Abstract
Serum total osteocalcin, a marker of bone formation, may regulate glucose metabolism and influence the risk of developing adverse metabolic outcomes. We conducted a systematic review and meta-analysis of published observational evidence, to assess and quantify the associations of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes [e.g., metabolic syndrome (MetS)]. Relevant studies were identified in a literature search of MEDLINE, EMBASE, Web of Science, and reference lists of relevant studies to May 2015. Mean differences and risk estimates (odds ratios or relative risks) with 95 % CIs were aggregated using random-effects models. Fifty-two observational (38 cross-sectional, eight cohort, five case–control, and one both cross-sectional and cohort) studies with data on 46,998 non-overlapping participants were included. Baseline serum total osteocalcin levels were significantly lower in type 2 diabetes compared with non-type 2 diabetes and in MetS compared with non-MetS in pooled analysis of cross-sectional evidence. Pooled risk estimates (95 % CIs) for type 2 diabetes in a comparison of extreme fourths of total osteocalcin levels were 0.23 (95 % CI 0.12, 0.46) and 0.89 (95 % CI 0.78, 1.01) for cross-sectional and cohort studies respectively. The corresponding estimate was 0.39 (0.27, 0.56) for MetS from cross-sectional evidence. In both cross-sectional and cohort studies, a unit increase in serum total osteocalcin levels was associated with a significant mean increase in HOMA-B and mean reduction in HbA1c; with significant mean reductions in fasting plasma glucose levels, HOMA-IR, and body mass index in only cross-sectional studies. Available evidence—mainly from cross-sectional studies, supports inverse associations of serum total osteocalcin with risk of adverse metabolic outcomes. Large-scale prospective studies are needed to establish whether serum total osteocalcin may be useful in the prevention of adverse metabolic outcomes such as type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteocalcin, a bone-derived protein secreted by osteoblasts, is a constituent of bone extracellular matrix [1] and is used as a biochemical indicator of bone resorption and formation [2]. Osteocalcin is secreted in a fully carboxylated form, demonstrated in vitro experiments to be the inactive form, which is then decarboxylated to an active biological form [1, 3, 4]. Circulating serum total osteocalcin comprises both carboxylated and undercarboxylated osteocalcin. In addition to its physiological functions, a growing body of evidence indicates that serum levels of osteocalcin may play a role in regulating glucose metabolism and fat mass. In animal studies, osteocalcin modulates insulin secretion and sensitivity, increases β-cell proliferation, and stimulates energy metabolism [3]. Lee et al. [3] demonstrated that mice lacking osteocalcin exhibited high blood glucose levels and insulin resistance. Conversely, mice treated with osteocalcin displayed decreased glycemia and increased insulin sensitivity [5].
Emerging evidence indicates that circulating levels of serum total ostecalcin may exhibit protective effects on adverse metabolic outcomes in humans, by causing increased insulin secretion and sensitivity, increased energy expenditure, reduced blood glucose levels, and decreased visceral fat. A number of studies have reported on the associations of serum total osteocalcin with adverse metabolic outcomes, but the data are sparse and conflicting; with some studies suggesting inverse associations [6–8], whereas others failed to establish any associations [9, 10]. Osteocalcin may hold potential for the prevention and treatment of obesity and adverse metabolic outcomes such as type 2 diabetes and metabolic syndrome (MetS); therefore, there is a need to evaluate its role in the development of these outcomes in greater detail. In this context, we performed a systematic review and meta-analysis of all available published observational evidence to clarify and quantify the extent of potential associations of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes. We also sought to identify gaps in the existing evidence.
Methods
Data sources and search strategy
We conducted this review using a predefined protocol and in accordance with PRISMA and MOOSE guidelines [11, 12] (Appendices 1, 2 of Electronic Supplementary Material). Two independent authors (S.K.K., T.A.A.) in duplication, searched MEDLINE, EMBASE, and Web of Science up to May 2015. The computer-based searches combined free and MeSH search terms and combination of key words related to the exposure (e.g., “osteocalcin”) and outcomes (e.g., “type 2 diabetes”, “MetS”, “glucose”, “insulin resistance”, “body mass index”, “adiponectin”, “leptin”). There were no restrictions on language or the publication date. Reference lists of retrieved articles were manually scanned for all relevant additional studies and review articles. We restricted the search to studies of humans. Further details on the search strategy are presented in Appendix 3 of Electronic Supplementary Material.
Eligibility criteria
We sought observational cohort, case–control, or cross-sectional population-based studies that had reported on associations of serum circulating levels of total osteocalcin with [1] type 2 diabetes and [2] intermediate metabolic phenotypes [MetS, insulin resistance [estimated by homeostasis model assessment of insulin resistance (HOMA-IR)], homeostasis model assessment of beta cell function (HOMA-B), non-alcoholic fatty liver disease (NAFLD), body mass index (BMI), fasting plasma glucose (FPG), fasting insulin, glycated haemoglobin (HbA1c), leptin, or adiponectin]. Studies conducted among individuals with type 1 diabetes only or gestational diabetes were excluded.
Data extraction and quality assessment
The data extraction and quality assessment were conducted by two independent reviewers (S.K.K., T.A.A.). A standardized predesigned data collection form was used for data extraction. Data were abstracted, where available, on study; publication date; geographical location; population source; year of baseline survey; sample population; study design; age range at baseline; duration of follow-up (for cohort studies); type of outcome; and degree of adjustment for potential confounders (defined as ‘+’ when risk estimates were unadjusted or adjusted for age and/or sex; ‘++’ further adjustment for established metabolic or type 2 diabetes risk factors such as age, sex, anthropometric indices such as BMI and waist circumference, smoking status, blood pressure, physical activity, and high-density lipoprotein cholesterol; and ‘+++’ additional adjustment for inflammation, FPG, or fasting insulin). Each article was assessed using the inclusion criteria above and any disagreement regarding eligibility of an article was discussed, and agreement reached by consensus with a third reviewer (J.A.L.). We contacted authors to obtain additional information, if study results were not reported in sufficient detail. For cohort and case–control studies, study quality was assessed based on the nine-star Newcastle-Ottawa Scale (NOS) [13] using three pre-defined domains namely: selection of participants (population representativeness), comparability (adjustment for confounders), and ascertainment of outcomes of interest. The NOS assigns a maximum of four points for selection, two points for comparability, and three points for outcome. Nine points on the NOS reflects the highest study quality. For cross-sectional studies, quality was evaluated using the NOS modified for cross-sectional studies [14], which was also modified for the purposes of the current review question. A maximum score of 8 reflected the highest study quality (Appendix 4 of Electronic Supplementary Material). Overall, a score of ≥5 indicated adequate quality for inclusion in the review.
Statistical analysis
Summary measures were presented as mean differences for continuous outcomes and risk estimates (risk ratios for cohort studies and odds ratios for cross-sectional studies) for categorical outcomes. For data reported as medians, ranges, and 95 % confidence intervals (CIs), means and standard deviations were calculated as described by Hozo and colleagues [15]. To enable a consistent approach to the meta-analysis and enhance interpretation of the findings, units of measurements were converted where appropriate and reported study-specific risk estimates (per-unit or standard deviation change, quintiles, or other groupings) were also transformed to involve comparisons between the top quartile and bottom quartile of each study population’s baseline distribution of total osteocalcin levels, using standard statistical methods [16, 17]. When reported risk estimates could not be transformed, we obtained the standardised estimates through correspondence with the study authors. The inverse variance weighted method was used to combine summary measures using random-effects models to minimise the effect of between-study heterogeneity [18]. Heterogeneity was assessed using the Cochrane χ 2 statistic and the I 2 statistic; and was distinguished as low (I 2 ≥ 25 %), moderate (25 % < I 2 ≥ 50 %) or high (I 2 ≥ 75 %) [19]. Publication bias was evaluated through funnel plots and Egger’s regression symmetry tests [20]. A narrative synthesis was performed for studies that could not be pooled. All tests were two-tailed and p values of 0.05 or less were considered significant. STATA release 13 (Stata Corp., College Station, TX, USA) was used for all statistical analyses.
Results
Study identification and selection
Our initial search identified 1134 potentially relevant citations. After screening based on titles and abstracts, 74 articles remained for further evaluation. Following detailed assessments, 22 articles were excluded. The remaining 52 articles based on 52 unique observational studies met our inclusion criteria and were included in the meta-analysis (Fig. 1; Appendix 5 of Electronic Supplementary Material).
Study characteristics and study quality
Table 1 summarizes the key characteristics of the studies included in the review. In aggregate, 46,998 unique participants were included in this review. However, not all studies provided relevant data that could be included in the meta-analysis. The majority of studies (n = 29) were conducted in Asian countries; 14 in Europe; 4 in North America (USA); 2 in Australia; 2 in South America (Brazil); and 1 in Africa (Morocco). The average baseline age of participants ranged from 39 to 76 years. The majority of studies (n = 38) were cross-sectional studies; seven were prospective cohorts; five case–controls; one had both cross-sectional and prospective cohort designs; and 1 was a retrospective cohort. The average follow-up for cohort studies ranged from 0.5 to 10.0 years. There was considerable variability in study populations which included healthy participants, pre- and post-menopausal women, as well as participants with pre-existing conditions such as MetS, type 2 diabetes, and participants at high cardiovascular risk. Among cohort, case–control, and cross-sectional studies, quality score ranged from 5 to 8. Table 1 additionally provides assay characteristics of measured levels of serum total osteocalcin from studies contributing to the analysis. Apart from 3 studies which did not provide details of type of assay used, all studies used conventional radioimmunoassays, human-specific radioimmunoassays, or electrochemiluminescence immunoassays which have been shown to be quite precise as they are able to recognize the fully carboxylated and noncarboxylated forms of osteocalcin with the same affinity [21].
Baseline serum total osteocalcin levels in type 2 diabetes, MetS, and non-alcoholic fatty liver disease
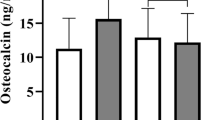
The pooled random-effects mean difference across 16 cross-sectional studies showed significantly lower circulating baseline levels of serum total osteocalcin −3.31 ng/ml (95 % CI −4.04, −2.57; p < 0.001) in type 2 diabetes compared with non-type 2 diabetes. A similar result was found for participants with MetS −2.90 ng/ml (95 % CI −3.61, −2.19; p < 0.001) compared with non-MetS in 12 cross-sectional studies. In pooled analysis of three cross-sectional studies, baseline circulating serum total osteocalcin level was non-significantly lower −1.58 ng/ml (95 % CI −4.38, 1.23; p = 0.270) comparing subjects with and without NAFLD (Fig. 2). In pooled analysis of 2 cohort studies, baseline circulating serum total osteocalcin level was significantly lower −2.14 ng/ml (95 % CI −2.30, 1.97; p < 0.001) in type 2 diabetes compared with non-type 2 diabetes. In the only cohort study, baseline circulating serum total osteocalcin level was non-significantly lower −1.33 ng/ml (95 % CI −2.95, 0.29; p = 0.107) comparing subjects with and without MetS [22]. In sex-stratified analysis of cross-sectional evidence, baseline circulating levels of serum total osteocalcin were significantly lower in type 2 diabetes compared with non-type 2 diabetes in both males and females (Appendix 6 of Electronic Supplementary Material). Similar findings were obtained for studies of MetS (Appendix 7 of Electronic Supplementary Material).
Mean differences in serum total osteocalcin levels comparing subjects with type 2 diabetes metabolic syndrome, and non-alcoholic fatty liver disease with their respective controls. Study references are provided in Appendix 5. The summary estimates presented were calculated using random effects models; CI confidence interval (bars)
Association of serum total osteocalcin with type 2 diabetes
Circulating serum total osteocalcin levels in relation to risk of type 2 diabetes was reported in six cross-sectional and three cohort (prospective or retrospective) studies. Pooled risk estimates for type 2 diabetes in a comparison of individuals in the top fourth versus those in the bottom fourths of the population distribution of serum total osteocalcin levels, adjusted for several established risk factors for type 2 diabetes were 0.23 (95 % CI 0.12, 0.46; p < 0.001) for cross-sectional studies (6974 participants and 1484 cases) and 0.89 (95 % CI 0.78, 1.01; p = 0.060) for cohort studies (1662 participants and 189 cases) (Fig. 3). Substantial between-study heterogeneity was found in the cross-sectional analysis: I2 = 84 % (95 % CI 68, 92 %; p < 0.001), with no evidence of heterogeneity in the cohort analyses: I2 = 0 % (0, 90 %; p = 0.640). In analyses stratified by degree of confounder adjustment for cross-sectional studies with relevant data, the pooled age and/or sex risk estimate of three studies for type 2 diabetes in a comparison of individuals in the top fourth versus those in the bottom fourths of the population distribution of serum total osteocalcin levels was 0.12 (95 % CI 0.04, 0.44; p = 0.001). The corresponding estimate for pooled analysis of two studies that adjusted for established risk factors plus FPG was 0.31 (95 % CI 0.15, 0.62; p = 0.001).
Associations of serum total osteocalcin with type 2 diabetes. Study references are provided in Appendix 5. The summary estimate presented was calculated using a random effects model; size of data markers are proportional to the inverse of the variance of the risk estimate; CI confidence interval (bars); degree of adjustment: + unadjusted or adjusted for age and/or sex, ++ further adjustment for established type 2 diabetes risk factors such as age, sex, anthropometric indices such as BMI and waist circumference, smoking status, blood pressure, physical activity, and high-density lipoprotein cholesterol, +++ additional adjustment for inflammation, FPG, or fasting insulin
Association of serum total osteocalcin with intermediate metabolic phenotypes
In pooled analyses of cross-sectional studies, risk estimates comparing individuals in the top versus bottom fourths of circulating levels of serum total osteocalcin, adjusted for several established metabolic risk factors and other potential confounders, were 0.39 (95 % CI 0.27, 0.56; p < 0.001) for MetS risk (12,644 participants, 3471 cases); 0.33 (95 % CI 0.07, 1.56; p = 0.162) for hyperglycemia risk (4133 participants, 678 cases); and 0.68 (95 % CI 0.31, 1.46; p = 0.320) for NAFLD (8750 participants, 366 cases) (Fig. 4). There was evidence of between-study heterogeneity in the MetS analysis, I2 = 85 % (95 % CI 75, 91 %; p < 0.001). In subgroup analysis by degree of confounder adjustment, the risk estimates for MetS were 0.44 (95 % CI 0.30, 0.64; p < 0.001) and 0.19 (95 % CI 0.05, 0.68; p = 0.011) in analyses adjusted for established metabolic risk factors and further for inflammation, FPG, or fasting insulin respectively (Appendix 8 of Electronic Supplementary Material). Due to differences in total osteocalcin categories, it was not possible to include one study in the pooled results for MetS. Yeap and colleagues reported an increased risk of MetS for men in the lowest two quintiles of serum total osteocalcin levels <30 ng/ml compared to men with serum total osteocalcin levels ≥30 ng/ml [8].
Association of serum total osteocalcin with metabolic syndrome, hyperglycaemia, and non-alcoholic fatty liver disease in cross-sectional studies. Study references are provided in Appendix 5. The summary estimates presented were calculated using random effects models; Size of data markers are proportional to the inverse of the variance of the relative ratio; CI confidence interval (bars); NAFLD non-alcoholic fatty liver disease; degree of adjustment: + unadjusted or adjusted for age and/or sex, ++ further adjustment for established metabolic risk factors such as age, sex, anthropometric indices such as BMI and waist circumference, smoking status, blood pressure, physical activity, and high-density lipoprotein cholesterol, +++ additional adjustment for inflammation, FPG, or fasting insulin
In pooled analyses, a unit (ng/ml) increase in serum total osteocalcin levels was associated with mean differences in FPG of −0.37 mg/ml (95 % CI −0.52, −0.23; p < 0.001) in cross-sectional analyses and −0.10 mg/ml (95 % CI −1.77, 1.57; p = 0.905) in cohort analyses (Appendix 9 of Electronic Supplementary Material). The corresponding mean differences were −0.02 µU/ml (95 % CI −0.07, 0.03; p = 0.533) and 0.57 µU/ml (95 % CI −0.89, 2.03; p = 0.446) respectively for fasting insulin (Appendix 10 of Electronic Supplementary Material); −0.09 percentage points (95 % CI −0.14, −0.03; p = 0.002) and −0.38 percentage points (95 % CI −0.67, −0.09; p = 0.010) respectively for HbA1c (Appendix 11 of Electronic Supplementary Material); −0.03 (95 % CI −0.05, −0.01; p = 0.004) and 0.43 (95 % CI −0.74, 1.60; p = 0.468) respectively for HOMA-IR (Appendix 12 of Electronic Supplementary Material); and 1.53 (95 % CI 0.77, 2.29; p < 0.001) and 0.97 (95 % CI 0.95, 0.99; p < 0.001) respectively for HOMA-B (Appendix 13 of Electronic Supplementary Material). In pooled analyses of 4 cross-sectional studies, a unit (ng/ml) increase in serum total osteocalcin levels was associated with a mean difference in BMI of −0.22 kg/m2 (95 % CI −0.43, −0.02; p = 0.031) (Appendix 14 of Electronic Supplementary Material). In the cross-sectional analyses, between-study heterogeneity was I2 = 82 % (95 % CI 67, 90 %; p < 0.001) for FPG; I2 = 79 % (95 % CI 59, 89 %; p < 0.001) for fasting insulin; I2 = 73 % (95 % CI 37, 88 %; p = 0.003) for HbA1c; I2 = 85 % (95 % CI 71, 92 %; p < 0.001) for HOMA-IR; I2 = 26 % (95 % CI 0, 70 %; p = 0.251) for HOMA-B; and I2 = 79 % (95 % CI 45, 92 %; p = 0.002) for BMI. One study assessed the association of serum total osteocalcin with adiponection in men and post-menopausal women [23]. A unit (ng/ml) increase in serum total osteocalcin levels was associated with a significant mean increase in adiponectin levels 0.36 µg/ml (95 % CI 0.04, 0.69; p = 0.028) in post-menopausal women and a mean difference of −0.10 µg/ml (95 % CI −0.29, 0.10; p = 0.327) in men.
Publication bias
Under visual examination, funnel plots for those analyses that involved 5 or more studies were mostly symmetrical, with possible exception of cross-sectional studies that evaluated associations with type 2 diabetes, FPG levels, and HOMA-B. However, Egger’s regression tests showed statistical evidence of publication bias for analyses involving HbA1c, HOMA-IR, and MetS (Appendix 15).
Comment
Summary of findings
We have conducted the first study that systematically reviews and summarises through a meta-analytical approach, available observational studies that have assessed the associations of serum circulating levels of total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes. Our results showed statistically significant lower baseline serum total osteocalcin levels in type 2 diabetes compared with non-type 2 diabetes and in MetS compared with non-MetS, with similar findings for both males and females. Cross-sectional evidence suggested an inverse association between serum total osteocalcin levels and risk of type 2 diabetes in fully adjusted models including potential mediators such as FPG and fasting insulin, but no significant association from prospective evidence (albeit limited number of studies) was demonstrated. In analyses limited to cross-sectional evidence, we found an inverse association between serum total osteocacin and MetS in fully adjusted models, but no significant association with hyperglycemia or NAFLD could be demonstrated. In both cross-sectional and cohort studies, a unit increase in serum total osteocalcin levels was associated with a significant mean increase in HOMA-B and mean reduction in HbA1c; with significant mean reductions in FPG levels, HOMA-IR, and BMI in only cross-sectional studies – the overall findings which are consistent with results from animal studies. In contrast, an increase in serum total osteocalcin levels was not significantly associated with fasting insulin in both cross-sectional and cohort studies.
Interpretation of findings
The current review with the results based largely on cross-sectional evidence, supports emerging evidence of a decreased risk of adverse metabolic outcomes such as type 2 diabetes and MetS with increased circulating levels of serum total osteocalcin. Apart from the well-known pro-osteoblastic functions of osteocalcin [24], there is emerging evidence of an endocrine function as well. Mechanistic evidence linking osteocalcin with decreased risk of adverse metabolic outcomes is its role in influencing insulin levels, glucose metabolism, insulin sensitivity, fat mass, beta cell proliferation, and energy expenditure, which has so far been established in animal models [3, 5, 25]. Though mechanistic studies for osteocalcin have mostly involved experimental mice, growing epidemiological and recent genetic evidence indeed suggest that multiple aspects of the biology of osteocalcin are similar for both humans and rodents [26, 27]; demonstrating that the metabolic functions of osteocalcin in animals are conserved in humans. Evidence from animal models also suggest that the active form of osteocalcin (undercarboxylated osteocalcin) may be of more importance in regulating glucose and energy metabolism [3, 5, 28] and therefore implicated in the development of adverse metabolic outcomes. However, this has not been established, as it is uncertain if uncarboxylated osteocalcin might be the active form in humans [26]. In addition, only few and smaller studies have assessed the relationship of undercarboxylated osteocalcin with metabolic outcomes, and have reported conflicting results [29–32]. Compared to serum total osteocalcin, which can be conveniently measured in large studies using automated immunoassays [33], assays for undercarboxylated osteocalcin are more cumbersome, labour intensive, or less precise [34].
Implications of findings
The role of osteocalcin in glucose and energy metabolism has been established experimentally in animal studies. Our findings based on observational evidence, provide further insight concerning the relationship between serum total osteocalcin and adverse metabolic outcomes in humans. They underscore a potentially protective role of increased serum total osteocalcin levels on the risk of type 2 diabetes and other adverse metabolic outcomes. Our review has also identified gaps in the literature concerning the relationships between serum total osteocalcin levels and metabolic outcomes. Very few and inadequately powered prospective cohort studies were available that evaluated these outcomes particularly for type 2 diabetes, limiting the review to mainly cross-sectional study designs. Thus, large-scale prospective studies are needed to confirm the current available evidence and additional research is required to address the existing gaps. Serum total osteocacin remains a promising though unproven strategy in the prevention or treatment of adverse metabolic outcomes such as type 2 diabetes. This therapeutic potential has only so far been demonstrated in animal experiments. Well designed randomised controlled trials or Mendelian randomisation experiments are also warranted to investigate these potential implications.
Strengths and limitations
The strengths and potential limitations of this review and meta-analysis deserve consideration. We implemented a comprehensive search strategy across multiple databases without language restriction, yielding several published studies on the topic. This review involved approximately 47,000 participants and evaluated the risk of a wide-range of metabolic outcomes in relation to circulating serum total osteocalcin. Our meta-analysis was robust as we were able to harmonize estimates from almost all available contributing studies (mean differences and risk estimates that compared extreme fourths of baseline distribution of serum total osteocalcin levels), allowing a consistent combination of estimates across studies. Because the present review was based on variably adjusted data reported by the eligible studies, there remained a risk of residual confounding as with meta-analyses involving published data. Majority of studies included in the review, however, reported estimates based on adjustment for a comprehensive panel of conventional risk factors and potential confounders. In addition, when studies that evaluated associations for type 2 diabetes and MetS were grouped by degree of adjustment, the significant associations remained consistent. Given the novelty and the lack of relevant clinical trials published on the topic, our review was based on only observational evidence (with majority limited to cross-sectional designs) which precluded the ability to make any causal inferences. There was evidence of substantial heterogeneity among contributing studies for some analyses, which could not be explored because of the limited number of studies and data on relevant study characteristics. Finally, given that tests for publication bias are unlikely to be useful for analysis involving limited number of studies [35], we were unable to adequately explore for publication bias.
In conclusion, available evidence—mainly from cross-sectional studies—supports inverse associations of serum total osteocalcin with risk of adverse metabolic outcomes. This review also highlights important gaps in the existing literature, with large-scale prospective studies in particular needed to establish whether serum total osteocalcin may be useful in the prevention of adverse metabolic outcomes such as type 2 diabetes.
References
Price PA. Gla-containing proteins of bone. Connect Tissue Res. 1989;21(1–4):51–7; discussion 7–60.
Gerdhem P, Isaksson A, Akesson K, Obrant KJ. Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos Int. 2005;16(12):1506–12. doi:10.1007/s00198-005-1877-5.
Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–69. doi:10.1016/j.cell.2007.05.047.
Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69(3):990–1047.
Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50(2):568–75. doi:10.1016/j.bone.2011.04.017.
Lerchbaum E, Schwetz V, Nauck M, Volzke H, Wallaschofski H, Hannemann A. Lower bone turnover markers in metabolic syndrome and diabetes: the population-based Study of Health in Pomerania. Nutr Metab Cardiovasc Dis. 2015;. doi:10.1016/j.numecd.2015.02.002.
Yeap BB, Alfonso H, Chubb SA, et al. Higher serum undercarboxylated osteocalcin and other bone turnover markers are associated with reduced diabetes risk and lower estradiol concentrations in older men. J Clin Endocrinol Metab. 2015;100(1):63–71. doi:10.1210/jc.2014-3019.
Yeap BB, Chubb SA, Flicker L, et al. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol. 2010;163(2):265–72. doi:10.1530/EJE-10-0414.
Liatis S, Sfikakis PP, Tsiakou A, et al. Baseline osteocalcin levels and incident diabetes in a 3-year prospective study of high-risk individuals. Diabetes Metab. 2014;40(3):198–203. doi:10.1016/j.diabet.2014.01.001.
Movahed A, Larijani B, Nabipour I, et al. Reduced serum osteocalcin concentrations are associated with type 2 diabetes mellitus and the metabolic syndrome components in postmenopausal women: the crosstalk between bone and energy metabolism. J Bone Miner Metab. 2012;30(6):683–91. doi:10.1007/s00774-012-0367-z.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology. J Am Med Assoc. 2000;283(15):2008–12. doi:10.1001/jama.283.15.2008.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097.
Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. www.ohri.ca/programs/clinical_epidemiology/oxford.asp. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 10 Mar 2015).
van Dijk GM, Maneva M, Colpani V, et al. The association between vasomotor symptoms and metabolic health in peri- and post-menopausal women: a systematic review. Maturitas. 2015;80(2):140–7. doi:10.1016/j.maturitas.2014.11.016.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi:10.1186/1471-2288-5-13.
Chêne G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144(6):610–21.
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi:10.1016/0197-2456(86)90046-2.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi:10.1136/bmj.327.7414.557.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Garnero P, Grimaux M, Demiaux B, Preaudat C, Seguin P, Delmas PD. Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J Bone Miner Res. 1992;7(12):1389–98. doi:10.1002/jbmr.5650071206.
Szulc P, Varennes A, Delmas PD, Goudable J, Chapurlat R. Men with metabolic syndrome have lower bone mineral density but lower fracture risk—the MINOS study. J Bone Miner Res. 2010;25(6):1446–54. doi:10.1002/jbmr.13.
Kanazawa I, Yamaguchi T, Tada Y, Yamauchi M, Yano S, Sugimoto T. Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone. 2011;48(4):720–5. doi:10.1016/j.bone.2010.12.020.
Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol. 2009;310(1–2):21–9. doi:10.1016/j.mce.2009.04.004.
Hinoi E, Gao N, Jung DY, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183(7):1235–42. doi:10.1083/jcb.200809113.
Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011;54(6):1291–7. doi:10.1007/s00125-011-2155-z.
Wei J, Karsenty G. An overview of the metabolic functions of osteocalcin. Curr Osteoporos Rep. 2015;. doi:10.1007/s11914-015-0267-y.
Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab. 2008;19(5):161–6. doi:10.1016/j.tem.2008.02.006.
Hwang YC, Jeong IK, Ahn KJ, Chung HY. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev. 2009;25(8):768–72. doi:10.1002/dmrr.1045.
Shea MK, Gundberg CM, Meigs JB, et al. Gamma-carboxylation of osteocalcin and insulin resistance in older men and women. Am J Clin Nutr. 2009;90(5):1230–5. doi:10.3945/ajcn.2009.28151.
Kanazawa I, Yamaguchi T, Yamauchi M, et al. Adiponectin is associated with changes in bone markers during glycemic control in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94(8):3031–7. doi:10.1210/jc.2008-2187.
Kanazawa I, Yamaguchi T, Yamauchi M, et al. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22(1):187–94. doi:10.1007/s00198-010-1184-7.
Takahashi M, Kushida K, Nagano A, Inoue T. Comparison of the analytical and clinical performance characteristics of an N-MID versus an intact osteocalcin immunoradiometric assay. Clin Chim Acta. 2000;294(1–2):67–76.
Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83(9):3258–66. doi:10.1210/jcem.83.9.5126.
Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. www.cochrane-handbook.org (Accessed 01 Mar 2013).
Acknowledgments
We thank Anil Baran Choudhury, PhD, Government Medical college Rajnandgaon, India; Rebeca Reyes, MD, PhD, Bone Metabolic Unit (RETICEF), Endocrinology Division, Hospital Universitario San Cecilio, Av. Dr. Oloriz 16, 18012 Granada, Spain; Weiping Jia, Department of Endocrinology and Metabolism, Shanghai Diabetes Institute, Shanghai Key Laboratory of Diabetes Mellitus, Shanghai Clinical Center for Diabetes, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shangai, China; Dong Hyun Sinn, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea; and Zhen-lin Zhang, Metabolic Bone Disease and Genetic Research Unit, Department of Osteoporosis and Bone Diseases, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China for readily providing data on request.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kunutsor, S.K., Apekey, T.A. & Laukkanen, J.A. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur J Epidemiol 30, 599–614 (2015). https://doi.org/10.1007/s10654-015-0058-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-015-0058-x