Abstract
Surface sediment samples from the Arabian Gulf coast of Saudi Arabia were collected by Van Veen grab sampler to identify the characteristics, distribution, and levels and to discriminate between natural and anthropogenic sources of the total extractable organic matter (TEOM). The dried and sieved sediments were extracted with a dichloromethane/methanol mixture for analysis by gas chromatography-mass spectrometry. The TEOM included n-alkanes (353.9 ± 283.8 ng.g−1), n-alkanols (283.2 ± 296.1 ng.g−1), fatty acid methyl esters (245.2 ± 353.7 ng.g−1), hopanes (100.7 ± 158.2 ng.g−1), steranes (58.5 ± 96.3 ng.g−1), triterpenoids (18.9 ± 21.1 ng.g−1), steroids (15.3 ± 17.0 ng.g−1), polycyclic aromatic hydrocarbons (PAHs) (0.48 ± 1.19 ng.g−1), as well as an unresolved complex mixture (UCM = 1633 ± 3151 ng.g−1) and petrochemicals (343.1 ± 424.2 ng.g−1). The major sources of these TEOM compound groups were anthropogenic (petroleum and petrochemical) and natural (lipids from higher plants, marine material, and microbiota) inputs. Anthropogenic contaminants from petroleum products ranged from 46.6 to 85.6% of the TEOM, whereas petrochemicals varied from 10.7 to 40.6%. The biogenic influx from terrestrial vegetation ranged from 5.7 to 19.3%, and marine biotic sources varied from 11.1 to 37.5%. The continuous accumulation of anthropogenic contaminants will ultimately affect the critical habitats of this marine coastal region. This provides a basis for further studies to understand human and developmental activities on input delivery, deposition processes, distribution, and biogeochemical alteration of organic matter in the coastal zones of the Arabian Gulf. Such studies are important for the sustainable development and protection of these key regional habitats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Arabian Gulf is a major source region for crude oil and an important shipping pathway for oil tankers. Its tropical marine habitats have been recognized by international organizations for their conversation, scientific, economic, and recreational values (Sheppard et al. 1992, 2010; Vogt 1995, 1996; George and John 1999; Qurban et al. 2011; Almahasheer 2018; Cusack et al. 2018). The need for conservation and protection arises from the rapid developmental activities in the region, with the concomitant introduction of a range of pollutants and the unplanned use of these habitats (Burt 2014; Burt and Bartholomew 2019). Petroleum exploitation and transport have been of particular concern in these coastal marine systems because of the obvious and detrimental short-term effects, although there is also evidence of subtle long-term consequences (Sadiq and McCain 1993; Burt et al. 2008; Issa and Vempatti 2018). However, it should be noted that there are considerable difficulties in distinguishing the subtle long-term effects of petroleum or any other pollution under field conditions due to the natural variability in ecosystems.
Previous studies have indicated that the main sources of contaminants in the Arabian Gulf are inputs from urbanization and tourism activities and from oil and industrial production activities (Vaughan et al. 2019). Much of the petroleum contaminants in the Arabian Gulf region are due to onshore and offshore oilfields, discharges from refineries and shipping traffic, petrochemical plants, regional recurrent wars, and possibly natural oil seeps (PME/UNEP 1989; Sadiq and McCain 1993; Michel et al. 1993; Soliman et al. 2019). The degree of oil pollution of shorelines around the Arabian Gulf has been a serious problem along major sections of the Gulf (Al-Arfaj and Alam 1993; Literathy 1993; Reynolds 1993; Massoud et al. 1998; Issa and Vempatti 2018; Soliman et al. 2019). Runoff from rivers and fallout of air particulate matter transported by wind are other major sources of organic components to the Arabian Gulf (Rushdi et al. 2009, 2010, 2014a, b). The Shatt al-Arab River, which is about 200 km in length, is the main river discharging into the Gulf. It deposits copious amounts of silt into the coastal zone of the northern part of the Gulf (Talling 1980; DouAbul et al. 1988), which ultimately introduces different amounts and types of organic and inorganic materials to the upper part of the Gulf (Rushdi et al. 2010, 2018). Dust storm episodes are ubiquitous on the Arabian Peninsula and extend offshore to impact the Arabian seas (Rushdi et al. 2017, 2019a). A wide variety of materials of different chemical compositions are introduced to oceanic ecosystems by atmospheric dust and fine aerosol components (Simoneit 1977, 1978, 2006).
Organic compositions and components of sediments are commonly applied to discriminate the various sources of chemical compounds (e.g., Rushdi and Simoneit 2002a, b; Birgel et al. 2004; Gogou and Stephanou 2004; Hopmans et al. 2004; Boot et al. 2006; Rushdi et al. 2006a, b, c, 2009). Source correlation of sediment organic matter has usually been utilized to distinguish specific inputs from point sources (Rushdi and Simoneit 2002a, b; Stiehl et al. 2005; Boot et al. 2006; Jaffé et al. 2006; Rushdi et al. 2019a, b).
Different organic sources will have different impacts on the biogeochemistry and biodiversity of the aquatic environment depending on the chemical components and levels of inputs. Most of the previous research on organic matter (OM) in the Gulf has focused mainly on oil pollution (Sadiq and McCain 1993; Zhao et al. 2015; Al-Saad et al. 2017; Marzooq et al. 2019). However, we still do not know the different fractions of natural and anthropogenic OM sources and their contributions to this coastal zone environment. Therefore, the objectives of this work are to characterize the compositions, levels, and distribution of the total extractable organic matter (TEOM) and to assess its sources in the surface sediments of the eastern coast of Saudi Arabia. This is based on key parameters and molecular marker analysis. Also, we will evaluate the possible impacts of these organic inputs on the habitats of this coastal ecosystem.
Study area
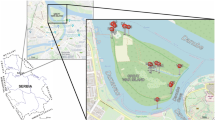
The coast of Saudi Arabia has about a 777 km shoreline from the Kuwait border to Salawa Bay at the border of Qatar (Fig. 1). The major ecosystems along this coastal zone include seagrasses, coral reefs, mangroves, saltmarshes, sabkha, rocky shores, and landfills (Price 1993; Price et al. 1991; Barth 2001). These habitats are important for maintaining the biological diversity of the marine environment of the region and providing precious natural and economic resources such as nursery areas for a variety of commercially important marine organisms. The coastal zone is also a significant fishery resource for the region, especially for shrimp (Price 1979, 1982; Burt and Bartholomew 2019; Vaughan et al. 2019).
Experimental procedures
Sampling
Shallow surface sediments were collected by Van Veen samplers from the Arabian Gulf along the coastal zone of Saudi Arabia, where twelve sites were selected for sample collection (Fig. 1). The location names, coordinates, and water depths of the selected sites, which ranged from 0.2 to 20.3 m, are listed in Table 1. The sites were divided into nearshore and offshore locations from Al-Khafji in the north to Al-Uqair in the south. About 35 g of each wet sediment subsample was taken, dried at room temperature, ground with mortar and pestle, and sieved to obtain < 125 µm fine particles.
Extraction
The extraction method was the same procedure as that described by Rushdi et al. (2014a, b). About 15 g of each dried and ground sample was extracted three times using ultrasonic agitation for a 15 min period each with 20 mL of dichloromethane (DCM) and 10 mL of methanol (MeOH). The extraction was carried out in a 150 mL precleaned beaker. A filtration unit with an annealed glass fiber filter (Whatman, GF/A filters, particle retention = 1.6 µm) was used to separate the sediment particles from the extract. We first concentrated the filtrate on a rotary evaporator and then reduced it to a volume of about 50 µL by a stream of dry nitrogen gas. The volume of the extract was then adjusted to 100 µL exactly by the addition of DCM:MeOH (2:1, v:v).
Instrumental analysis
Instrumental analysis by gas chromatography-mass spectrometry (GC–MS) was performed by an Agilent 6890 gas chromatography coupled to a 5973MSD operated in the electron impact mode at 70-eV ion source energy. The gas chromatography (GC) was fitted with a 30 m × 0.25 mm fused capillary column coated with 0.25-μm film thickness DB-5MS (Agilent). Helium was the carrier gas at a constant flow rate of 1.3 ml.min−1 (split ratio = 1:1.2), and the samples were injected in the splitless mode (splitless time of 30 s). The GC was temperature programmed from 65 (2-min initial hold) to 310 °C at 6 °C min−1 (isothermal for 20-min final time). The MS was operated in the electron impact mode at 70-eV ion source energy and scanned from 50 to 650 dalton at 1.27 scan s−1. Mass spectrometric data were acquired and processed using the GC–MS ChemStation software (NIST98-library) data system.
Before analysis by GC–MS, we derivatized an aliquot (50 uL) of each total extract with a silylating reagent [N,O-bis(trimethylsilyl)trifluoroacetamide, BSTFA, Pierce Chemical Co.] to replace the H in hydroxyl groups with a trimethylsilyl [(CH3)3Si, i.e., TMS] group for better GC resolution of polar compounds. Each sample was injected splitless with an injector temperature of 300 °C.
Identification and quantification
The identifications of n-alkanes, hopanes, steranes, polycyclic aromatic hydrocarbons (PAHs), plasticizers (mainly phthalates), fatty acid methyl esters (FAME), fatty alcohols, sterols, triterpenoids are based primarily on their key ion pattern and mass spectra (i.e., fragmentograms m/z 85, 191, 217/218, 128/178/202/228/252/276/300, 177, 149, 87, 103, and 189, respectively) and gas chromatographic retention times that were compared with those of external standards. Other anthropogenic compounds were identified from their mass spectra. Quantification was performed from the total ion current (TIC) GC profiles using the external standard method with authentic compounds of each homologous series (Rushdi et al. 2006a, b, 2014a, b).
The external standards, which are shown in Figure SM-1, were from Sigma-Aldrich and included n-alkanes (nC10–nC40; total concentration = 500 ng.µL−1), standard mixture (phenanthrene = 14 ng.µL−1, methyl hexadecanoate = 16 ng.µL−1, nonadecane-2-one = 12 ng.µL−1, and tetracosane = 14 ng.µL−1), chrysene-d-12 (100 ng.µL−1), cholesterol (248 ng.µL−1), friedelin (584 ng.µL−1), and hexamethylbenzene (25 ng.µL−1). We assessed the limit of detection (LoD) and limit of quantification (LoQ) the same way with the samples. The least-square method was used to fit the concentrations of the different standards versus their relative responses, and the correlations were significant with correlation coefficients (R2 = 0.91–0.98). The LODs were 0.05–0.8 ng.µL−1 for n-alkanes, 0.2–2.0 ng.µL−1 for PAHs (chrysene), 0.2–2.7 ng.µL−1 for friedelin, 0.01–0.01 ng.µL−1 for cholesterol. The software SPSS 16.0 (IBM-Statistical Package for Social Science, version 16.0) was used to treat the data.
We calculated the average response factors for each compound, and the peak areas of the compounds derived from the TIC trace were used for their quantifications. The integration parameters were selected from ChemStation integrator with an initial threshold of 10. Relative ion counts were converted to compound mass using the area counts of the external standards determined under the same instrumental operating conditions. The following formula: [C(s) (ng/g) = Cst (ng.µL). Vinj,st (µl).As (count). VTEXT (µL)]/[Ast (count). Vinj,s (µL). wtsed (g)] was used to calculate the concentration of each compound; where C, A, V, and wt are concentration, count, volume, and weight; the subscripts s, st. inj, and sed represent the sample, standard injected, and sediment, respectively.
Recovery blank and quality control
Fiber filters and sediment samples were spiked with n-tetracosane-d50 to test the recoveries, which were 92.8% and 72.9%, respectively. The measured concentrations were corrected accordingly. Both procedural blanks for sediments and solvents were analyzed and quantified to make sure there were no significant background interferences. We performed blank extracts with patches of three samples throughout the entire chemical analysis.
The technical accuracy of the research was one of the main considerations throughout this study and included sample collection, analytical methods, chemistry, geochemical interpretation, and biomarker tracer determination.
Results and discussion
Examples of the main features of the GC–MS data and the major compounds in the total extractable organic matter (TEOM), identified in the sediment extracts are shown in Fig. 2. The TEOM included lipids from both natural and anthropogenic sources. The major natural organic compounds consisted of wax n-alkanes, fatty acid methyl esters, and n-alkanols, steroids, and triterpenoids. The anthropogenic compounds included n-alkanes, hopane and sterane biomarkers, PAHs, miscellaneous petrochemicals, and a UCM.
GC–MS total ion current traces of total extracts from nearshore sediment samples of a station 2 (Al-Khafji) and b station 8 (Dammam), showing the major organic compounds (as TMS) (● n-alkanes, ○ methyl n-alkanoates, □ n-alkanols, DBP = dibutyl phthalate, DOP = dioctyl phthalate, A = 2,4-dioctyl phenol, B = 2-mercaptobenzothiazole, C = diethylene glycyl-p-(1,1,3,3-tetramethylbutyl)phenol, D = N-phenylbenzenesulfonamide, and E = 2,2′-methylenebis[6-(t-butyl)-4-methyl]phenol. The numbers indicate the carbon chain length
The presence, characteristics, and distribution patterns of the different compounds of TEOM from environmental samples can be used to classify their sources (Simoneit 1977, 1984, 1985; Bouloubassi et al. 2001; Rushdi et al. 2010, 2014a, b, 2017, 2018, 2019a). Therefore, known sources and mixtures of compounds in the environment can be compared. Accordingly, the TOEM compounds are reported and discussed here in order to define the sources of natural and anthropogenic inputs.
n-Alkanes
The dominant n-alkanes were in the range of C13 to C35 with maximum (Cmax) concentrations at lower molecular weights mainly at pentadecane, heptadecane, eicosane, docosane, or tetracosane (Cmax = 15, 17, 20, 22, or 24). The total concentrations ranged from 61 to 1080 ng.g−1 dry weight (dw) with a mean of 354 ± 284 ng.g−1 (Table 2). The lowest concentration was observed for the sediment from station 8 (Al-Jubail conservation area) and the highest from station 10 (near Dammam). The odd-numbered n-alkanes were dominant over the entire range for some samples and the carbon preference index (CPIo/e, Mazurek and Simoneit 1984) has been calculated using the following equation:
where ΣCi(o) and ΣCi(e) are the total concentrations of odd- and even-numbered n-alkanes, respectively. The CPI(o/e) values for the entire range varied from 0.75 to 3.42 (mean 1.63 ± 0.79). Pristane and phytane were not detected in any sample.
n-Alkanes are mainly derived from biogenic and anthropogenic sources and occur in all ecosystems. Their presence and distribution patterns are useful in assessing their sources and preservation conditions in the environment. The CPI and Cmax parameters of the most abundant n-alkane in the homologous series provide critical information about their sources and characteristics. Another useful parameter is the unresolved-to-resolved compound ratio (U:R), which can be applied to confirm the sources of hydrocarbons (Simoneit 1977, 1978, 2002; Mazurek and Simoneit 1984).
The different Cmax of the n-alkanes of these samples, mainly at 15, 17, 21, and 22 (Table 2) indicated that major sources were from marine biota, including algae and bacteria and petroleum input (Gelpi et al. 1970; Meyers 1997; Bouloubassi et al. 2001). The CPI(o/e) was used to assess the contribution of biogenic versus anthropogenic inputs (Scalan and Smith 1970; Simoneit 1989), where a high value (> 3.0) indicates a major input from terrestrial plants and a low value (~ 1.0) indicates that the major source is petroleum residues. The range has been divided into C13–C24 for marine inputs and C25–C35 for a higher plant wax contribution. The low range (C13–C24) CPI(o/e) values vary from 0.70 to 8.83 with an average of 2.15 ± 2.31 (Table 2) and indicate a mixture of both marine plants, bacterial residues, and petroleum inputs are major in these sediments. Except for station 10, nearshore to Dammam, its CPI(13–24) = 8.83 is comparatively high due to dominant input from coastal marine algae. The CPI(o/e) values for the C25–C35 range vary from 0.78 to 3.49 (mean 1.51 ± 0.75) and indicate that higher plant wax and petroleum are sources of these n-alkanes. The CPI(o/e) values for the entire n-alkane range (C13–C35) of these samples of 0.74–3.42 (mean 1.63 ± 0.79, Table 2), also confirm a mixture of natural and anthropogenic sources. The lowest CPI(o/e) values are observed for station 2 (CPI(o/e) = 0.89, near shore of Al-Khafji), station 3 (CPI(o/e) = 0.74, offshore Saffaniyah) and station 7 (CPI(o/e) = 0.98, Khursaniyah), indicating that petroleum is the major source of n-alkanes for these locations.
To estimate the relative input from different sources, the concentrations of terrestrial plant wax n-alkanes (i.e., C27, C29, C31, C33) were calculated according to Simoneit et al. (1991a) and found to range from 2.5 to 38.5 ng.g−1 (mean 17.1 ± 13.1 ng.g−1, Table 2). We have used the same method to estimate the natural n-alkane (NAx) contributions of marine algae (i.e., total of C15, C17, and C19) and bacteria (total of C16, C18, and C20) by the following equation:
where Ci is the Cmax of n-alkanes and Ci-1 and Ci+1 are the carbon numbers before and after the Ci. Thus, the concentrations of n-alkanes from marine algae and bacteria ranged from 4.0 to 101.4 ng.g−1 (mean 33.7 ± 26.6 ng.g−1) and from 0.0 to 6.7 ng.g−1 (mean 1.3 ± 2.1 ng.g−1), respectively.
Accordingly, the contributions from both marine and terrestrial natural sources range from 10.2 to 117.0 ng.g−1 (mean 52.1 ± 29.6 ng.g−1), and for n-alkanes from fossil fuel sources 53.9 to 936.0 ng.g−1 (mean 302.9 ± 261.3 ng.g−1, Table 2). Apparently, the major source of n-alkanes on the eastern coast of Saudi Arabia is crude oil and petroleum by-products. This is shown by the significant correlation (R2 = 0.99) between petroleum n-alkanes and the total n-alkanes of the samples (Fig. 3d). The insignificant correlations between marine bacterial and terrestrial plant n-alkanes versus total n-alkanes (R2 = 0.0 and 0.4, respectively; Fig. 3b, c) confirm that both bacterial and terrestrial inputs are not major in the sediments. The marine plant inputs have some contribution to n-alkanes of the sediments as indicated by the relatively significant correlation (R2 = 0.59) between marine n-alkanes and total n-alkanes (Fig. 3a). The concentrations of n-alkanes from the Arabian Gulf coast sediments were about the same as concentrations in other contaminated coastal zones, such as Jiaozhou Bay, Qingdao, China (500–8200 ng.g−1; Wang et al. 2006). These concentrations were much lower compared to those reported from the Niger Delta, Nigeria (20–16,840 ng.g−1 dw; Oyo-Ita et al. 2010), Patagonia, San Jorge Gulf (0–13,047 ng.g−1; Commendatore et al. 2000), and Santos, SP, Brazil (170–107,800 ng.g−1; Medeiros and Bicego 2004).
The percentages of anthropogenic n-alkanes from petroleum inputs on the eastern coast of Saudi Arabia ranged from 61 at station 8 to 94% at station 3 (mean 82 ± 9%, Table 2). The natural n-alkane percentages ranged from 4 at stations 1 and 3 to 33% at station 8 (mean 12 ± 8%) from marine algae, and from 1 at stations 3, 7, and 10 and 15% at station 1 (mean 6 ± 5%) from terrestrial plants. The n-alkanes from bacterial sources were 0.0% at many stations to 1.8% at station 7. This indicates that petroleum by-products and marine algae are the major sources of n-alkanes in this coastal zone, followed by terrestrial plant wax and marine bacteria.
Fatty acids and alcohols
The methyl n-alkanoates (i.e., fatty acid methyl esters), were not detected in the sample blank and evidently, they were esterified natural fatty acids during sample preparation and extraction. Their concentrations in the sediment samples ranged from 13 to 728 ng.g−1 (mean 245 ± 354 ng.g−1). The highest concentration was detected at station 1 and the lowest at station 8. The series from C14 to C30 had a Cmax at 16 as acid, and an even-to-odd carbon preference indices (CPI(e/o)) from 3.8 at station 6 to > 100 at stations 11 and 12 (mean 61.0 ± 77.9, Tables 2 and SM-1). The total n-alkanol concentrations ranged from 35 to 1137 ng.g−1 (mean 283 ± 296 ng.g−1), where the lowest and highest concentrations were also observed at stations 8 and 10, respectively. The Cmax was at 28 or 30, and the CPI(e/o) ranged from 6.4 to 15.0 (mean 9.5 ± 2.8, Table 2).
Methyl n-alkanoates are generally of natural origin or may be formed by transesterification in the extraction solvent from fatty acids or wax esters present. Their sources are similar to n-alkanoic acids from terrestrial vegetation, marine phytoplankton, microbial mats, and bacteria. The n-alkanoic acids from terrestrial plants are characterized by even carbon numbered homologs > C20, whereas those from algae, plankton, and diatoms are characterized by also even carbon-numbered and branched homologs < C20 (Simoneit 1978; Perry et al. 1979; Volkman et al. 1980; Kharlamenko et al. 1995; Budge and Parrish 1998). n-Alkanoic acids from bacterial mats are characterized by odd carbon-numbered and branched homologs < C20 (Volkman et al. 1980; Rajendran et al. 1993; Harvey and Marko 1997; Parrish et al. 2000; Oyo-Ita and Oyo-Ita 2012; Yang et al. 2014). Accordingly, we treated all fatty acids > C20 as inputs from terrestrial sources, and all < C20 from marine inputs. The contributions of terrestrial inputs ranged from 1.2 at station 8 to 19.8 ng.g−1 at station 1 (mean 5.9 ± 5.9 ng.g−1), whereas the marine contributions ranged from 11.3 at station 8 to 1162 ng.g−1 at station 10 (mean 239 ± 334 ng.g−1). The ratios of terrestrial/marine were small (0.061 ± 0.059, Table 2), indicating that the dominant sources of fatty acids in these sediments were from marine biota, where the high values of CPI(e/o) (61 ± 78) confirmed that they were mainly from marine algae and planktonic diatoms but not bacteria.
The occurrence of n-alkanols in the environment with Cmax at 28, 30, or 32 and a strong even carbon-numbered predominance confirmed an input of terrestrial plant wax from tropical to semitropical environments (Simoneit 1977, 1978, 1989; Mudge 2005; Rushdi et al. 2006b; Treignier et al. 2006). Here, the high concentrations of n-alkanols with Cmax at 28 or 30 and CPI(e/o) > 4 support that the main source of these compounds is a terrestrial wax plant (Table 2). Also, the presence of the short-chain (< C20) homologs are reported as markers of microbial sources (Bianchi 2007; Robinson et al. 1984). The ratios of terrestrial/marine n-alkanols in these sediments were relatively high (1.98 ± 0.14, Table 2), indicating that the major source was terrestrial plant wax.
Hopane and sterane biomarkers
The occurrence of hopanes and or steranes in the environment indicates contamination from petroleum residues (e.g., Simoneit 1984; Simoneit et al. 1991b; Medeiros et al. 2005; Rushdi et al. 2016). Both hopane and sterane biomarkers were detected in the sediment samples, except for stations 11 and 12. The hopanes ranged from C27 to C35 with Cmax at 30, 29, or 31 and concentrations from 0.0 to 491 ng.g−1 (mean 101 ± 158 ng.g−1, Table 2). The maximum concentration was found for station 10. Hopanes are usually resistant to degradation and alteration in the environment (Simoneit et al. 2009); thus, they are used to validate contamination by petroleum or coal in the environment (Peters and Moldowan 1993). The hopanes in the sediment samples had a dominance of the thermodynamically stable 17α(H),21β(H) epimers, Cmax at 30, and minor 17β(H), 21α(H)-hopanes (Table 2). These epimeric configurations occur in crude oils and mature sedimentary rocks and are derived from the diagenetic interconversion of the 17(H),21(H)-hopane precursors of bacterial origins (Peters and Moldowan 1993). The distribution of the hopanes ranged from C27 to C35 for the α,β-series, with the homologs > C30 present as the typically mature C-22 R/S pairs (Simoneit 1984; Simoneit et al. 1990).
Commonly, the hopane distributions from petroleum and engine exhaust show higher 22S hopane concentrations relative to the corresponding 22R epimers (Simoneit 1984, 1985). The C31 and C32 S/(S + R) ratios of the extended hopanes ranged from 0.58 to 0.68 (mean 0.64 ± 0.04) and from 0.32 to 0.79 (mean 0.43 ± 0.14), respectively (Table 2). These values are in the range of mature crude oil and petroleum hydrocarbons (Peters and Moldowan 1993; Rushdi and Simoneit 2002a, b), and therefore confirm that petroleum is the source of the hopanes in sediments.
Sterane hydrocarbons are rarely found in gasoline or diesel fuels and are introduced into the environment from petroleum spills or lubricants in emissions of vehicular engines (Abas and Simoneit 1996; Rushdi et al. 2016). Ship-washing discharges, refinery activities, and municipal wastewaters are additional inputs of petroleum components to marine sediments (Laws 1993; Rushdi et al. 2014b). Thus, steranes are useful in confirming biomarker indicators for pollution by oil-related products in the marine environment (e.g., Moldowan et al. 1986; Aboul-Kassim and Simoneit 1996; Barakat et al. 1999). Steranes were also detected in the sediment samples, with dominant amounts of the C27 and C28 homologs. Their concentrations were significantly lower than those of the hopanes, ranging from 0.0 for stations 2–4 to 12 to 291 ng.g−1 for station 10 (mean 58.4 ± 96.3 ng.g−1, Table 2). The homologs comprised mainly the 5α,14α,17β and minor 5α,14α,17α configurations, both occurring as 20S and 20R epimers. The S/(S + R) ratio of sterane epimerization at C-20 for C27 was 0.34 to 0.67 (mean 0.53 ± 0.16), and for C29 from 0.49 to 0.75 (mean 0.59 ± 0.10, Table 2). These values indicated that the steranes were derived from petroleum sources.
Steroids and triterpenoids
The steroids and triterpenoids were also major components of the TEOM in these sediments (Fig. 2). The steroid concentrations ranged from 1.4 to 64.5 ng.g−1 (mean 15.3 ± 17.1 ng.g−1). The highest concentration was at station 10 and the lowest at station 8. They ranged from C27 (cholesterol) to C29 (sitosterol) with a Cmax at 29.
Steroids occur in all ecosystems and are derived from the tissues of both fauna and flora (Akihisa et al. 1991). They have been used to classify the sources and identify the fate of organic matter in the environment (Volkman et al. 1981; Mudge and Norris 1997; Duan 2000; Rushdi et al. 2006b; Tolosa et al. 2014; Wisnieski et al. 2014). The major sterols in terrestrial plant lipids are campesterol, stigmasterol, and sitosterol (Moreau et al. 2002; Volkman et al. 2008), whereas the highest sterol in animal lipids and some phytoplankton and aquatic microbes is cholesterol (Volkman 1986; Bouloubassi et al. 1997; Voet and Voet 2004; Rampen et al. 2010). Brassicasterol, dinosterol, fucosterol, and minor cholesterol are dominant sterols in marine algae, diatoms, and dinoflagellates (Volkman and Smittenberg 2017 and references therein). The presence of campesterol, stigmasterol, and sitosterol in these samples supports an origin from terrestrial vascular higher plant sources (Barbier et al. 1981; Simoneit et al. 1983; Volkman 1986; Moreau et al. 2002; Volkman et al. 2008), whereas the occurrence of brassicasterol, dinosterol, and fucosterol is interpreted as input from marine biota (Bouloubassi et al. 1997; Volkman 1986; Rampen et al. 2010). The contributions of steroids from terrestrial vascular plants ranged from 1.0 at station 8 to 45.9 ng.g−1 at station 10 (mean 10.6 ± 12.1 ng.g−1). The steroid component from marine biota (i.e., algae, diatoms, and fauna) ranged from 0.4 at station 8 to 18.6 ng.g−1 at station 10 (mean 4.7 ± 5.0 ng.g−1, Table SM-1). Terrestrial higher plant detritus was the major source of steroids in these sediments as indicated by the elevated ratios of terrestrial/marine steroids, ranging from 1.79 to 3.37 (mean 2.32 ± 0.42).
The triterpenoids in these sediments were mainly tetrahymanol, tetrahymanone, and 17β(H), 21β(H)-bishomohopan-32-ol (Table SM-1) and ranged from 1.7 to 79.8 ng.g−1 (18.9 ± 21.1 ng.g−1, Table 2). The presence of these triterpenoids in the samples indicated a microbial origin, where the protozoan Tetrahymena pyriformis is considered to be the major source of tetrahymanol (Mallory et al. 1963; Holz and Conner 1973), excluding minor other sources. Therefore, tetrahymanol and its derivative tetrahymanone confirm that marine biota is an input of sedimentary organic detritus (Venkatesan 1989). The 17β(H),21β(H)-bishomohopan-32-ol is a known compound in sediments of aquatic environments as an early diagenetic product from bacteriohopanepolyol demonstrating oxidation or bacterial reworking of microbial organic detritus (Ourisson et al. 1979; Brassell et al. 1983).
Plasticizers and other petrochemicals
Plasticizers and other petrochemicals were significant components of these sediments (Fig. 2, Table 2). The major plasticizers included di-isobutyl phthalate (DiBP), dibutyl phthalate (DBP), and dioctyl phthalate (DOP). The petrochemicals were 2,4-dioctylphenol (CAS 1807–29-0), 2-mercaptobenzothiazole (CAS 149–30-4), diethylene glycyl p-(1,1,3,3-tetramethylbutyl)phenol (a Triton-X), N-phenylbenzenesulfonate (CAS 1678–25-7), and 2,2′-methylenebis(6-tert-butyl-4-methylphenol) (Advastab 405, CAS 119–47-1). Their total concentrations ranged from 27 to 2799 ng.g−1 (mean 491 ± 756 ng.g−1) of the TEOM. The antioxidant Advastab 405 was a major compound and ranged from 0.00 to 334 ng.g−1, with a maximum level at station 10 (Table SM-1). The major plasticizer was DOP ranging from 14 to 662 ng.g−1 (Table SM-1).
Elevated levels of phthalates have been detected in atmospheric total suspended particles (TSP) collected from the region (Rushdi et al. 2017). A recent study reported high concentrations of phthalates, non-phthalates (adipates and mellitates), phenyl phosphates, and polychlorinated biphenyls in atmospheric suspended particles from the Dhahran area in Saudia Arabia (Rushdi et al. 2022). The region is known as the largest industrial complex for the petrochemical and plastic industry in the Middle East (Picó et al. 2021) and is a major source of plasticizers, flame retardants, and pesticides in the regional environment (Rushdi et al. 2017; Saini et al. 2019). Littering is another source of these POPs (persistent organic pollutants) in the environment. Accordingly, the obvious sources of the plasticizer compounds in the Gulf coastal zones are emissions and spillage from the regional petrochemical industry, as well as leaching from plastic debris. These TSP plasticizers transported by wind and those leached from plastic debris in the coastal environment eventually accumulate in marine biota (Vered et al. 2019; Jebara et al. 2021; Sala et al. 2022; Hidalgo-Serrano et al. 2022) and can transfer across different trophic levels (Farrell and Nelson 2013; Setälä et al. 2014). Further studies are needed to investigate the plasticizer levels in marine organisms and their toxicity effects.
Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) were detected only in the sediments from the stations near Al-Khafji. The total concentrations were 1.86 ng.g−1 and station 2 to 3.87 ng.g−1 at station 1 (Table 2). The major PAHs detected in these sediments included fluoranthene, pyrene, benzo[g,h,i]fluoranthene, cyclopenta[c,d]pyrene, benzo[k]fluoranthene, benzo[a]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, and perylene (Table SM-1). The concentrations were in the range measured at other locations of the Gulf (Soliman et al. 2014, 2019; Gevao et al. 2016). The absence of low molecular weight aromatic and alkyl aromatic hydrocarbons (i.e., alkylnaphthalenes and phenanthrene/alkylphenanthrenes) is likely due to their removal as a result of their high volatility and water solubility (Kawka and Simoneit 1990). The summertime high temperature in the region causes considerable loss of PAHs, especially low molecular weight PAHs (Soliman et al. 2019). Also, the sediment properties and the water current direction affect the levels of PAHs in the Gulf, where higher concentrations have been reported in the semi-enclosed area such as bays and harbors (Gevao et al. 2016; Soliman et al. 2019).
The ratio of fluoranthene/(fluoranthene + pyrene) has been used to differentiate between petrogenic and combustion sources, where a low value (< 0.4) indicates petroleum inputs and a high value (> 0.5) implies combustion sources (Rogge et al. 1993; Yunker et al. 2002, 2012). Here the ratios were > 0.5, similar to the values obtained by others (Gevao et al. 2016; Soliman et al. 2019) and confirming that these PAHs were from combustion.
Unresolved complex mixture (UCM)
The UCM consists of branched and cyclic compounds above the baseline and under the envelope of the resolved compounds (Fig. 2). The UCM concentrations ranged from 14 at station 9 to 11,388 ng.g−1 at station 10 (mean 1633 ± 3151 ng.g−1, Table 2). The ratio U:R (UCM-to-resolved compound concentrations, Mazurek and Simoneit 1984) ranged from 0.03 to 1.61 (mean 0.57 ± 0.58). The lowest ratio was found offshore of Dammam (station 9) and the highest offshore of Al-Khafji (station 1). High levels of UCM were detected mainly in the semi-enclosed area, locales close to oil production, and near seaports such as Al-Khafji town in the north (stations 1 and 2) and Dammam city (stations 10 and 11). These locales are less affected by water currents, resulting in additional accumulation of organic matter in sediments.
The major sources of the UCM (Fig. 2) are oil spills and/or fossil fuel utilization (Simoneit 1984, 1985; Tolosa et al. 2004; Harji et al. 2008). The presence of a narrow UCM is distinctive for a gasoline source, whereas a broader UCM is exhibited by the input of diesel or lubricant oils, and both envelopes can be derived from refined crude oil (Simoneit 1984, 1985). Hydrocarbons from higher plants have no UCM (Simoneit and Mazurek 1982). Microbial detritus can produce a UCM with a Cmax at 19–22 under an anaerobic condition (Simoneit et al. 1979). The UCM derived exclusively from fossil fuel utilization was evaluated as a ratio of UCM to biogenic (marine and terrestrial) n-alkanes (U:nCB ratio) to assess the level of oil and petroleum contamination, where high values of the ratio suggest contamination by biodegraded petroleum residues (Peters and Moldowan 1993; Rushdi et al. 2014b). The U:nCB ratio ranged from 0 to 97 (mean 23 ± 28, Table 2) and a value > 3 was considered to be contaminated. Obviously, all sites were contaminated, except station 9 (offshore of Dammam), and the most polluted were the nearshore of Dammam city (station 10) and offshore of Al-Khafji (stations 1 and 2).
Station similarities and anthropogenic versus natural sources
The data of the related compound groups in TEOM were examined by principal component analysis (PCA), using Varimax rotation to examine the similarities between the different stations. Two significant components (C1 and C2) were identified by the PCA output explaining 94.62% of the variance at an eigenvalue of > 1 (Table SM-2, Fig. SM-2). We used factor loadings of > 0.75 for each component. The C1 revealed a variance of 81.54% with S10, S5, S1, and S2 suggesting that they had similar sources of organic matter. The variance of C2 was 13.08% with S9, S4, S3, S7, and S6 indicating that their organic matter sources were likely comparable but have been modified by physicochemical processes. These include wind and current directions and speeds, sediment deposition rates, oxygen concentration, and microbes at surface sediments (Hedges and Oades 1997; Zonneveld et al. 2010; Zakem et al. 2021).
Obviously, the contributions of anthropogenic versus natural biogenic sources of TEOM varied and depended on the locations of the sampling stations (Fig. 4). The estimated anthropogenic sources of n-alkanes ranged from 61 to 94% (mean 82 ± 9%), whereas the terrestrial biogenic sources ranged from 1.2 to 15.2% (mean 6.2 ± 4.5%), and the marine biota sources ranged from 5.2 to 32.5% (mean 12.3 ± 8.1%) (Fig. 4a). For TEOM (based on n-alkanes, hopanes, steranes, PAHs, plasticizers, and UCM), the anthropogenic sources were from 56.1 to 84.9% (mean 72.7 ± 8.7%), and the marine biogenic components were 9.4 to 24.1% (mean 16.7 ± 5.7%) and 4.9–19.7% (mean 10.9 ± 4.6%) for terrestrial and marine sources. The ternary plots of both n-alkanes and TEOM showed that anthropogenic inputs were dominant in this coastal zone, followed by marine and minor terrestrial higher plant contributions (Fig. 4). The results confirmed that marine traffic, crude oil industry, and human-related activities are the major detrital anthropogenic organic matter source and pollutants in this region. The low input from natural terrestrial sources (i.e., vegetation) to these sediments is attributed to the arid region with low vegetation around this coastal zone.
Environmental effects
Certain coastal areas are defined as critical habitats (Ray 1976; IUCN 1983; Dugan 1990) because they are productive locales providing breeding, feeding, nursing, or nesting places for marine organisms (Sheppard et al. 1992; Burt 2014). These critical habitats include fauna and flora sites (e.g., rocky shores), nesting locales for turtles and nursery floor for crustaceans (e.g., sandy beaches), as well as nursery grounds for shrimp (e.g., mangrove stands) (Vaughan et al. 2019). The ecosystems of the Arabian Gulf coastal zone including benthic macroalgae, mangrove stands, coral reefs, and seagrasses are very important sources of nutrients in the region (Ogden and Gladfelter 1983; Crossland et al. 1987). For example, benthic macroalgal productivity is significantly greater than of both microalgae and phytoplankton (Valiela et al. 1997).
The environmental effects of pollution from petroleum, plasticizers, and other petrochemicals on these critical habitats of the region are detrimental and expected (Al-Hurban 2013). Benthic species and largely their early life stages such as eggs and larvae are susceptible to such toxic waste. Coastal spawning and nursery floors are adversely affected by the presence of oil pollutants (Basson et al. 1977; Price 1979, 1982). Petroleum product inputs from oil transfer docks, oil refineries, oil tankers, and sewage treatment plants likely distress the coastal ecosystems and associated groups of species (Loya 1975; Rinkevich and Loya 1979; Dicks 1987). Studies have shown that the presence of petroleum reduced colonization in reefs (Dicks 1987); nevertheless, there is no concrete evidence that the reefs have declined due to oil pollution in the region (IUCN/UNEP 1985). The petroleum pollution effects on coral reefs and marine coastal ecosystems need further study. The inorganic nutrients exchange with dissolved organic matter (DOM) and particulate organic matter (POM), as well as animal migrations, can also be affected by the presence and elevated levels of organic pollutants (IUCN 1983; Por and Dor 1984).
Conclusions
The total solvent-extractable organic matter (TEOM) of the sediments from the Arabian Gulf coast of Saudi Arabia has been characterized using GC–MS techniques. The analyses showed that anthropogenic and biogenic sources both contributed to their organic matter contents. The presence of UCM, n-alkanes with CPI ~ 1, steranes, and hopanes from petroleum production and utilization in the region such as offshore oilfields, discharges from refineries and tanker traffic, and the possibility of natural oil seeps, are the major sources of TEOM. The anthropogenic sources of compounds from petroleum, its products, and plastic waste accumulation comprised 72.7 ± 8.7% of the TEOM. The abundance of anthropogenic organic compounds in the sediments of the Arabian Gulf coast depended on the location of the sampling site and the types of regional urban activities. Thus, the environmental effects of anthropogenic organic matter on the critical habitats of the coastal Arabian Gulf are possible and need more investigation.
The natural sources of organic compounds including n-alkanes (in part), n-alkanols, n-alkanoic acids, steroids, and triterpenoids are mostly from marine biota at 21.8 ± 9.4% of TEOM. The terrestrial detritus from higher plant influx to the TEOM is 16.7 ± 5.7%.
References
Abas MRB, Simoneit BRT (1996) Composition of extractable organic matter of air particles from Malaysia: initial studies. Atmos Environ 15:2779–2793
Aboul-Kassim TAT, Simoneit BRT (1996) Lipid geochemistry of surficial sediments from coastal environment of Egypt I. Aliphatic hydrocarbons - characterization and sources. Mar Chem 54:135–158
Akihisa T, Kokke WCMC, Tamura T, Matsumoto T (1991) Sterols of Kalanchoe pinnata: first report of the isolation of both C-24 epimers of 24-alkyl-Δ 25-sterols from a higher plant. Lipids 26:660–665
Al-Arfaj AA, Alam IA (1993) Chemical characterization of sediments from the Gulf area after the 1991 oil spill. Mar Poll Bull 27:97–101
Al-Hurban A (2013) Effects of recent anthropogenic activities on the surface deposits of Kuwait. Arab J Geosci 7:665–691
Almahasheer H (2018) Spatial coverage of mangrove communities in the Arabian Gulf. Environ Monit Assess 190:85
Al-Saad HT, Karem DS, Kadhim HA (2017) Total petroleum hydrocarbons in the soil of west Qurna-2 oil field southern Iraq. Intern J Mar Sci 7:51–58
Barakat AO, Mostafa AR, Rullkötter J, Hegazi AR (1999) Application of a multimolecular marker approach to fingerprint petroleum pollution in the marine environment. Mar Pollut Bull 38:535–544
Barbier M, Tusseau D, Marty JC, Saliot A (1981) Sterols in aerosols, surface microlayer and subsurface water in the northeastern tropical Atlantic. Oceanol Acta 4:77–84
Barth H-J (2001) Understanding coastal fluctuations at the Arabian Gulf leading to the “lost city of Gerrha.” Palaeoecol Africa 27:291–303
Basson PW, Burchard JE, Hardy JT, Price ARG (1977) Biotopes of the western Arabian Gulf. Aramco, Dhahran, 248pp
Bianchi TS (2007) Biogeochemistry of Estuaries. Oxford University Press Inc, New York
Birgel D, Stein R, Hefter L (2004) Aliphatic lipids in recent sediments of the Fram Strait/Yermak Plateau (Arctic Ocean): composition, sources and transport processes. Mar Chem 88:127–160
Boot CS, Ettwein VJ, Maslin MA (2006) A 35,000 year record of terrigenous and marine lipid in Amazon Fan sediments. Org Geochem 37:208–219
Bouloubassi I, Lipiatou E, Saliot A, Tolosa I, Bayona JM, Albaigés J (1997) Carbon sources and cycle in the western Mediterranean - the use of molecular markers to determine the origin of organic matter. Deep-Sea Res 2 Top Stud Oceanogr 44:781–799
Bouloubassi I, Fillaux J, Saliot A (2001) Hydrocarbons in surface sediments from the Changjiang (Yangtze River) Estuary, East China Sea. Mar Pollut Bull 42:1335–1346
Brassell SC, Eglinton G, Maxwell JR (1983) The geochemistry of terpenoids and steroids. Biochem Soc Trans 11:575–586
Budge SM, Parrish CC (1998) Lipid biogeochemistry of plankton, settling matter and sediments in Trinity Bay, Newfoundland II. Fatty acids. Org Geochem 29:1547–1559
Burt JA (2014) The environmental costs of coastal urbanization in the Arabian Gulf. City 18:760–770
Burt JA, Bartholomew A (2019) Towards more sustainable coastal development in the Arabian Gulf: opportunities for ecological engineering in an urbanized seascape. Mar Pollut Bull 142:93–102
Burt J, Bartholomew A, Usseglio P (2008) Recovery of corals a decade after bleaching in Dubai, United Arab Emirates. Mar Biol 154:27–36
Commendatore MG, Esteves JL, Colombo JC (2000) Hydrocarbons in coastal sediments of Patagonia, Argentina: levels and probable sources. Mar Pollut Bull 40:989–998
Crossland CJ, Dawson, Sheperd A, Smith S, Marshall JI (1987) Saudi Arabia: an analysis of coastal and marine habitats of the Red Sea. Saudi Arabia Marine Conservation Programme. Synoptic Report. National Union for Conservation of Nature, Geneva
Cusack M, Saderne V, Arias-Ortiz A, Masque P, Krishnakumar PK, Rabaoui L, Qurban MA, Qasem AM, Prihartato P, Loughland RA, Elyas AA (2018) Organic carbon sequestration and storage in vegetated coastal habitats along the western coast of the Arabian Gulf. Environ Res Lett 13:074007
Dicks B (1987) Pollution. In: Edwards A, Head SM (eds) Key Environments: The Red Sea. Pergamon Press, Oxford, pp 383–404
DouAbul A, Al-Saad H, Al-Timari A, Al-Rakabi H (1988) Tigris-Euphrates delta: a major source of pesticides to the Shatt al-Arab River (Iraq). Arch Environ Con Tox 17:405–418
Duan Y (2000) Organic geochemistryof recent marine sediments from the Nansha Sea, China. Org Geochem 31:159–167
Dugan PJ (ed) (1990) Wetland conservation: a review of current issues and required action. IUCN, Gland, 96 pp
Farrell P, Nelson K (2013) Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ Pollut 177:1–3
Gelpi E, Schneider H, Mann J, Oró J (1970) Hydrocarbons of geochemical significance in microscopic algae. Phytochem 9:603–612
George JD, John DM (1999) High sea temperatures along the coast of Abu Dhabi (UAE), Arabian Gulf—their impact upon coral and macroalgae. Reef Encounter 25:21–23
Gevao B, Boyle EA, Carrasco GG, Ghadban AN, Zafar J, Bahloul M (2016) Spatial and temporal distributions of polycyclic aromatic hydrocarbons in the northern Arabian Gulf sediments. Mar Pollut Bull 112:218–224
Gogou A, Stephanou EG (2004) Marine organic geochemistry of the Eastern Mediterranean 2. Polar biomarkers in Cretan Sea sturfacial sediments. Mar Chem 85:1–25
Harji RR, Yvenat A, Bhosle NB (2008) Sources of hydrocarbons in sediments of the Mandovi estuary and the Marmugoa harbour, west coast of India. Environ Intern 34:959–965
Harvey RH, Marko SA (1997) Kinetics of phytoplankton decay during simulated sedimentation: changes in lipids under oxic and anoxic conditions. Org Geochem 27:129–140
Hedges JI, Oades JM (1997) Comparative organic geochemistries of soils and marine sediments. Org Geochem 27:319–361
Hidalgo-Serrano M, Borrull F, Marcé RM, Pocurull E (2022) Phthalate esters in marine ecosystems: analytical methods, occurrence and distribution. TrAC Trends Anal Chem 151:116598
Holz Jr GG, Conner RL (1973) The composition, metabolism and roles of lipids in Tetrahymena. Biology of Tetrahymena, pp. 99–122
Hopmans EC, Weijers JWH, Schefuβ E, Herfort L, Sinninghe Damsté JS, Schouten S (2004) A novel proxy for terrestrial organic matter in sediments based on branched and isoprenoid tetraether lipids. Earth Planet Sci Lett 224:107–116
Issa N, Vempatti SS (2018) Oil spills in the Arabian Gulf: a case study and environmental review. Environ Nat Resources Res 8:144–153
IUCN (1983) Global status of mangrove ecosystems. IUCN Commission on Ecology Papers No. 3, 88 pp
IUCN/UNEP (1985) The management and conservation of renewable marine resources in the Indian Ocean Region, in the Red Sea and Gulf of Aden region. UNEP Regional Seas Reports & Studies, No. 64
Jaffé R, Rushdi AI, Medeiros PM, Simoneit BRT (2006) Natural product biomarkers as indicators of sources and transport of sedimentary organic matter in a subtropical river. Chemosphere 64:1870–1884
Jebara A, Albergamo A, Rando R, Potortì AG, Turco VL, Mansour HB, Di Bella G (2021) Phthalates and non-phthalate plasticizers in Tunisian marine samples: occurrence, spatial distribution and seasonal variation. Mar Pollut Bull 163:111967
Kawka OE, Simoneit BRT (1990) Polycyclic aromatic hydrocarbons in hydrothermal petroleums from the Guaymas Basin spreading center. Appl Geochem 5:17–27
Kharlamenko VI, Zhukova NV, Khotimchenko SV, Svetashev VI, Kamanev M (1995) Fatty acids as markers of food sources in a shallow-water hydrothermal ecosystem (Kratemaya Bight, Yankich Island, Kurile Islands). Mar Ecol Prog Ser 120:231–241
Laws EA (1993) Oil pollution. Aquatic Pollution: An Introductory Text, 2nd edn. Wiley, p 417–458
Literathy P (1993) Considerations for the assessment of environmental consequences of the 1991 Gulf War. Mar Pollut Bull 27:349–356
Loya Y (1975) Possible effects of water pollution on the community structure of Red Sea corals. Mar Biol 29:177–185
Mallory FB, Gordon JT, Conner RL (1963) The isolation of a pentacyclic triterpenoid alcohol from a protozoan. J Amer Chem Soc 85:1362–1363
Marzooq H, Naser HA, Elkanzi EM (2019) Quantifying exposure levels of coastal facilities to oil spills in Bahrain. Arabian Gulf Environ Monit Assessment 191:160–176
Massoud MS, Al-Abdali F, Al-Ghadban AN (1998) The status of oil pollution in the Arabian Gulf by the end of 1993. Environ Int 24:11–22
Mazurek MA, Simoneit BRT (1984) Characterization of biogenic and petroleum derived organic matter in aerosols over remote, rural and urban areas. In: Kieth LH (ed) Identification and Analysis of Organic Pollutants in Air. American Chemical Society Symposium. Ann Arbor Science/Butterworth Publishers, Woburn, pp 353–370
Medeiros PM, Bicego MC (2004) Investigation of natural and anthropogenic inputs in sediments using geochemical markers: I. Santos, SP- Brazil. Mar Pollut Bull 49:761–769
Medeiros PM, Bicego MC, Castelao RM, Rosso DC, Fillmann G, Zamboni AT (2005) Natural and anthropogenic hydrocarbon inputs to sediments of Patos Lagoon estuary, Brazil. Environ Int 31:77–87
Meyers PA (1997) Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org Geochem 27:213–250
Michel J, Hayes MO, Keenan RS, Sauer TC, Jensen JR, Narumalani S (1993) Contamination of nearshore subtidal sediments of Saudi Arabia from the Gulf War oil spill. Mar Pollut Bull 27:109–116
Moldowan JM, Sundararaman P, Schoell M (1986) Sensitivity of biomarker properties to depositional environment and/or source input in the Lower Toarcian of SW-Germany. Org Geochem 10:915–926
Moreau RA, Whitaker BD, Kicks KB (2002) Phytosterols, phytostanols and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Prog Lipid Res 41:457–500
Mudge SM (2005) Fatty alcohols - a review of their natural synthesis and environmental distribution. Soap Deterg Assoc 132:1–141
Mudge SM, Norris CE (1997) Lipid biomarkers in the Conwy Estuary (North Wales, U.K.): a comparison between fatty alcohols and sterols. Mar Chem 57:61–84
Ogden JC, Gladfelter EH (1983) Coral reefs, seagrass beds and mangroves: their interaction in the coastal zones of the Caribbean. UNESCO 23, 133 pp
Ourisson G, Albrecht P, Rohmer M (1979) The hopanoids: palaeochemistry and biochemistry of a group of natural products. Pure Appl Chem 51:709–729
Oyo-Ita OE, Oyo-Ita IO (2012) Fatty acid and alcohol distributions and sources in surface sediments of Imo River, southeast Niger Delta, Nigeria. Environ Nat Resour Res 2:101–113
Oyo-Ita OE, Ekpo BO, Oros DR, Simoneit BRT (2010) Distributions and sources of aliphatic hydrocarbons and ketones in surface sediments from the Cross River estuary, S.E. Niger Delta, Nigeria. J Appl Sci Environ Sanit 5:24–34
Parrish CC, Abrajano TA, Budge SM, Helleur RJ, Hudson ED (2000) Chapter 8: Lipid and phenolic biomarkers in marine ecosystems: analysis and applications. In: Wangersky P (ed) The Handbook of Environmental Chemistry. Marine Chemistry, Springer-Verlag, Berlin, vol. 5-D, pp. 193–223
Perry GJ, Volkman JK, Johns RB, Bavor HJ Jr (1979) Fatty acids of microbial origin in contemporary marine sediments. Geochim Cosmochim Acta 43:1715–1725
Peters KE, Moldowan JM (1993) The Biomarker Guide: Interpreting Molecular Fossils in Petroleum and Ancient Sediments. Prentice-Hall, Englewood Cliffs
Picó Y, Soursou V, Alfarhan AH, El-Sheikh MA, Barceló D (2021) First evidence of microplastics occurrence in mixed surface and treated wastewater from two major Saudi Arabian cities and assessment of their ecological risk. J Hazard Mater 416:125747
PME/UNEP (1989) Environmental Impact Assessment- Basic Processes. The Presidency of Meteorology and Environment (PME) and United Nations Environmental Programme (UNEP), PME Press, Jeddah, 45 p
Por FD, Dor I (1984) Hydrobiology of the Mangal. Dr. W. Junk Publishers, The Hague, 260 pp
Price ARG (1979) Temporal variations in abundance of penaeid shrimp larvae and oceanographic conditions off Ras Tanura, western Arabian Gulf. Estuar Coast Mar Sci 9:451–465
Price ARG (1982) Distribution of penaeid shrimp larvae along the Arabian Gulf coast of Saudi Arabia. J Nat Hist 16:745–757
Price ARG (1993) The Gulf: human impacts and management initiatives. Mar Pollut Bull 27:17–27
Price AR, Wrathall TJ, Medley PA, Al-Moamen AH (1991) Broadscale changes in coastal ecosystems of the western Gulf following the 1991 Gulf war. Mar Pollut Bull 27:143–147
Qurban MA, Krishnakumar PK, Joydas TV, Ashraf M, Manikandan TT, Abdulkader KP, Loughland KA (2011) Overview of Gulf marine habitats marine atlas of the Saudi Arabian waters of the Arabian Gulf. Khaled A, Al-Abdulkader RAL (eds). Saudi Aramco, Dhahran
Rajendran N, Suwa Y, Urushigawa Y (1993) Distribution of phospholipid ester-linked fatty acid biomarkers for bacteria in the sediment of Ise Bay, Japan. Mar Chem 42:39–56
Rampen SW, Abbas BA, Schouten S, Sinninghe Damsté JS (2010) A comprehensive study of sterols in marine diatoms (Bacillariophyta): implications for their use as tracers for diatom productivity. Limnol Oceanogr 55:91–105
Ray GC (1976) Critical marine habitats. In: IUCN. An international conference on marine parks and reserves. IUCN Publication New Series No. 37, pp. 15–60
Reynolds RN (1993) Physical oceanography of the Gulf, Strait of Hormuz, and the Gulf of Oman: results of the Mt. Mitchell expedition. Mar Pollut Bull 27:35–59
Rinkevich B, Loya Y (1979) Laboratory experiments on the effects of crude oil on the Red Sea coral Stylophora pistillate. Mar Pollut Bull 10:328–330
Robinson N, Cranwell PA, Finlay BJ, Eglinton G (1984) Lipids of aquatic organisms as potential contributors to lacustrine sediments. Org Geochem 6:143–152
Rogge WF, Mazurek MA, Hildemann LM, Cass GR, Simoneit BRT (1993) Quantification of urban organic aerosols at a molecular level: identification, abundance and seasonal variation. Atmos Environ 27A:1309–1330
Rushdi AI, Simoneit BRT (2002a) Hydrothermal alteration of organic matter in sediments of the Northeastern Pacific Ocean: Part 1. Middle Valley, Juan De Fuca Ridge. Appl Geochem 17:1401–1428
Rushdi AI, Simoneit BRT (2002b) Hydrothermal alteration of organic matter in sediments of the Northeastern Pacific Ocean: Part 2. Gorda Ridge, Escanaba Trough. Appl Geochem 17:1467–1494
Rushdi AI, DouAboul AA, Mohammed SS, Simoneit BRT (2006a) Distribution and sources of extractable organic matter in the Mesopotamian wetland marsh sediments of Iraq: I- aliphatic lipids. Environ Geol 50:857–866
Rushdi AI, DouAbul AA, Mohammed SS, Simoneit BR (2006b) Compositions and sources of extractable organic matter in Mesopotamian marshland surface sediments of Iraq: II. Polar compounds. Environ Geol 50:1171–1181
Rushdi AI, Al-Zarban S, Simoneit BRT (2006c) Chemical compositions and sources of organic matter in fine particles of soils and sands from the vicinity of Kuwait city. Environ Monit Assessment 120:537–557
Rushdi AI, Kassim TATA, Simoneit BRT (2009) Sources of organic tracers in sediments from the coastal zone of Ras Abu el-Darag, Gulf of Suez. Environ Geol 58:1675–1687
Rushdi AI, Al-Mutlaq KF, Simoneit BRT, Al-Azri A, DouAbul AAZ, Al-Zarban Sh, Al-Yamani F (2010) Characteristics of lipid tracers to the Arabian Gulf by runoff from rivers and atmospheric dust transport. Arab J Geosci 3:113–131
Rushdi AI, Simoneit BRT, DouAbul AAZ, Al-Mutlaq KF, El-Mubarak AH, Qurban M, Goni MA (2014a) Occurrence, distribution and sources of polar lipid tracers in sediments from the Shatt al-Arab River, Iraq – Polar compounds. Sci Tot Environ 470–471:180–192
Rushdi AI, DouAbul AAZ, Simoneit BRT, El-Mubarak AH, Al-Mutlaq KF, Qurban M, Goni M (2014b) Occurrence and sources of non-polar lipid tracers in sediments from the Shatt al-Arab River of Iraq and the northwestern Arabian Gulf. Arab J Geosci 7:5495–5508
Rushdi AI, Al-Mutlaq KF, El-Mubarak AH, Al-Saleh MA, El-Otaibi MT, Ibrahim SMM, Simoneit BRT (2016) Lipid tracers for organic matter sources in surface soils from Riyadh city, Saudi Arabia. Environ Pollut 208:696–703
Rushdi AI, El-Mubarak AH, Lijotra L, Al-Otaibi MT, Qurban MA, Al-Mutlaq KF, Simoneit BR (2017) Characteristics of organic compounds in aerosol particulate matter from Dhahran city, Saudi Arabia. Arab J Chem 10:S3532–S3547
Rushdi AI, DouAbul AAZ, Al-Maarofi SS, Simoneit BRT (2018) Impacts of Mesopotamian wetland re-flooding on the lipid biomarker distributions in sediments. J Hydrol 558:20–28
Rushdi AI, Chase Z, Simoneit BRT, Paytan A (2019a) Sources of organic tracers in atmospheric dust, surface seawater particulate matter and sediment of the Red Sea. In: Rasul NMA, Stewart ICF (eds) Oceanographic and Biological Aspects of the Red Sea, Springer Oceanography, pp. 75–88
Rushdi AI, Rasul N, Bazyad A, Dumenden R (2019b) Distribution and sources of hydrocarbon compounds in sediments from Obhur Lagoon: Red Sea coast of Saudi Arabia. In: Rasul NMA, Stewart ICF (eds) Oceanographic and Biological Aspects of the Red Sea, pp. 133–146
Rushdi AI, Simoneit BRT, Lijotra L, Bazeyad AY, Dumenden R, El-Mubarak AH, Qurban MA, Al-Mutlaq KF (2022) Phthalates, non-phthalates, polychlorinated biphenyls and phenyl phosphates in atmospheric suspended particulate matter of Dhahran City, Saudi Arabia: levels and seasonal variation. International Journal of Environmental Science and Technology (in press)
Sadiq M, McCain J (eds) (1993) A marine wildlife sanctuary for the Arabian Gulf. Environmental research following the 1991 Gulf War oil spill. NCWCD, Riyadh and Senckenberg Research Institute, Frankfurt, Germany
Saini A, Clarke J, Jariyasopit N, Rauert C, Schuster JK, Halappanavar S, Evans GJ, Su Y, Harner T (2019) Flame retardants in urban air: a case study in Toronto targeting distinct source sectors. Environ Pollut 247:89–97
Sala B, Giménez J, Fernández-Arribas J, Bravo C, Lloret-Lloret E, Esteban A, Bellido J, Coll M, Eljarrat E (2022) Organophosphate ester plasticizers in edible fish from the Mediterranean Sea: marine pollution and human exposure. Environ Pollut 292:118377
Scalan ES, Smith JE (1970) An improved measure of the odd-even predominance in the normal alkanes of sediment extracts and petroleum. Geochim Cosmochim Acta 34:611–620
Setälä O, Fleming-Lehtinen V, Lehtiniemi M (2014) Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut 185:77–83
Sheppard CRC, Price ARG, Roberts CM (1992) Marine Ecology of the Arabian Region: Patterns and Processes in Extreme Tropical Environments. Academic Press, London, p 257
Sheppard C, Al-Husiani M, Al-Jamali F, Al-Yamani F, Baldwin R, Bishop J, Benzoni F, Dutrieux E, Dulvy NK, Durvasula SRV, Jones DA (2010) The Gulf: a young sea in decline. Mar Pollut Bull 60:13–38
Simoneit BRT (1977) Diterpenoid compounds and other lipids in deep-sea sediments and their geochemical significance. Geochim Cosmochim Acta 41:463–476
Simoneit BRT (1978) The organic chemistry of marine sediments. In: Riley JP, Chester R (eds) Chemical Oceanography, 2nd edn. Academic Press, New York, pp 233–311
Simoneit BRT (1984) Organic matter of the troposphere-III. Characterization and sources of petroleum and pyrogenic residues in aerosols over the Western United States. Atmos Environ 18:51–67
Simoneit BRT (1985) Application of molecular marker analysis to vehicular exhaust for source reconciliation. Int J Environ Anal Chem 22:203–233
Simoneit BRT (1989) Organic matter of troposphere -V: application of molecular analysis to biogenic emissions into troposphere for source reconciliations. J Atmos Chem 8:251–275
Simoneit BRT (2002) Biomass burning – a review of organic tracers for smoke from incomplete combustion. Appl Geochem 17:129–162
Simoneit BRT, Mazurek MA (1982) Organic matter of the troposphere-II. Natural background of biogenic lipid matter in aerosols over the rural Western United States. Atmos Environ 16:2139–2159
Simoneit BRT, Mazurek MA, Brenner S, Crisp PT, Kaplan IR (1979) Organic geochemistry of recent sediments from Guaymas Basin, Gulf of California. Deep-Sea Res 26A:879–891
Simoneit BRT, Mazurek MA, Reed WE (1983) Characterization of organic matter in aerosols over rural sites: phytosterols. In: Bjorøy M et al (eds) Advances in Organic Geochemistry 1981. Wiley, Chichester, pp 355–361
Simoneit BRT, Cardoso JN, Robinson N (1990) An assessment of the origin and composition of higher molecular weight organic matter in aerosols over Amazonia. Chemosphere 21:1285–1301
Simoneit BRT, Crisp PT, Mazurek MA, Standley LJ (1991a) Composition of extractable organic matter of aerosols from the Blue Mountains and southeastcoast of Australia. Environ Int 17:405–419
Simoneit BRT, Sheng G, Chen X, Fu J, Zhang J, Xu Y (1991b) Molecular marker study of extractable organic matter in aerosols from urban areas of China. Atmos Environ 25A:2111–2129
Simoneit BR, Deamer DW, Kompanichenko V (2009) Characterization of hydrothermally generated oil from the Uzon caldera, Kamchatka. Appl Geochem 24:303–309
Simoneit BRT (2006) Atmospheric transport of terrestrial organic matter to the sea. In: Volkman JK (ed) The Handbook of Environmental Chemistry, vol. 2. Part N. Marine organic matter, biomarkers, isotopes and DNA. Springer, Berlin, pp 165–208
Soliman YS, Al Ansari EMS, Wade TL (2014) Concentration, composition and sources of PAHs in the coastal sediments of the exclusive economic zone (EEZ) of Qatar, Arabian Gulf. Mar Pollut Bull 85:542–548
Soliman YS, Alansari EM, Sericano JL, Wade TL (2019) Spatio-temporal distribution and sources identifications of polycyclic aromatic hydrocarbons and their alkyl homolog in surface sediments in the central Arabian Gulf. Sci Tot Environ 658:787–797
Stiehl T, Rullkötter J, Nissenbaum A (2005) Molecular and isotopic characterization of lipids in cultured halophilic microorganisms from the Dead Sea and comparison with the sediment record of this hypersaline lake. Org Geochem 36:1242–1251
Talling JF (1980) Water characteristics. In: Rzoska J (ed) Euphrates and Tigris, the Mesopotamian ecology and density. Junk, W., The Hague, pp. 122
Tolosa I, de Mora S, Sheikholeslami MR, Villeneuve JP, Bartocci J, Cattini C (2004) Aliphatic and aromatic hydrocarbons in coastal Caspian Sea sediments. Mar Pollut Bull 48:44–60
Tolosa I, Mesa M, Alonso-Hernandez CM (2014) Steroid markers to assess sewage and other sources of organic contaminants in surface sediments of Cienfuegos Bay, Cuba. Mar Pollut Bull 86:84–90
Treignier C, Derenne S, Saliot A (2006) Terrestial and marine n-alcohol inputs and degradation processes relating to a sudden turbidity current in the Zaire canyon. Org Geochem 37:1170–1184
Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, Foreman K (1997) Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol Oceanogr 42:1105–1118
Vaughan GO, Al-Mansoori N, Burt JA (2019) World Seas: An Environmental Evaluation (2nd ed). pp. 1–23, Chapter 1 In Volume II: the Indian Ocean to the Pacific
Venkatesan MI (1989) Tetrahymanol: its widespread occurrence and geochemical significance. Geochim Cosmochim Acta 53:3095–3101
Vered G, Kaplan A, Avisar D, Shenkar N (2019) Using solitary ascidians to assess microplastic and phthalate plasticizers pollution among marine biota: a case study of the Eastern Mediterranean and Red Sea. Mar Pollut Bull 138:618–625
Voet D, Voet JG (2004) Biochemistry. John Wiley, New York
Vogt IP (1995) Coral reefs in Saudi Arabia: 3.5 years after the Gulf War oil spill. Coral Reefs 14:271–273
Vogt H (1996) Investigations on coral reefs in the Jubail wildlife sanctuary using under water video recordings and digital image analysis. A marine wildlife sanctuary for the Persian Gulf. NCWCD Riyadh and Senckenbergische Naturforschende Gesellschaft, Frankfurt, pp.302–327
Volkman JK (1986) A review of sterol markers for marine and terrigenous organic matter. Org Geochem 9:83–99
Volkman JK, Smittenberg RH (2017) Lipid biomarkers as organic geochemical proxies for the paleoenvironmental reconstruction of estuarine environments. Applications of Paleoenvironmental Techniques in Estuarine Studies. Springer, Dordrecht, pp 173–212
Volkman JK, Johns RB, Gillian FT, Perry GI (1980) Microbial lipids of an intertidal sediment. I. Fatty acids and hydrocarbons. Geochim Cosmochim Acta 44:1133–1143
Volkman JK, Smith DJ, Eglinton G, Forsberg TEV, Corner EDS (1981) Sterol and fatty acid composition of four marine haptophycean algae. J Mar Biol Assoc 61:509–527 (U.K)
Volkman JK, Revill AT, Holdsworth DG, Fredericks D (2008) Organic matter sources in an enclosed coastal inlet assessed using lipid biomarkers and stable isotopes. Org Geochem 39:689–710
Wang X-C, Sun S, Ma H-Q, Liu Y (2006) Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Jiaozhou Bay, Qingdao, China. Mar Pollut Bull 52:129–138
Wisnieski E, Bícego MC, Montone RC, Figueira RCL, Ceschim LMM, Mahiques MM, Martins CC (2014) Characterization of sources and temporal variation in the organic matter input indicated by n-alkanols and sterols in sediment cores from Admiralty Bay, King George Island, Antarctica. Polar Biol 37:483–496
Yang H, Ding WH, Xie SC (2014) Distribution of microbial fatty acids and fatty alcohols in soils from an altitude transect of Mt. Jianfengling in Hainan, China: implication for paleoaltimetry and paleotemperature reconstruction. Spec Top Front Geobiol 57:999–1012
Yunker MB, Mcdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in Fraser River basin: a critical appraisal of PAH ratios as indicators of PAHs source and composition. Org Geochem 33:489–515
Yunker MB, Perreault A, Lowe CJ (2012) Source apportionment of elevated PAH concentrations in sediments near deep marine outfalls in Esquimalt and Victoria, BC, Canada: is coal from an 1891 shipwreck the source? Org Geochem 46:12–37
Zakem EJ, Cael BB, Levine NM (2021) A unified theory for organic matter accumulation. Proc Natl Acad Sci 118(6):e2016896118
Zhao J, Temimi M, Al Azhar M, Ghedira H (2015) Satellite-based tracking of oil pollution in the Arabian Gulf and the Sea of Oman. Can J Remote Sens 41:113–125
Zonneveld KA, Versteegh GJ, Kasten S, Eglinton TI, Emeis KC, Huguet C, Koch BP, de Lange GJ, de Leeuw JW, Middelburg JJ, Mollenhauer G (2010) Selective preservation of organic matter in marine environments: processes and impact on the sedimentary record. Biogeosciences 7:483–511
Acknowledgements
Financial support from the National Plan for Sciences and Technology-King Saud University (project number: 09-ENV842-02) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rushdi, A.I., El-Mubarak, A.H., Simoneit, B.R.T. et al. Natural and anthropogenic sources of extractable organic matter in sediments from the coastal zone of the Arabian Gulf in Saudi Arabia. Arab J Geosci 15, 1446 (2022). https://doi.org/10.1007/s12517-022-10731-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10731-0