Abstract
Chronological age alone is not a sufficient measure of the true physiological state of the body. The aims of the present study were to: (1) quantify biological age based on a physiological biomarker composite model; (2) and evaluate its association with death and age-related disease onset in the setting of an elderly population. Using structural equation modeling we computed biological age for 1699 individuals recruited from the first and second waves of the Rotterdam study. The algorithm included nine physiological parameters (c-reactive protein, creatinine, albumin, total cholesterol, cytomegalovirus optical density, urea nitrogen, alkaline phosphatase, forced expiratory volume and systolic blood pressure). We assessed the association between biological age, all-cause mortality, all-cause morbidity and specific age-related diseases over a median follow-up of 11 years. Biological age, compared to chronological age or the traditional biomarkers of age-related diseases, showed a stronger association with all-cause mortality (HR 1.15 vs. 1.13 and 1.10), all-cause morbidity (HR 1.06 vs. 1.05 and 1.03), stroke (HR 1.17 vs. 1.08 and 1.04), cancer (HR 1.07 vs. 1.04 and 1.02) and diabetes mellitus (HR 1.12 vs. 1.01 and 0.98). Individuals who were biologically younger exhibited a healthier life-style as reflected in their lower BMI (P < 0.001) and lower incidence of stroke (P < 0.001), cancer (P < 0.01) and diabetes mellitus (P = 0.02). Collectively, our findings suggest that biological age based on the biomarker composite model of nine physiological parameters is a useful construct to assess individuals 65 years and older at increased risk for specific age-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a complex gradual process that varies in its occurrence rate in humans [1]. Chronological age as a function of calendar years, does not reflect the underlying state of physiological breakdown and body degradation [2]. A more precise estimate of aging entails incorporation of physiological and other biological parameters to explain variations not captured by calendar years alone.
Over the past decades, aging research has focused on studying single diseases. This approach has been successful in increasing life expectancy but left many individuals living longer with disabilities and functional dependence [3]. The increasing challenges related to aging populations globally, created a need to start addressing biological aging as a general health indicator of morbidity and mortality [4].
The concept of biological age as a biomarker composite was first introduced in 1969 [5]. Methods for calculating biological age evolved over time, and Klemera and Doubal (KDM), were the first to define the relationship between chronological age and biological age by modeling biological age as a function of chronological age [6].
Biological age as a predictor of mortality has been assessed in previous studies [7]. However, the relationship between biological age and morbidity onset in terms of specific age-related diseases and the differences between individuals based on their biological age, remains to be unclear. The availability of continuous follow-up in the Rotterdam Study provides an opportunity to validate and assess the role of biological age in predicting morbidity and mortality.
The aims of the present study were to: (1) quantify biological age based on a physiological biomarker composite model; and (2) evaluate its association with death and age-related disease onset in the setting of an elderly population.
Methods
Study settings
The Rotterdam Study
This study included participants from the Rotterdam Study, a prospective population-based cohort study [8]. In 1990, residents aged 55 years and older residing in Ommoord, a district of Rotterdam, the Netherlands, were invited to participate in the study. Of 10,215 invited inhabitants, 7983 agreed to participate in the baseline examinations. In 2000, 3011 participants (of 4472 invitees) who had reached 55 years of age or moved into the study district since the start of the study were added to the cohort. In 2006, a further extension of the cohort was started in which 3932 participants, of 6057 invited, aged at least 45 years living in Ommoord were included. Follow-up examinations take place every 3–4 years. For the purpose of this study, 2000 individuals were randomly selected from Rotterdam Study-I and Rotterdam Study-II. The criteria for inclusion included the availability of informed consent and valid serum samples that were both available in the fourth visit in the Rotterdam Study (Fig. 1).
NHANES
The reference population included participants from the third National Health and Nutrition Examination Survey (NHANES III), a nationally representative, cross-sectional study conducted by the National Center for Health Statistics conducted between 1988 and 1994. Data collection was done through at-home interviews and examinations. Further details on study population and design are available through the Centers for Disease Control and Prevention [9].
Calculation of biological age
Selection of biomarkers
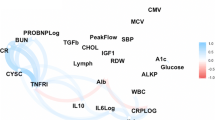
Nine biomarkers were selected based on their independent correlation with chronological age, use in previous studies and their availability in the Rotterdam Study data. The biomarkers represent six systems as follows: total cholesterol (metabolic function); systolic blood pressure (cardiac function); forced expiratory volume (lung function); serum creatinine and serum urea nitrogen (kidney function); serum alkaline phosphatase and serum albumin (liver function) and C-reactive protein, cytomegalovirus optical density (immune function and inflammation). Biomarkers were measured using serum.
Application of Klemera and Doubal method (KDM) through structural equation modeling
A key characteristic of KDM is the use of chronological age as a standard biomarker. Biological age is a latent intermediate between chronological age and the biomarkers, as opposed to the traditional approach of including chronological age as a dependent variable. Biomarkers are responses to biological age. KDM uses only regression functions of individual biomarkers that can be interpreted as functions of biological age as well as functions of chronological age. Biological age was calculated as the weighted mean of the nine physiological parameters. Two separate models were used for men and women (Supplementary Fig. 1).
Validation and calibration
Given biomarkers of whole blood samples (as HBA1c) were not available in our data, we compared the nine versus ten biomarkers algorithm in NHANES and the results showed similar results of hazard ratios and model performance based on Akaike Information Criterion (AIC) (Supplementary Table 1). Further, biological age calculations using weights of the Rotterdam study showed better model performance compared to NHANES, based on AIC and strength of association with the end-points (Supplementary Table 2).
Statistical analysis
Statistical analysis was done in two stages. First, the association between biological age and time from baseline to death, all-cause morbidity and specific age-related diseases, was assessed using Cox proportional hazard models. In this first stage, a model to be selected needed to meet two criteria: (1) a statistically significant association with the endpoint at alpha = 0.05; (2) a better model performance based on Akaike’s information criterion (AIC). All models were adjusted for gender. For each incident morbidity outcome, the corresponding number of individuals with prevalent disease were excluded from the model for this endpoint (e.g. prevalent stroke excluded from time to stroke analysis). Given the variation in follow-up in specific diseases, follow-up was truncated on January 1st 2012 in the Cox-models of all-cause morbidity and cause-specific morbidities to allow comparable follow-up duration for the different outcomes.
Receiver operating characteristic curve analysis
Area under the receiver operating characteristic curve (AUC) analysis was conducted to assess the sensitivity (true positive rate) and specificity (true false rate) of biological age, chronological age and selected traditional biomarkers of age-related disease (systolic blood pressure and total cholesterol) as predictors of death and disease. All AUC analyses were adjusted for gender. Further, systolic blood pressure and total cholesterol observations were adjusted for blood pressure and lipid lowering medication use, using censored regression.
Results
Population characteristics
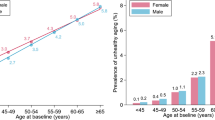
Our final sample included 1699 individuals, excluding individuals with missing data on biomarkers due to failed patches (Fig. 1). Median age was 70 years (IQR = 65–76), with the majority being females (57%), past smokers (56%) and free of morbidity at baseline (64%). The median follow-up duration was 11 years, during which the following events occurred: 586 (35%) deaths, 389 (23%) all-cause morbidity, 97 (5%) strokes, 138 (8%) diabetes mellitus, 176 (10%) dementia, 120 (7%) Alzheimer, 303 (18%) cancers, 112 (7%) coronary heart disease (Table 1).
Individual biomarkers and delta biological age
All the individual biomarkers were age-dependent. Further all biomarkers showed a positive correlation with age except forced expiratory volume and total cholesterol that were negatively correlated with chronological age (Supplementary Fig. 2).
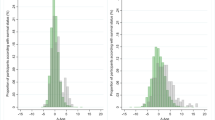
Chronological age showed a similar distribution (P = 0.92) between those who were biologically younger versus older based on delta biological age. Delta biological age was calculated as the difference between biological age and chronological age at baseline (Supplementary Fig. 3). Based on the difference between biological age and chronological age at baseline, individuals who were biologically younger exhibited a healthier life-style as reflected in their lower BMI and less smoking compared to those were biologically older. Further, biologically older individuals had significantly higher incidences of stroke (P < 0.001), cancer (P < 0.01) and diabetes mellitus (P = 0.02).
Association of biological age with death and disease
Compared to chronological age alone or combined with the individual biomarkers, in adjusted Cox proportional hazard models, biological age showed a stronger association with the following end-points (in order of strength of association and magnitude): diabetes mellitus (aHR = 1.12 vs. 1.01), stroke (aHR = 1.17 vs. 1.08), cancer (aHR = 1.07 vs. 1.04) and mortality (aHR = 1.15 vs. 1.13). In contrast, chronological age was a better predictor for dementia (1.13 vs. 1.00) and Alzheimer’s disease (1.15 vs. 0.99). Biological age and chronological age showed similar sensitivity in prediction of coronary heart disease and all-cause morbidity (Table 2).
Biological age as a predictor of death and disease
Diseases that showed association with biological age in the Cox proportional hazards model were included for prediction assessment, this included: mortality, all-cause morbidity, stroke, cancer, diabetes mellitus and coronary heart disease.
Biological age predictive ability exceeded that of chronological age and traditional biomarkers with varying degrees, in strength and magnitude of association as follows: stroke (AUC biological age vs. chronological age: 0.67 vs. 0.63), mortality (0.81 vs. 0.79), cancer (0.56 vs. 0.54) and morbidity (0.63 vs. 0.62). This however was not the case for diabetes mellitus, where systolic blood pressure was the strongest predictor compared to biological age, chronological age and total cholesterol (Supplementary Table 3).
Discussion
Identification of individuals at higher risk of morbidity and mortality before they enter a disease state is key to extend health span in the light of the escalating burden of aging populations worldwide [3]. Over a median follow-up of 11 years, biological age, calculated at baseline was able to predict, better than chronological age alone and traditional biomarkers, mortality, morbidity and onset of specific diseases such as stroke and cancer. In contrast, traditional biomarkers of age-related diseases such as systolic blood pressure remained a better predictor of diabetes mellitus compared to biological age and chronological age. These observations suggest that the biological age construct could be a useful measure to identify individuals at higher risk of specific diseases that are dependent on aging as reflected by physiological deterioration with increasing age.
A key barrier in biological age investigations is its reproducibility. We used validated common criteria of biological age to allow comparisons with other settings [10]. The nine biomarkers we used in the biological age calculations are known to be correlated with age and have been tested in other settings [7, 11, 12]. A key difference in our study compared to previous studies that adopted the same biological age composite biomarker model, is age distribution. As the mean age in our population is 71 years compared to the young population of the Dunedin Study [11] and mixed age in NHANES [7]. This variation might explain differences in the relationship between chronological age and the individual biomarkers, as total cholesterol, that is negatively correlated with chronological age in our sample (r = − 0.12) of elderly, also as documented in the literature [13].
Unlike studying single diseases, quantifying biological aging requires methods that take into consideration the underlying age complexities as reflected by the different interrelated body functions. Therefore, we opted to use the Klemera and Doubal method that has been tested for its predictive sensitivity compared to traditional modeling methods [6, 14,15,16,17,18,19,20,21,22].
Several measures have been proposed to quantify biological age, such as telomere length, DNA-methylation data and composites of clinical biomarkers [23,24,25,26]. These measures vary in the number of assays required (single versus multiple), design (cross-sectional versus longitudinal) and the biological level of implementation (tissues versus body systems) [26]. However, the impression that these methods measure the same features of aging is questioned [26]. The biological age algorithm of clinical biomarkers has the advantage of being based on continuously measured physiological parameters using standard quantitative techniques, thus less susceptible to variation related to the method of measurement [10].
In our study, the difference in biological age at baseline was reflected in the risk and age of disease onset and death during follow-up. Such finding aligns with the fact that the biomarker algorithm mirrors age-related biological changes of multiple body systems [27]. Biological age predicted, more accurately than chronological age alone, risk of all-cause morbidity and mortality. Biologically older individuals at baseline had higher incidence of stroke, diabetes mellitus, cancer, COPD and death. These observations suggest that the biological age algorithm could be a useful construct to predict death and disease onset in the setting of an elderly population.
We observed the strongest association with stroke, in particular. Biologically older individuals had almost three times higher risk of stroke compared to their biologically younger counterparts. Models with chronological age and single biomarkers including stroke-related factors (i.e. total cholesterol and systolic blood pressure) were less sensitive to predict stroke. Such observation further supports the notion that the KDM method allows for capturing aging-related complexities better than chronological age alone [19, 20, 22].
On the other hand, the set of biomarkers we used were not able to predict Alzheimer’s or Dementia. This comes as no surprise given the absence of neurological biomarkers in our algorithm other than chronological age. In the Dunedin study, the investigators reported an association between pace of aging and outcomes as cognitive decline in a homogenous sample of young adults [11]. The lack of longitudinal data on biomarkers restricted our measurement of biological age to baseline, however biological age was shown to agree with pace of aging in prediction of disease [11]. Whether adding other organ-specific biomarkers in our algorithm would translate to a better predictive ability for onset of specific disorders compared to chronological age alone, requires further investigation.
Those who were biologically older had higher BMI and were more often current smokers. This observation draws insights on possible explanations related to life style that could explain the variation in physiological deterioration between individuals.
Biological age as a baseline measurement could be useful to identify individuals at higher risk of disease and disability that are core elements to advance investigations on antiaging therapies [28,29,30]. This could be supported by the hypothesis that increasing age is closely linked to increased morbidity due to deterioration in multiple body systems [31, 32]. As such, studying the role of biological aging, as an indicator of disease, is key to unravel aging-related pathways of disease and disability.
Our study has several limitations. First, longitudinal biomarkers data was not available. Thus, we did not calculate pace of aging that could further confirm the rate of change in biological age. Second, biomarkers of whole blood samples (as HBA1c) were not available; however, the results are comparable as we validated nine versus ten biomarkers in NHANES. Third, selection bias owing to the included sample is possible. However, the included participants were randomly selected and sample size was determined a priori for measurement of the specified biomarkers. Lastly, the inclusion of factor loadings from a latent variable as biological age in the context of standard Cox-regression analyses may have influenced the overall predictive sensitivity of biological age.
Collectively, our results confirm the reproducibility of the Klemera and Doubal method and the value of the biological age algorithm in the elderly. This builds on the important observations of previous studies in samples of mixed-age and young individuals. Further work is needed to further develop the biological age construct and its application to identify and monitor individuals at higher risk of specific diseases at a later age, offering opportunities for primary prevention before onset of disease in healthy individuals.
References
Hayflick L. The future of ageing. Nature. 2000;408(6809):267–9.
Sebastiani P, Thyagarajan B, Sun F, et al. Biomarker signatures of aging. Aging Cell. 2017;16(2):329–38.
Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. https://doi.org/10.1001/jama.2013.13805.
Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol Ser A, Biol Sci Med Sci. 2014;69(Suppl 1):S1–3. https://doi.org/10.1093/gerona/glu041.
Comfort A. Test-battery to measure ageing-rate in man. Lancet. 1969;294(7635):1411–5.
Klemera P, Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127(3):240–8.
Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol Ser A: Biomed Sci Med Sci. 2012;68(6):667–74.
Ikram MA, Brusselle GGO, Murad SD, et al. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32:807–50.
Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National health and nutrition examination survey data. Hyattsville, MD: CDC. http://www.cdc.gov/nchs/nhanes/nh3data.htm. Accessed May 2018.
Levine ME. Response to Dr. Mitnitski’s and Dr. Rockwood’s letter to the editor: biological age revisited. J Gerontol Ser A: Biomed Sci Med Sci. 2013;69(3):297–8.
Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112(30):E4104–10. https://doi.org/10.1073/pnas.1506264112.
Yoo J, Kim Y, Cho ER, Jee SH. Biological age as a useful index to predict seventeen-year survival and mortality in Koreans. BMC Geriatr. 2017;17(1):7.
Kreisberg RA, Kasim S. Cholesterol metabolism and aging. Am J Med. 1987;82(1b):54–60.
Hollingsworth JW, Hashizume A, Jablon S. Correlations between tests of aging in Hiroshima subjects—an attempt to define “physiologic age”. Yale J Biol Med. 1965;38(1):11–26.
Takeda H, Inada H, Inoue M, Yoshikawa H, Abe H. Evaluation of biological age and physical age by multiple regression analysis. Med Inform = Medecine et informatique. 1982;7(3):221–7.
Kroll J, Saxtrup O. On the use of regression analysis for the estimation of human biological age. Biogerontology. 2000;1(4):363–8.
Bae CY, Kang YG, Kim S, et al. Development of models for predicting biological age (BA) with physical, biochemical, and hormonal parameters. Arch Gerontol Geriatr. 2008;47(2):253–65. https://doi.org/10.1016/j.archger.2007.08.009.
Hofecker G, Skalicky M, Kment A, Niedermuller H. Models of the biological age of the rat. I. A factor model of age parameters. Mech Ageing Dev. 1980;14(3–4):345–59.
Skalicky M, Hofecker G, Kment A, Niedermuller H. Models of the biological age of the rat. II. Multiple regression models in the study on influencing aging. Mech Ageing Dev. 1980;14(3–4):361–77.
Nakamura E, Miyao K, Ozeki T. Assessment of biological age by principal component analysis. Mech Ageing Dev. 1988;46(1–3):1–18.
Nakamura E, Miyao K. A method for identifying biomarkers of aging and constructing an index of biological age in humans. J Gerontol Ser A, Biol Sci Med Sci. 2007;62(10):1096–105.
MacDonald SW, Dixon RA, Cohen AL, Hazlitt JE. Biological age and 12-year cognitive change in older adults: findings from the Victoria longitudinal study. Gerontology. 2004;50(2):64–81. https://doi.org/10.1159/000075557.
Barzilai N, Guarente L, Kirkwood TB, Partridge L, Rando TA, Slagboom PE. The place of genetics in ageing research. Nat Rev Genet. 2012;13(8):589–94. https://doi.org/10.1038/nrg3290.
Heyn H, Li N, Ferreira HJ, et al. Distinct DNA methylomes of newborns and centenarians. Proc Natl Acad Sci USA. 2012;109(26):10522–7. https://doi.org/10.1073/pnas.1120658109.
Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–9.
Belsky DW, Moffitt TE, Cohen AA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2017;187(6):1220–30.
Crimmins E, Vasunilashorn S, Kim JK, Alley D. Biomarkers related to aging in human populations. Adv Clin Chem. 2008;46:161–216.
Longo VD, Antebi A, Bartke A, et al. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14(4):497–510. https://doi.org/10.1111/acel.12338.
de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157(7):1515–26. https://doi.org/10.1016/j.cell.2014.05.031.
Moffitt TE, Belsky DW, Danese A, Poulton R, Caspi A. The longitudinal study of aging in human young adults: knowledge gaps and research agenda. J Gerontol Ser A, Biol Sci Med Sci. 2017;72(2):210–5. https://doi.org/10.1093/gerona/glw191.
Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol. 2013;48(1):1–5. https://doi.org/10.1016/j.exger.2012.11.014.
Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408(6809):233–8.
Acknowledgements
The dedication and commitment by which study participants, general practitioners, and pharmacists of the Ommoord district contribute to the Rotterdam Study are gratefully acknowledged. We thank all staff at the Rotterdam Study research center, facilitating assessment of participants throughout the years, and Frank J.A. van Rooij as data manager.
Funding
The Rotterdam Study is sponsored by the Erasmus Medical Centre and Erasmus University Rotterdam, The Netherlands Organization for Scientific Research (NWO), The Netherlands Organization for Health Research and Development (ZonMW), the Research Institute for Diseases in the Elderly (RIDE), The Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. Further support was obtained from the Netherlands Consortium for Healthy Ageing. This study is supported by Janssen Prevention Centre.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Waziry, R., Gras, L., Sedaghat, S. et al. Quantification of biological age as a determinant of age-related diseases in the Rotterdam Study: a structural equation modeling approach. Eur J Epidemiol 34, 793–799 (2019). https://doi.org/10.1007/s10654-019-00497-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-019-00497-3