Abstract
To study inheritance of Malus sieboldii-derived apple proliferation resistance, 14 cross combinations were performed with the tetraploid apomictic M. sieboldii and first and second generation parental lines as donor of resistance and Malus x domestica scion cultivars and apple rootstocks as donor of pomological traits. In the progeny examined mainly three classes were present consisting of mother-like plants with the allele composition of the maternal apomict (ML), hybrids based on fertilization of an unreduced egg cell (hybrid I), and fully recombinant plants (hybrid II). Two-year screening of inoculated plants in the nursery revealed that progeny classes ML and H I responded similarly to infection and that about half of the progeny showed satisfactory resistance. No appropriate resistance was identified in progeny class H II. This might be due to the fact that in fully recombinant offspring M. sieboldii haplotypes have been reduced from 4n to 1-2n or were entirely lost. Following nursery-growing, promising trees were evaluated for six more years in the orchard. Nearly all of them showed satisfactory resistance but were mostly less productive and more vigorous than trees on clonal standard rootstock M9. However, mainly among the offspring of progeny 4608 × M9, resistant genotypes were identified showing pomological properties similar to M9.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apple proliferation (AP) disease is caused by the uncultured wall-less bacterium ‘Candidatus (Ca.) Phytoplasma mali’ of the class Mollicutes. The pathogen induces a range of symptoms including witches’ brooms, enlarged stipules, rosetting, foliar reddening or yellowing, growth suppression and undersized, unmarketable fruits. Due to crop losses and severely affected fruit quality, AP is of considerably economic importance in several major apple growing areas of Europe. The causal phytoplasma is mainly spread by the psyllids Cacopsylla picta and C. melanoneura (Frisinghelli et al. 2000; Tedeschi and Alma 2004) and may also be transmitted by root bridges from neighboring trees (Ciccotti et al. 2007). The disease is difficult to control as there is no direct treatment of the phytoplasma in infected trees. The recommended preventive measures such as the use of healthy planting material, uprooting of diseased trees to reduce infection sources, and control of insect vectors can reduce AP incidence but their effect is often not satisfactory.
A more promising approach to control AP would be the use of resistant rootstocks. Previous work showed that ‘Ca. P. mali’ is eliminated in the aerial parts during winter due to degeneration of phloem sieve tubes on which the pathogen is depending. Overwintering occurs in the roots where functional sieve elements are present throughout the year. From the roots, the aerial parts are recolonized in spring when new phloem is being formed (Schaper and Seemüller 1982, 1984; Seemüller et al. 1984). This fluctuation in colonization pattern has led to the presumption that growing the usually susceptible scion cultivars on resistant rootstocks may prevent disease or reduce its impact. However, examination of many established and experimental stocks has shown that there is no satisfactory resistance in this group (Kartte and Seemüller 1991). Screening of a large number of Malus taxa not used as rootstocks revealed that they often are more susceptible to infection than the rootstocks examined. Many of them showed a high mortality rate. However, satisfactory resistance was observed in some experimental rootstock selections consisting of a specific genotype of apomictic Malus sieboldii and interspecific hybrids of this type with the nonapomictic species M. x domestica or M. x purpurea. Resistant plants either never developed symptoms or recovered from the mild symptoms in the following season. Few hybrids of apomictic species M. sargentii showed a similar feature while others were severely affected (Kartte and Seemüller 1991; Seemüller et al. 1992).
To further examine the M. sieboldii-based AP resistance, progeny derived from open pollination of M. sieboldii and 15 M. sieboldii-derived experimental rootstock selections were compared in several long-term field trials with 10 established clonal rootstocks, consisting mainly of hybrids of M. x domestica. Similar results were obtained in all trials, regardless whether the plants were grown under natural infection conditions only or whether they were artificially inoculated prior to the field trial. Satisfactory resistance was shown by progeny of M. sieboldii and by 11 of the 15 M. sieboldii hybrid progeny tested. Within progeny, considerable differences in resistance were observed. However, the number of severely affected trees was low in the progeny of resistant seed parents and high in the progeny of susceptible seed parents (Bisognin et al. 2008; Seemüller et al. 2008). The variability in the progeny of apomictic seed parents is mainly due to the fact that apomixis is not obligate in the genus Malus. Thus, progeny may contain a variable percentage of hybrids from reduced and unreduced maternal gametes. Their resistance is influenced by recombination events and the genetic background of the unknown pollen parent associated with open pollination (Seemüller and Harries 2010).
From the above it is evident that there is satisfactory AP resistance in parental lines of M. sieboldii and to a lesser extent of M. sargentii. However, under the aspect of commercial apple growing, most M. sieboldii lines are inferior to dwarfing standard stock M9 as they are in most cases more vigorous and have a lower yield potential. Another problem is the sensitivity of some M. sieboldii genotypes and particular of M. sargentii and derived hybrids of these species to infection by latent apple viruses. Symptoms ranging from mild to a high mortality rate induced by these groups that include apple chlorotic leaf spot virus (ACLSV), apple stem pitting virus (ASPV) and apple stem grooving virus (ASGV) have been reported (Schmidt 1972; Seemüller et al. 2008). To improve pomological needs and to select virus-tolerant offspring, a breeding program was initiated in 2001. In this project, M. sieboldii and first, second and third generation M. sieboldii hybrids were selected that showed resistance to AP disease in previous work. Information on the first part of the results of this project consisting of the description of the parental lines used, the crossing procedure and the use of simple sequence repeat (SSR) markers to identify each progeny class were reported previously (Bisognin et al. 2009). The SSR markers were applied to infer mode of reproduction, genomic constitution, and ploidy level of the progeny. In addition, ploidy data were confirmed by flow cytometry. Furthermore, information on the number of seedlings obtained in the various cross combinations and their assignment to each progeny class was provided (Bisognin et al. 2009). In focus of the current work was to analyze in nursery and field trials the resistance of the breeding products to AP disease and their suitability for commercial apple growing.
Materials and methods
Plant material
The M. sieboldii selection used, first generation hybrids 4551 and 4608, and second generation hybrids C1907, D2118, D2212, H0801 and H0909 of this genotype were provided by late Hanna Schmidt, formerly Federal Research Station for Horticultural Plant Breeding, Ahrensburg (Germany) (Schmidt 1964). The M. sieboldii genotype and the hybrids listed above derive from a single accession originating from Japan and grown in the botanical garden of Lund (Sweden) (Oldén 1953; Schmidt 1964). Flowers of rootstock P22 and cv. Gala were provided by Bundessortenamt, Wurzen (Germany). Rootstocks Supporter 1 and M9 were obtained from commercial nurseries and were grown at the Dossenheim institute. From 2001 to 2005, 14 successful cross combinations were carried out with these genotypes (Table 1). As described above, the resulting seedlings were characterized and classified by simple sequence repeat (SSR) genotyping (Bisognin et al. 2009).
Screening for resistance and pomological traits
The seedlings were grown in pots and were graft-inoculated in late summer of the first year with cv. Golden Delicious scions taken from symptomatic trees infected by isolates GDH, 3/93, 12/93, 14/93 and 3/4 of ‘Ca. P. mali’ that proved highly virulent in previous work (Seemüller and Schneider 2007). The grafted plants were grown for another year in pots and were then transplanted to the nursery where they were maintained for one more year. In the two years after inoculation, resistance was estimated by recording foliar symptoms and terminal growth in early fall using a rating scheme from 0 to 3. Symptom rating categories were: slight reddening or mild yellowing = 0.5; severe reddening or yellowing, premature leaf drop, leaf roll, and enlarged stipules = 1; reduced vigor or rosetting = 2; witches’-broom or severe stunting = 3. Ratings of the two years were added to obtain the cumulative disease index (CDI).
Following nursery growing, plants showing no or only very mild symptoms were selected and transplanted to the field where they were maintained under standard growing conditions for six years. In this period, the trees were observed for foliar symptom appearance as described above. In addition, fruit symptoms were recorded applying a scheme from 1 to 3 for the reduction of fruit size. At the end of the observation period, the annual ratings were added to obtain the CDI over the entire observation period of eight years. In addition, the vigor of the trees and the effect of infection on vegetative growth were determined by measuring the trunk diameter 40 cm above ground. Furthermore, to assess the production potential, the relative yield was determined by dividing the total yield of each tree by the cross section value (cm2) of the trunk.
Determination of phytoplasma titer
Offspring of selected progeny were examined at the end of the field evaluation period for phytoplasma titer. Shoots and roots of the trees (three samples of each location per tree) were sampled in autumn when the phytoplasma titer is highest (Baric et al. 2011). Total DNA was extracted from shoot and root phloem as described by Ahrens and Seemüller (1994). Quantitative real-time PCR was performed according to the procedure described by Bisognin et al. (2008). The results were expressed as phytoplasma cells per gram of extracted phloem tissue.
Detection of latent apple viruses
When it became evident that some of the resistent lines respond highly sensitive to latent apple virus infection (Seemüller et al. 2008), the virus status of the inoculum was determined using the procedure described by Rott and Jelkmann (2001). The isolates used for inoculation were examined for the presence of ASPV, ASGV or/and ACLSV. Clearly affected progeny were excluded from screening for AP resistance and pomological traits. In the following, virusfree isolates were used for inoculation. However, all progeny inoculated with such inoculum were in parallel also examined for virus sensitivity to ensure that only virus-tolerant progeny were examined for AP resistance and pomological traits.
Statistical analysis
Linear models (LM) were used to analyze the effect of progeny classes from different cross combinations inoculated with strains of ‘Ca. P. mali’ on disease severity, yield and tree vigor. The combination of progeny class, cross combination and inoculum was used to define groups which were compared using the lsmeans function from lsmeans package (Lenth 2016). For multiple comparisons, P values were adjusted by the method of Bonferroni. Resistance values were log transformed to achieve normal distribution of residuals. Statistical analyses were performed using R (R Core Team 2016).
Results
Progeny classes and inoculation
As reported in the previous publication on this breeding program, the offspring of the 14 progeny examined are composed of up to four different genetic classes (Bisognin et al. 2009). They consist of: (1) tri- or tetraploid mother-like plants having the same set of SSR alleles as the seed parent; (2) tetra- or pentaploid H I hybrids deriving from tri- or tetraploid apomictic seed parents and include the whole marker profile of the seed plant plus one allele of the diploid pollen parent; (3) fully recombinant triploid hybrids H II that exhibit half of the specific alleles of both seed and pollen parent; and (4) plants that putatively derived from autopollination. These tetraploid individuals, which mainly occur in progeny W, may represent new genetic recombinations of the maternal genome. Most or all of the breeding products that were classified as hybrid I and hybrid II and a subset of mother-like offspring and seedlings derived from autopollination were examined for AP resistance. Table 1 shows the cross combinations included in the works, the number of plants examined and the progeny class they were assigned to.
Examining graft-inoculation efficiency by symptom assessment on AP-sensitive plants revealed that nearly 100% of them were infected. Similar results were obtained by random PCR testing of non-symptomatic resistant plants after nursery growing. Evidence of the high inoculation efficiency is also provided in Table 5 which shows the results of PCR detection 8 years post inoculation. Also, all inoculated M9 controls developed symptoms and remained symptomatic during the entire observation period.
Virus sensitivity
When it became evident that inoculated offspring of a few progeny are highly susceptible to latent apple virus infection, the virus status of the different phytoplasma inocula was tested. It turned out that the inoculum 14/93 was contaminated by ASPV, inoculum 3/93 by ASPV and ACLSV, and incolum GDH by ASGV, ASPV and ACLSV. Nearly 100% of the offspring of cross combinations 4551 × M9 and M9 × 4551 died following inoculation with phytoplasma isolates contaminated with ASGV, ASPV and/or ACLSV. The plants of cross combination M9 × D2212 inoculated with isolate GDH showed a mortality rate of about 50%. In addition, most of the remaining plants were severely affected. In contrast, the reciprocal cross D2212 × M9 inoculated with the same isolate was not affected by the virus contamination. No or only insignificant effects of latent apple viruses were observed in other crosses. This is evidenced by the finding that progeny I, Y and BB, which were inoculated both with virus-free and virus-contaminated isolates did not differ significantly in the response to infection (Table 2). Because AP resistance of progeny severely suffering from virus infection cannot be evaluated when inoculated with virus-contaminated inoculum, progeny N and M were excluded from further examination.
Assessing resistance in nursery
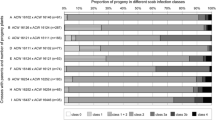
The resistance of the remaining 12 breeding products was examined using the five highly virulent isolates 3/4, 3/93, 12/93, 14/93 and GDH of ‘Ca. P. mali’ (Table 2). In the two years of pot and nursery screening, similar average values were observed in progeny classes ML and HI of the same cross whereas this value was distinctly higher in class H II. The results were also similar, when the same progeny was inoculated with two different isolates. In contrast, the inoculated seedlings of the 12 crosses responded differently to infection and showed highly variable results. Despite this variability, it could be shown that ML offspring of progeny B (inoculum 3/93) and L, and H I offspring of progeny B (both inocula) had significantly lower CDI values (P ≤ 0.5) than most other groups. Only the CDI values of ML offspring of progeny A, B (inoculum GDH), C, AA, CC and EE, and H I offspring of progeny A, I, and L (Table 2) were not statistically different. A largely similar evaluation of the results was obtained when highly resistant genotypes were distinguished from less resistant groups by a CDI median threshold of about 1 in the graphical data presentation (Fig. 1). This threshold was chosen as it applies for plants showing no or only very mild symptoms in the first two years. As the median of 1 signifies that 50% or more of the progeny population exhibits a CDI between 0 and 1, it was chosen as criterion of selection. This requirement is fulfilled by ML offspring of progeny A, B (inoculum 3/93) and L, and by H I offspring of progeny A and B (both inocula), L and I. In these progeny, the majority of seedlings were not or only little affected. No satisfactory resistance was identified in H II offspring where the CDI means in particular of progeny W, AA and BB were close to the mean CDI value of the infected M9 control. Even higher CDI values showed autopollination-derived offspring of progeny W (Table 2).
Cumulative disease index of offspring of the progeny classes used as rootstock in nursery growing. Box and whisker plots with median as line, interquartile ranges are represented by boxes, whiskers extend to samples laying within 1.5 times the interquartile range and dots represent outliers. 1 Mother-like due to apomixis. 2 Contains the marker profile of seed plant plus one paternal allele. 3 Exhibits half of the alleles of both seed and pollen parent. 4 Number of trees examined. 5 Inoculum: a = 3/93; b = 14/93; c = 12/93; d = GDH; e = 3/4. 6 Progeny code. 7 Cumulative disease index
Resistance in orchard growing and pomological traits
Offspring of promising resistance were selected from six cross combinations after two years of pot and nursery growing. These trees showed this trait also in six years of orchard growing. This is evidenced by the fact that there was no or only a slight increase of the CDI values during this period (Table 3). In addition, the majority of CDI values over all progeny classes did not differ significantly from each other, indicating satisfactory resistance throughout the selected progeny. The lowest value (0.8) was shown by H I hybrids of progeny B exhibiting a significantly lower average value than several other groups. On the other hand, the H II hybrids of progeny W showed a significantly higher value than the majority of the other groups. However, the average CDI value of 11.6 of infected trees on standard stock M9 was still significantly higher than the CDI of all progeny examined (average value 2.7 ranging from 0.8 to 6.1) (Table 3, df = 12; F value = 26.5; p < 0.05; r2 = 45.6%).
The productivity of the breeding products differed also distinctly. The average relative yield over the various crosses and progeny classes ranged from 1.83 to 2.97 kg/cm2. However, the differences between these values were in most cases statistically not significant (Table 3, df = 14; F value = 15.0; p < 0.05; r2 = 41.9%). Such differences occurred mainly between the low-yielding trees on mother-like and H I roots of progeny A and the significantly higher values on H I rootstocks of progeny B. However, the average yield of trees on these latter rootstocks was significantly lower than that of trees on standard stock M9 (2.97 versus 4.45 kg/cm2) (Table 3, df = 14; F value = 15.6; p < 0.05; r2 = 42.8%). This high value of trees on M9 roots was associated with low vigor, expressed by trunk diameter. While healthy trees on M9 showed an average trunk diameter of 4.9 cm, the average diameter of the inoculated breeding products ranged from 5.1 to 8.3 cm (Table 3).
To analyze the pomological potential of the breeding products, the range of variation of both yield and vigor was determined (Table 4). Considering only not or scarcely affected trees (CDI ≤ 3.0), the most promising genotypes were mainly identified in progeny B. Among the trees on ML and H 1 roots of this progeny, three and six individuals were identified showing an average relative yield of 6.81 and 4.67 kg/cm2, respectively, and a trunk diameter ranging from 3.8 and 5.6 cm. These values are close to the data for healthy trees on M9. In trees on rootstocks of other progeny, in particular on offspring of progeny W, individuals were identified showing a similar yield potential. In this case, however, the high yield was mostly associated with higher CDI scores that resulted in reduced vigor and was associated with a reduced fruit size. Related to these findings on the effect of infection on vigor and yield is the comparison of healthy trees with infected trees on susceptible rootstock M9 and class ML of fairly resistant progeny W. Whereas the vigor of infected trees was reduced by 22 and 11%, respectively, the effect on yield is much more pronounced by being 52% for infected trees on M9 whereas the yield of trees on mother-like roots of progeny W was not affected by the infection (Table 3).
Phytoplasma concentration
Quantitative real time PCR was employed to determine presence and concentration of ‘Ca. P. mali’ in the taxonomically different plant tissues and plant parts. Of the M. sieboldii-related progeny examined, six groups of samples from roots and four groups from shoots were selected to be compared with samples from trees grown on standard stock M9 (Table 5). Of these selections, trees on M9 showed the highest titer that was similar in roots and the cv. Golden Delicious top. In contrast, in trees on M. sieboldii-related stocks a great variation of the phytoplasma titer was identified. It was low in progeny CC and Y and highest in hybrids II of progeny W. The phytoplasma concentration in the roots affected in most cases also the titer in the shoots. Another finding was that mainly in resistant rootstocks of progeny B the pathogen was not detectable, supposedly because of too low titer or phytoplasma eradication.
Discussion
In Europe, almost all rosaceous fruit trees are prone to phytoplasma infection. Particularly apple, pear, apricot, peach and Japanese plum can severely be affected by apple proliferation, pear decline and European stone fruit yellows (ESFY) caused by ‘Ca. P. mali’, ‘Ca. P. pyri’ and ‘Ca. P. prunorum’, respectively. It has been observed that species within the genus of the impaired host differ considerably in susceptibility to the corresponding pathogen. Distinct differences also occur within the same species. For instance, Pyrus betulifolia, P. elaeagrifolia and some genotypes of P. communis were little affected while P. ussuriensis and P. pyrifolia are very susceptible (Batjer and Schneider 1960; Seemüller et al. 2009; Westwood and Lombard 1982). Within the genus Prunus, sweet cherry, sour cherry and their rootstocks are not or little affected, like plums on Prunus domestica stocks such as Ackermanns and Brompton and on P. cerasifera stocks. In contrast, peach trees on peach rootstocks Montclar, Rutgers Red Leaf and St. Julien 2 (P. insititia) showed high susceptibility with up to 100% mortality (Dosba et al. 1991; Kison and Seemüller 2001; Morvan 1977). Similar differences in susceptibility of stone fruit scion cultivars have been reported (Dosba et al. 1991; Jarausch et al. 2000).
As described in the introductory section, our previous work revealed that there is no satisfactory AP resistance in M. x domestica, the culinary apple. The same applies for most or all established apple rootstocks, which often are interspecific hybrids of M. x domestica, and the majority of wild and ornamental Malus species examined. Satisfactory resistance was mainly identified in apomictic M. sieboldii and experimental apomictic rootstock selections consisting of interspecific hybrids of M. sieboldii with the nonapomictic taxa M. x domestica or M. x purpurea ‘Eleyi’. They include selections 4551, 4608, C1907, D2118, D2212, H0801, and H0909 that were also included in this study. The resistance level of this group was usually significantly higher than that of trees on M. x domestica-related stocks (Bisognin et al. 2008; Seemüller et al. 2008). These data indicate that the M. sieboldii-based resistance is well established and a segregating trait. However, AP resistance is not a universal feature of M. sieboldii as shown by a second genotype of M. sieboldii that was examined in parallel to this study and proved highly susceptible to AP infection following inoculation with a virus-free isolate (data not shown). High susceptibility was previously also identified in a different genotype of M. sieboldii (Kartte and Seemüller 1988).
The resistance to ‘Ca. P. mali’ is provided by a defined tetraploid and apomictic accession of M. sieboldii (Oldén 1953; Bisognin et al. 2009). These features made it difficult to develop an AP-resistant rootstock with agronomic value by classical breeding. However, the apomixis of the tetraploid M. sieboldii is not complete but facultative and may vary from year to year according to climatic influences (Schmidt 1964). Therefore, a certain number of hybrids are being formed after pollination, the products of which can easily be distinguished from motherlike offspring by their phenotype (Schmidt 1964). Facultative apomixis was also inherited to varying degree to first and second generation hybrids produced between the 1950s and 1970s (Schmidt 1964, 1977). The application of SSR markers by Bisognin et al. (2009) confirmed these data and revealed three classes of recombinants: hybrids I and II as well as a rearrangement of the tetraploid genotype by autopollination. These recombinants are characterized by a varying number of M. sieboldii haplotypes which ranges from 0 to 2. Thus, a certain number of H II and autopollinated offspring has entirely lost the M. sieboldii genome. Although the SSR analyses allowed no precise assignment of allelic configuration, the data indicated a free pairing of the chromosomes in the recombinant offspring (Bisognin et al. 2009). It can therefore be assumed that the breeding progeny received different parts of the tetraploid genome of M. sieboldii. This is of great importance for the interpretation of the data regarding the inheritance of AP resistance. However, the relatively low number of recombinant offspring due to apomixis and their varying degree of ploidy limited a deeper analysis of the inheritance of the resistance.
From the first screening step at nursery scale it is evident that the resistance of mother-like and hybrid I progeny of most crosses is distinctly higher than that of the M9 controls whereas comparable levels of resistance are rare in progeny class hybrid II. This may be explained by the fact that hybrids I contain two haplotypes of the M. sieboldii genome whereas hybrids II may have only one or even no haplotype of M. sieboldii. This indicates that AP resistance is a quantitative trait. Furthermore, different resistance levels were also found among second and third generation hybrids which possess in addition to two haplotypes of the tetraploid M. sieboldii genome one or two haplotypes of the susceptible M. x domestica genome. One important exception is the parental genotype 4608 which is a hybrid with M. purpurea ‘Eleyi’ whose AP susceptibility is unknown but whose H I progeny showed the highest resistance regardless of the inoculum used.
The data of mother-like progeny confirm previous rankings of resistance levels for parental M. sieboldii hybrids (Seemüller et al. 2008) where 4608, 4551 and D2212 were found to be highly resistant. These ML genotypes and also their H I progeny showed in our study a higher resistance than the tetraploid M. sieboldii ML genotype as well as some ML genotypes of third generation parentals, e.g. BB and CC. This indicates that AP resistance may be linked to specific M. sieboldii alleles, combination of alleles or recombination events. This question can only be answered when molecular markers for the resistance trait or resistance genes are identified. However, the variability in disease response in combination with the different ploidy levels of the material hampered up to now the development of molecular markers for AP resistance (Moser et al. 2011). Thus, classical resistance screening in the field is still the only way to select suitable resistant genotypes.
Extending the screening of six progenies by growing for six more years under orchard conditions confirmed the high level of resistance of the selected trees by showing significantly lower CDI values than trees on M9. In most cases the average value of M9 trees was three or more times higher than the means of most breeding products. These data confirm previous results (Bisognin et al. 2008; Seemüller et al. 2008) that AP resistance is stable after experimental inoculation as well as under natural infection condition.
Resistance is usually defined by no symptom appearance and low titer of the pathogen (Cooper and Jones 1983). This was confirmed for most of the selected genotypes which had after eight years of observation low CDI values and low phytoplasma concentrations. Both features are confirming the results obtained in previous work (Bisognin et al. 2008) where similar low phytoplasma concentrations were found for parental genotypes D2212, 4608 and 4551. As shown by progeny B, the phytoplasma titer in resistant plants can be reduced below the detection level or to the elimination of the pathogen.
Our results confirm previous findings that M. sieboldii genotypes may respond highly sensitive to latent apple virus infection (Schmidt 1972). This aspect was studied recently in more detail using in vitro plants (Liebenberg et al. 2013). This work showed that ASGV and ASPV are causing hypersensitive reactions in specific M. sieboldii hybrids like 4551. The high virus susceptibility of the progeny of 4551 has been confirmed in parallel work with micropropagated ex vitro plants (Ciccotti et al. 2011). On the other hand, these studies and our screening data confirmed that 4608 and its progeny are virus-tolerant exhibiting no hypersensitive reactions to virus inoculation. This has also been reported for genotype 4608 by Schmidt (1972) and Seemüller et al. (2008).
Our data indicate that the M. sieboldii-based AP resistance works efficiently. Of similar importance is the effect of the rootstocks on productivity and vigor. Thus, the objective of the breeding program started in 2001 was to introduce these agronomic traits into AP-resistant genotypes by crossing them with rootstock M9. It was expected that recombinant offspring with a variable contribution of the M9 genome will offer a good basis for the selection of the best performing resistant rootstock genotype (Bisognin et al. 2009). However, our results demonstrate that sufficient AP resistance is only maintained in H I offspring that have two M. sieboldii haplotypes. Therefore, mainly these genotypes were examined further for their pomological traits. This may explain why the average data of the progeny examined are in general significantly inferior to M9 as far as yield potential and dwarfism are concerned. However, analysis of the data of individual trees revealed that mainly in progeny B selections occur with pomological traits comparable with M9. Therefore, the nine selections of progeny B described in the result section are under further evaluation in field trials at different sites. Other genotypes are under examination for purposes where more vigorous rootstocks are needed as, for example, in processing.
Another criterion of selection is the ability for multiplication by routine methods. However, attempts to propagate these genotypes by standard methods were not successful. By contrast, micropropagation as established by Ciccotti et al. (2008) has been experienced to be the most efficient way for propagating these M. sieboldii hybrids.
In conclusion, our results are one of the rare examples of successful breeding for genetic resistance to phytoplasma diseases (Seemüller and Harries 2010). However, and despite considerable efforts, the basis of this resistance is not well understood and further fundamental research is needed to elucidate the mechanism of the resistance. Our results have provided the first selected AP-resistant genotypes for further pomological evaluation. In addition, the data indicate which progeny can be used for further breeding: H I offspring of progeny B exhibits the highest resistance levels, the best pomological traits, is virus-tolerant and is tetraploid which favors a high amount of recombinant offspring.
References
Ahrens, U., & Seemüller, E. (1994). Detection of mycoplasmalike organisms in declining oaks by polymerase chain reaction. European Journal of Forest Pathology, 24, 55–63.
Baric, S., Berger, J., Cainelli, C., Kerschbamer, C., Letschka, T., & Dalla Via, J. (2011). Seasonal colonisation of apple trees by ‘Candidatus Phytoplasma mali’ revealed by a new quantitative TaqMan real-time PCR approach. European Journal of Plant Pathology, 129, 455–467.
Batjer, L. P., & Schneider, H. (1960). Relation of pear decline to rootstocks and sieve-tube necrosis. Proceedings of the American Society of Horticultural Science, 76, 85–97.
Bisognin, C., Schneider, B., Salm, H., Grando, M. S., Jarausch, W., Moll, E., & Seemüller, E. (2008). Apple proliferation resistance in apomictic rootstocks and its relationship to phytoplasma concentration and simple sequence repeat genotypes. Phytopathology, 98, 153–158.
Bisognin, C., Seemüller, E., Citterio, S., Velasco, R., Grando, M. S., & Jarausch, W. (2009). Use of SSR markers to assess sexual vs. apomictic origin and ploidy level of breeding progeny derived from crosses of apple proliferation-resistant Malus sieboldii and its hybrids with Malus x domestica cultivars. Plant Breeding, 128, 507–513.
Ciccotti, A. M., Bianchedi, P. L., Bragagna, P., Deromedi, M., Filippi, M., Forno, F., & Mattedi, L. (2007). Transmission of ‘Candidatus Phytoplasma mali’ by root bridges under natural and experimental conditions. Bulletin of Insectology, 60, 387–388.
Ciccotti, A. M., Bisognin, C., Battocletti, I., Salvadori, A., Herdemertens, M., & Jarausch, W. (2008). Micropropagation of apple proliferation-resistant apomictic Malus sieboldii genotypes. Agronomy Research, 6, 445–458.
Ciccotti, A. M., Bisognin, C., Battocletti, I., Deromedi, M., Bragagna, P., & Filippi, M. (2011). Response of apple proliferation-resistant Malus sieboldii hybrids to multiple infections with latent apple viruses. Bulletin of Insectology, 64(Suppl), S273–S274.
Cooper, J. I., & Jones, A. T. (1983). Responses of plants to viruses: proposals for the use of terms. Phytopathology, 73(Suppl. 2), 127–128.
Dosba, F., Lansac, M., Mazy, K., Garnier, M., & Eyquard, J. P. (1991). Incidence of different diseases associated with mycoplasma-like organisms in different species of Prunus. Acta Horticulturae, 283, 311–320.
Frisinghelli, C., Delaiti, L., Grando, M. S., Forti, D., & Vindimian, M. E. (2000). Cacopsylla costalis (Flor 1861), as a vector of apple proliferation in Trentino. Journal of Phytopathology, 148, 425–431.
Jarausch, W., Eyquard, J. P., Lansac, M., Mohns, M., & Dosba, F. (2000). Susceptibility and tolerance of new French Prunus domestica cultivars to European stone fruit yellows phytoplasmas. Journal of Phytopathology, 148(148), 489–493.
Kartte, S., & Seemüller, E. (1988). Variable response within the genus Malus to the apple proliferation disease. Journal of Plant Disease and Plant Protection, 95, 25–34.
Kartte, S., & Seemüller, E. (1991). Susceptibility of grafted Malus taxa and hybrids to apple proliferation disease. Journal of Phytopathology, 131, 137–148.
Kison, H., & Seemüller, E. (2001). Differences in strain virulence of the European stone fruit yellows phytoplasma and susceptibility of stone fruit trees on various rootstocks to this pathogen. Journal of Phytopathology, 149, 533–541.
Lenth, R. V. (2016). Least-squares means: the R package lsmeans. Journal of Statistical Software, 69, 1–33.
Liebenberg, A., Kappis, A., Barth, J., Weiter, M., Herdemertens, M., Wetzel, T., & Jarausch, W. (2013). Use of micropropagated Malus to study latent apple viruses. Petria, 22, 393–398.
Morvan, G. (1977). Apricot chlorotic leaf roll. EPPO Bulletin, 7, 37–55.
Moser, M., Musetti, R., Velasco, R., & Jarausch, W. (2011). Gene expression analysis and cytochemical investigations in ‘Candidatus Phytoplasma mali’-resistant and -susceptible Malus genotypes grown in vitro. Bulletin of Insectology, 64, S161–S162.
Oldén, E. J. (1953). Sexual and apomictic seed formation in M. sieboldii Rehd. Botaniska Notisser, 1, 105–128.
R Core Team. (2016). R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing URL https://www.R-project.org.
Rott, M. E., & Jelkmann, W. (2001). Characterization and detection of serveral filamentous viruses of cherry: adaptation of an alternative cloning method (DOP-PCR), and modification of an RNA extraction protocol. European Journal of Plant Pathology, 107, 411–420.
Schaper, U., & Seemüller, E. (1982). Condition of the phloem and the persistence of mycoplasmalike organisms associated with apple proliferation and pear decline. Phytopathology, 72, 736–742.
Schaper, U., & Seemüller, E. (1984). Recolonization of the stem of apple proliferation and pear decline-diseased trees by the causal organisms in spring. Journal of Plant Disease and Plant Protection, 91, 608–613.
Schmidt, H. (1964). Beiträge zur Züchtung apomiktischer Apfelunterlagen. I. Zytogenetische und embryogische untersuchungen. Zeitschrift für Pflanzenzüchtung, 52, 27–102.
Schmidt, H. (1972). Reaction of 25 apomictic apple rootstock selections to inoculation with mixtures of ‘latent’ viruses. Journal of Horticultural Science, 47, 151–157.
Schmidt, H. (1977). Contributions on the breeding of apomictic apple stocks. 4. On the inheritance of apomixis. Zeitschrift für Pflanzenzüchtung, 78, 3–12.
Seemüller, E., & Harries, H. (2010). Plant resistance. In P. G. Weintraub & P. Jones (Eds.), Phytoplasmas: genomes, plant hosts and vectors (pp. 147–169). Wallingford: CABI Publishing.
Seemüller, E., & Schneider, B. (2007). Differences in virulence and genomic features of strains of ‘Candidatus Phytoplasma mali’, the apple proliferation agent. Phytopathology, 97, 964–970.
Seemüller, E., Schaper, U., & Zimbelmann, F. (1984). Seasonal variation in the colonization patterns of mycoplasmalike organisms associated with apple proliferation and pear decline. Journal of Plant Disease and Plant Protection, 91, 371–382.
Seemüller, E., Kartte, S., & Kunze, L. (1992). Resistance in established and experimental apple rootstocks to apple proliferation disease. Acta Horticulturae, 309, 245–251.
Seemüller, E., Moll, E., & Schneider, B. (2008). Apple proliferation resistance of Malus sieboldii-based rootstocks in comparison to rootstocks derived from other Malus species. European Journal of Plant Pathology, 121, 109–119.
Seemüller, E., Moll, E., & Schneider, B. (2009). Pear decline resistance in progenies of Pyrus taxa used as rootstocks. European Journal of Plant Pathology, 123, 217–223.
Tedeschi, R., & Alma, A. (2004). Transmission of apple proliferation phytoplasma by Cacopsylla melanoneura (Homoptera: Psyllidae). Journal of Economic Entomology, 97, 8–13.
Westwood, M., & Lombard, P. B. (1982). Rootstocks for pear. Proceedings of the Oregon Horticultural Society, 73, 64–79.
Acknowledgments
We are indebted to late Hanna Schmidt for providing most of the apomictic genotypes used in this project and her engaged support of the breeding work. We thank Stella Grando and Claudia Bisognin (Fondazione E. Mach, San Michele, Italy) for providing unpublished SSR data. We also thank Felix Hergenhahn for his excellence in growing and inoculating the planting material and Constance Berwarth for technical assistance.
Funding
This study was in part supported by the Province of Trentino, Italy (Project Scopazzi del Melo – Apple Proliferation, SMAP). The major part was funded by the institutions of the authors themselves.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manuscript has not been submitted to another journal for simultaneous consideration.
The manuscript has not been published previously. However, several extended abstracts on the breeding approach have been published between 2007 and 2010. These abstracts do not contain any concrete data. Thus, the present manuscript contains the entire data which are true and accurate to the knowledge of the authors.
A single study is not split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time.
Any work (data, text, or theories) of others besides the authors has been properly acknowledged.
All authors (including their responsible authorities) listed in the title page agreed to be named as authors on this manuscript.
Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
Authors ensure the correct author group, corresponding author, and order of authors at submission.
Authors are aware that changes of authorship or in the order of authors are not accepted after acceptance of a manuscript.
Authors are aware that upon request they should be prepared to send relevant documentation or data in order to verify the validity of the results.
Authors are aware of the COPE guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Seemüller, E., Gallinger, J., Jelkmann, W. et al. Inheritance of apple proliferation resistance by parental lines of apomictic Malus sieboldii as donor of resistance in rootstock breeding. Eur J Plant Pathol 151, 767–779 (2018). https://doi.org/10.1007/s10658-017-1412-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1412-5