Abstract
Two new fungicides, flutianil and pyriofenone were introduced into the Japanese market in 2013 and 2014, to control powdery mildew on cucumber. Isolates of Podosphaera xanthii, the causal agent of powdery mildew, were collected in Ibaraki Prefecture, Japan, between 2017 and 2019 from cucumber greenhouses with a history of flutianil and pyriofenone usage. They were then tested for sensitivity to both fungicides by the leaf disc test. First, the sensitivity of three baseline reference isolates to each fungicide was determined. Minimum inhibitory concentrations values were 0.0063 μg/ml for flutianil and 10 μg/ml for pyriofenone, while 50% effective concentration (EC50) values of these fungicides were 0.00013–0.00035 μg/ml and 0.39–0.70 μg/ml, respectively. To determine the current sensitivity of P. xanthii in detail, 23 single-spore isolates were then sampled from five greenhouses and tested. Nineteen isolates showed high resistance (Flu-HR/Pyr-HR) with EC50 values of >100 μg/ml for flutianil and > 1000 μg/ml for pyriofenone. The low sensitivity of Flu-HR/Pyr-HR isolates was stable even after 46 subcultures on fungicide-untreated cotyledons. Additionally, two isolates showed moderate resistance (Flu-MR/Pyr-MR) to flutianil (EC50: mean 0.8 μg/ml) and pyriofenone (mean 58.7 μg/ml). The results from foliar inoculation tests on potted cucumber plants confirmed low efficacies of flutianil and pyriofenone against resistant isolates. Among a total of 122 isolates sampled from 13 greenhouses, 89 isolates (73.0%) were categorized as Flu-HR/Pyr-HR and two isolates (1.6%) as Flu-MR/Pyr-MR. This is the first report on flutianil resistance in any pathogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Ibaraki Prefecture, Japan, cucumber is cultivated almost all year in greenhouses, mainly in two cropping periods, summer-fall (July to November) and winter-spring (December to May) cultivation, each year. During these periods, powdery mildew is one of the major diseases affecting cucumber production, which is reportedly caused by Podosphaera xanthii or Golovinomyces orontii in Japan (Sato et al. 2013). According to the Fungicide Resistance Action Committee (FRAC), P. xanthii is a pathogen with a high-risk of developing resistance, the same as Botrytis cinerea and Pseudoperonospora cubensis (Russell 2004). The development of resistance in this pathogen to various fungicides, such as sterol demethylation inhibitors (DMIs, FRAC code 3) (Ohtsuka et al. 1988), quinone outside inhibitors (QoIs, FRAC code 11) (Ishii et al. 2001), cyflufenamid (FRAC code U06) (Hosokawa et al. 2006) and succinate dehydrogenase inhibitors (SDHIs, FRAC code 7) (Miyamoto et al. 2010; Ishii et al. 2011), has also been reported in Japan.
To control powdery mildew, the mixed or alternating application of various fungicides with different modes of action is being used in cucumber cultivation. Flutianil, the only fungicide in the cyano-methylene thiazolidine group (FRAC code U13), was introduced into the market in 2013 to control powdery mildew on cucumber. The following year, another fungicide that also controls this disease, pyriofenone, which belongs to the aryl-phenyl-ketone group (FRAC code 50), was introduced. Both fungicides play important roles in rotation spraying with various fungicides, as a strategy to delay the development of resistance.
The mode of action of the cyano-methylene thiazolidine group is unknown, and there is no report of development of resistance to this group. Similarly, the mode of action of the aryl-phenyl-ketone group is unknown, but recent studies have suggested that metrafenone, which is not currently registered for use in Japan, belongs to the same group as pyriofenone and interrupts the organisation of the actin cytoskeleton (Opalski et al. 2006; Schmitt et al. 2006). The emergence of isolates that are less sensitive to metrafenone was first reported in wheat powdery mildew (Blumeria graminis f. sp. tritici) in Europe (Felsenstein et al. 2010). Such sensitive isolates were assigned to two phenotypes; moderately adapted isolates, which were still controlled by the registered doses, and resistant isolates, that were not fully controlled. Recently, the resistance to metrafenone was reported in Erysiphe necator in European vineyards; the fungicide did not control the pathogen and the isolates showed cross-resistance to pyriofenone (Kunova et al. 2016; Graf 2017). According to the FRAC code list (Fungicide Resistance Action Committee 2019), the intrinsic risk for developing resistance to the aryl-phenyl-ketone group is estimated to be medium. Graf (2017) carried out whole genome sequencing to compare genome sequences among three fungi with different levels of sensitivity to metrafenone, including B. graminis f. sp. tritici. Graf (2017) found some non-synonymous single nucleotide polymorphisms (SNPs) resulting in amino acid alterations but did not observe any association between them and a phenotype related to metrafenone sensitivity.
The objectives of the current study were as follows: (i) to monitor flutianil and pyriofenone sensitivity of powdery mildew isolates collected from cucumber greenhouses that had a history of use of both fungicides in Ibaraki Prefecture, Japan, and (ii) to investigate the stability of the resistance of isolates after a series of consecutive subcultures on fungicide-untreated detached leaves.
Materials and methods
Collection of cucumber powdery mildew isolates
From March 2017 to May 2019, leaves with sporulating mildew were collected from cucumber plants commercially grown in 13 greenhouses in three cities, Jousou, Ishioka, and Omitama, in Ibaraki Prefecture, Japan. Cucumber was cultivated twice a year, and flutianil and pyriofenone were used to control powdery mildew in all of the greenhouses examined. A total of 122 single-spore isolates, derived from a single lesion sampled randomly in a greenhouse, were obtained. These isolates were maintained on new cotyledons of cucumber (Cucumis sativus cv. High green 22; Saitama Gensyu Ikuseikai, Saitama, Japan) placed on 0.5% agar plate with 2% mannitol and 1% sucrose (MS medium) in Petri dish at 25 °C under fluorescent light at 12-h photoperiod and subcultured at 10- to 20-day intervals on new cotyledons until used for sensitivity tests. To differentiate whether the powdery mildew had been caused by P. xanthii or by G. orontii, the lesions that developed on inoculated cotyledons were observed under a light microscope and the characteristics of the conidia, namely, the presence of fibrosin bodies and the production of conidia in chains (Uchida et al. 2009), were examined.
In addition, two isolates, N1 and N2, which were collected from melon greenhouses in 1997 with no prior history of flutianil and pyriofenone usage and maintained at the Plant Biotechnology Institute, Ibaraki Agricultural Centre, Ibaraki Prefecture, and one isolate K-7-2, which was kindly supplied by ZEN-NOH Agricultural R&D Centre (Kanagawa, Japan), were used as sensitive reference isolates. These isolates were maintained on detached healthy cotyledons of cucumber (cv. High green 22), on MS medium in Petri dishes as described above.
Determination of sensitivity with leaf disc test
To determine the sensitivity to flutianil and pyriofenone of field isolates of P. xanthii, 23 chosen single-isolates obtained from five greenhouses with a history of both fungicides usage were initially tested by leaf disc tests using a method (Ohara and Tsutsumi, 2015) developed for testing penthiopyrad, which belongs to the SDHIs. Leaf discs (8 mm in diameter), cut with a cork borer from healthy cucumber true leaves, were temporarily placed on a moist filter paper in a Petri dish. Then, the discs were immersed for a few seconds into fungicide suspensions and placed with the upper side up on a 0.6% agar plate in a square dish (96 × 96 mm). Using a modified inoculation method, the conidia from a lesion on cotyledon was transferred to the center of each disc using a platinum hook. The concentrations of fungicides used for the sensitivity tests were 0, 0.01, 0.1, 1, 10 and 100 μg/ml, and 0, 0.1, 1, 10, 100 and 1000 μg/ml of active ingredient (a. i.) for flutianil (Gatten™; OAT Agrio, Tokyo, Japan) and pyriofenone (Property™; ISK Biosciences, Tokyo, Japan), respectively, at which the second highest concentrations were equivalent to the doses registered for practical use in controlling cucumber powdery mildew. Moreover, to assess the sensitivity in more detail, additional tests were carried out with the following fungicide concentrations; 0, 0.0001, 0.0004, 0.0016, 0.0063, 0.025 and 0.1 μg/ml for flutianil and 0, 0.01, 0.04, 0.16, 0.63, 2.5 and 10 μg/ml for pyriofenone against sensitive (S) isolates, and 0, 0.098, 0.391, 1.56, 6.25, 10, 25 and 100 μg/ml for flutianil and 0, 0.977, 3.91, 15.6, 62.5, 100, 250 and 1000 μg/ml for pyriofenone against moderately resistant (MR) isolates.
Ten to eleven days after inoculation, mildew development on each leaf disc (five leaf discs in total) was recorded with a stereoscopic microscope, using the following index; 0 = no visible mildew development, 1 = >0 to 5%, 2 = >5 to 25%, 3 = >25 to 50%, 4 = >50 to 75% and 5 = >75% of the disc surface covered with mildew. To avoid effects of outliers, the highest and lowest values of disease indexes were removed. Subsequently, disease severity (DS) was calculated from the sets of remaining three values: [(5A + 4B + 3C + 2D + E)/5F] × 100, where A, B, C, D and E are the numbers of leaf discs corresponding to indexes of 5, 4, 3, 2, and 1, respectively, and F is the total number of leaf discs assessed. The percentage of growth inhibition was then calculated in relation to the DS in the control treatment: (each DS/control DS) × 100. The 50% effective concentration (EC50) value of each fungicide for growth inhibition was calculated using log-linear model software kindly supplied by ZEN-NOH (Tokyo, Japan). The sensitivity tests were repeated three times for each isolate.
To investigate the stability of the resistance, the minimum inhibitory concentration (MIC) and EC50 values of both fungicides were evaluated for five resistant isolates (S-1sp, N-C2, N-E4, M1-D1 and M2-E) after the 10th, 27th and 46th subcultures from the first isolation on fungicide untreated cotyledons by the above-mentioned leaf disc test.
Sensitivity test with foliar spray inoculation
An inoculation test was performed using four true-leaf stage potted cucumber plants (cv. High Green 22) to evaluate the controlling effects of flutianil and pyriofenone on the isolates resistant to both fungicides. Three isolates highly resistant (HR) against flutianil and pyriofenone (Flu-HR/Pyr-HR) (N-C2, M1-D1 and M2-E), two MR isolates (Flu-MR/Pyr-MR) (H-B1 and Ic1905–1) and three S isolates (Flu-S/Pyr-S) (N2, N1 and K-7-2) were used in this test. The inoculation test was replicated two times for each isolate. The first to third true leaves were each sprayed with suspensions of flutianil at 2.5 and 10 μg/ml or pyriofenone at 25 and 100 μg/ml, as 1/4 and the manufacturer’s registered field doses, respectively. Mepanipyrim (Frupica™; Kumiai Chemical Industry, Tokyo, Japan), an anilinopyrimidine fungicide, at 200 μg/ml and tap water were sprayed as positive and negative control, respectively. Five replicate plants were prepared for each treatment. After the treatments, the plants were air-dried, followed by inoculation with the above-mentioned isolates by spraying the spore suspensions (ca. 2 × 104/ml suspended in distilled water containing 0.01% Tween-20). Plants were kept in a greenhouse, at temperatures ranging from about 25 °C to 30 °C. Disease development on each leaf was recorded 12 days after inoculation using the same index as described previously. Disease indexes of 15 leaves sprayed with each fungicide and tap water for each isolate were compared using a Kruskal-Wallis tests followed by a post hoc Steel-Dwass test at the 5% significance level. These analyses were performed with EZR (Saitama Medical Center, Jichi Medical University) (Kanda 2013), which is a graphical user interface for R (The R Foundation for Statistical Computing).
Monitoring study with the leaf disc test
A monitoring study was performed using the leaf disc test with 99 isolates that were not used in sensitivity test described above. The concentrations of fungicides were 0, 0.1, 1 and 100 μg/ml for flutianil and 0, 10 and 1000 μg/ml for pyriofenone. To assess levels of resistance to flutianil and pyriofenone, isolates were categorized according to their sensitivity to each fungicide: flutianil; Flu-S (complete inhibition at 0.1 μg/ml), Flu-MR (growth at 1 μg/ml but complete inhibition at 100 μg/ml) and Flu-HR (growth at 100 μg/ml), pyriofenone; Pyr-S (complete inhibition at 10 μg/ml), Pyr-MR (growth at 10 μg/ml but more than 80% inhibition at 1000 μg/ml) and Pyr-HR (<50% inhibition at 1000 μg/ml).
Field survey of fungicide application history and change in powdery mildew incidence from 2016 to 2018 cropping seasons
In four greenhouses, Jousou-A, and -B, Omitama and Ishioka-A, the records for the application of fungicide spray were surveyed from 2016 to 2018 for each farmer. Additionally, in three greenhouses, Jousou-A, Omitama and Ishioka-A, changes in the incidence of powdery mildew were investigated every month during the same period. The disease incidence was calculated based on the observation of 40 arbitrarily selected cucumber plants in each greenhouse, 10 leaves from the middle part of each plant, and 400 leaves per greenhouse in total.
Results
Sensitivity of single-spore isolates to flutianil and pyriofenone
In the leaf disc assays, disease development of the sensitive reference isolates, N1, N2 and K-7-2, which had never been treated with both fungicides, was completely inhibited at 0.0063 μg/ml of flutianil and 10 μg/ml of pyriofenone, respectively, and the EC50 values for these fungicides were 0.00013–0.00035 μg/ml (mean 0.00027 μg/ml) and 0.39–0.70 μg/ml (mean 0.54 μg/ml), respectively (Table 1).
The sensitivities of 23 single-spore isolates to both fungicides were determined initially. Two isolates collected from Ishioka-A, H-1 and H-2, showed sensitivity similar to that of sensitive reference isolates (Table 2). The remaining 21 isolates were divided into two resistant phenotypes, Flu-HR/Pyr-HR and Flu-MR/Pyr-MR. All of the 19 isolates from Jousou-A, -B and Omitama were categorized as Flu-HR/Pyr-HR, with MIC and EC50 values of >100 μg/ml for flutianil and > 1000 μg/ml for pyriofenone. Isolates H-B1 from Ishioka-A and Ic1905–1from Ishioka-G were Flu-MR/Pyr-MR, with MIC value of 25 μg/ml for flutianil and > 1000 μg/ml for pyriofenone and EC50 value of 0.61 and 0.99 μg/ml, and 51.0 and 66.3 μg/ml, respectively. Inhibition rate of mildew growth at the highest concentrations by flutianil was 100% against Flu-MR and ranged from 5.5–27.0% (mean 17.8%) against Flu-HR isolates. That value by pyriofenone was 90.5 and 93.0% against Pyr-MR and ranged from 8.3–32.7% (mean 21.3%) against Pyr-HR isolates.
When five Flu-HR/Pyr-HR isolates (S-1sp, N-C2, N-E4, M1-D1 and M2-E) were subcultured repeatedly on cucumber cotyledons in the absence of both fungicides, their resistances were maintained even after subculturing 10, 27 and 46 times (Table 3). Upon subculturing 46 times, more than 29 months had passed since the most recent exposure to the fungicides.
In foliar spray tests, disease development of isolate N2, N1 and K-7-2 was almost completely inhibited at 2.5 and 10 μg/ml of flutianil and 25 and 100 μg/ml of pyriofenone, as well as at 200 μg/ml of mepanipyrim, which was used as the reference fungicide (Fig. 1). In contrast, efficacy of flutianil and pyriofenone was low even at registered doses against three Flu-HR/Pyr-HR isolates, N-C2, M1-D1 and M2-E, unlike mepanipyrim. Against Two Flu-MR/Pyr-MR isolates, H-B1 and Ic1905–1, both fungicides showed the control efficacy dependent on the concentration, however a decreased response at full registered doses.
Efficacies of flutianil (2.5 and 10 μg/ml), pyriofenone (25 and 100 μg/ml), and mepanipyrim against cucumber powdery mildew inoculated with Podosphaera xanthii isolates in foliar spray tests. Two experiments (a and b) were independent runs with the same experimental design. Error bars represent the standard error of the means. Different letters indicate significantly different efficacies within the same isolate according to a Kruskal-Wallis test employing a post hoc Steel-Dwass test (P < 0.05)
Monitoring study of sensitivity to flutianil and pyriofenone
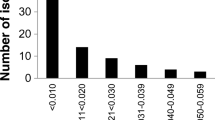
Sensitivity to flutianil and pyriofenone was tested using 99 single-spore isolates collected from the 13 cucumber greenhouses in addition to the previously described 23 isolates for a total of 122 isolates (Table 4). Ninety-one isolates (73.0%) sampled from all of the greenhouses showed resistance to both fungicides. Out of the 91 isolates, only two isolates from Ishioka-A and Ishioka-G were identified as Flu-MR/Pyr-MR and the remaining 89 isolates from 12 greenhouses as Flu-HR/Pyr-HR. Detection frequencies of Flu-HR/Pyr-HR isolates exceeded 50% in 10 greenhouses. In this study, no isolate with reduced sensitivity to only one fungicide was detected (Fig. 2). Observation with a light microscope showed that all of the isolates obtained from these greenhouses were classified as P. xanthii.
History of spraying with flutianil, pyriofenone and other fungicides, and change in disease incidence of powdery mildew
The total number of fungicide applications for control of powdery mildew from 2016 to 2018 was 7–13 per winter-spring cultivation and 13–20 times per summer-fall cultivation in the greenhouses of Jousou-A and -B and Omitama (Table 5). In Jousou-A, flutianil and pyriofenone were applied once or twice in each of the four cultivation periods except for pyriofenone in winter-spring cultivation in 2017 (no spray). In Jousou-B, flutianil was applied twice in summer-fall in the 2017 cultivation period and pyriofenone was sprayed only once in the winter-spring in 2016. In Omitama, spraying was performed twice with flutianil and once with pyriofenone in the summer-fall of 2016–2017. In Jousou-B and Omitama, following our recommendations, neither of the fungicides was used since the winter-spring cultivation in 2017, due to the detection of resistant isolates in the summer-fall cultivation in 2017 (Table 4). In Ishioka-A, the total number of fungicide sprays was two to three in the winter-spring cultivation and five to six in the summer-fall. Flutianil was used only once in the summer-fall cultivation in 2016–2017, and pyriofenone once in the summer-fall cultivation in 2016–2017 and in 2017–2018.
The development of powdery mildew was almost completely inhibited during the four cultivation periods in Jousou-A and Omitama, except for the summer-fall cultivation from 2016 to 2017 in Omitama (Fig. 3). In contrast, in Ishioka-A, severe disease incidence was recorded in every cultivation during this study.
Discussion
The leaf disc and foliar spray tests in this study clearly showed the occurrence of resistance to flutianil and pyriofenone in P. xanthii isolates. In addition, the present study suggests that the resistance to both fungicides have already developed and spread among many greenhouses with a history of spraying; these fungicides appear to have lost their efficacies to control powdery mildew on cucumber. This is the first report on resistance to flutianil in P. xanthii and other pathogen.
To avoid the development of fungicide resistance, the growers in Jousou-A, and -B and Omitama followed the registered spray dose and the number of usages for flutianil and pyriofenone per cultivation and adopted rotational spray patterns with other fungicides with different modes of action. Additionally, in Jousou-A, powdery mildew disease was almost completely controlled during the research period, so it was deduced that the pathogen populations exposed to both fungicides were very small. Despite these anti-resistance strategies, a grower (at Jousou-A) complained of a decline in the efficacy of both fungicides against powdery mildew in March 2017. This complaint led to the detection of rapid and extensive development of resistance to both fungicides. Considering the results of the present study, it is suggested that when pyriofenone and flutianil are used in greenhouses where cucumber is cultivated in two cultivation periods per year, there is a high risk of resistance development. In cucumber cultivation in Ibaraki Prefecture, extensive development of resistance to other fungicides, such as QoIs, DMIs, and SDHIs, was also observed in P. xanthii (Miyamoto, unpublished data). Consequently, it has been recommended that growers discontinue the use of flutianil and pyriofenone, in addition to the other fungicides mentioned, for the control of cucumber powdery mildew, and instead apply protectant fungicides such as iminoctadine albesilate, quinoxaline or mepanipyrim. Incidentally, the reason why powdery mildew was almost controlled in Jousou-A was that these effective protectants were sprayed regularly, in combination with cultural management, such as the clearance of diseased leaves and the use of an appropriate planting density.
Resistance factors (RF; the mean EC50 value for resistant isolates / mean EC50 value for sensitive isolates) for MR and HR isolates were 3015 and > 375,000 for flutianil, and 108 and > 1840 for pyriofenone, respectively. Therefore, it could be easily distinguished as S, MR and HR to each fungicide. About levels of resistance to metrafenone, which belongs to the same aryl-phenyl-ketone group as pyriofenone, there are a few reports in powdery mildew fungi on other crops. E. necator isolates that had developed resistance to metrafenone showed two levels of resistance (Kunova et al. 2016). Sensitivity to metrafenone in B. graminis f. sp. tritici was categorized into moderately adapted and resistant phenotypes (Felsenstein et al. 2010; Graf 2017). The moderately adapted phenotype of B. graminis f. sp. tritici showed RF of 55, which was similar to the RF value of Pyr-MR isolates of P. xanthii in our study, however this phenotype was still well controlled by metrafenone at the recommended field dose (375 μg/ml). The differences could be due to the EC50 value of metrafenone for sensitive isolates of B. graminis f. sp. tritici being about one-sixth the value of pyriofenone for P. xanthii isolates and the registered field dose being 3.75 times higher for metrafenone to wheat than pyriofenone to cucumber. Additionally, frequency of Pyr-MR and Pyr-HR isolates was 1.6% and 73.0% in cucumber greenhouses, respectively, however the B. graminis f. sp. tritici phenotype moderately adapted to metrafenone was more frequently isolated (up to 34%) than the resistant phenotype (0.3–1.5%) in European wheat growing regions (Graf 2017). This difference indicates the unique development of resistance to the aryl-phenyl-ketone group in each powdery mildew fungus.
No isolate that was resistant to only one fungicide was identified in this study. It is possible that resistance to both flutianil and pyriofenone developed as a result of the application of both fungicides at similar frequencies to control powdery mildew. Moreover, it can be inferred that there is, unfortunately, similar risks of resistance developing to both fungicides. To the best of our knowledge, no reports have described that isolates resistant to metrafenone or pyriofenone were tested for their sensitivity to flutianil. Nonetheless, an analysis such as whole genome sequencing of the flutianil resistant isolates obtained in this study may elucidate the mechanism of action of the fungicide, which remains unknown.
Our results show that Flu-HR/Pyr-HR isolates maintain the resistance even after consecutive subculture on fungicide-untreated detached cotyledons for more than two years and can also persist in the cucumber greenhouse in the absence of application of either fungicide for at least one year. A fitness study was performed for B. graminis f. sp. tritici isolates with reduced sensitivity to metrafenone (Stammler et al. 2014). Mixtures that contained combinations of sensitive isolates along with moderately adapted or resistant isolates, were cultured for several cycles on fungicide untreated wheat leaves. The results revealed that the sensitive isolates dominated after several transfers. Moreover, in the same report, it was described that single E. necator isolates resistant to metrafenone were fully sensitive after several cycles of subculture on untreated grapevine leaves (Stammler et al. 2014). However, a more detailed study on the competitiveness of this fungus showed that the change in the development of metrafenone resistance was dependent on the sensitive and resistant isolates used (Graf 2017). We hypothesise that the high frequency of resistant P. xanthii isolates in cucumber greenhouses where flutianil and pyriofenone applications and their selection pressure had been discontinued might have been caused by a similar fitness level in comparison with pyriofenone-sensitive strains, or by less influx of sensitive isolates from surrounding cucurbits. From the results of this study, it can be inferred that flutianil-resistant isolates also dominated in the greenhouses for the same reasons as isolates resistant to pyriofenone.
As mentioned above, it is recommended that growers avoid the use of flutianil and pyriofenone in greenhouses with two cultivation periods per year in Ibaraki Prefecture. In 2018, flutianil and pyriofenone were introduced onto the Japanese market as products mixed with other effective fungicides with different modes of action, namely, iminoctadine albesilate and mepanipyrim, respectively. These strategies are expected to play an essential role in reducing the risk of developing fungicide resistance. Furthermore, cucumber is also cultivated once a year in open fields or greenhouses in rotation with other crops, such as tomato. When cucumber is cultivated in this way, there is a possibility that the level of disease pressure and the influx of pathogens differ from those in the greenhouses surveyed in this study, and that the development of resistance might be delayed. Therefore, it is necessary to continue to carefully monitor pathogen sensitivity to flutianil and pyriofenone after the introduction of mixed products to control cucumber powdery mildew in such cultivation conditions.
References
Felsenstein, F., Semar, M., & Stammler, G. (2010). Sensitivity of wheat powdery mildew (Blumeria graminis f. sp. tritici) towards metrafenone. Gesunde Pflanzen, 62(1), 29–33.
Fungicide Resistance Action Committee (2019) FRAC Code List© 2019: Fungal control agents sorted by cross resistance pattern and mode of action (including FRAC Code numbering). http://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2019.pdf?sfvrsn=98ff4b9a_2. Accessed 3 October 2019.
Graf, S. (2017) Characterisation of metrafenone and succinate dehydrogenase inhibitor resistant isolates of grapevine powdery mildew Erysiphe necator. PhD Dissertation, Technische Universität Kaiserslautern, Kaiserslautern, German.
Hosokawa, H., Yamanaka, H., Haramoto, M., Sano, S., Yokota, C., & Hamamura, H. (2006). Occurrence and biological properties of cyflufenamid-resistant Sphaerotheca cucurbitae (abstract in Japanese). Japanese Journal of Phytopathology, 72(4), 260–261.
Ishii, H., Fraaije, B. A., Sugiyama, T., Noguchi, K., Nishimura, K., Takeda, T., et al. (2001). Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology, 91(12), 1166–1171.
Ishii, H., Miyamoto, T., Ushio, S., & Kakishima, M. (2011). Lack of cross-resistance to a novel succinate dehydrogenase inhibitor, fluopyram, in highly boscalid-resistant isolates of Corynespora cassiicola and Podosphaera xanthii. Pest Management Science, 67(4), 474–482.
Kanda, Y. (2013). Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplantation, 48, 452–458.
Kunova, A., Pizzatti, C., Bonaldi, M., & Cortesi, P. (2016). Metrafenone resistance in a population of Erysiphe necator in northern Italy. Pest Management Science, 72(2), 398–404.
Miyamoto, T., Ishii, H., & Tomita, Y. (2010). Occurrence of boscalid resistance in cucumber powdery mildew in Japan and molecular characterization of the iron-sulfur protein of succinate dehydrogenase of the causal fungus. Journal of General Plant Pathology, 76(4), 261–267.
Ohara, T., & Tsutsumi, K. (2015). Sensitivity testing method of cucumber powdery mildew caused by Podosphaera xanthii for penthiopyrad (in Japanese). Monthly Plant Protection Journal, 69(9), 563–568.
Ohtsuka, N., Sou, K., Amano, T., Ojima, M., Nakazawa, Y., & Yamada, Y. (1988). Decreased sensitivity of cucumber powdery mildew (Sphaerotheca fuliginea) to ergosterol biosynthesis inhibitors. Annuals of the Phytopathological Society of Japan, 54(5), 629–632.
Opalski, K. S., Tresch, S., Kogel, K. H., Grossmann, K., Köhle, H., & Hückelhoven, R. (2006). Metrafenone: Studies on the mode of action of a novel cereal powdery mildew fungicide. Pest Management Science, 62(5), 393–401.
Russell, P. E. (2004) Sensitivity baselines in fungicide resistance research and management. Brussels, Belgium: Crop life international: FRAC monograph 3. http://www.frac.info/docs/default-source/publications/monographs/monograph-3.pdf?sfvrsn=629d419a_8. Accessed 3 October 2019.
Sato, Y., Hoshi, H., Kagiwada, S., Nishio, T., & Horie, H. (2013). First record of Golovinomyces orontii on cucumber in Japan (abstract in Japanese). Japanese Journal of Phytopathology, 79(3), 187.
Schmitt, M. R., Carzaniga, R., Cotter, H. V. T., O’Connell, R., & Hollomon, D. (2006). Microscopy reveals disease control through novel effects on fungal development: A case study with an early-generation benzophenone fungicide. Pest Management Science, 62(5), 383–392.
Stammler, G., Semar, M., & Strobel, D. (2014) Resistance management of metrafenone in powdery mildews. In Dehne, H. W., Deising, H. B., Fraaije, B., Gisi, U., Hermann, D., Mehl, A., et al. (Ed) Modern fungicides and antifungal compounds VII. Proceedings of the 17th international Reinhardsbrunn symposium, April 21–25, 2013, Friedrichroda, Germany (pp 179–184). Braunschweig: Deutsche Phytomedizinische Gesellschaft e.V. Verlag.
Uchida, K., Takamatsu, S., Matsuda, K., So, K., & Sato, Y. (2009). Morphological and molecular characterization of Oidium subgenus Reticuloidium (powdery mildew) newly occurred on cucumber in Japan. Journal of General Plant Pathology, 75(2), 92–100.
Acknowledgements
We express our thanks to Dr. H. Ishii (Kibi International University) for his valuable comments on and his critical review of this study, and Dr. S. Takamatsu (Mie University) for the identification of powdery mildew pathogen. We also thank K. So, N. Ishihama, K. Maeda, R. Mori and Y. Ando (ZEN-NOH) for providing P. xanthii isolate K-7-2 and software for this work, M. Kuzuya (Ibaraki Plant Biotechnology Institute) for providing isolates N1 and N2, and Dr. D. Wari (Ibaraki Horticultural Research Institute) for carefully proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Human and animal rights statement
This article does not describe any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Miyamoto, T., Hayashi, K. & Ogawara, T. First report of the occurrence of multiple resistance to Flutianil and Pyriofenone in field isolates of Podosphaera xanthii, the causal fungus of cucumber powdery mildew. Eur J Plant Pathol 156, 953–963 (2020). https://doi.org/10.1007/s10658-020-01946-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-01946-6