Abstract
In three trials carried out over a period of 24 years, open-pollinated seedlings of Malus sieboldii and M. sargentii and 22 apomictic rootstock selections with either M. sieboldii, M. sargentii or M. hupehensis in their parentage were examined for apple proliferation (AP) resistance in comparison to clonal M. x domestica-based rootstocks M 9, M 11, M 13, stocks of the B (Budagovski) and the Polish P series and M. robusta seedlings. Following experimental inoculation or natural infection the Golden Delicious-grafted trees on most of the M. sieboldii-derived progenies showed a high level of AP resistance expressed by low cumulative disease indices, a high percentage of non or little affected trees, low incidence of the small fruit symptom and non or little effect on vigour. Trees on M 9 and M 11, B 118 and M. robusta seedlings were moderately susceptible while trees on progenies with M. sargentii and M. hupehensis parentage, rootstocks of the P series, B 9, B 490 and M 13 proved highly susceptible. The screening also showed that rootstocks with M. sieboldii and M. sargentii parentage are often highly susceptible to latent apple viruses. Trees on most of the M. sieboldii-based progenies were more vigorous than trees on standard stock M 9, whereas the vigour of some progenies from selections with M. sargentii parentage was in the range of M 9 or even lower. Productivity was often correlated with the vigour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytoplasmas are wall-less bacteria of the class Mollicutes that cause diseases in more than a thousand plant species (Seemüller et al. 2002). Many of these diseases are of great economic importance. In Europe, one of the most damaging phytoplasmal diseases is apple proliferation (AP). This disease is present in several major fruit growing areas of western and central Europe and is caused by ‘Candidatus Phytoplasma mali’ (Seemüller and Schneider 2004). The pathogen induces a range of symptoms that are either specific such as witches-brooms, enlarged stipules and rosettes, or non-specific such as foliar reddening, yellowing and growth suppression. Economically most important is the deleterious effect of the disease on size, colour and taste of the fruits, which often makes the crop unmarketable. The AP pathogen is mainly spread by two psyllids, Cacopsylla picta and C. melanoneura (Frisinghelli et al. 2000; Tedeschi and Alma 2004). The leafhopper Fieberiella florii is also reported to vector the pathogen (Krczal et al. 1988; Tedeschi and Alma 2006). Furthermore, infection via natural root grafts has been demonstrated (Ciccotti et al. 2005).
AP is difficult to control. Phytosanitary measures such as the use of healthy planting material and uprooting of diseased trees are often unsatisfactory, because infections by insect vectors are difficult to prevent. Currently, spraying against the vectors is the most promising method to reduce infection. However, sprays cannot always be applied, for example during flowering, so that insecticide treatment is often not fully efficient. The most promising approach to control AP would be the use of resistant plants. Previous work has shown that ‘Ca. P. mali’ is eliminated in the aerial parts during winter due to degeneration of phloem sieve tubes on which the pathogen depends. Overwintering occurs in the roots where intact sieve tubes are present throughout the year. From the roots the stem may be recolonized in spring when new phloem is being formed (Schaper and Seemüller 1982, 1984b; Seemüller et al. 1984). This fluctuation in the colonization pattern has led to the presumption that growing scion cultivars on resistant rootstocks can prevent the disease or reduce its impact.
However, extensive studies with many established and experimental rootstocks, which were mainly based on Malus x domestica, have shown that there is no satisfactory resistance in this group. They frequently develop symptoms, remain permanently infected and show, as estimated by DAPI (4′-6-diamidino-2-phenylindole) fluorescence staining and quantitative real-time PCR, high phytoplasma titers (Bisognin et al. 2008; Seemüller et al. 1992). Screening of a large number of wild and ornamental Malus taxa revealed that they often are more susceptible to infection than M. x domestica genotypes. Many of them showed a high mortality rate. Resistance was observed in some experimental apomictic rootstock selections derived from crosses between the apomictic species M. sieboldii and genotypes of the non-apomictic species M. x domestica or M. purpurea. Resistant plants either never developed symptoms or temporarily only mild symptoms. The pathogen was difficult to detect in these genotypes by fluorescence microscopy, indicating a low phytoplasma titer (Kartte and Seemüller 1991; Seemüller et al. 1992). The low phytoplasma concentration in apomictic rootstocks was recently confirmed in a real-time PCR study (Bisognin et al. 2008).
Apomixis is a form of asexual reproduction characterized by the formation of seeds that are genetically identical to the usually female parent. It occurs in several plant families including the Rosaceae. In the genus Malus apomixis is, among others, present in the Asian tetraploid species M. sieboldii and M. sargentii and in the triploid species M. hupehensis. However, apomixis is not obligate in these species. Progenies from open pollination contain a variable percentage of hybrids from unreduced and reduced maternal gametes. For example, from a tetraploid apomictic female parent and a diploid non-apomictic male parent, pentaploid hybrids are obtained from unreduced female gametes by having an allele at each locus segregating from the male parent. Triploid recombinants result when both gametes are reduced (Bisognin et al. 2004; Schmidt 1988). Attempts to develop apomictic apple rootstocks were undertaken for easy propagation by seeds, virus-free plants, better anchorage and higher resistance to some fungal and bacterial diseases than dwarfing M. x domestica-derived stocks (Schmidt 1988).
In our previous work on AP resistance the appearance of foliar symptoms, mortality, phytoplasma titer and phytoplasma persistence in trees on apomictic rootstock selections were compared with trees on standard and experimental rootstocks (Kartte and Seemüller 1988, 1991; Seemüller et al. 1992). In extending this work, three field trials were established. In these not only apomictic and non-apomictic genotypes were included that were not tested previously but also other traits such as the incidence of undersized fruits, vigour and the effect of the disease on vigour, and productivity were evaluated. Furthermore, tests were carried out under both experimental inoculation and natural infection conditions.
Trials under natural infection conditions cover several aspects that cannot be investigated when inoculation occurs by grafting. These include exposure of the trees to a wider virulence spectrum of the pathogen. Also, the inoculum dose is much lower which may affect the response of the host. In addition, infections by latent apple viruses, to which some apomictic rootstocks respond very sensitively (Schmidt 1972, 1988), can be excluded under natural infection conditions because these viruses are not insect-transmissible (Fridlund 1989).
Materials and methods
Plant material
Open-pollinated seedlings from 22 apomictic rootstock selections and the apomictic parents M. sieboldii and M. sargentii were obtained from H. Schmidt, formerly Bundesanstalt für Züchtungsforschung an Kulturpflanzen, Ahrensburg, Germany. The apomictic seed parent selections, their parentage and ploidy level are listed in Table 1. Seedlings that phenotypically differed from their mother’s phenotype were rogued. Clonal rootstocks of the Budagovsky series B and Polish series P were obtained from T. Orlikowska, Research Institute of Pomology and Floriculture, Skierniewice, Poland. Clonal rootstock M 13 and seeds from M. robusta were provided by R. A. Smith, Horticulture International, East Malling, UK. Standard stocks M 9 and M 11 were propagated at Dossenheim.
Inoculation and disease rating
For experimental inoculation, seedlings or clonal rootstocks were grown in pots and inoculated in July or August of the first or second year by top-grafting scions from symptomatic current season’s shoots of cv. Golden Delicious. Only when enlarged stipules as specific symptom developed on the growing scion, was inoculation considered to be successful and the trees were used further. In the spring of the following year the plants were transferred to the nursery where they were kept for 2 years. Non-symptomatic and slightly to moderately affected plants were then transplanted to the field at standard spacing for further observation. Severely affected and declining plants were discarded. Plants to be used as healthy controls or to be evaluated under natural infection conditions were grown in the nursery and budded with healthy Golden Delicious in the same year the other tests plants were inoculated. For evaluation under natural infection conditions in trial II (see below), a subset of the plants of most rootstocks were budded with cv. Elstar. Two years after budding suitable plants were transferred to the field as described.
Over the entire observation period, foliar symptoms (reddening, yellowing, enlarged stipules, witches-brooms, rosettes), terminal growth and fruit size of the trees were recorded annually in the late summer/early fall using a rating system from 0 (no symptoms) to 3 (severe symptoms). Symptom rating categories were: slight reddening or mild yellowing = 0.5; enlarged stipules, premature leaf drop or severe reddening or yellowing = 1; reduced vigour or rosetting = 2; witches-brooms, undersized fruits, or severe stunting = 3. Mortality was rated as a 10. Incidence of the small fruit symptom was also recorded separately. At the end of the observation period the annual rating values were added so that accumulated disease indices were obtained. Yield was either estimated using a rating system from 0 to 3 or was determined by weighing the fruits. Trunk diameters were measured 40 cm above ground at the end of the observation period. For statistical analysis the differences between means were tested for significance using the Tukey-Kramer test with a significance level of P = 0.05.
Trials
Three trials were carried out. In trial I the plants were graft-inoculated between 1982 and 1985 and were observed until the fall of 1999. Trial II was carried out under natural infection conditions. Nursery trees were transplanted to the field in spring of 1993 where they were observed until the fall of 2005. In trial III evaluation was performed under both experimental inoculation and natural infection conditions. Inoculated and non-inoculated trees were transplanted to the field in the spring of 1997 where they were observed until the fall of 2006. In trials I and III, for each inoculated rootstock–scion combination 4 to 6 non-inoculated trees were planted as healthy controls. Maintenance of the trials including plant protection measures was similar to that of commercial orchards.
Nucleic acid extraction and PCR assays
To examine presence and persistence of the AP phytoplasma, root samples were collected at the end of the trials. Phloem was prepared as described (Ahrens and Seemüller 1994) and DNA was extracted from 0.2 to 0.8 g of phloem tissue according to Doyle and Doyle (1990). PCR amplification was performed using AP phytoplasma group-specific primers fO1/rO1 or fAT/rAS as previously described (Marcone et al. 1996; Smart et al. 1996).
For the detection of Apple chlorotic leaf spot virus (ACLSV), Apple stem grooving virus (ASGV) and Apple stem pitting virus (ASPV) total RNA was extracted from 300 mg phloem tissue according to a modified silica-capture method described by Rott and Jelkmann (2001). Quantity and quality of the total RNA was verified by agarose gel electrophoresis. Reverse transcription was performed using the RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas) according to the instructions of the manufacturer. An aliquot (usually 1 µl) of the transcribed DNA was subjected to PCR amplification using the following parameters: one cycle at 95°C for 5 min followed by 35 cycles consisting of 1 min at 95°C, 1 min at 62° (for ACLSV 60°C) and 1 min at 70°C. A final step at 70°C for 5 min was added. The primers for reverse transcription and PCR amplification were as described by Menzel et al. (2002).
Results
Verification of infection
Nearly all trees on M. x domestica-based clonal rootstocks of the B, M and P series and on M. robusta seedlings showed at least temporarily specific AP symptoms such as witches-brooms, enlarged stipules or rosetting. Thus, from this group, only roots of a representative number of trees on rootstocks B 118, M 9, M 11 and M 13 were examined for the presence of the AP pathogen using a PCR assay (Table 2). All experimentally inoculated trees of this group tested phytoplasma-positive, such as 14 of 18 trees on M 9 that were grown under natural infection conditions. Specific symptoms were not developed by trees grown on progenies of the apomictic selections with M. sieboldii, M. sargentii or M. hupehensis parentage. Therefore, between 6 and 18 trees of each rootstock that was tested in the three trials were examined for AP phytoplasma infection (Table 2). Following experimental inoculation 113 out of 121 trees tested phytoplasma-positive. Similarly, 177 of 187 trees grown under natural infection condition proved to be infected by ‘Ca. P. mali’. In the PCR of samples from apomictic selections usually faint DNA bands were obtained, indicating low phytoplasma concentrations in these stocks. The bands obtained from clonal stocks were much more pronounced (data not shown).
AP resistance
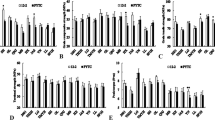
The rootstocks examined showed considerable differences in AP resistance (Tables 3, 4 and 5). Also, within most apomictic rootstocks and a few clonal rootstocks, a great variability in resistance was observed. On all these stocks both no or little, and severely affected trees occurred. However, there were great quantitative differences in the occurrence of the two severity classes among the rootstocks. On some apomictic selections and most clonal rootstocks, all or nearly all trees were severely affected. The cumulative disease indices, the percentage of no or slightly affected trees, the cumulative undersized fruit values, and the effect of the disease on vigour allowed the classification of the rootstocks examined into three resistance groups. Resistant rootstocks were characterized by low disease and undersized fruit values, at least 80% of no or little affected trees, and a negative effect on vigour of not more than 10%. The rootstocks with lower values were divided into a group of moderately affected trees and a group of severely affected trees. Both of these were not sufficiently resistant and will not be further defined here.
In trial I the by far highest level of resistance were shown by the trees on the progenies of M. sieboldii-derived selections 4608, D2212, 4551 and 4556 (Table 3). Considerably more affected were trees on clonal stock B 118 and the C0725 progeny (M. sieboldii and M. sargentii as parents). Most susceptible were trees on the rootstocks of the P series, on B 9, B 490 and M 13 and on seedlings of the M. hupehensis-based selection D1718.
Under the natural infection conditions of trial II with Golden Delicious as the scion cultivar, the differences in the level of resistance were smaller than in trial I, probably due to the fact that infections occurred when trees were older and thus less susceptible. However, the same tendency occurred (Table 4). Least affected by AP were trees on progenies of M. sieboldii and selections 4608, D2212, 4551 and D1131 with M. sieboldii parentage. The progeny of selections 3432 that has both M. sieboldii and M. sargentii as parents performed similarly well. Slightly more suffering from disease were trees on M. robusta seedlings, M 11 and M 9. However, trees on M 9 showed significantly higher incidence of the small fruit symptom than trees on the resistant M. sieboldii-based stocks. Most severely damaged were trees on progenies of M. sargentii and the M. sargentii-derived selections D1111 and D1828 as well as the selection 20186 with M. sieboldii parentage. Similarly poor values were shown by trees on the C0725 progeny.
In trial III, following experimental inoculation, trees on a group of progenies with M. sieboldii parentage consisting of selections 4637, 4551, Gi477/4, H0909 and C1907 showed a high level of resistance (Table 5). Trees on M. sieboldii-derived progenies H0801 were slightly more affected, being in the range of M. sargentii-derived selection C1812. Trees on other progenies with M. sargentii parentage such as C1825 and D1105, M. hupehensis-derived progeny D2009, and M. sieboldii-based selection H0901 were most severely damaged in this trial.
Of the non-inoculated trees in trial III, 4 of the 12 trees on M 9 stocks showed foliar reddening for the first time in 2002 (Table 5). Trees on seedlings of selections C1907, D2118 and H0801 started to show symptoms only in 2004. The disease values for trees on seedlings of C1907 and D2118 were similar to those of trees on M 9, whereas trees on H0801 seedlings were more affected. This corresponds with the higher susceptibility of H0801 than C1907 observed after experimental inoculation.
Sensitivity to latent apple viruses
Under natural infection conditions in trial II, the plants on most rootstocks budded with cv. Elstar developed poorly. Most severely affected were seedlings of M. sieboldii and M. sargentii and selections 4551, 20186, C1828 and C0725. On these stocks the plants developed so poorly that they died in the nursery or in the orchard or were severely stunted (Table 6). Less affected were the progenies of D2212, 4332 and M. robusta that also showed mortality and severe stunting but of which 20 to 26% of the trees developed well. Seedlings of 4608 were the only apomictic progeny on which the trees, like those on M 9 and M 11, did not show any virus-induced symptoms.
To investigate the reason of this severe problem, 10 affected and 10 unaffected Elstar trees grown on several rootstocks were examined for the presence of latent apple viruses. All of them were infected with ACLSV and most of them also with ASGV or ASPV or both. The same number of trees with scion cv. Golden Delicious on the same rootstocks was also examined. No virus infection was detected in these trees. These results indicate that the cv. Elstar scion was infected by latent apple viruses and that these viruses are responsible for the poor development of the Elstar trees.
Vigour and productivity
Among the apomictic rootstocks examined, trees on progenies of M. sieboldii and most apomictic rootstocks with M. sieboldii parentage were similar in vigour and were more vigorous than trees on M 9 (Tables 3, 4 and 5). However, the differences were not always statistically significant. Within the group of resistant rootstocks only the progeny of selection D1131 was sufficiently dwarfed for commercial apple growing. Slightly more dwarfed than the majority of resistant M. sieboldii-based stocks were trees on the two selections with M. hupehensis parentage. Distinctly less vigorous were trees on progenies with M. sargentii parentage. Several of these stocks can be classified in the vigour class of M 9 or they induced even lower vigour such as C1825 or D1105. Most vigorous were trees on M 11 and M. robusta seedlings.
Although the fruits of diseased trees were often smaller than normal, affected trees were often heavily-bearing. Thus, there were no significant differences in the relative yield between infected and non-infected trees based on cross-section of the trunk or the estimated fruit set. In productivity there is a clear correlation between parentage and vigour within the apomictic rootstocks (Tables 3, 4 and 5). Among the apomictic rootstocks, trees on the more dwarfing stocks with M. sargentii parentage showed productivity similar to M 9 and P 22. The yield of this group was usually significantly higher than that of trees on the more vigorous M. sieboldii-derived stocks. Productivity of trees on the two rootstocks with M. hupehensis parentage was in the same range as that of trees on M. sieboldii stocks. Poorest cropping were trees on M 11, M 13, P 18 and B 490 rootstocks.
Discussion
In the three trials carried out, different sets of apomictic seedling rootstocks were examined for AP resistance in comparison to various non-apomictic clonal rootstocks. The overall results obtained were similar. They showed that, depending on the stock, the resistance of the grafted trees differed greatly. A high level of resistance was only induced by progenies of M. sieboldii and most selections with M. sieboldii in their parentage. They include selections 3432, 4551, 4556, 4608, 4637, C1907, D1131, D2212, Gi477/4 and H0909. Thus, M. sieboldii appears to be the only resistance source known at present. More affected were trees on progenies of apomictic selections H0801, C1812, C1825 and clonal stocks M 9, M 11 and B 118 and M. robusta seedlings. High susceptibility was shown by trees on progenies of M. sargentii, most selections with M. sargentii parentage, M. hupehensis-derived selections and clonal stocks of the P series, B 9, B 490 and M 13. In all resistance criteria the group of resistant M. sieboldii-derived stocks performed better than the clonal stocks although the differences were not always statistically significant. Of particular importance is that the incidence of undersized fruits on trees on resistant apomicts is significantly lower than of trees on M 9, the major commercial rootstock in Europe. The results obtained in this study largely confirm the data of previous work in which, however, mainly other criteria including foliar symptoms, phytoplasma persistence and phytoplasma concentration were examined (Bisognin et al. 2008; Kartte and Seemüller 1988, 1991; Seemüller et al. 1992).
In most rootstocks considerable differences in disease severity were observed. In the case of clonal stocks this variation may be explained by differences in virulence of the infecting phytoplasma. Recent work by Seemüller and Schneider (2007) has shown that phytoplasma strains differ strongly in this respect. Of the 24 sources examined, about one third of the strains were either avirulent to weakly virulent, moderately virulent, or severely virulent, respectively. Differences in strain virulence are certainly also involved in the variability of trees on apomictic rootstocks. However, in apomictic rootstocks several combinations of the parental genetic contribution were deduced from profiles of co-dominant microsatellite (SSR) markers (Bisognin et al. 2004, 2008). These genetic differences appeared to also account for variation. It could be shown that progenies of the triploid selections 4608 and 4551 trees on mother-like plants are more severely affected than trees on hybrids having one allele at each locus segregating from the male parent. This phenomenon may be due to the unknown father’s genetic contribution. On the other hand, hybrids of selection D2212 that resulted from two reduced gametes showed a higher susceptibility than trees on mother-like stocks, indicating segregation of the resistance trait of a susceptible male parent (Bisognin et al. 2008). The variation within progenies from apomict parents shows that such progenies even from resistant genotypes are unsuitable for being directly used as rootstocks. Rather, suitable genotypes have to be carefully selected and then vegetatively propagated for use in commercial apple growing.
In the screening of apomictic rootstocks the sensitivity of some to latent apple viruses has to be taken into account (Schmidt 1988). Such viruses do not cause recognizable symptoms in most commercial apple cultivars if the scions are grown on tolerant rootstocks. According to Schmidt (1972, 1988) some of the seed parents used in our work are sensitive to latent apple viruses. For this reason we attempted to use virus-free scionwood and inoculum. The results show that the Golden Delicious scion and inoculum we used were free from viruses whereas the Elstar scion source was contaminated. This contamination severely affected the trees on most apomictic rootstocks used in trial II. The results obtained confirm the findings of Schmidt (1972, 1988) about the high sensitivity of M. sargentii, M. sieboldii and selections 4551 and C0725, and the tolerance of selection 4608. Other sensitive selections used in our work include 4637 and D1111 whereas selections 4556 and genotypes with M. hupehensis parentage are reported to be tolerant (Schmidt 1972, 1988). However, these selections were not exposed to virus infection in our screening.
A recent study on possible factors involved in AP resistance of apple rootstocks revealed that the phytoplasma titer in established standard stocks based on M. x domestica is 100 to 5,000 times higher than in apomictic rootstocks. However, there were no significant differences in the phytoplasma concentrations between resistant apomicts with M. sieboldii parentage and susceptible genotypes with M. sargentii parentage (Bisognin et al. 2008). From these data it appears that host suitability per se as expressed in phytoplasma titer is obviously not the only defining factor for resistance. However, it could be shown that only a minority of trees on resistant M. sieboldii-derived stocks were colonized in the top section, in contrast to trees on M 11 and some selections with M. sargentii parentage where most or all trees were infected in the scion. Also, the phytoplasma titer in the top-infected scion of trees on resistant rootstocks was much lower than in top-infected trees on M 9 and M 11 roots (Bisognin et al. 2008). The low infection rate and the low titer in the stem of trees on resistant apomictic stocks seem to result from low phytoplasma concentrations in the roots. The low starting concentration in and poor host suitability of apomictic rootstocks may have a negative effect on the spread of the pathogen from the roots into the scion during recolonization of the stem in spring. Thus, the low titer in the roots is likely to contribute to the resistance of M. sieboldii-derived stocks. It is well established that severe symptoms such as witches-brooms and undersized fruits only develop when the phytoplasma concentration in the stem is high (Schaper and Seemüller 1984a).
Our results show that trees on most resistant rootstocks with M. sieboldii in their parentage are larger and have lower yields than trees on M 9. Trees on these apomicts were generally similar to M 7 and M 4 in size and also seem to be similar to these rootstocks in their yield potential. Our results largely agree with the findings of Schmidt (1977, 1988) and Ferree (1998) who examined several of the selections included in our screening. These drawbacks of the M. sieboldii-based AP-resistant apomicts will make it difficult to introduce them into European apple growing that is dominated by M 9. For this reason a breeding programme has been initiated with the aim to reduce vigour and improve yield of trees on AP-resistant apomicts by crossing such selections with M 9 and other dwarfing stocks (Jarausch et al. 2005).
Abbreviations
- ACLSV:
-
Apple chlorotic leaf spot virus
- AP:
-
apple proliferation
- ASGV:
-
Apple stem grooving virus
- ASPV:
-
Apple stem pitting virus
- DAPI:
-
4′-6-diamidino-2-phenylindole
- PCR:
-
polymerase chain reaction
References
Ahrens, U., & Seemüller, E. (1994). Detection of mycoplasmalike organisms in declining oaks by polymerase chain reaction. European Journal of Forest Pathology, 24, 55–63.
Bisognin, C., Jarausch, W., Seemüller, E., & Grando, M. S. (2004). Pedigree analysis through SSR markers of Malus progenies obtained by crossing with apomictic species. Italus Hortus, 10(Suppl. 4), 242–245 (in Italian).
Bisognin, C., Schneider, B., Salm, H., Grando, M. S., Jarausch, W., Moll, E., et al. (2008). Apple proliferation resistance in apomictic rootstocks and its relationship to phytoplasma concentration and SSR genotypes. Phytopathology, in press.
Ciccotti, A. M., Bianchedi, P. L., Bragagna, P., Deromedi, M., Filippi, M., Forno, F., et al. (2005). Trasmissione di apple proliferation tramite anastomosi radicali. Petria, 15, 169–171.
Doyle, J. J., & Doyle, J. L. (1990). Isolation of plant DNA from fresh tissue. Focus (BRL), 12, 13–15.
Ferree, D. C. (1998). Performance of eight apomictic selections as apple rootstocks. HortScience, 33, 641–643.
Fridlund, P. R. (1989). Virus and viruslike diseases of pome fruits and simulating noninfectious disorders (330 pp). Pullman (WA):: Washington State University.
Frisinghelli, C., Delaiti, L., Grando, M. S., Forti, D., & Vindimian, M. E. (2000). Cacopsylla costalis (Flor 1861), as a vector of apple proliferation in Trentino. Journal of Phytopathology, 148, 425–431.
Jarausch, W., Bisognin, C., Peccerella, T., & Seemüller, E. (2005). Control of apple proliferation through the use of resistant rootstocks. Petria, 15, 129–131.
Kartte, S., & Seemüller, E. (1988). Variable response within the genus Malus to the apple proliferation disease. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 95, 25–34.
Kartte, S., & Seemüller, E. (1991). Susceptibility of grafted Malus taxa and hybrids to apple proliferation disease. Journal of Phytopathology, 131, 137–148.
Krczal, G., Krczal, H., & Kunze, L. (1988). Fieberiella florii (Stal), a vector of apple proliferation agent. Acta Horticulturae, 235, 99–106.
Marcone, C., Ragozzino, A., & Seemüller, E. (1996). Association of phytoplasmas with the decline of European hazel in southern Italy. Plant Pathology, 45, 857–863.
Menzel, W., Jelkmann, W., & Maiss, E. (2002). Detection of four apple viruses by multiplex RT-PCR assays with coamplification of plant mRNA as internal control. Journal of Virological Methods, 99, 81–92.
Rott, M. E., & Jelkmann, W. (2001). Characterization and detection of several filamentous viruses of cherry: Adaptation of an alternative cloning method (DOP-PCR), and modification of an RNA extraction protocol. European Journal of Plant Pathology, 107, 411–420.
Schaper, U., & Seemüller, E. (1982). Condition of the phloem and the persistence of mycoplasmalike organisms associated with apple proliferation and pear decline. Phytopathology, 72, 736–742.
Schaper, U., & Seemüller, E. (1984a). Effect of the colonization behavior of the apple proliferation and pear decline causal agents on their detectability by fluorescence microscopy. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes, 36, 21–25 (in German).
Schaper, U., & Seemüller, E. (1984b). Recolonization of the stem of apple proliferation and pear decline-diseased trees by the causal organisms in spring. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 91, 608–613.
Schmidt, H. (1972). The effect of ‘latent’ virus infections on the yield of maiden trees on 20 apomictic apple seedling rootstocks. Journal of Horticultural Science, 47, 159–163.
Schmidt, H. (1977). Unterlagenzüchtung am Beispiel apomiktischer Apfelunterlagen. Erwerbsobstbau, 19, 114–117.
Schmidt, H. (1988). Criteria and procedures for evaluating apomictic rootstocks for apple. HortScience, 23, 104–107.
Seemüller, E., Garnier, M., & Schneider, B. (2002). Mycoplasmas of plants and insects. In S. Razin, & R. Herrmann (Eds.) Molecular biology and pathology of mycoplasmas (pp. 91–116). London: Kluwer.
Seemüller, E., Kartte, S., & Kunze, L. (1992). Resistance in established and experimental apple rootstocks to apple proliferation disease. Acta Horticulturae, 309, 245–251.
Seemüller, E., Schaper, U., & Zimbelmann, F. (1984). Seasonal variation in the colonization patterns of mycoplasmalike organisms associated with apple proliferation and pear decline. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 91, 371–382.
Seemüller, E., & Schneider, B. (2004). ‘Candidatus Phytoplasma mali’, ‘Candidatus Phytoplasma pyri’ and ‘Candidatus Phytoplasma prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. International Journal of Systematic and Evolutionary Microbiology, 54, 1217–1226.
Seemüller, E., & Schneider, B. (2007). Differences in virulence and genomic features of strains of Candidatus Phytoplasma mali, the apple proliferation agent. Phytopathology, 97, 964–970.
Smart, C. D., Schneider, B., Blomquist, C. L., Guerra, L. J., Harrison, N. A., Ahrens, U., et al. (1996). Phytoplasma-specific PCR primers based on sequences of the 16S–23S rRNA spacer region. Applied and Environmental Microbiology, 62, 2988–2993.
Tedeschi, R., & Alma, A. (2004). Transmission of apple proliferation phytoplasma by Cacopsylla melanoneura (Homoptera: Psyllidae). Journal of Economic Entomology, 97, 8–13.
Tedeschi, R., & Alma, A. (2006). Fieberiella florii (Homoptera: Auchenorrhyncha) as a vector of “Candidatus Phytoplasma mali”. Plant Disease, 90, 284–290.
Acknowledgements
We thank Hanna Schmidt for providing apomictic seedlings and information about their parentage and ploidy level. We also thank Teresa Orlikowska, and R. A. Smith for providing rootstocks or seeds. We are grateful to Wolfgang Jarausch for critical reading of the manuscript and helpful suggestions. The assistance of Constanze Berwarth in data processing and PCR analysis is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seemüller, E., Moll, E. & Schneider, B. Apple proliferation resistance of Malus sieboldii-based rootstocks in comparison to rootstocks derived from other Malus species. Eur J Plant Pathol 121, 109–119 (2008). https://doi.org/10.1007/s10658-007-9250-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-007-9250-5