Abstract
Gray mold caused by Botrytis cinerea is the major postharvest disease in table grapes grown in the Central Valley of California. Preharvest use of fungicide sprays may provide an alternative to the control of postharvest gray mold. However, fungicide resistance in B. cinerea can result in the failure of disease control. In this study, 212 isolates of B. cinerea were collected from table grape vineyards in three table grape-producing counties in the region and tested for resistance to selected fungicides on fungicide-amended media. In addition, 80 isolates were tested to establish baseline sensitivity to the newer fungicide fluopyram. Seven fungicide-resistant phenotypes were detected; 85.0%, 23.1%, 13.7%, and 94.8% of the isolates were resistant to boscalid, cyprodinil, fenhexamid, and pyraclostrobin, respectively. All isolates were sensitive to fludioxonil. Only 5.2% of the isolates were sensitive to all fungicides tested, whereas 8.9%, 56.1%, 23.6% and 6.1% were resistant to one, two, three, and four modes-of-action fungicides, respectively. Of the 80 isolates tested, all were sensitive to fluopyram with EC50 values ranging from 0.001 to 0.054 μg/mL. Most fungicides failed to control gray mold on detached table grapes inoculated with respective fungicide-resistant phenotypes. Our results suggest that alternation of sprays using different classes of fungicides will be needed to control postharvest gray mold, and that fludioxonil and fluopyram could be effective fungicides integrated into a preharvest fungicide spray program for control of gray mold in table grapes in the Central Valley of California.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gray mold caused by Botrytis cinerea is the major postharvest disease limiting storage and shelf life of table grapes (Gabler et al. 2003). Sulfur dioxide fumigation is a common postharvest measure practiced by the table grape industry in California to control gray mold during storage (Smilanick et al. 2010). In the past, various alternative measures to sulfur dioxide fumigation, including biological agents; natural antimicrobials such as salts, chitosan and plant extract; GRAS (generally regarded as safe) substances such as acetic acid, electrolyzed oxidizing water and ethanol; and physical means such as UV-C irradiation and hyperbaric treatment (Feliziani et al. 2014; Romanazzi et al. 2009; Romanazzi et al. 2012) have been explored. However, in California, table grapes are harvested by hand and commonly packed in the field. This practice is common due to the fragility of grape berries and other logistic issues such as the cost of indoor or shed packing (Romanazzi et al. 2012; Smilanick et al. 2010). Despite various alternative measures being tested, sulfur dioxide fumigation remains the major postharvest tool for control of gray mold on table grapes in California. Sulfur dioxide, however, can cause bleaching injuries to grape berries of SO2-susceptible table grape cultivars (Nelson 1985) and alters the flavor of the grapes (Lichter et al. 2005); and its use raises issues of regulatory concerns such as sulfite sensitivity in some consumers, and worker safety (Lichter et al. 2005). Taking into account the table grape harvest practices and potential issues associated with SO2 use, preharvest fungicide applications in the vineyard may serve as a viable alternative as it can be readily integrated into the current harvest and postharvest handling practices.
Chemical control has long been an important tool for control of diseases caused by B. cinerea. Several site-specific fungicides with different modes of action are available for gray mold management, including anilinopyrimidines (APs), dicarboximides, hydroxyanilides, quinone outside inhibitors (QoIs), phenylpyrroles, and succinate dehydrogenase inhibitors (SDHIs). However, the emergence of fungicide-resistant isolates renders chemical control ineffective. In fact, B. cinerea field isolates resistant to multiple classes of fungicides have been reported in various economically important crops (Angelini et al. 2014; Fernández-Ortuño et al. 2015; Li et al. 2014; Panebianco et al. 2015; Saito et al. 2016a; Veloukas et al. 2014; Walker et al. 2013; Weber 2010; Yin et al. 2012). Smilanick et al. (2010) have reported sensitivities to seven commercial fungicides, pyrimethanil, cyprodinil, fenhexamid, pyraclostrobin, boscalid, thiabendazole, and iprodione, in B. cinerea isolates collected in 2007 and 2008 from table grape fields in the Central Valley of California. However, B. cinerea is a high risk pathogen for the development of fungicide resistance. Fungicide resistance can be selected in the pathogen populations over the years (Veloukas et al. 2014; Walker et al. 2013), and new fungicides have been released in the meantime. Therefore, determining the frequency and phenotypes of fungicide resistance in the current B. cinerea populations is important to the development of effective chemical control programs.
Fluopyram is a fungicide belonging to SDHIs. It is effective against all stages of fungal growth and its activity spectrum includes several agricultural important pathogens, including B. cinerea. Although the pre-mixed commercial fungicide of fluopyram with pyrimethanil (trade name: Luna Tranquility) have been registered in some crops such as apple, potato, and wine grape in California, it has not been registered yet for table grapes. Although the baseline sensitivity of B. cinerea to fluopyram has already been reported on table grapes in Italy, strawberry in Florida, and various tree fruit in Greece (Amiri et al. 2014; Veloukas and Karaoglandis 2012; Vitale et al. 2016), no research has been conducted for B. cinerea populations in California. Such information is of great importance for estimating the possible risk of resistance selection against specific chemical groups of fungicides and enables the establishment of effective fungicide application programs regionally (Russell 2004).
The objectives of this study were: (i) to determine fungicide-resistant phenotypes of B. cinerea isolates collected from table grapes in the Central Valley of California, (ii) to determine the baseline sensitivity to fluopyram of B. cinerea isolates from table grape, and (iii) to evaluate the effectiveness of commercial fungicides in controlling gray mold on detached table grape berries inoculated with B. cinerea isolates belonging to different fungicide-resistant phenotypes.
Material and methods
Isolates of Botrytis spp.
In total, 442 isolates of Botrytis spp. were obtained from table grapes during the fall and winter in 2012 (Saito et al. 2016b). Briefly, after harvest, table grape berries that were left on the vines or on the vineyard floor, exhibiting typical gray mold symptoms (characteristic sporulation of Botrytis spp.), were collected from 10 commercial table grape vineyards in three major table grape-producing counties in the Central Valley of California. All isolates were then identified to the species level based on morphological and molecular characteristics (Saito et al. 2016b). In the present study, 212 B. cinerea isolates arbitrarily selected from vineyards located in seven cities (Madera, Fresno, Reedley, Exeter, Pixley, Earlimart and Delano) (at least 30 isolates from each sampling city) were used for fungicide resistance tests.
Fungicides
For in vitro tests, the technical grades of six fungicides were used. Boscalid (99% active ingredient [a.i.]; BASF Corporation, Research Triangle Park, NC, USA), cyprodinil (98% a.i.; Syngenta Crop Protection, Greensboro, NC, USA), fludioxonil (93% a.i.; Syngenta Crop Protection,), fluopyram (97.8% a. i.; Bayer CropScience, Research Triangle Park, NC, USA), and pyraclostrobin (98% a.i.; BASF) were dissolved in 100% acetone, and fenhexamid (95% a.i.; Arysta LifeScience, Cary, NC, USA) was dissolved in 100% ethanol, to prepare stock solutions.
Nine formulated fungicides and their concentrations used for the in vivo inoculation tests described below were Scala at 0.703 ml/L (37.4% a.i.; pyrimethanil, BASF), Elevate 50WDG at 0.60 g/L (50% a. i.; fenhexamid, Arysta LifeScience Corporation), Cabrio EG at 0.550 g/L (20% a. i.; pyraclostrobin, BASF), Endura 70WG at 0.890 g/L (70% a. i.; boscalid, BASF), Scholar SC at 0.524 mL/L (20.4% a. i.; fludioxonil, Syngenta Crop Protection), Pristine at 0.859 g/L (12.8 and 25.2% a. i.; pyraclostrobin and boscalid, BASF), Switch at 0.530 g/L (37.5 and 25% a. i.; cyprodinil and fludioxonil, Syngenta Crop Protection), Luna Tranquility at 0.938 g /L (11.3 and 33.8% a. i.; fluopyram and pyrimethanil, Bayer CropSciences LP) and Luna Privilege at 0.255 ml/L (41.5% a. i.; fluopyram, Bayer CropSciences LP). Although Scholar SC, Luna Privilege and Luna Tranquility are not registered for table grapes in California, they were included for research purposes. Concentrations of Cabrio EG, Elevate 50WDG, Endura 70WG, Pristine, Scala and Switch were based on the recommended label rates for table grapes, while the concentration of Luna Tranquility was based on the advice from the manufacturer. Concentrations of Luna Privilege and Scholar SC were adjusted to the same concentrations as one of the two active ingredients in fungicide mixture, Luna Tranquility or Switch, respectively.
Phenotypic characterization of fungicide resistance
All isolates were tested for resistance to five fungicides, cyprodinil, fludioxonil, boscalid, pyraclostrobin and fenhexamid, following the procedures originally developed by Weber and Hahn (2011) and modified by Saito et al. (2016a).
Fenhexamid and fludioxonil were tested on 1% malt extract agar (MEA). For pyraclostrobin, the alternative oxidase inhibitor salicyl hydroxamic acid (SHAM) was added to MEA at a concentration of 100 μg/mL (Mondal et al. 2005; Wise et al. 2008). In order to exclude amino acids from the cyprodinil assay (Myresiotis et al. 2007), 0.5% sucrose agar (SA) was used instead of MEA. For boscalid, 0.5% yeast extract agar (YEA) was used to avoid the interference of sugars with the assay. For fluopyram, YBA media was used as described below. Each medium without amending it with the respective fungicide was used as control.
Conidia were harvested by flooding 1- to 2-week-old cultures of B. cinerea growing on 9-cm diameter potato dextrose agar (PDA) petri dishes with sterile scraper and suspended in sterile distilled water. The resultant conidial suspension was filtered through autoclaved gauze. The conidial concentration was then quantified microscopically with a hemocytometer and diluted to the concentration of 1.0 × 105 spores/ml. Conidial suspensions were prepared immediately prior to the experiment and placed on ice until use.
An aliquot of 10 μl of conidial suspension of each isolate was streaked on the agar plate with or without fungicide. All the inoculated plates were incubated at 20 °C in the dark for 14 to 16 h with the exception of the one containing fluopyram that were incubated for 18 h under the same conditions. Germ tube elongation on each plate was measured under a microscope. All experiments were performed twice.
The discriminatory concentrations of each fungicide and the criteria used for the classification of fungicide resistance were based on those previously published (Saito et al. 2016a; Weber and Hahn 2011). Briefly, two different discriminatory concentrations for each fungicide were used as follows: for boscalid, 1 and 50 μg/mL; for cyprodinil, 1 and 25 μg/mL; for fenhexamid, 1 and 50 μg/mL; for fludioxonil, 0.1 and 10 μg/mL; for pyraclostrobin, 0.1 and 10 μg/mL. For boscalid, isolates with a fully grown germ tube at 50 μg/mL and more than 30% of growth relative to that grown on the control were classified as resistant, while sensitive isolates showed less than 50% of growth at 1 μg/mL. Since cyprodinil and pyrimethanil belong to the same class, AP fungicides, and the cross resistance among the APs is a known fact in B. cinerea (Hilber and Schüepp 1996), fungicide resistance to AP fungicides was tested only on cyprodinil. For cyprodinil, isolates that showed more than 50% of growth at 1 μg/mL and almost no growth at 25 μg/mL relative to that grown on the control were classified as resistant, while sensitive isolates grew less than 50% at 1 μg/mL and no growth at 25 μg/mL. For fludioxonil, isolates that grew more than 50% at 0.1 μg/mL and almost no growth at 10 μg/mL were classified as less sensitive, while sensitive isolates showed almost no growth at 0.1 μg/mL. For fenhexamid, isolates that showed more than 50% of growth at 0.1 μg/mL and less than 50% at 50 μg/mL were classified as resistant, while sensitive isolates showed less than 50% of growth at 0.1 μg/mL and little or no growth at 50 μg/mL. For pyraclostrobin, isolates with a fully grown germ tube at 10 μg/mL were classified as resistant, while isolates that grew less than 50% at 0.1 μg/mL were classified as sensitive.
Baseline sensitivity to fluopyram
In total, 80 B. cinerea isolates (at least 10 isolates from each of seven sampling cities) were used in this study to obtain the baseline sensitivity to fluopyram. Those isolates were collected in 2012 prior to the registration of fluopyram for use in table grape fields. Sensitivity to fluopyram was assessed in germ tube elongation tests in YBA agar medium (10 g of yeast extract, 10 g of bacto peptone, 20 g of sodium acetate and 15 g of agar per liter) as previously described (Veloukas and Karaoglandis 2012). YBA agar was amended with fluopyram at concentrations of 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1.0 and 5.0 μg/mL. YBA without the fungicide was used as control.
Conidia were harvested as described above. For each isolate, an aliquot of 10 μl of conidial suspension was streaked out on the YBA plate with or without fungicide. After 18 h of incubation at 20 °C in the dark, the germ-tube growth of 20 representative conidia was measured under a microscope (OLYMPUS BH-2; Oympus America Co., Center Valley, PA). All experiments were performed twice. Effective concentrations that inhibit growth at 50% (EC50) were determined for individual isolates using regression equations from the resulting log-liner dose-response curves (Leroux et al. 1999). Minimum inhibitory concentrations (MICs; the concentration at which fungal growth is completely inhibited) were also recorded for each isolate.
For fluopyram, based on the baseline sensitivity tests conducted in this study, 0.5 μg/mL was considered as minimum inhibitory concentration (MIC) as most of isolates did not grow at this concentration. Therefore this concentration was used as a single discriminatory concentration for fluopyram test.
Fungicide activities on inoculated table grapes
Based on the in vitro fungicide sensitivity tests, eight B. cinerea isolates with different fungicide-resistant phenotypes were used in this test. Their fungicide-resistant phenotypes were shown in Table 1. Conidial suspension of each isolate was prepared prior to the inoculation tests as described above.
Organically grown mature table grapes (V. vinifera cv. Crimson Seedless) were used for the inoculations to assess fungicide activities on grape berries against selected fungicide-resistant phenotypes of B. cinerea. Prior to the experiments, grape berries were detached from the rachis by cutting the bottom of the receptacle area using secateurs. Grape berries were then dipped into 0.5% of sodium hypochlorite for 2 min and rinsed with sterile water three times, and allowed to air dry in a fume hood. The grape berries were then taped onto Petri dishes with their cheek side up. Each plate comprised 10 berries. Four hours prior to inoculation with B. cinerea conidial suspension, three replicate plates with grape berries were sprayed with each fungicide at the concentration described above using a hand sprayer. Approximately three milliliters of each fungicide per plate were sprayed. The fruit were again allowed to dry at room temperature in a fume hood. Grape berries sprayed with sterile distilled water were used as non-treated controls.
Before inoculation, a wound was made on the cheek of each fruit by sticking a sterile needle in two-millimeter depth. An aliquot of 1 μl of B. cinerea conidial suspension was delivered onto each wound using a micropipette. After inoculation, the petri dishes without lids were transferred onto sterile paper towels in transparent plastic containers. Paper towels were saturated with sterile distilled water to establish high relative humidity in the container, and the containers were covered with the lids and sealed and incubated for 5 days at 20 °C in the dark. Disease severity on grape berries was examined as described previously. Briefly, disease severity was rated visually according to the following scale: 0 = no symptoms; 0.5 = <12.5%; 1 = <25%; 2 = 25% to 50%; 3 = 50% to 75%; 4 = 75% to 100% of the grape fruit surface decayed. The average of score was calculated for each plate. Disease incidence was calculated by dividing the number of fruit exhibiting disease symptom by the total number of fruit (n = 30). All experiments were performed twice.
In a separate test, based on the baseline sensitivity to fluopyram conducted in this study, seven B. cinerea isolates with varying degrees of sensitivity to fluopyram were used in an attempt to evaluate the effectiveness of fluopyram on detached table grape berries. Three isolates, X403, X524 and X698, were selected arbitrarily among the 10 most sensitive isolates to fluopyram (EC50 < 0.010) with the MICs value of 0.05 to 0.1 μg/mL. The other three isolates, X461, X704 and X731, were selected arbitrarily among the 10 least sensitive isolates to fluopyram (EC50 > 0.031) with the MICs value of 0.5 μg/mL. The isolate, X764, was selected as this was the only isolate that grew on the YBA plates amended with 0.5 μg/mL of fluopyram.
Statistical analyses
For the first assay, means of six averages (three replicates × two separate experiments) for each of two treatments were separated using an analysis of variance, followed by Waller-Duncan K-ratio tests in order to differentiate disease severity among seven isolates variably sensitive to fluopyram. Similarly, for the second assay, means separation was performed as described above to evaluate the effects of fungicide treatments within the same isolate. Data in percentage were arcsine-root square transformed prior to analysis (Perveen and Hussain 2012). All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Fungicide resistance profiles
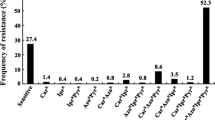
Of the 212 B. cinerea isolates tested for fungicide resistance, 49 (23.1%), 180 (85.0%), 201 (94.8%) and 29 isolates (13.7%) were resistant to cyprodinil, boscalid, pyraclostrobin, fenhexamid, respectively, and no isolates resistant to fludioxonil or fluopyram were detected (Table 1). Seven fungicide-resistant phenotypes were detected, with dual resistance to boscalid and pyraclostrobin being the predominant phenotype (55.2%) (Table 1). Frequencies of resistance to one, two, three or four mode-of-action fungicides were 9.0%, 56.1%, 23.6% and 6.1%, respectively; while 5.2% of the isolates were sensitive to all fungicides (Table 1).
Sensitivity to fluopyram
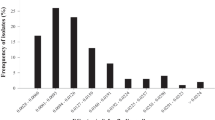
Eighty B. cinerea isolates were used in this study to establish the baseline sensitivity to fluopyram. EC50 values of fluopyram for 80 B. cinerea isolates ranged from 0.001 to 0.054 μg/mL (Fig. 1). EC50 values of 44 isolates (55%) were less than 0.010 μg/mL (Fig. 1). With the exception of the isolate X764, the MIC of fluopyram for the remaining 79 isolates ranged from 0.05 to 0.5 μg/mL. The isolate X764 showed 25% of relative growth on YBA media containing 0.5 μg/mL and recorded the highest MIC with 1.0 μg/mL.
Effectiveness of fungicides on detached grapes
Pyrimethanil significantly reduced gray mold incidence and severity on the fruit inoculated with all CYPS isolates compared to the non-treated control but failed to control the CYPR isolates, X471, X463 and X501 (Table 2). Fludioxonil significantly reduced gray mold incidence and severity for all of the isolates tested regardless of the phenotypes as all isolates were sensitive to fludioxonil (Table 2). The mixture of cyprodinil and fludioxonil significantly reduced gray mold severity on the inoculated fruit regardless of the isolates, but not the incidence for some isolates (Table 2). Boscalid, pyraclostrobin, and their mixture were effective against the isolates sensitive to respective fungicides, but the mixture failed to control the isolate resistant to both fungicides (Table 2). No decay developed on the fruit that were inoculated with fenhexamid-sensitive isolates and treated with fenhexamid, but fenhexamid failed to control gray mold on the fruit inoculated with fenhexamid-resistant isolates (Table 2). The mixture of fluopyram and pyrimethanil appeared to be the best treatment in controlling all isolates tested, significantly reducing both gray mold incidence and severity (Table 2).
On the fruit that were inoculated with isolates with varying degrees of sensitivity to fluopyram and treated with fluopyram, gray mold severity and incidence were evaluated after five-days incubation at 20 °C in the dark. There was a significant difference in incidence between control and fluopyram treated berries within the same isolate (P < 0.0001), except the isolate X764 (P = 0.34) (Table 3). There was a significant difference in severity between control and fluopyram treated berries within the same isolate (P < 0.0001) (Table 3). Although the disease severity of the isolate X764 on fluopyram treated berries was significantly higher than those of the other isolates (P < 0.0001), the disease severity was about 35% compared to that on the control berries (Table 3), thus the isolate X764 was considered to be controllable by the fungicide.
Discussion
High frequencies of resistance to two or more modes-of-action fungicides were observed among B. cinerea isolates collected from table grapes. Multiple resistance in B. cinerea has been reported on various crops in the world (Amiri et al. 2013; Fernández-Ortuño et al. 2015; Konstantinou et al. 2011; Rupp et al. 2017; Saito et al. 2016a). In this study, seven fungicide-resistant phenotypes were detected with dual fungicide-resistant phenotypes being most predominant, followed by triple fungicide-resistant phenotypes. Similar phenomena were observed in B. cinerea populations collected from blueberry in the Central Valley of California, and from strawberry fields in Florida where dual resistance to boscalid and pyraclostrobin was the most common fungicide-resistant phenotype (Amiri et al. 2013; Saito et al. 2016a). These previous studies and the present study suggested that the fungicide mixture of boscalid and pyraclostrobin (Pristine) is likely ineffective for controlling gray mold due to the high frequency of dual resistance to boscalid and pyraclostrobin. Fitness study of the dual-resistant isolates to boscalid and pyraclostrobin showed, however, a competitive disadvantage over the dual-sensitive isolates when successively transferred on apple fruit (Kim and Xiao 2011). Furthermore, the dual-resistant isolates disappeared after 5 years of discontinued use of the fungicides in kiwifruit fields in Greece (Veloukas et al. 2014). These findings suggest that the discontinued use of these two fungicides may help sensitive isolates increase in the population, and thereby may improve efficacy of the fungicides.
Fluopyram is a recently developed fungicide and has no history of use in table grape fields in California until 2015. For future resistance monitoring purpose, the baseline sensitivity of fluopyram was established in 80 B. cinerea isolates collected from seven commercial table grape fields prior to the use of the fungicide. Although fluopyram resistant B. cinerea isolates have been reported for strawberry fields in Florida and apple orchards in Washington (Amiri et al. 2014, 2017), in the present study no resistant isolates to fluopyram were detected among 212 B. cinerea isolates collected from table grape in the Central Valley, prior to the use of fungicide. Luna Privilege, fungicide that contains fluopyram as active ingredient, effectively controlled B. cinerea isolates. Luna Tranquility, the mixture of fluopyram and pyrimethanil, also showed effective control against all of the fungicide-resistant phenotypes tested in this study.
Although fluopyram belongs to SDHIs, the same fungicide group as boscalid, structural differences between the SDHIs thought to engender differences in binding properties to the target enzyme, and cross-resistance between boscalid and fluopyram has not been observed in Alternaria alternata, B. cinerea, Didymella bryoniae, M. graminicola, C. cassiicola and Podosphaera xanthii (Avenot et al. 2008; Fraaije et al. 2012; Ishii et al. 2011; Veloukas et al. 2011; Vitale et al. 2016). However, recent molecular studies revealed possible cross-resistance patterns between fluopyram and other SDHI fungicides, such as boscalid, fluxapyroxad and penthiopyrad in B. cinerea isolates that harbor certain amino acid substitution in the target gene, SdhB subunit (Amiri et al. 2014; Veloukas et al. 2013). To avoid possible cross-resistance among SDHI fungicides, judicious use of SDHI fungicides is needed to prevent occurrence of fluopyram resistance and to maintain efficacy of SDHI fungicides.
The presence of B. cinerea isolates resistant to AP fungicides, cyprodinil and pyrimethanil has been reported in the USA. For instance, 47% and 53% of the isolates collected from strawberry fields between 2010 and 2012 in Carolinas and in Florida, respectively, were resistant to AP fungicides (Amiri et al. 2013; Fernández-Ortuño et al. 2013). In the Central Valley of California, 20.1% were resistant to cyprodinil among 249 B. cinerea isolates collected from blueberry (Saito et al. 2016a). Although the frequency of resistance to AP fungicides in table grape isolates (23.1%) was similar to that of B. cinerea isolates from blueberry collected in the same region, high frequency of resistance was found in the southern part of the Central Valley. The same trend was observed for other classes of fungicides. Overall, the table grape isolates from the southern part of the Central Valley are resistant to more classes of fungicides than are the isolates from the northern part. This might reflect the fact that more diverse susceptible crops such as pistachio, almond, stone fruit, blueberry, are grown more intensively in the southern part of the Central Valley, thereby B. cinerea table grape isolates from the southern part of the Valley might have been exposed to fungicides more often than in the northern part.
Resistance to fludioxonil has been reported in B. cinerea from strawberries in southeastern United States but at very low frequency, and only four isolates showed low and moderately resistance to fludioxonil among 412 B. cinerea isolates (Li et al. 2014), but no resistance to fludioxonil was detected in 392 strawberry isolates in Florida (Amiri et al. 2013) and 249 blueberry isolates in the Central Valley of California (Saito et al. 2016a). Of the 106 blueberry isolates collected from Washington State, 70% of the isolates showed reduced-sensitivity to fludioxonil, but that was not enough to render fungicide ineffective to control gray mold on detached blueberry fruit (Saito et al. 2016a). Although fludioxonil appeared the most effective fungicide for gray mold control on inoculated berries in the lab tests, limited applications of fludioxonil, and monitoring of its resistance are of great importance to maintaining its fungicidal efficacy for gray mold control.
Fenhexamid-resistant isolates were phenotyped by full germ-tube growth on the MEA containing 1 mg/L and 50 mg/L of fenhexamid (Weber and Hahn 2011; Grabke et al. 2013). In the present study, we did not find any isolates that can meet this criterion. For all the isolates with a fully grown germ tube at 1 mg/L, relative germ tube growth on 50 mg/L ranged from 15 to 40% with the average of 24.0% (data not shown). However, when two of those isolates, X337 and X501, were used to inoculate fenhexamid-treated table grape berries, the averages of disease severity relative to that of the control were 99.4 and 85.2%, and the disease incidence was 100% on the fruit inoculated with these two isolates (Table 2), indicating that fenhexamid appeared to be ineffective against these two isolates. Therefore, the isolates with full germ-tube growth at 1 mg/L and around 15 to 40% growth at 50 mg/L were considered as fenhexamid resistant isolates in this paper, which was consistent with a previous report (Saito et al. 2016a). Fenhexamid resistance in B. cinerea field isolates has been reported in the Eastern US with frequency ranging from 4.5 to 44.4% (Amiri and Peres 2014; Grabket al., 2013; Saito et al. 2014). Although frequency of fenhexamid resistance in table grape isolates (13.7%) was lower than that of blueberry isolates collected in the same region (29.3%) (Saito et al. 2016a), a proper use of fenhexamid in the context of resistance management is needed. A field study on wine grape showed that the number of fenhexamid applications per year had impact on gray mold control, but not the selection for resistant isolates (Saito et al. 2014). Similarly, a long-term monitoring study of strawberry grown in Florida showed no clear correlation between the application frequency of fenhexamid and the frequency of resistant isolates (Amiri and Peres 2014). This suggests that regardless of frequency of resistance to the fungicide, efficacy of fenhexamid could be maintained if appropriate fungicide application programs and management strategies are implemented.
In the present study, the frequency of isolates resistant to pyraclostrobin (94.8%) was the highest among all fungicides tested. Frequency of QoI resistance in B. cinerea populations from various crops and geographical regions varied greatly in previous reports. For example, 19.5% of the isolates of B. cinerea from apple in Washington State were resistant to pyraclostrobin (Yin et al. 2012); of the1060 B. cinerea isolates collected from strawberry fields from seven southern states in the USA in 2013, 59% were resistant to pyraclostrobin (Fernández-Ortuño et al. 2014). In Germany, frequency of QoIs resistance was 81.2% among B. cinerea isolates collected from strawberry fields between 2009 and 2011 (Leroch et al. 2013). Walker et al. (2013) reported that 33% of B. cinerea isolates were found to be resistant to QoI fungicides in 2008, and resistance to the same fungicide has then become ubiquitous in French vineyards. It appears that the QoI usage patterns affect the frequency of QoI resistance in the pathogen populations. In addition, genetic diversity of the pathogen populations may also affect the QoI resistance frequency. The point mutation G143A in the cytochrome b (cytb) gene confers resistance to QoI fungicides in many fungal species (Ishii et al. 2009). However, the presence of the Bcbi-143/144 intron in the cytb gene is believed to prevent the development of resistance to QoI (Yin et al. 2012). Previously, the presence of Bcbi-143/144 intron was examined in 249 blueberry isolates collected from the Central Valley of California and found that 11.8% of the isolates carried the intron as opposed to 40.0% among 108 blueberry isolates collected in Washington (Saito et al. 2016a), suggesting that inherent risk for the development of resistance to QoI fungicides is higher in California than at least in Washington. Although the presence of the Bcbi-143/144 intron was not examined in the present study, this may explain the higher frequency of isolates resistant to pyraclostrobin in the Central Valley of California.
In summary, we reported the presence of several fungicide-resistant phenotypes in B. cinerea populations in table grape vineyards in California. Knowing the profiling of fungicide resistance in the pathogen populations is the first step for designing effective control programs. The findings from this study will help table grape growers implement relevant fungicide spray programs for control of gray mold in table grapes.
References
Amiri, A., & Peres, N. A. (2014). Diversity in the erg27 gene of Botrytis cinerea field isolates from strawberry defines different levels of resistance to the hydroxianilide fenhexamid. Plant Disease, 98, 1131–1137.
Amiri, A., Heath, S. M., & Peres, N. A. (2013). Phenotypic characterization of muti fungicide resistance in Botrytis cinerea isolates from strawberry fields in Florida. Plant Disease, 97, 393–401.
Amiri, A., Heath, S. M., & Peres, N. A. (2014). Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Disease, 98, 532–539.
Amiri, A., Mulvaney, K. A., Pandit, L. K., & Angelis, D. R. (2017). First report of resistance to fluxapyroxad and fluopyram in Botrytis cinerea from commercial apple orchards in Washington state. Plant Disease, 101, 508.
Angelini, R. M. D. M., Rotolo, C., Masiello, M., Gerin, D., Pollastro, S., & Faretra, F. (2014). Occurrence of fungicide resistance in populations of Botryotinia fuckeliana (Botrytis cinerea) on table grape and strawberry in southern Italy. Pest Management Science, 70, 1785–1796.
Avenot, H. F., Sellam, A., Karaoglanidis, G., & Michailides, T. J. (2008). Characterization of mutations in the iron-Sulphur subunit of succinate dehydrogenase correlating with boscalid resistance in Alternaria alternata from California pistachio. Phytopathology, 98, 736–742.
Feliziani, E., Romanazzi, G., & Smilanick, J. L. (2014). Application of low concentrations of ozone during the cold storage of table grapes. Postharvest Biology and Technology, 93, 38–48.
Fernández-Ortuño, D., Chen, F., & Schnabel, G. (2013). Resistance to cyprodinil and lack of fludioxonil resistance in Botrytis cinerea isolates from strawberry in north and South Carolina. Plant Disease, 97, 81–85.
Fernández-Ortuño, D., Grabke, A., Bryson, P. K., Amiri, A., Peres, N. A., & Schnabel, G. (2014). Fungicide resistance profiles in Botrytis cinerea from strawberry fields of seven southern U. S. States. Plant Disease, 98, 825–833.
Fernández-Ortuño, D., Grabke, A., Li, X., & Schnabel, G. (2015). Independent emergence of resistance to seven chemical classes of fungicides in Botrytis cinerea. Phytopathology, 105, 424–432.
Fraaije, B. A., Bayon, C., Atkins, S., Cools, H. J., Lucas, J. A., & Fraaije, M. W. (2012). Risk assessment studies on succinate dehydrogenase inhibitors, the new weapons in the battle to control Septoria leaf blotch in wheat. Molecular Plant Pathology, 13, 263–275.
Gabler, F. M., Smilanick, J. L., Mansour, M., Ramming, D. W., & Mackey, B. E. (2003). Correlations of morphological, anatomical, and chemical features of grape berries with resistance to Botrytis cinerea. Phytopathology, 93, 1263–1273.
Grabke, A., Fernández-Ortuño, D., & Schunabel, G. (2013). Fenhexamid resistance in Botrytis cinerea from strawberry fields in the Carolinas is associated with four target gene mutations. Plant Disease, 97, 271–276.
Hilber, U. W., & Schüepp, H. (1996). A reliable method for testing the sensitivity of Botryotinia fuckeliana to anilinopyrimidines in vitro. Pesticide Science, 47, 241–247.
Ishii, H., Fountaine, J., Chung, W.-H., Kansako, M., Nishimura, K., Takahashi, K., & Oshima, M. (2009). Characterisation of QoI-resistant field isolates of Botrytis cinerea from citrus and strawberry. Pest Management Science, 65, 916–922.
Ishii, H., Miyamoto, T., Ushio, S., & Kakishima, M. (2011). Lack of cross-resistance to a novel succinate dehydrogenase inhibitor, fluopyram, in highly boscalid-resistant isolates of Corynespora cassiicola and Podosphaera xanthii. Pest Management Science, 67, 474–482.
Kim, Y. K., & Xiao, C. L. (2011). Stability and fitness of pyraclostrobin- and boscalid-resistant phenotypes in field isolates of Botrytis cinerea from apple. Phytopathology, 101, 1385–1391.
Konstantinou, S., Karaoglanidis, G. S., Bardas, G. A., Minas, I. S., Doukas, E., & Markoglou, A. N. (2011). Postharvest fruit rots of apple in Greece: Pathogen incidence and relationships between fruit quality parameters, cultivar susceptibility, and patulin production. Plant Disease, 95, 666–672.
Leroch, M., Plesken, C., Weber, R. W. S., Kauff, F., Scalliet, G., & Hahn, M. (2013). Gray mold populations in German strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Applied and Environmental Microbiology, 79, 159–167.
Leroux, P., Chapeland, F., Desbrosses, D., & Gredt, M. (1999). Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop Protection, 18, 687–697.
Li, X., Fernandez-Ortuño, D., Grabke, A., & Schnabel, G. (2014). Resistance to fludioxonil in Botrytis cinerea isolates from blackberry and strawberry. Plant Disease, 104, 724–732.
Lichter, A., Zutahy, Y., Kaplonov, T., Aharoni, N., & Lurie, S. (2005). The effect of ethanol dip and modified atmosphere on prevention of Botrytis rot of table grapes. HortTechnology, 15, 9–16.
Mondal, S. N., Bharia, A., Shilts, T., & Timmer, L. W. (2005). Baseline sensitivities of fungal pathogens of fruit and foliage of citrus to azoxystrobin, pyraclostrobin, and fenbuconazole. Plant Disease, 89, 1186–1194.
Myresiotis, C. K., Karaoglanidis, G. S., & Tzavella-Klonari, K. (2007). Resistance of Botrytis cinerea isolates from vegetable crops to anilinopyrimidine, phenylpyrrole, hydroxyanilide, benzimidazole, and dicarboximide fungicides. Plant Disease, 91, 407–413.
Nelson, K. E. (1985). Harvesting and handling California table grapes for market. Bulletin. 1913, University of California Division of Agriculture and Natural Resources.
Panebianco, A., Castello, I., Cirvilleri, G., Perrone, G., Epifani, F., Ferrara, M., Polizzi, G., Walters, D. R., & Vitale, A. (2015). Detection of Botrytis cinerea field isolates with multiple fungicide resistance from table grape in Sicily. Crop Protection, 77, 65–73.
Perveen, F., & Hussain, Z. (2012). Review: Use of statistical techniques in analysis of biological data. Basic Research Journal of Agricultural Science, 1, 1–10.
Romanazzi, G., Gabler, F. M., Margosan, D., Mackey, B. E., & Smilanick, J. L. (2009). Effect of chitosan dissolved in different acids on its ability to control postharvest gray mold of table grape. Phytopathology, 99, 1028–1036.
Romanazzi, G., Lichter, A., Gabler, M. F., & Smilanick, J. L. (2012). Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biology and Technology, 63, 141–147.
Rupp, S., Weber, R. W. S., Rieger, D., Detzel, P., & Hahn, M. (2017). Spread of Botrytis cinerea strains with multiple fungicide resistance in German horticulture. Frontiers in Microbiology, 7, 2075.
Russell, P. E. (2004). Sensitivity baselines in fungicide resistance research and management. FRAC, Monograph. No. 3, Brussels, Belgium: Crop Life International.
Saito, S., Cadle-Davidson, L., & Wilcox, F. W. (2014). Selection, fitness and control of Botrytis cinerea isolates from grapes variably sensitive to fenhexamid. Plant Disease, 98, 233–240.
Saito, S., Michailides, T. J., & Xiao, C. L. (2016a). Fungicide resistance profiling in Botrytis cinerea populations from blueberry in California and Washington and their impact on control of gray mold. Plant Disease, 100, 2087–2093.
Saito, S., Margosan, D., Michailides, T. J., & Xiao, C. L. (2016b). Botrytis californica, a new cryptic species in the B. cinerea species complex ausing gray mold in blueberries and table grapes. Mycologia, 108, 330–343.
Smilanick, J. L., Mansour, M. F., Gabler, F. M., Margosan, D. A., & Hashim-Buckey, J. (2010). Control of postharvest gray mold of table grapes in the San Joaquin Valley of California by fungicides applied during the growing season. Plant Disease, 94, 250–257.
Veloukas, T., & Karaoglandis, G. S. (2012). Biological activity of the succinate dehydrogenase inhibitor fluopyram against Botrytis cinerea and fungal baseline sensitivity. Pest Management Science, 68, 858–864.
Veloukas, T., Leroch, M., Hahn, M., & Karaoglandis, G. S. (2011). Detection and molecular characterization of boscalid-resistant Botrytis cinerea isolates from strawberry. Plant Disease, 95, 118–122.
Veloukas, T., Markoglou, N. A., & Karaoglanidis, G. S. (2013). Differential effect of SdhB gene mutations of the sensitivity to SDHI fungicides in Botrytis cinerea. Plant Disease, 97, 118–122.
Veloukas, T., Kalogeropoulou, P., Markoglou, A. N., & Karaoglanidis, G. S. (2014). Fitness and competitive ability of Botrytis cinerea field isolates with dual resistance to SDHI and QoI fungicides, associated with several sdhB and the cytb G143A mutations. Phytopathology, 104, 347–356.
Vitale, A., Panebianco, A., & Polizzi, G. (2016). Baseline sensitivity and efficacy of fluopyram against Botrytis cinerea from table grape in Italy. Annals of Applied Biology, 169, 36–45.
Walker, A.-S., Micoud, A., Rémuson, F., Grosman, J., Gredt, M., & Leroux, P. (2013). French vineyards provide information that opens ways for effective resistance management of Botrytis cinerea (grey mould). Pest Management Science, 69, 667–678.
Weber, R. W. S. (2010). Occurrence of Hyd R3 fenhexamid resistance among Botrytis isolates in northern German soft fruit production. Journal of Plant Disease and Protection, 117, 177–179.
Weber, R. W. S., & Hahn, M. (2011). A rapid and simple method for determining fungicide resistance in Botrytis. Journal of Plant Disease and Protection, 118, 17–25.
Wise, K. A., Bradley, C. A., Pasche, J. S., Gudmestad, N. C., Dugan, F. M., & Chen, W. (2008). Baseline sensitivity of Ascochyta rabiei to azoxystrobin, pyraclostrobin, and boscalid. Plant Disease, 92, 295–300.
Yin, Y. N., Kim, Y. K., & Xiao, C. L. (2012). Molecular characterization of pyraclostrobin resistant and structural diversity of the cytochrome be gene in Botrytis cinerea from apple. Phytopathology, 102, 315–322.
Acknowledgments
We thank Sean Pelham for technical assistance and personnel of the vineyards for assistance in sample collection. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendations or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Funding
This research was funded in part by the California Table Grape Commission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
No human and/or animal participants were involved in this research.
Informed consent
All authors consent to this submission.
Disclaimer
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendations or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Saito, S., Michailides, T.J. & Xiao, C.L. Fungicide-resistant phenotypes in Botrytis cinerea populations and their impact on control of gray mold on stored table grapes in California. Eur J Plant Pathol 154, 203–213 (2019). https://doi.org/10.1007/s10658-018-01649-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-01649-z