Abstract
The levels, spatial distribution, and sources of petroleum hydrocarbons and phthalates were assessed in surface sediment samples from the urban lagoon of Obhur near Jeddah, the largest city on the Red Sea coast of Saudi Arabia. The lagoon was divided into the inner zone, middle zone, and outer zone based on its geomorphological features and developmental activities. n-Alkanes, hopane and sterane biomarkers, and unresolved complex mixture were the major petroleum hydrocarbon compounds of the total extractable organic matter. Phthalates were also measured in the sediment samples. In the three zones, n-alkanes ranged from 89.3 ± 88.5 to 103.2 ± 114.9 ng/g, whereas the hopane and sterane biomarkers varied from 69.4 ± 75.3 to 77.7 ± 69.9 ng/g and 72.5 ± 77.9–89.5 ± 82.2 ng/g, respectively. The UCM concentrations ranged from 821 ± 1119 to 1297 ± 1684 ng/g and phthalates from 37.4 ± 34.5 65 ± 68 ng/g. The primary origins of these anthropogenic hydrocarbons in the lagoon sediments were petroleum products (boat engine discharges, boat washing, lubricants, and wastewater flows) and plasticizers (plastic waste and litter). The proportions of anthropogenic hydrocarbons derived from petroleum products in the sediment’s TEOM ranged from 43 ± 33 to 62 ± 15%, while the percentages for plasticizers varied from 2.9 ± 1.2 to 4.0 ± 1.6%. The presence and inputs of these contaminants from petroleum and plastic wastes in the lagoon’s sediments will eventually have an impact on its habitats, including the benthic nursery and spawning areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal areas are mostly affected by anthropogenic sources from petroleum hydrocarbons (Gao et al., 2022; Ndungu et al., 2017; Uddin et al., 2021). These sources include onshore oilfield operations, liquid released by refineries, accidental spills, petrochemical plants and shipping, natural oil seeps, and sewage discharges (Alsharhan, 2003; El Nemr et al., 2004; El Sayed, 2002a, 2002b; Rushdi et al., 2009, 2022a). Petroleum residues in the Red Sea are largely attributed to oil tankers and oil terminals (Dicks, 1987; El-Sikaily et al., 2003; Rushdi et al., 2010), where the coastal ecosystems of the Red Sea shorelines are seriously contaminated with oil-related products (Alhudhodi et al., 2022; Jamoussi et al., 2022; Kostianaia et al., 2020; Periáñez, 2020; Rushdi et al., 1994; Shetaia et al., 2016). In the coastal environment of Saudi Arabia’s Red Sea, oil pollution can also originate from sewage, leisure activities, and littering (El Sayed, 2002a, 2002b; Wilson et al., 2017). Sewage is frequently discharged to the coastal zones, especially into nearby lagoons, around large cities such as the city of Jeddah (Badr et al., 2009). It is also discharged from offshore platforms, ship traffic, tour boats, ferries, and private yachts (Hees, 1977). Transported atmospheric particulate matter, wind-resuspended soil, and seasonal runoff and floods can significantly contribute to petroleum-related products in the coastal environment (Rushdi et al., 2010, 2022b). The marine life of the distinct ecosystems of the Red Sea coast is facing significant danger due to contamination from oil-related sources (Batang et al., 2016; UNEP, 1985; Ziegler et al., 2016).
Jeddah has a population of about 4.7 million, making it the biggest city on the western coast of Saudi Arabia. It is projected to reach 5.3 million in 2023 (WPR, 2023). The city is highly industrialized and hosts one of the largest Saudi Aramco refineries. However, the presence of other facilities, including municipal waste and sewage, electrical power generation, refinery plants, and desalination, has negative effects on the environment along the shoreline (Badr et al., 2009). According to (Durmus, 2019), approximately 40% of the total 1.9 million tons of solid waste in the city is organic waste. Unfortunately, some of this waste can end up polluting the environment and reaching the coastlines through runoff or particulate matter transported by wind (Al-Khion, et al., 2021; Al-Lihaibi, et al., 2019; Rushdi et al., 2010, 2022a, 2022b). Plastics, which make up most of the marine litter, are a major source of contaminants in the marine environment (Diem et al., 2023; Ryan, 2015) and have different impacts on marine biota based on the size of the plastic litter (Barboza et al., 2019; Bellou et al., 2021). Obhur Creek near Jeddah, also known as Sharm Obhur, is a well-known tourist spot situated on the western coast of Saudi Arabia. It acts as a link between inland and marine ecosystems and provides visitors with resorts, harbors, docks, and recreational activities like boat trips. Nevertheless, the water outflow from the lagoon can introduce land source pollutants to the Red Sea.
The concentrations and contribution of anthropogenic petroleum hydrocarbons and plastic-related compounds in the sediment of the lagoon are still unknown. Therefore, the main objectives of this work are to: (1) determine the levels and spatial distribution of n-alkanes, hopanes, steranes, and phthalates in sediments from Sharm Obhur on the Red Sea coast of Saudi Arabia; (2) identify their potential sources in the sediments based on biomarkers and geochemical indices; and (3) describe their possible environmental impacts.
Experimental
Study area, sampling sites, and sample preparation
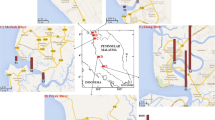
The study area (Fig. 1) is Obhur Lagoon (also locally known as Sharm Obhur). It is located about 35 km north of Jeddah City and is around 11 km long and 1.5 km wide, with a depth of 35 m near its mouth on the Red Sea and about 6 m near the head (Rasul, 2015). Sharm Obhur is a highly favored recreational spot in Jeddah, known for its abundance and popularity. It is connected to the Red Sea proper through a narrow outlet, spanning 264 m in width, situated at its southwestern end. According to Basaham et al., (2006), the hydrographic structure displays a two-layer flow pattern at its entrance. The incoming water is low in salinity and occurs at both surface and intermediate depths. The outgoing water is more saline and found at the bottom. The water temperature and salinity range from 24.4 °C to 32.2 °C and from 39.10 to 40.2 between winter and summer, respectively (Alsaafani et al., 2017; Basaham & El-Shater, 1994). The inflow of surface water from the sea to the lagoon is about 30 psu salinity, whereas the outflow of deep water to the sea from the lagoon is about 39 psu (Abdulla & Al-Subhi, 2020; Albarakati, 2009; Alsaafani et al., 2017). The amount of water coming into the area varies between approximately 151 m3/sec in November and 302 m3/sec in February. Similarly, the amount of water flowing out of the area ranges from about 151 m3/sec in November to 402 m3/sec in February (El-Rayis & Eid, 1997).
Surface sediment samples from forty-two stations were collected from the lagoon in May 2014 (Fig. 1) by Van Veen grab samplers where thirty stations covering and representing the different zones and the entire lagoon were selected and chemically analyzed. The samples were stored in ice boxes and transferred to a freezer in the lab after 4–5 h. About 10 g from each sediment sample was taken by a clean spatula, defrosted, and dried at room temperature, then ground and sieved to obtain < 125 µm fine particles. We divided the lagoon into three zones based on its physical feature and developmental activities (Fig. 1). They were the inner inland zone (IZ), middle zone (MZ), and outer zone (OZ).
Extraction and Instrumental analysis
150 mL precleaned beakers were used for the sediment sample extraction. About 5 g of each sieved sediment sample was extracted with a mixture of dichloromethane (DCM = 30 ml) and methanol (MeOH = 10 ml) three times (15 min each) using ultrasonic agitation (Rushdi et al., 2014a, 2014b, 2018). A filtration unit containing an annealed glass fiber filter was used to remove the sediment particles. First, the filtrate was concentrated by a rotary evaporator and then reduced to about 200 µL by a flow of dry nitrogen gas. Then, the volume of the total extract was corrected to exactly 500 µL by adding DCM:MeOH (3:1, v:v) mixture.
Gas chromatography-mass spectrometry (GC–MS) with a Hewlett-Packard 6890 GC coupled to a 5975 Mass Selective Detector (Agilent) was used for total extract analysis. An Agilent DB-5MS fused silica capillary column (30 m long, 0.25 mm internal diameter, and 0.25 um film thickness) and helium as carrier gas were used. The setting of the GC oven temperature was 65 °C with an initial hold for 2 min, then increased to 310 °C at 6 °C/min and isothermal final hold for 20 min. The ion source energy for the MS was the electron impact mode at 70 eV. The mass spectrometric data were acquired using the ChemStation data system.
Identification, quantification, and quality control
The hydrocarbon compounds identification was based on the similarity of the retention times of each compound with the external standard and the GC–MS data. The identifications of n-alkanes, hopanes, steranes, and phthalates are based principally on GC retention times and their key ion patterns and mass spectra (i.e., key ion fragmentograms m/z 85, 191, 217/218, and 149, respectively). The quantification was performed from the GC profiles utilizing the external standard method with authentic compounds of each homologous series (Rushdi et al., 2006, 2010, 2014a). The external standards were n-alkanes (C8–C40 (90–100%); SupelcoR -Sigma-Aldrich, USA)), hopanes (17α(H),21β(H)-hopane, C30H52; (90–100%); SupelcoR -Sigma-Aldrich, USA)), and bis(2-ethylhexyl)phthalate; (100%), Sigma-Aldrich, USA). The average response factors were computed for each compound. The peak areas of the compounds derived from the TIC traces were used for quantifications.
During the course of this study, the quality control consisted of analytical chemistry and biomarker method application. n-Tetracosane-d50 was added to both the fiber filter and sediment samples to test recoveries, which were 92.8% and 72.9%, respectively, and the measured concentrations were adjusted accordingly. We measured the limit of detection (LoD) and limit of quantification (LoQ) the same way with the samples where the least-square method was applied to fit the relative responses of the different standards versus their concentrations. The correlations were significant with correlation coefficients (R2 = 0.91–0.98). The LODs were 0.04–0.7 ng/µL for n-alkanes, 0.1–1.5 ng/µL for Hopane, and 0.1–1.6 ng/µL for phthalate. We have tested all reagents and solvents used for extraction for possible contaminations. Procedural blanks were examined to assess background contamination introduced by laboratory practices. Throughout the entire procedure, we furthermore performed blank extracts after batches of three samples.
Results and discussion
The sediment samples had a range of total extractable organic matter (TEOM) concentrations from 119 to 5924 ng/g (Fig. 2a). The average values were 1428 ± 1448 ng/g for the inner/inland zone (IZ), 1982 ± 1710 ng/g for the middle zone (MZ), and 2080 ± 2229 ng/g the outer zone (OZ), as shown in Table 1, and Fig. 3a. The data revealed that there were significant amounts of hydrocarbon compounds, including biomarkers, present in the TEOM (Table 1.). The detected compounds included n-alkanes, hopane and sterane biomarkers, phthalates, and an unresolved complex mixture (UCM) of highly branched and cyclic hydrocarbons (Table 1 and SM1), which are the emphasis of the discussion in this work.
The existence, dispersal patterns, amounts, and characteristics of homologous series of n-alkanes, hopanes, and steranes in the environment can be applied to identify their major sources in the environment (; Bouloubassi et al., 2001; Rushdi et al., 2006, 2010, 2014a; Simoneit, 1977a, 1984, 1985). Therefore, similarities are likely between the observed organic compound mixtures in the environmental samples and the recognized sources of those compounds. Here, merely non-polar hydrocarbons and phthalates in the TEOM are explained and discussed.
n-Alkanes
The sediment samples’ TEOM contained n-alkanes ranging from C17 to C34 (Table 1), with the highest concentration found at C31 (hentriacontane). The total concentration ranged from 5.7 ng/g to 464.6 ng/g, with average values of 140.6 ± 196.4 ng/g in sediments from IZ, 89.3 ± 88.5 ng/g in MZ, and 103.2 ± 114.9 ng/g in OZ (Table 1 and MS1, Fig. 3b). The IZ had the highest concentration range followed by OZ and MZ as shown in Fig. 2a, indicating that the topography and hydrography of the lagoon, as well as human activities around it, impact the distribution of these alkanes. These concentrations were relatively similar to the levels measured in Jiaozhou Bay of China (0.5–8.2 µg/g; Wang et al., 2006), Gulf of Suez-Egypt (34–553 ng/g; El Nemr et al., 2014), Yellow River estuary-China (0.356–0.572 mg/kg; Wang et al., 2018), Patos Lagoon estuary-Brazil (0.28–36.4 µg/g; Neves et al., 2023). But they were lower than the values reported in other regions such as the Caspian coast-Iran (249–3900 µg/g; Shirneshan et al., 2016), Eastern Mediterranean Coast (1.6 14.7 µg/g; Tsapakis et al., 2010), Northern Arabian Gulf (5654–20,942 ng/g; Rushdi et al., 2014b).
The sources of n-alkanes in the environment are mainly biogenic and anthropogenic contributions, and their sources can be identified based on the distribution pattern of their homologous series. The existence and detection the n-alkanes in ecosystems are valuable markers to assess the sources, transport, and preservation of organic matter in the environment. The focal parameters coupled with the n-alkane characteristics and sources are the carbon number maximum (Cmax) of the most abundant n-alkane in the homologous series and the carbon preference index (CPI; Bray & Evans, 1961). The CPIo/e (Mazurek & Simoneit, 1984) was estimated using the equation:
The major samples had maximum concentrations at C31 (Table 1 and SM1), suggesting a contribution from higher terrestrial plant waxes of grassy flora (Eglinton & Hamilton, 1967; Ficken et al., 2000; Simoneit, 1978; Zhang et al., 2006). This higher molecular weight n-alkane was also detected in the sediments from the Arabian Gulf (Rushdi et al., 2010, 2022a) signifying that plant waxes of tropical vegetation have a high Cmax (Simoneit, 1978). Frequently, the n-alkane CPI(o/e) has been employed to assess the impact and influence of biogenic and anthropogenic inputs (Al-Khion et al., 2021; Bray & Evans, 1961; Colombo et al., 1989; Commendatore & Esteves, 2004; Diefendorf et al., 2014; Rushdi et al., 2022a; Simoneit, 1989). In the sediment of the lagoon, the CPI(o/e) values for n-alkanes with carbon chains ranging from C17–C35 were estimated between 0.8 and 5.7 (Table SM1). Specifically, the values for sediment in IZ ranged from 0.8 to 3.5 (average = 2.0 ± 0.7), for MZ it was 1.1 to 4.4 (average = 1.9 ± 0.9), and for OZ it was 1.1 to 5.7 (average = 2.1 ± 1.7) as shown in Table 1. These values confirmed that n-alkanes in the sediments were originally derived predominantly from petroleum-related products with small amounts from higher plant waxes.
To assess the relative inputs of the different sources, the n-alkane concentrations from plant wax were calculated following the method of Simoneit et al., (1991a). The plant wax concentrations varied from 1.1 to 132 ng/g of total n-alkanes of the lagoon. They were ranging from 3.1 to 98.5 ng/g (average = 31.2 ± 34.4 ng/g) in the IZ, 0.6–60.1 ng/g (average = 23.4 ± 29.0 ng/g) in the MZ, and from 1.5 to 57.2 ng/g (average = 28.5 ± 45.1 ng/g) in the OZ (Table 1 and SM1). The TARalk ratios (terrestrial-to-marine n-alkanes; TARalk = [(nC27 + nC29 + nC31)/(nC15 + nC17 + nC19)]; Bourbonniere & Meyers, 1996) were calculated and found to range from 9.3 ± 10.1 for ZII to 28.2 ± 22.4 for ZI (Table SM1). These high values indicated that terrestrial plant sources were prevalent in these sediments. The petroleum n-alkane inputs were then estimated by deducting the calculated plant wax n-alkanes from the total n-alkane concentrations. Their total concentrations ranged from 4.0 to 444.2 ng/g in the sediment samples of the lagoon. They ranged from 4.0 to 444.2 ng/g (average = 109.4 ± 191.9 ng/g) in the IZ, 7.8–188.6 ng/g (average = 65.9 ± 65.8 ng/g) in MZ, and from 2.7 to 272.7 ng/g (average = 74.4 ± 72.0 ng/g) in the OZ (Table 1 and SM1). The relatively low concentrations of n-alkanes and the absence of the isoprenoid pristane and phytane in the lagoon sediments are probable due to biodegradation and high oxidation processes.
Hopanes and steranes
Both hopane and sterane biomarkers were detected in the lagoon sediment samples. Hopanes, which were observed to range from C27 to C35 had Cmax at 29 and 30 (Table 1), were measured with concentrations ranging from 4.2 to 197.0 ng/g (Table SM1). As shown in Table 1 and Figs. 2c, 3, the average concentrations were roughly similar in the three zones (i.e., 74.9 ± 54.6 ng/g in IZ, 69.4 ± 75.3 ng/g in MZ, and 77.7 ± 69.9 ng/g in OZ). These concentrations were in the range of the levels reported in the sediments of the estuary of Paranagaue Bary-Southeast Atlantic (41.2–198 ng/g; Garcia et al., 2019); they were higher than the concentrations in the coastal sediments of Qatar (0.0–32.9 ng/g; Rushdi et al., 2017a, 2017b), and lower than the values determined in the coastal canal of Thailand (1510–17114 ng/g; Boonyatumanond et al., 2006), Iranian coast of Arabian/Persian Gulf (189–3713 ng/g, 42–3864 ng/g; Jafarabadi et al., 2019), and estuary in Malaysia (023–2.45 mg/kg; Keshavarzifard et al., 2022) Steranes total concentrations ranged from 6.2 to 194.3 ng/g with averages of 89.5 ± 82.2 ng/g, 72.5 ± 77.9 ng/g, and 81.4 ± 76.8 ng/g for sediments from IZ, MZ, and OZ, respectively (Figs. 2d and 3d). They ranged from C27 to C29 with a Cmax at 29 and major amounts at C27 (Table 1). The concentrations of these sediments were lower than the levels measured in the sediments of the Arabian/Persian coast of Iran coast (42–3864 ng/g; Jafarabadi et al., 2019), similar to the values in Leizhou Peninsula-South China (20–230 ng/g; Gao et al., 2021), and relatively higher than the levels in the coastal zone of Qatar (0.7–36.3 ng/g; Rushdi et al., 2017a, 2017b).
The occurrence of hopane and sterane biomarkers in the environment suggests that the source of organic matter was fossil fuel residues (e.g., Jafarabadi et al., 2019; Medeiros et al., 2005; Simoneit, 1984; Simoneit et al., 1991b). The positive correlations between hopane and sterane concentrations versus TEOM were significant (Fig. 4; R2 = 0.74–0.85, 0.69–0.72, and 0.70–0.8 for IZ, MZ, and OZ respectively), confirming that petroleum residues were also major sources to the sediment of the lagoon. Hopanes are usually applied as biomarker tracers for fossil fuel contributions in the environment since they resist alteration and degradation processes (Ten Bouloubassi et al., 2001; Haven et al., 1988; Moldowan et al., 1985; Peters & Moldowan, 1993). Therefore, we utilized them to differentiate the incidence of petroleum-related products in the environment. The maximum detected hopanes were at the C30 and/or C29 homologues, with the thermodynamically stable 17α(H),21β(H) configuration and minor 17β(H),21α(H)-hopanes (Table 1 and SM1). These isomeric configurations normally take place in crude oils and mature sedimentary rocks due to the diagenetic interconversion of the 17β(H),21β(H)-hopane precursors of bacterial origins (Peters & Moldowan, 1993). The hopane distribution ranged from C27 to C35 for the α,β-series with typically mature C-22 R/S (sinister/rectus enantiomers at carbon 22) pairs of the predominant homologs > C30 (Simoneit, 1984; Simoneit et al. 1990; Peters & Moldowan, 1993). High concentration distributions of the 22S hopane relative to the equivalent 22R isomer have been generally related to petroleum and vehicle engine exhaust (Simoneit, 1984, 1985). The 22S/(S + R) ratios for the extended hopanes of C31 and C32 varied from 0.29 to 0.8 in the sediments of the lagoon. They were 0.56 ± 0.11, 0.65 ± 0.06, and 0.62 ± 0.05 for sediments from IZ, MZ, and OZ, respectively. These ratios were comparable to the values reported for petroleum hydrocarbons and mature crude oil (Kvenvolden et al., 1990; Rushdi & Simoneit, 2002a, 2002b). Consequently, these ratios verified that the sources of the hopane compound series in the lagoon sediments were petroleum-related inputs.
The detection of steranes in the environment is generally related to petroleum emissions and or releases of lubricants from vehicular engines (Abas & Simoneit, 1996). Refinery activities, ship-washing discharges, and municipal wastewaters are also possible sources of these biomarkers (Laws, 1993). Hence, the sterane biomarkers are similarly valuable supporting tracers for oil-product contamination in coastal and urban environments (e.g., Aboul-Kassim & Simoneit, 1996; Albaiges & Cuberes, 1980; Rushdi et al., 2017a, 2017b, 2022a). The steranes in the sediments of Obhur lagoon ranged from C27 to C29 with primarily the 5α,14β,17β-series and minor 5α,14α,17α configurations and both occurring as the 20S and 20R epimers. The epimerization ratio of steranes at C-20, S/(S + R) for these samples, ranged from 0.10 to 0.91 for C27 and from 0.14 to 0.87 for C29. They were 0.48 ± 0.11 in IZ sediments, 0.42 ± 0.12 in MZ, 0.46 ± 0.10 in OZ sediments for C27, and 0.65 ± 0.15 in IZ, 0.0.62 ± 0.28 in MZ, and 0.66 ± 0.22 in OZ for C29 (Table 1). These ratios revealed that the sources of these biomarkers were petroleum residues (Peters & Moldowan, 1993; Simoneit, 1984).
Phthalates
The phthalates in the sediment samples of the lagoon were major compounds, including diisobutyl- dibutyl and dioctyl- phthalates. Their total concentrations ranged from 3.7 ng/g to 201.2 ng/g, with average concentrations of 37.4 ± 34.5 ng/g in the IZ, 58.6 ± 40.0 ng/g in the MZ, and 65 ± 68 ng/g in the OZ sediments (Table 1, and SM1, Fig. 3e). Dioctyl phthalate was the predominant plasticizer detected in the lagoon sediments. These man-made plasticizer compounds were not detected in the solvents, analytical blanks, and sample containers, indicating that they were the main components of the lagoon sediments. The total concentrations of these phthalates in the lagoon sediments were much lower than the levels measured in Campeche-Mexico (18292–21702 ng/g; Ramirez et al., 2019), Asalouyeh harbor coast of Iran (mean = 5180 ng/g; Arfaeinia et al., 2019), Arabian/Persian Gulf of Saudi Arabia coast (31–2799 ng/g; Rushdi et al., 2022a), similar to values in Santos Bay-Brazil (0.0–182 ng/g; Cesar et al., 2014), and relatively higher than the levels in the coast of Qatar (7.8 ± 0.7 ng/g; Rushdi et al., 2017a, 2017b).
Plastic waste and litter in marine ecosystems have been recognized as a serious environmental concern (Andrady, 2011; Eriksen et al., 2013; Fan et al., 2023; Isobe & Iwasaki, 2022; Jang et al., 2023; Peng et al., 2020; Perkins, 2015; Sivadas et al., 2022; Vegter et al., 2014). About 269,000 tons of plastic objects have been predicted floating on oceanic surface waters (Xanthos & Walker, 2017), which represent 60–95% of marine debris (Derraik, 2002; Surhoff & Scholz-Bottcher, 2016; Walker et al., 1997, 2006). Plasticizers, mainly phthalates, make up the mass chemical composition of plastics and are very stable compounds in the environment (Dong et al., 2019; Zhang et al., 2021). They are hazardous and harmful substances to marine life (Squillante et al., 2023; Tranganida et al., 2023).
Unresolved complex mixture (UCM)
Commonly, the GC traces of TEOM of environmental samples show the unresolved complex mixture (UCM) of branched and cyclic compounds above the baseline with the superimposing of the resolved compounds (Frysinger et al., 2003; Killops & Al-Juboori, 1990; Simoneit, 1984, 1985). The concentrations of UCM in the sediment samples of the lagoon were relatively high, ranging from ~ 0.0 to 4571.4 ng/g (Table SM1) with high concentration ranges in the three zones (Figs. 2f and 3f). They ranged from 27 to 2755 ng/g (average = 821 ± 1119 ng/g) in IZ, 0.0–4571.4 ng/g (average = 1041 ± 1539 ng/g) in MZ, and from 21 to 4433 ng/g (average = 1297 ± 1684 ng/g) in OZ (Table 1). These concentrations were lower than the values reported in sediments from the Arabian/Persian Gulf coasts of United Arab Emirates (10000–670000 ng/g; Tolosa et al., 2005), Iraq (71753–92540 ng/g; Rushdi et al., 2014a, 2014b), Saudi Arabia (14–11388 ng/g, Rushdi et al., 2022a), and relatively similar to Qatar (38–609 ng/g; Rushdi et al., 2017a, 2017b).
Fossil fuel utilization and oil spills are the main sources of the UCM. Generally, the refining process of crude oil produces diesel and gasoline, with the former having a broader UCM and the latter having a narrower UCM (Frysinger et al., 2003; Gough & Rowland, 1990; Simoneit, 1984, 1985). A TEOM of a sample with only biogenic sources such as terrestrial plants has no UCM (Simoneit & Mazurek, 1982). Thus, the UCM-to-resolved compound ratio (U:R ratio, Simoneit & Mazurek, 1982) is employed to evaluate the level of contamination from the input of petroleum-related products and oil spills. A U:R ratio value of > 1.0 suggests contamination by biodegraded petroleum-related products in sediments. The U:R ratios varied from 0.01 to 3.5 (Table SM1) and the average values were 1.00 ± 0.99 for IZ, 0.86 ± 1.17 MZ, and 1.23 ± 1.33 for OZ (Table 1). The estimated values of the U:R of the sediment samples of the lagoon indicated that the lagoon was comparatively contaminated with petroleum residues.
The ratio of UCM/n-Alkanes has been used to confirm and distinguish the sources of petroleum hydrocarbons in the environment, where the values > 2.0, > 4.0, and > 10 indicate petroleum, lubricating oil, and degraded petroleum, respectively (Harji et al., 2008; Tolosa et al., 2004). The ratio values ranged from 0.1 to 32.2 for the sediment samples of the lagoon. The averages were 6.8 ± 6.6 in IZ, 8.5 ± 7.6 in MZ, and 10.8 ± 10.9 in OZ. We have applied the ratios of UCM/n-Alkanes versus CPI(o/e) to validate and differentiate the origins of hydrocarbons in the sediments of the lagoon (Fig. 5). Obviously, the main sources of these hydrocarbons in the lagoon were petroleum hydrocarbons.
The percentages of petroleum-related compounds, including petroleum n-alkanes, hopanes, steranes, and UCM, were relatively high ranging from 1 to 97% of the TEOM of the lagoon sediment. They were from 39.5 to 83.8% (average = 62 ± 15%) in IZ, 7.7–97.2% (average = 43 ± 33%) in MZ, and 18.8% to 90.2% (average = 54 ± 28%) in OZ. The fractions of phthalates from plastics varied from 0.70 to 5.6% in the sediments and from 1.8 to 4.7% (average = 2.9 ± 1.2%) in IZ, 0.7–4.7% (average = 3.2 ± 1.1%) in MZ, and from 1.3 to 6.3% (average = 4.0 ± 1.6%) in OZ (Fig. 5). The plasticizer fractions were similar to the values reported from the Gulf of Suez (3.3 ± 2.0%, Rushdi et al., 2009) and higher than in the Qatar coast (0.42 ± 0.72%, Rushdi et al., 2017a, 2017b).
Environmental effects
Coastal lagoons are important sources of organic and inorganic nutrients to marine environments (Por, 2012) with estimated primary production similar to upwelling areas (Knoppers et al., 1991). They are naturally very important and ultimate ecosystems for aquaculture projects but, are still, extremely influenced by anthropogenic inputs and developmental activities (Kjerfve, 1994). Urbanization and human activities have influenced the well-being conditions of the lagoons of the Red Sea coasts (Albarakati & Ahmad, 2019; Basaham et al., 2019). They affect key habitats, such as mangroves, coral reefs, seagrass stands, and bottom water fish nursing areas of the lagoons (Alamri et al., 2021; Aljahdali et al., 2021).
To evaluate the potential warning effects of these sediment hydrocarbons and phthalates, we've utilized the sediment quality assessment guidelines (SQAGs) approach developed by Long and Morgan (1990). This method involves testing various sediment contaminant concentrations against two ranges: the threshold effects level (TEL), which is the minimum level that could cause an effect, and the probable effects level (PEL), which is the lower limit range of contaminant concentrations that could cause damage to the biological system. Although n-alkanes have been reported to be non-toxic (Nilsen et al., 1988), there are no guidelines available for their regulation. Thus, we have used phthalates to assess the sediment quality of the lagoon. The TEL and PEL values for phthalate esters are 182 µg/kg and 2647 µg/kg, respectively, as reported by Long and Morgan (1990) and Long et al. (1995). For DBP, based on the characteristics of toxicology and environmental chemistry, Van Wezel et al. (2000) established environmental risk limits (ERLs), which was set at 0.7 mg/kg for DBP. The acceptable exposure threshold (AETs) for DNBP is 71 1400 µg/Kg, according to PTI (1988) and Bellar et al. (1986). The TEL for DBP is 2200 ng/g and the PEL is 17000 ng/g, as determined by Kingsley and Witthayawirasak (2020). Accordingly, based on the works mentioned above, the levels of DBP and total phthalates found in the sediments of the lagoon were below the TEL values. This indicates that the measured concentrations of phthalates should not have any significant harmful effects.
Increase in petroleum-related products and plasticizer wastes from many sources such as oil transfer docks, oil refineries, municipal sewage treatment plants, and public littering is anticipated to have impacts on the various habitats and associated species groups in the lagoons (Dicks, 1987; Loya, 1975; Rinkevich & Loya, 1979). The most sensitive early life stages of biota such as egg, larva, and young genera will be disturbed by these toxic chemicals. Other marine species, especially benthic species that depend on the lagoon for reproduction, growth, and protection, are harshly impacted. Thus, the presence, characteristics, distributions, and sources of crude oil contamination and other man-made chemical pollutants in the Obhur lagoon need further studies. These studies will facilitate the control and preventive measures to be undertaken to avert or at least reduce the inputs of these pollutants into the lagoons.
Conclusion
The TEOM hydrocarbons in the sediments of Sharm Obhur were mainly from petroleum residues (43 ± 33–62 ± 15% of the TEOM) with fewer inputs from vascular higher plants (23.4 ± 29.0–31.2 ± 34.4% of TEOM). The sources of petroleum hydrocarbons were largely from oil-related operations, spills, boat engines, oil transfer docks, oil refineries, and untreated water discharges into the lagoon. Other sources of pollutants in the sediments of the lagoon were phthalates from plastic littering. These petroleum residues and plasticizers will in due course have severe effects on the ecosystems of the lagoon and the adjoining Red Sea coastal environments.
Further studies are necessary to monitor and investigate the occurrence, characteristics, and distribution of organic and inorganic pollutants in the Red Sea coastal lagoons and their impacts on the key habitats of the area. These research and monitoring activities are critical to protecting the important ecosystems of the lagoons.
References
Abas, M. R. B., & Simoneit, B. R. T. (1996). Composition of extractable organic matter of air particles from Malaysia: Initial studies. Atmospheric Environment, 15, 2779–2793.
Abdallah, R. I., Khalil, N. M., & Roushdie, M. I. (2015). Monitoring of pollution in Egyptian Red Sea. Egypt J Petroleum, 24(1), 59–70.
Abdulla, C. P., & Al-Subhi, A. M. (2020). Sea level variability in the Red Sea: A persistent east-west pattern. Remote Sensing, 12(13), 2090.
Aboul-Kassim, T. A. T., & Simoneit, B. R. T. (1996). Lipid geochemistry of surficial sediments from coastal environment of Egypt I. Aliphatic hydrocarbons - characterization and sources. Marine Chemistry, 54, 135–158.
Alamri, D. A., Al-Solaimani, S. G., Abohassan, R. A., Rinklebe, J., & Shaheen, S. M. (2021). Assessment of water contamination by potentially toxic elements in mangrove lagoons of the Red Sea. Saudi Arabia. Environmental Geochemistry and Health, 43(11), 4819–4830.
Albaiges, J., & Cuberes, M. R. (1980). On the degradation of petroleum residues in the marine environment. Chemosphere, 9, 539–545.
Albarakati, A., Ahmad, F. (2019). Red Sea Coastal Lagoons: Their dynamics and future challenges. In: Oceanographic and Biological Aspects of the Red Sea, (pp. 123–131). Springer, Cham
Albarakati, A. (2009). Water exchange of Sharm Obhur, Jeddah. Red Sea. JKAU: Marine Scienes, 20, 49–58.
Alhudhodi, A. H., Alduwais, A. K., Aldhafeeri, Z. M., Al-Shamsi, M. A. S., & Alharbi, B. H. (2022). Contamination assessment of mangrove ecosystems in the Red Sea coast by polycyclic aromatic hydrocarbons. International Journal of Environmental Research and Public Health, 19(9), 5474.
Ali, A. M., Rønning, H. T., Al Arif, W. M., Kallenborn, R., & Al-Lihaibi, S. S. (2017). Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere, 175, 505–513.
Aljahdali, M. O., Munawar, S., & Khan, W. R. (2021). Monitoring mangrove forest degradation and regeneration: Landsat time series analysis of moisture and vegetation indices at Rabigh Lagoon, Red Sea. Forests, 12(1), 52.
Al-Khion, D. D., Al-Ali, B. S., Al-Saad, H. T., & Rushdi, A. I. (2021). Levels and source of aliphatic hydrocarbons in marine fishes from coast of Iraq based on biomarkers and biogeochemical indices. Indian Journal of Ecology, 48(2), 536–544.
Al-Lihaibi, S., Al-Mehmadi, A., Alarif, W. M., Bawakid, N. O., Kallenborn, R., & Ali, A. M. (2019). Microplastics in sediments and fish from the Red Sea coast at Jeddah (Saudi Arabia). Environmental Chemistry, 16(8), 641–650.
Alsaafani, M. A., Alraddadi, T. M., & Albarakati, A. M. (2017). Seasonal variability of hydrographic structure in Sharm Obhur and water exchange with the Red Sea. Arabian Journal Geoscience, 10(14), 315.
Alsharhan, A. S. (2003). Petroleum geology and potential hydrocarbon plays in the Gulf of Suez rift basin. Egypt Bulletin Am Assoc Petrol Geol, 87(1), 143–180.
Ameen, F., Moslem, M., Hadi, S., & Al-Sabri, A. E. (2016). Biodegradation of diesel fuel hydrocarbons by mangrove fungi from Red Sea Coast of Saudi Arabia. Saudi Journal Biological Sciences, 23(2), 211–218.
Andrady, A. L. (2011). Microplastics in the marine environment. Marine Pollution Bulletin, 62(8), 1596–1605.
Arfaeinia, H., Fazlzadeh, M., Taghizadeh, F., Saeedi, R., Spitz, J., & Dobaradaran, S. (2019). Phthalate acid esters (PAEs) accumulation in coastal sediments from regions with different land use configuration along the Persian Gulf. Ecotoxicology and Environmental Safety, 169, 496–506.
Badr, N. B., El-Fiky, A. A., Mostafa, A. R., & Al-Mur, B. A. (2009). Metal pollution records in core sediments of some Red Sea coastal areas, Kingdom of Saudi Arabia. Environ Monitoring Assessment, 155(1), 509–526.
Barboza, L.G.A., C ́ozar, A., Gimenez, B.C.G., Barros, T.L., Kershaw, P.J., Guilhermino, L. (2019). Macroplastics pollution in the marine environment. In: World Seas: An Environmental Evaluation. Elsevier, pp. 305–328. https://doi.org/10.1016/B978-0-12-805052-1.00019-X
Basaham, A. S., El-Sayed, M. A. (2006). Sharm Obhur: environmental consequences of 20 years of uncontrolled coastal urbanization. Marine Scienes, 17(1)
Basaham, A. S., & El-Shater, A. (1994). Textural and mineralogical characteristics of the surficial sediments of Sharm Obhur, Red Sea coast of Saudi Arabia. Marine Scienes, 5(1), 51–71.
Basaham, A. S., Ghandour, I. M., & Haredy, R. (2019). Controlling factors on the geochemistry of Al-Shuaiba and Al-Mejarma coastal lagoons, Red Sea Saudi Arabia. Open Geosciences, 11(1), 426–439.
Batang, Z. B., Alikunhi, N., Gochfeld, M., Burger, J., Al-Jahdali, R., Al-Jahdali, H., Aziz, M. A., Al-Jebreen, D., & Al-Suwailem, A. (2016). Congener-specific levels and patterns of polychlorinated biphenyls in edible fish tissue from the central Red Sea coast of Saudi Arabia. Sci Total Environment, 572, 915–925.
Bellar, H., Barrick, R. C., Becker, S. (1986). Development of sediment quality values for Puget Sound. Prepared by Tetra Tech, Inc. for Resource Planning Associates/U.S. Army Corps of Engineers. Seattle District for the Puget Sound Dredged Disposal Analysis Program. Tetra Tech Inc. Bellevue, Washington
Bellou, N., Gambardella, C., Karantzalos, K., Monteiro, J. G., Canning-Clode, J., Kemna, S., Arrieta-Giron, C. A., & Lemmen, C. (2021). Global assessment of innovative solutions to tackle marine litter. Nature Sustainability, 4, 516–524. https://doi.org/10.1038/s41893-021-00726-2
Boonyatumanond, R., Wattayakorn, G., Togo, A., & Takada, H. (2006). Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand. Marine Pollution Bulletin, 52(8), 942–956.
Bouloubassi, I., Fillaux, J., & Saliot, A. (2001). Hydrocarbons in surface sediments from the Changjiang (Yangtze River) Estuary, East China Sea. Marine Pollution Bulletin, 42, 1335–1346.
Bourbonniere, R. A., & Meyers, P. A. (1996). Sedimentary geolipid records of historical changes in the watersheds and productivities of Lakes Ontario and Erie. Limnology and Oceanography, 41(2), 352–359.
Bray, E. E., & Evans, E. D. (1961). Distribution of n-paraffins as a clue to recognition of source beds. Geochimica Et Cosmochimica Acta, 22(1), 2–15.
Bryan, G. T. (1976). Heavy metal contamination in the sea. Marine Pollution, 3, 185–302.
Cesar, A., Lia, L. R. B., Pereira, C. D. S., Santos, A. R., Cortez, F. S., Choueri, R. B., & Rachid, B. R. F. (2014). Environmental assessment of dredged sediment in the major Latin American seaport (Santos, São Paulo—Brazil): An integrated approach. Science of the Total Environment, 497, 679–687.
Colombo, J. C., Pelletier, E., Brochu, C., Khalil, M., & Catoggio, J. A. (1989). Determination of hydrocarbon sources using n-alkane and polyaromatic hydrocarbon distribution indexes. Case study: Rio de la Plata estuary Argentina. Environmental Science & Technology, 23(7), 888–894.
Commendatore, M. G., & Esteves, J. L. (2004). Natural and anthropogenic hydrocarbons in sediments from the Chubut river (Patagonia, Argentina). Marine Pollution Bulletin, 48, 910–918.
Derraik, J. G. B. (2002). The pollution of the marine environment by plastic debris: A review. Marine Pollution Bulletin, 44(9), 842–852.
Dicks, B. (1987). Pollution. In A. Edwards & S. M. Head (Eds.), Key Environments: The Red Sea (pp. 383–404). Pergamon Press.
Diefendorf, A. F., Freeman, K. H., & Wing, S. L. (2014). A comparison of terpenoid and leaf fossil vegetation proxies in Paleocene and Eocene Bighorn Basin sediments. Organic Geochemistry, 71, 30–42.
Diem, A., Tesfaldet, Y. T., Hocherman, T., Hoon, V., & Zijlemans, K. (2023). Marine litter in the Red Sea: Status and policy implications. Marine Pollution Bulletin, 187, 114495.
Dong, C. D., Huang, C. P., Nguyen, T. B., Hsiung, C. F., Wu, C. H., Lin, Y. L., & Hung, C. M. (2019). The degradation of phthalate esters in marine sediments by persulfate over iron–cerium oxide catalyst. Science of the Total Environment, 696, 133973.
DouAbul, A. A., Heba, H. M., & Fareed, K. H. (1997). Polynuclear aromatic hydrocarbons (PAHs) in fish from the Red Sea Coast of Yemen. Asia-Pacific Conference on Science and Management of Coastal Environment (pp. 251–262). Springer.
Durmuş, K. A. Y. A. (2019). Sustainable domestic solid waste management in Jeddah, Saudi Arabia. Journal of International Environmental Application and Science, 14(4), 193–198.
Eerkes-Medrano, D., Thompson, R. C., & Aldridge, D. C. (2015). Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Research, 75, 63–82.
Eglinton, G., & Hamilton, R. J. (1967). Leaf epicuticular waxes. Science, 156, 1322–1335.
El Nemr, A., El-Sadaawy, M. M., Khaled, A., & El-Sikaily, A. (2014). Distribution patterns and risks posed of polycyclic aromatic hydrocarbons contaminated in the surface sediment of the Red Sea coast (Egypt). Desalination and Water Treatment, 52(40–42), 7964–7982.
El Nemr, A., El-Sikaily, A., Khaled, A., Said, T. O., & Abd-Alla, A. M. (2004). Determination of hydrocarbons in mussels from the Egyptian Red Sea coast. Environmental Monitoring Assessment, 96(1–3), 251–261.
El Sayed, M. A. (2002a). Distribution and behavior of dissolved species of nitrogen and phosphorus in two coastal Red Sea lagoons receiving domestic sewage. Marine Sciences, 13, 47–73.
El Sayed, M. A. (2002b). Nitrogen and phosphorus in the effluent of a sewage treatment station on the eastern Red Sea coast: Daily cycle, flux and impact on the coastal area. International Journal Environmental Studies, 59(1), 73–94.
El-Rayis, O. A., & Eid, F. M. (1997). Hydrography and water budget of Obhur creek, Red Sea. Marine Scienes, 8, 29–45.
El-Sikaily, A., Khaled, A., El Nemr, A., Said, T. O., & Abd-Alla, A. M. A. (2003). Polycyclic aromatic hydrocarbons and aliphatics in the coral reef skeleton of the Egyptian Red Sea Coast. Bulletin Environment Contamination Toxicology, 71(6), 1252–1259.
Eriksen, M., Mason, S., Wilson, S., Box, C., Zellers, A., Edwards, W., Farley, H., & Amato, S. (2013). Microplastic pollution in the surface waters of the Laurentian Great Lakes. Marine Pollution Bulletin, 77(1–2), 177–182.
Fan, S., Yan, Z., Qiao, L., Gui, F., Li, T., Yang, Q., & Ren, C. (2023). Biological effects on the migration and transformation of microplastics in the marine environment. Marine Environmental Research, 185, 105875.
Ficken, K. J., Li, B., Swain, D. E., & Eglinton, G. (2000). An n-alkanes proxy for the sedimentary input of submerged/floating fresh water aquatic macrophytes. Organic Geochemistry, 31, 745–759.
Frysinger, G. S., Gaines, R. B., Xu, L., & Reddy, C. M. (2003). Resolving the unresolved complex mixture in petroleum-contaminated sediments. Environmental Sci Technol, 37(8), 1653–1662.
Gao, L., Wang, Z., Peng, X., Su, Y., Fu, P., Ge, C., & Peng, L. (2022). Occurrence and spatial distribution of microplastics, and their correlation with petroleum in coastal waters of Hainan Island China. Environmental Pollution, 294, 118636.
Gao, Y., Han, Y., Xia, J., Tan, J., Wang, Y. P., & Wang, S. (2021). Composition and distribution of aliphatic hydrocarbon compounds and biomarkers in seafloor sediments from offshore of the Leizhou Peninsula (South China). ACS Omega, 6(50), 34286–34293.
Garcia, M. R., Cattani, A. P., da Cunha Lana, P., Figueira, R. C. L., & Martins, C. C. (2019). Petroleum biomarkers as tracers of low-level chronic oil contamination of coastal environments: A systematic approach in a subtropical mangrove. Environmental Pollution, 249, 1060–1070.
Gough, M. A., & Rowland, S. J. (1990). Characterization of unresolved complex mixtures of hydrocarbons in petroleum. Nature, 344, 648–650.
Harji, R. R., Yvenat, A., & Bhosle, N. B. (2008). Sources of hydrocarbons in sediments of the Mandovi estuary and the Marmugoa harbour, west coast of India. Environment International, 34(7), 959–965.
Hees, W. (1977). Sewage discharges from ships transiting coastal salt waters. J Am Water Resources Assoc, 13(2), 215–230.
Isobe, A., & Iwasaki, S. (2022). The fate of missing ocean plastics: Are they just a marine environmental problem? Science of the Total Environment, 825, 153935.
Jafarabadi, A. R., Dashtbozorg, M., Bakhtiari, A. R., Maisano, M., & Cappello, T. (2019). Geochemical imprints of occurrence, vertical distribution and sources of aliphatic hydrocarbons, aliphatic ketones, hopanes and steranes in sediment cores from ten Iranian Coral Islands, Persian Gulf. Marine Pollution Bulletin, 144, 287–298.
Jamoussi, B., Chakroun, R., & Al-Mur, B. (2022). Assessment of total petroleum hydrocarbon contamination of the red sea with endemic fish from Jeddah (Saudi Arabia) as bioindicator of Aquatic environmental pollution. Water, 14(11), 1706.
Jang, M., Shim, W. J., Han, G. M., Cho, Y., & Hong, S. H. (2023). Plastic debris as a mobile source of additive chemicals in marine environments: In-situ evidence. Science of the Total Environment, 856, 158893.
Keshavarzifard, M., Zakaria, M. P., Sharifinia, M., Grathwohl, P., Keshavarzifard, S., Sharifi, R., & Mehr, M. R. (2022). Determination of hydrocarbon sources in major rivers and estuaries of peninsular Malaysia using aliphatic hydrocarbons and hopanes as biomarkers. Environmental Forensics, 23(3–4), 255–268.
Killops, S. D., & Al-Juboori, M. A. H. A. (1990). Characterisation of the unresolved complex mixture (UCM) in the gas chromatograms of biodegraded petroleums. Organic Geochem, 15(2), 147–160.
Kingsley, O., & Witthayawirasak, B. (2020). Deterministic assessment of the risk of phthalate esters in sediments of U-Tapao Canal Southern Thailand. Toxics, 8(4), 93.
Kjerfve, B. (1994). Coastal lagoons. In: Elsevier oceanography series (Vol. 60, pp. 1–8). Elsevier
Knoppers, B., Kjerfve, B., & Carmouze, J. P. (1991). Trophic state and water turn-over time in six choked coastal lagoons in Brazil. Biogeochemistry, 14(2), 149–166.
Kostianaia, E. A., Kostianoy, A., Lavrova, O. Y., Soloviev, D. M. (2020). Oil pollution in the Northern Red Sea: A threat to the marine environment and tourism development. In: Environmental Remote Sensing in Egypt (pp. 329–362). Springer, Cham
Kvenvolden, K. A., Rapp, J. B., & Hostettler, F. D. (1990). Hydrocarbon geochemistry of hydrothermal generated petroleum from Escanaba Trough, offshore California, U.S.A. Applied Geochemistry, 5, 83–91.
Laws, E. A. (1993). Oil Pollution. Aquatic Pollution: An Introductory Text (2nd ed., pp. 417–458). Wiley.
Long, E. R., & Morgan, L. G. (1990). The potential for biological effects of sediment-sorbed contaminants tested in the National Status and Trends Program. NOAA Technical Memorandum NOS OMA 52 (p. 175). National Oceanic and Atmospheric Administration Seattle.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
Loya, Y. (1975). Possible effects of water pollution on the community structure of Red Sea corals. Marine Biology, 29, 177–185.
Malins, D. C. (1977). Metabolism of aromatic hydrocarbons in marine organisms. Annals New York Acad Sci, 298(1), 482–496.
Mandura, A. S. (1997). A mangrove stand under sewage pollution stress: Red Sea. Mangroves Salt Marshes, 1(4), 255–262.
Mazurek, M. A., & Simoneit, B. R. T. (1984). Characterization of biogenic and petroleum derived organic matter in aerosols over remote, rural and urban areas. In L. H. Kieth (Ed.), Identification and Analysis of Organic Pollutants in Air (pp. 353–370). Ann Arbor Science/Butterworth Publishers.
Medeiros, P. M., Bicego, M. C., Castelao, R. M., Rosso, D. C., Fillmann, G., & Zamboni, A. T. (2005). Natural and anthropogenic hydrocarbon inputs to sediments of Patos Lagoon estuary, Brazil. Environment International, 31, 77–87.
Moldowan, J. M., Seifert, W. K., & Gallegos, E. J. (1985). Relationship between petroleum composition and depositional environment of petroleum source rocks. Bulletin - American Association of Petroleum Geologists, 69(8), 1255–1268.
Ndungu, K., Beylich, B. A., Staalstrøm, A., Øxnevad, S., Berge, J. A., Braaten, H. F. V., & Bergstrøm, R. (2017). Petroleum oil and mercury pollution from shipwrecks in Norwegian coastal waters. Science of the Total Environment, 593, 624–633.
Neves, P. A., Costa, P. G., Portz, L. C., Garcia, M. R., & Fillmann, G. (2023). Levels and sources of hydrocarbons in the Patos Lagoon estuary and Cassino Beach mud bank (South Atlantic, Brazil): Evidence of transference between environments. Environmental Monitoring and Assessment, 195(4), 484.
Nilsen, O. G., Haugen, O. A., Zahlsen, K., Halgunset, J., Helseth, A., Aarset, H., & Eide, I. (1988). Toxicity of n-C9 to n-C13 alkanes in the rat on short term inhalation. Pharmacology & Toxicology, 62(5), 259–266.
Peng, L., Fu, D., Qi, H., Lan, C. Q., Yu, H., & Ge, C. (2020). Micro-and nano-plastics in marine environment: Source, distribution and threats—A review. Science of the Total Environment, 698, 134254.
Periáñez, R. (2020). A Lagrangian oil spill transport model for the Red Sea. Ocean Engineering, 217, 107953.
Perkins, S. (2015). Nearly every seabird may be eating plastic by 2050. Retrieved from: http://www.sciencemag.org/news/2015/08/nearly-every-seabird-may-be-eating-plastic-2050
Peters, K. E., & Moldowan, J. M. (1993). The Biomarker Guide: Interpreting Molecular Fossils in Petroleum and Ancient Sediments. Prentice-Hall.
Por, F. D. (2012). Lessepsian migration: The influx of Red Sea biota into the Mediterranean by way of the Suez Canal. Springer.
PTI (PTI Environmental Services; Barrick R, Becker S, Brown L, Beller H, Pastorok R). (1988). Sediment quality values refinement: 1988 update and evaluation of Puget Sound AET. Volume 1. PTI Environmental Services. Bellevue, Washington. Pp. 173
Ramirez, M. M. B., Caamal, R. D., & von Osten, J. R. (2019). Occurrence and seasonal distribution of microplastics and phthalates in sediments from the urban channel of the Ria and coast of Campeche, Mexico. Science of the Total Environment, 672, 97–105.
Rasul, N. M. A. (2015). Lagoon sediments of the Eastern Red Sea: Distribution processes, pathways and patterns. In N. M. A. Rasul & I. C. F. Stewart (Eds.), The Red Sea: The Formation, Morphology, Oceanography and Environment of a Young Ocean Basin (pp. 281–316). Springer.
Rinkevich, B., & Loya, Y. (1979). Laboratory experiments on the effects of crude oil on the Red Sea coral Stylophora pistillate. Marine Pollution Bulletin, 10, 328–330.
Rushdi, A. I., Abubakr. M. M., Hebba, H. (1994). Marine habitats of Alurj-Alsalif and Dubab-Yakhtul areas: Ecology, environment and management. Final Report, Department of Oceanography, Sana'a University, pp. 117
Rushdi, A. I., Simoneit, B. R., Lijotra, L., Bazeyad, A. Y., Dumenden, R., El-Mubarak, A. H., & Al-Mutlaq, K. F. (2022b). Phthalates, non-phthalates, polychlorinated biphenyls, and phenyl phosphates in atmospheric suspended particulate matter of Dhahran City, Saudi Arabia: levels and seasonal variation. International Journal of Environmental Science and Technology, 20(4), 1–16.
Rushdi, A. I., Al-Mutlaq, K. F., Simoneit, B. R. T., Al-Azri, A., DouAbul, A. A. Z., Al-Zarban, S., & Al-Yamani, F. (2010). Characteristics of lipid tracers to the Arabian Gulf by run-off from rivers and atmospheric dust transport. Arabian Journal of Geosciences, 3, 113–131.
Rushdi, A. I., Al-Shaikh, I., El-Mubarak, A. H., Alnaimi, H. A., Al-Shamary, N., Hassan, H. M., & Abou Assali, M. (2017b). Characteristics and sources of anthropogenic and biogenic hydrocarbons in sediments from the coast of Qatar. Marine Pollution Bulletin, 124(1), 56–66.
Rushdi, A. I., Al-Shaikh, I., El-Mubarak, A. H., Alnaimi, H. A., Al-Shamary, N., Hassan, H. M., & Assali, M. A. (2017a). Characteristics and sources of anthropogenic and biogenic hydrocarbons in sediments from the coast of Qatar. Marine Pollut Bull, 124(1), 56–66.
Rushdi, A. I., DouAboul, A. A., Mohammed, S. S., & Simoneit, B. R. T. (2006). Distribution and sources of extractable organic matter in the Mesopotamian wetland marsh sediments of Iraq: I - aliphatic lipids. Environmental Geology, 50, 857–866.
Rushdi, A. I., DouAbul, A. A., Al-Maarofi, S. S., & Simoneit, B. R. (2018). Impacts of Mesopotamian wetland re-flooding on the lipid biomarker distributions in sediments. Journal of Hydrology, 558, 20–28.
Rushdi, A. I., DouAbul, A. A., Simoneit, B. R., El-Mubarak, A. H., Al-Mutlaq, K. F., Qurban, M. A., & Goni, M. A. (2014b). Nonpolar lipid tracers in sediments from the Shatt al-Arab River of Iraq and the northwestern Arabian Gulf. Arabian Journal of Geosciences, 7, 5495–5508.
Rushdi, A. I., El-Mubarak, A. H., Simoneit, B. R., Goni, M. A., Qurban, M. A., Bazeyad, A. Y., & Al-Mutlaq, K. F. (2022a). Natural and anthropogenic sources of extractable organic matter in sediments from the coastal zone of the Arabian Gulf in Saudi Arabia. Arabian Journal of Geosciences, 15(17), 1–17.
Rushdi, A. I., Kassim, T. A. T. A., & Simoneit, B. R. T. (2009). Sources of organic tracers in sediments from the coastal zone of Ras Abu el-Darag, Gulf of Suez. Environmental Geology, 58, 1675–1687.
Rushdi, A. I., & Simoneit, B. R. T. (2002a). Hydrothermal alteration of organic matter in sediments of the northeastern Pacific Ocean: Part 1. Middle Valley. Juan De Fuca Ridge. Appl Geochem, 17, 1401–1428.
Rushdi, A. I., & Simoneit, B. R. T. (2002b). Hydrothermal alteration of organic matter in sediments of the northeastern Pacific Ocean: Part 2. Gorda Ridge. Escanaba Trough. Appl Geochem, 17, 1467–1494.
Rushdi, A. I., Simoneit, B. R. T., DouAbul, A. A. Z., Al-Mutlaq, K. F., El-Mubarak, A. H., Qurban, M., & Goni, M. A. (2014a). Occurrence, distribution and sources of polar lipid tracers in sediments from the Shatt al-Arab River, Iraq – Polar compounds. Sci Tot Environ, 470–471, 180–192.
Ryan, P.G., (2015). A brief history of marine litter research. In: Marine Anthropogenic Litter. Springer, pp. 1–25. https://doi.org/10.1007/978-3-319-16510-3_1
Salem, D. M. A., Morsy, F. A. E. M., El Nemr, A., El-Sikaily, A., & Khaled, A. (2014). The monitoring and risk assessment of aliphatic and aromatic hydrocarbons in sediments of the Red Sea. Egypt. Egypt Journal Aquatic Research, 40(4), 333–348.
Shetaia, Y. M., Mohamed, T. M., Farahat, L. A., & ElMekawy, A. (2016). Potential biodegradation of crude petroleum oil by newly isolated halotolerant microbial strains from polluted Red Sea area. Marine Pollution Bull, 111(1), 435–442.
Shirneshan, G., Bakhtiari, A. R., & Memariani, M. (2016). Distribution and origins of n-alkanes, hopanes, and steranes in rivers and marine sediments from Southwest Caspian coast, Iran: Implications for identifying petroleum hydrocarbon inputs. Environmental Science and Pollution Research, 23, 17484–17495.
Simoneit, B. R. T. (1977a). Diterpenoid compounds and other lipids in deep-sea sediments and their geochemical significance. Geochimica Et Cosmochimica Acta, 41, 463–476.
Simoneit, B. R. T. (1977b). Organic matter in eolian dusts over the Atlantic Ocean. Marine Chemistry, 5, 443–464.
Simoneit, B. R. T. (1978). The organic chemistry of marine sediments. In J. P. Riley & R. Chester (Eds.), Chemical Oceanography (2nd ed., pp. 233–311). Academic Press.
Simoneit, B. R. T. (1984). Organic matter of the troposphere - III. Characterization and sources of petroleum and pyrogenic residues in aerosols over the western United States. Atmospheric Environment, 18, 51–67.
Simoneit, B. R. T. (1985). Application of molecular marker analysis to vehicular exhaust for source reconciliation. Int J Environ Analyt Chem, 22, 203–233.
Simoneit, B. R. T. (1989). Organic matter of troposphere – V: Application of molecular analysis to biogenic emissions into troposphere for source reconciliations. Journal of Atmospheric Chemistry, 8, 251–275.
Simoneit, B. R. T., Crisp, P. T., Mazurek, M. A., & Standley, L. J. (1991a). Composition of extractable organic matter of aerosols from the Blue Mountains and southeast coast of Australia. Environment International, 17, 405–419.
Simoneit, B. R. T., & Mazurek, M. A. (1982). Organic matter of the troposphere - II. Natural background of biogenic lipid matter in aerosols over the rural western United States. Atmospheric Environment, 16, 2139–2159.
Simoneit, B. R. T., Sheng, G., Chen, X., Fu, J., Zhang, J., & Xu, Y. (1991b). Molecular marker study of extractable organic matter in aerosols from urban areas of China. Atmospheric Environment, 25A, 2111–2129.
Sivadas, S. K., Mishra, P., Kaviarasan, T., Sambandam, M., Dhineka, K., Murthy, M. R., & Hoehn, D. (2022). Litter and plastic monitoring in the Indian marine environment: A review of current research, policies, waste management, and a roadmap for multidisciplinary action. Marine Pollution Bulletin, 176, 113424.
Squillante, J., Scivicco, M., Ariano, A., Nolasco, A., Esposito, F., Cacciola, N. A., & Cirillo, T. (2023). Occurrence of phthalate esters and preliminary data on microplastics in fish from the Tyrrhenian sea (Italy) and impact on human health. Environmental Pollution, 316, 120664.
Surhoff, T. J., & Scholz-Bottcher, B. M. (2016). Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics - a lab experiment. Marine Pollution Bulletin, 102(1), 84–94.
Ten Haven, H. L., De Leeuw, J. W., Damsté, J. S., Schenck, P. A., Palmer, S. E., & Zumberge, J. E. (1988). Application of biological markers in the recognition of palaeohypersaline environments. Special Publication of the Geological Society of London, 40(1), 123–130.
Tolosa, I., De Mora, S. J., Fowler, S. W., Villeneuve, J. P., Bartocci, J., & Cattini, C. (2005). Aliphatic and aromatic hydrocarbons in marine biota and coastal sediments from the Gulf and the Gulf of Oman. Marine Pollution Bulletin, 50(12), 1619–1633.
Tolosa, I., de Mora, S., Sheikholeslami, M. R., Villeneuve, J. P., Bartocci, J., & Cattini, C. (2004). Aliphatic and aromatic hydrocarbons in coastal Caspian Sea sediments. Marine Pollution Bulletin, 48(1–2), 44–60.
Tranganida, A., Hall, A. J., Armstrong, H. C., Moss, S. E., & Bennett, K. A. (2023). Consequences of in vitro benzyl butyl phthalate exposure for blubber gene expression and insulin-induced Akt activation in juvenile grey seals. Environmental Pollution, 316, 120688.
Tsapakis, M., Dakanali, E., Stephanou, E. G., & Karakassis, I. (2010). PAHs and n-alkanes in Mediterranean coastal marine sediments: Aquaculture as a significant point source. Journal of Environmental Monitoring, 12(4), 958–963.
Uddin, S., Fowler, S. W., Saeed, T., Jupp, B., & Faizuddin, M. (2021). Petroleum hydrocarbon pollution in sediments from the Gulf and Omani waters: Status and review. Marine Pollution Bulletin, 173, 112913.
UNEP. (1985). The management and conservation of renewable marine resources in the Indian Ocean Region in the Red Sea and Gulf of Aden region. UNEP Regional Seas Reports & Studies, No. 64
van Wezel, A. P., van Vlaardingen, P., Posthumus, R., Grommentijn, G. H., & Sijm, D. T. H. (2000). Environmental risk limits for two phthalates with special emphasis on endocrine disruptive properties. Ecotox Environment Safe, 46, 305–321.
Vegter, A. C., Barletta, M., Beck, C., Borrero, J., Burton, H., Campbell, M. L., Costa, M. F., Eriksen, M., Eriksson, C., Estrades, A., & Gilardi, K. V. (2014). Global research priorities to mitigate plastic pollution impacts on marine wildlife. Endanger Species Res, 25, 225–247.
Walker, T. R., Grant, J., & Archambault, M. C. (2006). Accumulation of marine debris on an intertidal beach in an urban park (Halifax Harbour, Nova Scotia). Water Quality Research Journal of Canada, 41, 256–262.
Walker, T. R., Reid, K., Arnould, J. P. Y., & Croxall, J. P. (1997). Marine debris surveys at Bird Island, South Georgia 1990–1995. Marine Pollution Bulletin, 34, 61–65.
Wang, S., Liu, G., Yuan, Z., & Da, C. (2018). n-Alkanes in sediments from the Yellow River Estuary, China: Occurrence, sources and historical sedimentary record. Ecotoxicology and Environmental Safety, 150, 199–206.
Wang, X. C., Sun, S., Ma, H. Q., & Liu, Y. (2006). Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Jiaozhou Bay, Qingdao. China. Marine Pollution Bulletin, 52(2), 129–138.
Wilson, J. J., Al-Sofyani, A. A., & Marimuthu, N. (2017). Current biodiversity and ecological status of scleractinian corals of Sharm Obhur, Jeddah, Saudi Arabian coast of the Red Sea. Marine Biodiversity. https://doi.org/10.1007/s12526-017-0784-2
WPR (World Population Review). (2023). https://worldpopulationreview.com/
Xanthos, D., & Walker, T. R. (2017). International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Marine Pollution Bulletin, 118(1–2), 17–26.
Zhang, H., Zhao, C., & Na, H. (2021). Stability Enhancement of a Plastic Additive (dimethyl Phthalate, dMP) with Environment-Friendly Based on 3d-QSAR Model. Polish Journal of Environmental Studies, 30(4), 3885–3896.
Zhang, Z., Zhao, M., Eglinton, G., Lu, H., & HuangC-Y,. (2006). Leaf wax lipids as paleovegetational and paleoenvironmental proxies for the Chinese Loess Plateau over the last 170 kyr. Quaternary Science Reviews, 25(5–6), 575–594.
Ziegler, M., Roik, A., Porter, A., Zubier, K., Mudarris, M. S., Ormond, R., & Voolstra, C. R. (2016). Coral microbial community dynamics in response to anthropogenic impacts near a major city in the central Red Sea. Marine Pollution Bulletin, 105(2), 629–640.
Acknowledgements
This study was funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (NPST 13-ENV2233-02-R).
Author information
Authors and Affiliations
Contributions
HA contributed to conceptualization, methodology, investigation, data curation, writing—original draft, visualization, project administration, and funding acquisition. AR contributed to Methodology, investigation, formal data analysis, and writing—review & editing. NR contributed to Methodology, investigation, data curation, and writing—review & editing. AB contributed to methodology, investigation, and writing—review & editing. KA contributed to investigation and writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alharbi, H.A., Rushdi, A.I., Rasul, N. et al. Levels, spatial distributions, and provision of petroleum hydrocarbons and phthalates in sediments from Obhur lagoon, Red Sea coast of Saudi Arabia. Environ Geochem Health 46, 22 (2024). https://doi.org/10.1007/s10653-023-01793-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10653-023-01793-4