, 7(8), 224. https://doi.org/10.3390/horticulturae7080224
Aguilar, C. G., Mazzola, M., & Xiao, C. L. (2018). Control of bull’s-eye rot of applecaused by Neofabraea perennans and Neofabraea kienholzii using pre-and postharvest fungicides. Plant Disease, 102(5), 905–910. https://doi.org/10.1094/PDIS-09-17-1363-RE
Ahmad, A., Hashmi, M. S., Durrani, Y., Khan, N. A., Khan, M. R., Siddiqi, M. Z., Riaz, A., Alam, M., & Rahman, W. U. (2023). Synergy of 1-MCP and hypobaric treatments prevent fermented flavour and improve consumers’ acceptability of ‘Shughri’pear. Journal of Food Science and Technology, 60(1), 200–210. https://doi.org/10.1007/s13197-022-05605-y
Ahmad, F., Zaidi, S., & Arshad, M. (2021). Postharvest quality assessment of apple during storage at ambient temperature. Heliyon, 7(8), 07714. https://doi.org/10.1016/j.heliyon.2021.e07714
Ali, A., Muhammad, M. T. M., Sijam, K., & Siddiqui, Y. (2011). Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chemistry, 124(2), 620–626. https://doi.org/10.1016/j.foodchem.2010.06.085
Çalhan, Ö., Onursal, C. E., Güneyli, A., Eren, İ, & Koyuncu, M. A. (2014). Effects of different storage techniques and 1-MCP application on quality of “Granny Smith” apple. Acta Horticulturae, 1120, 123–130. https://doi.org/10.17660/ActaHortic.2016.1120.18
Chen, H., Yang, H., Gao, H., Long, J., Tao, F., Fang, X., & Jiang, Y. (2013). Effect of hypobaric storage on quality, antioxidant enzyme and antioxidant capability of the Chinese bayberry fruits. Chemistry Central Journal, 7(1), 1–7. https://doi.org/10.1186/1752-153X-7-4
Chen, J. Y., Wen, P. F., Kong, W. F., Pan, Q. H., Zhan, J. C., Li, J. M., Wan, S. B., & Huang, W. D. (2006). Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biology and Technology, 40(1), 64–72. https://doi.org/10.1016/j.postharvbio.2005.12.017
de Oliveira, K. Á. R., da Conceição, M. L., de Oliveira, S. P. A., Lima, M. D. S., de Sousa Galvão, M., Madruga, M. S., Magnani, M., & de Souza, E. L. (2020). Postharvest quality improvements in mango cultivar Tommy Atkins by chitosan coating with Mentha piperita L. essential oil. The Journal of Horticultural Science and Biotechnology, 95(2), 260–272. https://doi.org/10.1080/14620316.2019.1664338
Dong, H., Cheng, L., Tan, J., Zheng, K., & Jiang, Y. (2004). Effects of chitosan coating on quality and shelf life of peeled litchi fruit. Journal of Food Engineering, 64(3), 355–358. https://doi.org/10.1016/j.jfoodeng.2003.11.003
Głos, H., Bryk, H., Michalecka, M., & Puławska, J. (2022). The recent occurrence of biotic postharvest diseases of apples in Poland. Agronomy, 12(2), 399. https://doi.org/10.3390/agronomy12020399
González, F., Salinas, C., Pinilla, B., & Castillo, A. (2020). First report of apple bull’s-eye rot caused by Neofabraea perennans in Chile. Plant Disease, 104, 1537. https://doi.org/10.1094/PDIS-12-19-2561-PDN
Hashmi, M. S., East, A. R., Palmer, J. S., & Heyes, J. A. (2013a). Pre-storage hypobaric treatments delay fungal decay of strawberries. Postharvest Biology and Technology, 77, 75–79. https://doi.org/10.1016/j.postharvbio.2012.11.008
Hashmi, M. S., East, A. R., Palmer, J. S., & Heyes, J. A. (2013b). Hypobaric treatment stimulates defence-related enzymes in strawberry. Postharvest Biology and Technology, 85, 77–82. https://doi.org/10.1016/j.postharvbio.2013.05.002
Hashmi, M. S., East, A. R., Palmer, J. S., & Heyes, J. A. (2016). Hypobaric treatments of strawberries: A step towards commercial application. Scientia Horticulturae, 198, 407–413. https://doi.org/10.1016/j.scienta.2015.12.017
Huan, C., Li, H., Jiang, Z., Shen, S., & Zheng, X. (2021). Effect of hypobaric treatment on off-flavour development and energy metabolism in ‘Bruno’kiwifruit. LWT, 136, 110349. https://doi.org/10.1016/j.lwt.2020.110349
Huang, Y., Sun, C., Guan, X., Lian, S., Li, B., & Wang, C. (2021). Butylated hydroxytoluene induced resistance against Botryosphaeria dothidea in apple fruit. Frontiers in Microbiology, 11, 599062. https://doi.org/10.3389/fmicb.2020.599062
Jianglian, D., & Shaoying, Z. (2013). Application of chitosan based coating in fruit and vegetable preservation: A review. Journal of Food Processing and Technology, 4(5), 227. https://doi.org/10.4172/2157-7110.1000227
Kabir, M. S. N., Ali, M., Lee, W. H., Cho, S. I., & Chung, S. O. (2020). Physicochemical quality changes in tomatoes during delayed cooling and storage in a controlled chamber. Agriculture, 10(6), 196. https://doi.org/10.3390/agriculture10060196
Karagöz, Ş, & Demirdöven, A. (2019). Effect of chitosan coatings with and without Stevia rebaudiana and modified atmosphere packaging on quality of cold stored fresh-cut apples. LWT, 108, 332–337. https://doi.org/10.1016/j.lwt.2019.03.040
Khalifa, I., Barakat, H., El-Mansy, H. A., & Soliman, S. A. (2016). Improving the shelf-life stability of apple and strawberry fruits applying chitosan-incorporated olive oil processing residues coating. Food Packaging and Shelf Life, 9, 10–19. https://doi.org/10.1016/j.fpsl.2016.05.006
Kou, X., Wu, J. Y., Wang, Y., Chen, Q., Xue, Z., Bai, Y., & Zhou, F. (2016). Effects of hypobaric treatments on the quality, bioactive compounds, and antioxidant activity of tomato. Journal of Food Science, 81(7), H1816–H1824. https://doi.org/10.1111/1750-3841.13360
Kulcu, R. (2018). Determination of the effects of different packaging methods and materials on storage time of dried apple. Matter International Journal of Science and Technology, 4(2), 238–255. https://doi.org/10.20319/mijst.2018.42.238255
Li, H., James, A., He, X., Zhang, M., Cai, Q., & Wang, Y. (2019). Effect of hypobaric treatment on the quality and reactive oxygen species metabolism of blueberry fruit at storage. CyTA-Journal of Food, 17(1), 937–948. https://doi.org/10.1080/19476337.2019.1674925
Liu, C., Chen, L., Zhao, R., Li, R., Zhang, S., Yu, W., ... & Shen, L. (2019). Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. Journal of Agricultural and Food Chemistry, 67(22), 6116–6124. https://doi.org/10.1021/acs.jafc.9b00058
Mahajan, P. V., Caleb, O. J., Singh, Z., Watkins, C. B., & Geyer, M. (2014). Postharvest treatments of fresh produce. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 372(2017), 20130309. https://doi.org/10.1098/rsta.2013.0309
Misir, J., Brishti, F. H., & Hoque, M. M. (2014). Aloe vera gel as a novel edible coating for fresh fruits: A review. American Journal of Food Science and Technology, 2(3), 93–97. https://doi.org/10.12691/ajfst-2-3-3
Rahman, W. U., Hashmi, M. S., Durrani, Y., Shah, S., Ahmad, A., Alam, S., & Ali, W. (2022). Hypobaric treatment augments the efficacy of 1-MCP in apple fruit. Journal of Food Science and Technology, 59(11), 4221–4229. https://doi.org/10.1007/s13197-022-05481-6
Ren, G., Ran, X., Zeng, R., Chen, J., Wang, Y., Mao, C., Wang, X., Fang, Y., & Yang, G. (2021). Effects of sodium selenite spray on apple production, quality, and sucrose metabolism-related enzyme activity. Food chemistry, 339, 127883. https://doi.org/10.1016/j.foodchem.2020.127883
Romanazzi, G., Nigro, F., & Ippolito, A. (2008). Effectiveness of a short hyperbaric treatment to control postharvest decay of sweet cherries and table grapes. Postharvest Biology and Technology, 49(3), 440–442. https://doi.org/10.1016/j.postharvbio.2008.01.021
Romanazzi, G., Nigro, F., Ippolito, A., & Salerno, M. (2001). Effect of short hypobaric treatments on postharvest rots of sweet cherries, strawberries and table grapes. Postharvest Biology and Technology, 22(1), 1–6. https://doi.org/10.1016/S0925-5214(00)00188-5
Shah, S., & Hashmi, M. S. (2020). Chitosan–aloe vera gel coating delays postharvest decay of mango fruit. Horticulture, Environment, and Biotechnology, 61, 279–289. https://doi.org/10.1007/s13580-019-00224-7
Shah, S., Hashmi, M. S., Qazi, I. M., Durrani, Y., Sarkhosh, A., Hussain, I., & Brecht, J. K. (2021). Pre-storage chitosan-thyme oil coating control anthracnose in mango fruit. Scientia Horticulturae, 284, 110139. https://doi.org/10.1016/j.scienta.2021.110139
Shao, X. F., Tu, K., Tu, S., & Tu, J. (2012). A combination of heat treatment and chitosan coating delays ripening and reduces decay in “Gala” apple fruit. Journal of Food Quality, 35(2), 83–92. https://doi.org/10.1111/j.1745-4557.2011.00429.x
Shiekh, R. A., Malik, M. A., Al-Thabaiti, S. A., & Shiekh, M. A. (2013). Chitosan as a novel edible coating for fresh fruits. Food Science and Technology Research, 19(2), 139–155. https://doi.org/10.3136/fstr.19.139
Thakker, J. N., Patel, S., & Dhandhukia, P. C. (2013). Induction of defense-related enzymes in banana plants: Effect of live and dead pathogenic strain of Fusarium oxysporum f. sp. cubense. International Scholarly Research Notices, 2013. https://doi.org/10.5402/2013/601303
Vasylieva, N., & Harvey, J. (2021). Production and trade patterns in the world apple market. Innovative Marketing, 17(1), 16–25. https://doi.org/10.21511/im.17(1).2021.02
Wang, J., You, Y., Chen, W., Xu, Q., Wang, J., Liu, Y., Song, L., & Wu, J. (2015). Optimal hypobaric treatment delays ripening of honey peach fruit via increasing endogenous energy status and enhancing antioxidant defence systems during storage. Postharvest Biology and Technology, 101, 1–9. https://doi.org/10.1016/j.postharvbio.2014.11.004
Wei, H., Seidi, F., Zhang, T., Jin, Y., & Xiao, H. (2021). Ethylene scavengers for the preservation of fruits and vegetables: A review. Food Chemistry, 337, 127750. https://doi.org/10.1016/j.foodchem.2020.127750
Zhang, Y., Shi, X., Li, B., Zhang, Q., Liang, W., & Wang, C. (2016). Salicylic acid confers enhanced resistance to Glomerella leaf spot in apple. Plant Physiology and Biochemistry, 106, 64–72. https://doi.org/10.1016/j.plaphy.2016.04.047
Zucoloto, M., Ku, K. M., Kim, M. J., & Kushad, M. M. (2017). Influence of 1-methylcyclopropene treatment on postharvest quality of four scab (Venturia inaequalis)-resistant apple cultivars. Journal of Food Quality, 2017. https://doi.org/10.1155/2017/5951041
Acknowledgements
We are thankful to Higher Education Commission, Pakistan for funding this project under National Research Program for Universities (NRPU-6301). This work was carried out as a requirement of M.Sc. (Hons) degree of the first author.
Author information
Authors and Affiliations
Contributions
The research was designed by Majid Suhail Hashmi, carried out by Syed Umair Shah, data analysis was done by Syed Umair Shah, and the paper was formatted by Ayaz Ahmad and Aysha Riaz. Muhammad Rafiullah Khan, Yosef Al Shoffe, Antonio Ippolito, Muhammad Ayub and Said Wahab helped in editing the manuscript.
Corresponding author
Ethics declarations
Ethical guidelines
Ethics approval was not required for this study.
Conflicts of interest
The authors have no conflicts of interest to declare.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, S.U., Hashmi, M.S., Khan, M.R. et al. Integration of chitosan coating and short-term hypobaric treatment extends postharvest life and upregulates defense-related enzymes in apple fruit. Eur J Plant Pathol 168, 231–247 (2024). https://doi.org/10.1007/s10658-023-02749-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02749-1