Abstract
The suffering from arsenic toxicity is a long-standing concern in Asian countries. The role of the key factors (arsenic intake, age and sex) regulating arsenic toxicity is aimed to evaluate for a severely exposed population from Murshidabad district, West Bengal. Mean arsenic concentrations in drinking water supplied through tube well, Sajaldhara treatment plant and pipeline were observed as 208, 27 and 54 µg/l, respectively. Urinary arsenic concentration had been observed as < 3–42.1, < 3–56.2 and < 3–80 µg/l in children, teenagers and adults, respectively. Mean concentrations of hair and nail arsenic were found to be 0.84 and 2.38 mg/kg; 3.07 and 6.18 mg/kg; and 4.41 and 9.07 mg/kg, respectively, for the studied age-groups. Water arsenic was found to be associated with hair and nail (r = 0.57 and 0.60), higher than urine (r = 0.37). Arsenic deposition in biomarkers appeared to be dependent on age; however, it is independent of sex. Principal component analysis showed a direct relationship between dietary intake of arsenic and chronic biomarkers. Nail was proved as the most fitted biomarker of arsenic toxicity by Dunn’s post hoc test. Monte Carlo sensitivity analysis and cluster analysis showed that the most significant factor regulating health risk is ‘concentration of arsenic’ than ‘exposure duration’, ‘body weight’ and ‘intake rate’. The contribution of arsenic concentration towards calculated health risk was highest in teenagers (45.5–61.2%), followed by adults (47.8–49%) and children (21–27.6%). Regular and sufficient access to arsenic-safe drinking water is an immediate need for the affected population.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic contamination in groundwater is a natural hazard affecting countries worldwide, especially the south-eastern countries like India, China, Bangladesh, Vietnam, etc. (Ravenscroft et al., 2011). Recently, several other countries have been exposed to high groundwater arsenic such as Bolivia, Uruguay, Ecuador, Nicaragua, Honduras and El Salvador (Shaji et al., 2021). The average arsenic concentration in igneous and sedimentary rocks is 2 mg/kg, although elevated concentrations were found in finer-grained argillaceous sediments and phosphorites (Mandal & Suzuki, 2002). Over the world, groundwater arsenic is majorly linked with rocks and sediments degradation, rainwater-mediated deposits of weathered sulphide ore, desorption from arsenic-enriched pyrites and hydroxides, which are influenced by several anions, dissolved organic carbon (DOC), pH and redox reactions (Alexakis & Gamvroula, 2014; Alexakis et al., 2021; Casentini et al., 2011; Chakraborty et al., 2015; Golfinopoulos et al., 2021; Harvey et al., 2002). So far, 20 states and 4 UT (Union Territories) in India are affected by groundwater arsenic contamination (Shaji et al., 2021). The suffering of more than 500 million people living in the Ganga–Meghna–Brahmaputra (GMB) plain, covering approximately 569,749 sq. km area from arsenic contamination, is a prolonged matter of concern (Chakraborti et al., 2004, 2013; Chowdhury et al., 2000). In the state of West Bengal, India, arsenic contamination has turned into the worst-case scenario in districts like Murshidabad, Nadia, North 24 Parganas, South 24 Parganas (Chakraborti et al., 2009; Santra et al., 2013). A huge amount of arsenic contaminated groundwater is irrigated for cultivation in these aforementioned districts throughout the year (Chowdhury et al., 2018; Das et al., 2021a; Roychowdhury et al., 2005). Average arsenic concentrations in domestic/community tube wells and agricultural tube wells in Raninagar II block, Murshidabad, were reported as 63.8 µg/l (range < 3–995 µg/l, n = 366) and 138 µg/l (range < 3–990 µg/l, n = 67), respectively (Das et al., 2021a). The annual withdrawal of arsenic through irrigational water was reported as nearly 12.5 tonnes, and its deposition rate in the cultivated fields of the studied block was estimated as 0.23–4.70 kg/ha annually. Surface soil arsenic concentrations in paddy fields from Murshidabad and North 24 Parganas districts were observed as 9.5–19.4 mg/kg and 22.4–60.2 mg/kg, respectively (Chowdhury et al., 2018; Roychowdhury et al., 2005). Moreover, rice grain being a significant cultivable crop, its bio-accumulation value of arsenic showed the capacity of hyper-accumulation (Das et al., 2021a; Samal et al., 2021). Therefore, besides the consumption of drinking water, arsenic-contaminated foodstuffs, especially rice grains, increase people’s health risk (Bhattacharya et al., 2010; Das et al., 2021a; Joardar et al., 2021a). Rice being the staple food in West Bengal contributes a substantial amount of arsenic through the daily diets and poses a significant health risk to the populations residing in both arsenic exposed and unexposed areas (Biswas et al., 2019; Joardar et al., 2021a). Even, animal by-products used as foods from arsenic endemic areas are observed to cause human health risk considerably (Das et al., 2021b).
While acute arsenic exposure causes respiratory troubles, cardiovascular problems, abdominal pain, diarrhoea or lungs dysfunction, neurological disorders, etc., chronic toxicity poses serious carcinogenic health complications (Chakraborti et al., 2017). Arsenic-induced toxicity is also reported to affect the reproductive system adversely; pre-mature birth, stillbirth, neonatal and perinatal mortality are some of the instances (Chakraborti et al., 2013). Continuous intake of arsenic causes thickening of the skin, hyper-pigmentation, keratosis, melanosis, black-foot disease, and gangrene followed by cancer (Abdul et al., 2015; Rahman et al., 2009; Ratnaike, 2003). The toxicity level is diagnosed by estimation of arsenic concentration in the biomarkers of the human body, i.e. urine, hair and nail (RGI, 2003; Vahter et al., 1994).
Several research reports found that arsenic exposure is limited neither to arsenic endemic areas nor to any specific age-groups (Biswas et al., 2019; Joardar et al., 2021a, 2021b). The severity of arsenic exposure depends on various factors like daily dietary intake through food and drinking water (Joardar et al., 2021a; Roychowdhury et al., 2003), exposure frequency, age and sex of the population (Rahman et al., 2006), genetic susceptibility, nutritional status, socioeconomic strata (Chakraborti et al., 2018; Rahman et al., 2018), occupational status (Brima et al., 2006; Hata et al., 2007), etc. School children aged between 5 to 15 years have been seen to be affected by arsenic toxicity in both exposed and apparently control areas of West Bengal (Das et al., 2021c; Joardar et al., 2021b). Health effects and risk assessment due to arsenic toxicity have been assessed among the mothers (aged 23–31 years old) and their children (7 months–4 years old) in an arsenic-exposed village in North 24 Parganas district, which shows that the populations are sub-clinically affected (Joardar et al., 2021c). While Joardar et al. (2021a) showed that the studied adults in the differently arsenic exposed villages have higher arsenic concentrations in urine or hair than children, Bibi et al. (2015) suggested that children and elderly people have higher arsenic accumulation in hair samples than adults, which might be a result of weaker metabolism and more susceptibility towards diseases. However, age and gender both were reported to be non-significant variables towards the affected people’s hair arsenic concentration (Das et al., 2018; Hadi & Praveen, 2004; Wu & Chen, 2010). It is pertinent to mention here that inorganic arsenic (As-V and As-III) is more toxic than organic arsenic. Metabolism of arsenic turns inorganic arsenic into organic one in the human body, which includes reduction of arsenate to arsenite followed by methylation into different species, MMA and DMA (monomethylarsonic acid and dimethylarsinic acid) (Vahter, 1999). A higher concentration of inorganic arsenic reduces the methylation capacity of arsenic resulting in higher inorganic urinary arsenic (Torres-Sánchez et al., 2016). Arsenic metabolism in human systems is also reliant on sex and age differences (Bozack et al., 2018; Torres-Sánchez et al., 2016). The government has taken several mitigation measures for abatement of groundwater arsenic contamination in Murshidabad, which include installation of the new hand pumps, big diameter deeper aquifer tube wells, arsenic removal plants and surface water-based pipeline water supply. A number of 80 dug wells are installed in the affected areas till the year 2007 under the second action plan and 34 numbers under state-funded action plan (Halder, 2019). However, Das et al. (2021a) found the incompetence of the governmental mitigation strategies in the district as the pipeline water and treated water from arsenic removal plants are found to be arsenic-contaminated. Therefore, it is important to investigate the state of the inhabitants after the administrative interventions in the past few years.
The present study aims to assess the extent of arsenic exposure to the affected population from sixty families including three different age groups (children, teenagers and adults) residing in the Raninagar II block based on three main hypotheses related to arsenic intake through drinking water and rice grain (staple food), age and sex. The distribution pattern of arsenic in intake (drinking water) and excrete (urine, hair, nail) has been studied through probability distribution (Q–Q) plots. Arsenic accumulation in the biological indices has been evaluated statistically considering gender and age differences and the relationship between arsenic intakes and excretes. Moreover, the study is a unique statistical attempt on identifying the most efficient biomarker of arsenic toxicity. The differences in all kinds of available drinking water sources have been collated to understand and define the safest source of drinking water. An extensive health risk assessment (cancer and non-cancer) has been performed for the studied population through consumption of arsenic-contaminated water and rice grain. Finally, inspecting the relative significance of the key factors behind health risk (cancer or non-cancer) of different studied age-groups is another prime focus of this study, which has been evaluated through Monte Carlo simulation and cluster analysis. The novelty of this work embraces drawing the attention of the policy makers with highlighting the present scenario after several mitigation strategies undertaken by the authorities.
Materials and methods
Study area
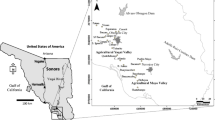
Murshidabad, the district located at the east bank of the river Bhagirathi, on the border between West Bengal and Bangladesh is well reported for arsenic contamination in groundwater (Chakraborti et al., 2009; Das, 2013; Rahman et al., 2005a). The study area has been selected covering certain villages from one of the worst arsenic-affected blocks in Murshidabad district of West Bengal, under Domkal sub-division, named Raninagar II (Supplementary Fig S1). Raninagar II is reportedly one of the worst arsenic-affected blocks in the Murshidabad district where 1091 villagers were registered with distinct arsenical skin lesions among total of 5827 examined villagers (Mukherjee et al., 2005). The community development block consists of 9 g panchayats (clusters of villages) covering a population of nearly 1.91 lakhs (Census, 2011). A targeted population from sixty families including three different age groups (children: ≤ 10 years, teenagers: > 10–20 years, and adults: > 20–80 years) from severely arsenic-affected three gram panchayats (Malibari-I, Katlamari-I and Raninagar-II) of Raninagar II block have been studied under the present work.
Sample collection and preservation
The study was performed by collecting the drinking water, raw rice grain, and biological samples (scalp hair, nail, and urine) from the family members of the three affected gram panchayats including three different age groups; children (≤ 10 years), teenagers (> 10–20 years) and adults (> 20–80 years). The samples were collected during September–October 2019 without maintaining any seasonal variation. All kinds of available drinking water samples (tube well, pipeline and treated water from the Sajaldhara treatment plant) were collected from the houses of the families in polyethylene containers that were pre-washed with distilled water. The collected water samples were preserved by adding 0.1% (v/v) concentrated nitric acid, kept in a cooled icebox during transportation and stored in a refrigerator at 4 °C in the laboratory for arsenic analysis (Das et al., 2020, 2021a). Rice as the staple food in Bengal (Biswas et al., 2021) was collected from the families in individual sterile zip-locks and preserved at room temperature. Human biological samples like urine, hair and nail were collected from each of the family members for estimation of acute and chronic arsenic exposure in their health. Urine samples were collected individually in sterile polyethylene containers and transported to the laboratory placed in a cooled ice-box and stored at —20 °C in the refrigerator. All the urine samples were diluted (1:1) with double distilled water and filtered to diminish matrix effects and to eliminate colloidal particles before analysis of arsenic. The scalp hair and nail samples were collected in separate polyethylene zip-locks and conserved at room temperature. To remove the external contamination of arsenic, the hair and nail samples were washed carefully using double distilled water, followed by acetone on a magnetic stirrer and dried at 50 °C overnight in a hot air oven. The detailed information on sample collection, preservation, storage and processing of solid and liquid samples was discussed in our previous reports (Das et al., 2021b; Joardar et al., 2021a, 2021b).
Chemicals and reagents
All the chemicals used in this study were of analytical grade. Double distilled water was used throughout the analytical work. Concentrated HNO3 (69%) and H2O2 (30% v/v) were used for digestion of the solid samples, while for the estimation of total arsenic concentration, 0.6% NaBH4 (in 0.5% NaOH) and 5–10 M HCl from Merck, Mumbai, India, were used in the hydride generation-atomic absorption spectrometry (HG-AAS) method. About 10% of KI solution (aq.) and 8% of concentrated HCl were added during sample preparation, and the mixed solution was allowed to settle down for 45 min before analysis of arsenic. Detailed information on chemicals and reagents was described earlier (Das et al., 2021a).
Digestion
The solid samples (scalp hair, nail and rice grain) were digested with a mixture solution of concentrated HNO3 and H2O2 (2:1) in Teflon bomb at 120 °C for 6 h within the hot air oven. The volume of digested solution was reduced on the hot plate at about 90 °C for 1 h. The evaporated samples were made up to a final volume of 2–5 ml with double distilled water and filtered through a suction filter (Millipore 0.45 µm). The filtered solutions were stored in fresh polyethylene containers for the estimation of total arsenic. Detailed information on the digestion procedure was discussed in Joardar et al., (2021a, 2021b). No digestion process was maintained for liquid samples (drinking water and urine) (Das et al., 2021b).
Analysis
The arsenic concentration of targeted samples was estimated using atomic absorption spectrophotometer (Varian AA140, USA) coupled with Vapor Generation Accessory (VGA-77, Agilent Technologies, Malaysia) with software version 5.1 in the HG-AAS method. More information on the instrumentation and its methodology are described in our earlier publications (Das et al., 2020; Joardar et al., 2021a, b).
Quality control and quality assurance
The quality of the analytical work was maintained by digesting about 30–35% of solid samples on the hot plate. To validate the analytical results, arsenic concentration in standard reference material (SRM) was also measured using both the Teflon bomb and hot-plate digestion methods. The SRM samples used in this study were rice flour 1568a (NBS, Gaithersburg, MD, USA), and Human hair ERM-DB001 (European Commission, JRC, IRMM, Retieseweg, Geel, Belgium) which showed 94 and 90%, recovery through Teflon Bomb digestion against their certified values (0.29 and 0.044 µg/g). The same SRM samples showed 82 and 81% recovery, respectively, in the hot-plate digestion method. The accuracy of the generated data was also verified by analysing duplicates (± 5% variation) and through recovery calculation of spiked digested samples. The comprehensive information on digestion and recovery of arsenic in SRM samples are well documented in Joardar et al. (2021a) and Das et al. (2021a).
Statistical analysis
Probability distribution of samples through Q–Q (Quantile–Quantile) plots, hypothesis-based Chi-squared test and a ‘two-sample paired t test’ for sex and age dependence have been performed using Excel 2016 (Microsoft Office). Spearman correlation, principal component analysis (PCA), hypothesis-based Kruskal–Wallis test and Dunn’s test were done with the help of PAST (version 4.09) to understand the relation between arsenic intake and excrete. The sensitivity analysis study has been evaluated for health risk assessment through Monte Carlo simulation technique using ‘sensitivity’ package (version 1.27.0)1 in R version 3.6.32. The cluster analysis has been measured for understanding the influence of the variables in health risk assessment using Origin 2017.
Data analysis
Health risk assessment
Human health risk assessment was performed to calculate the cancer and non-cancer risk on the different-aged studied populations based on the USEPA (2001) model of health risk assessment. At first, the daily exposure from arsenic-contaminated drinking water and rice grain has been estimated through average daily dose (ADD) calculation:
where C = concentration of arsenic through drinking water (µg/l) and rice grain (µg/kg); IR = oral intake rate per day (children: 2 l, range 2–3 l; teenager: 3 l, range 2–4.5 l; adult: 4 l; range 2–5 l for drinking water and children: 200 g, range 200–400 g; teenager: 350 g, range 200–600 g; adult: 400 g; range 300–600 g for rice grain); ED = exposure duration in years (individual age for the participants in the three studied age group of populations); EF = exposure frequency (365 days); BW = body weight (children: 19 kg, range 7–47 kg; teenager: 45 kg, range 20–50 kg; adult: 65 kg; range 32–75 kg) and AT = average lifetime (365*65 = 23,725 days).
Cancer risk (CR) is calculated by multiplying ADD with cancer slope factor (CSF), and non-carcinogenic risk or hazard quotient (HQ) is determined dividing ADD by reference dose (RfD).
(CSF of arsenic = 1.5 per mg/kg bw/day) (USEPA, 2005)
(RfD of arsenic = 0.0003 mg/kg bw/day) (USEPA, 2005).
Results and discussion
Arsenic concentration status in drinking water and rice grain
The present study took place considering the selected population of the three worst affected gram panchayats named Malibari- I, Katlamari- I and Raninagar- II, where approximately 70.6, 60 and 71.4% of drinking water samples were found to be arsenic contaminated with higher than the permissible limit in drinking water (Fig. 1a). The range and mean arsenic concentrations in drinking water of the three gram panchayats were observed to be (< 3–598 µg/l, n = 20), (< 3–754 µg/l, n = 20), (< 3–847 µg/l, n = 11) and 115, 178 and 294 µg/l, respectively. On a cumulative approach, the mean arsenic concentration of drinking water samples (range < 3–847 µg/l, n = 51) in the three studied sites is 17.6 times higher than the WHO (2011)-provided recommended limit of arsenic, i.e. 10 µg/l (Supplementary Table S1). The population residing in the study area majorly depends on groundwater withdrawn by tube wells for drinking purposes, while there are some alternative drinking water sources available too like pipeline supplied water or treated water from arsenic removal plants (Das et al., 2021a). The mean arsenic concentration was found to be 208 ± 259 µg/l (range < 3–847 µg/l, n = 35) in the tube well water, 54 ± 81.2 µg/l (range 4.1–250 µg/l, n = 12) in Sajaldhara plant treated water and 27 ± 29 µg/l (range 3–68 µg/l, n = 5) in pipeline supplied water, respectively (Fig. 1b). Also, it was observed that approximately 77.1, 60 and 45.5% of drinking water samples from tube well, pipeline and Sajaldhara water treatment plants contain arsenic concentration higher than its recommended value in drinking water (Fig. 1b). Unlawfully tapping of pipeline for irrigation or any other domestic purpose is pretty frequent in these rural belts, which ultimately cause damage of the pipelines during supply of water. Also, in some places, it is seen that the foundation of accessible pipeline water is not surface treated water; however, direct withdrawal of groundwater is supplied after storing in reservoirs overnight (Das et al., 2021a). More to it, the poor efficiency of arsenic-iron removal plants installed in West Bengal causes the population to drink arsenic-contaminated water unknowingly. Our previous study found the mean arsenic concentration in pipeline supplied water as 21 ± 14.3 µg/l (range < 3–48.1 µg/l, n = 10) and in Sajaldhara-treated water as 13.6 ± 16.8 µg/l (< 3–56.1 µg/l, n = 16) from Raninagar II block, Murshidabad (Das et al., 2021a). This clearly explains the lack of maintenance in the water treatment plants as well as no regular monitoring of pipeline water quality before supplying it to the villagers, which results in their poor health. The adverse health effect of arsenic is observed both pre-clinically and clinically. Spotted and diffuse melanosis, diffuse or nodular keratosis along with oedema of the feet, liver and spleen enlargement, diarrhoea are frequently observed (Mandal et al., 2022; Mukhopadhyay et al., 2020; Rahman et al., 2005b). The agony and despair observed in the community responses are ineffable.
The rice grain samples collected from the families for the present study showed an arsenic concentration range of 52–668 µg/kg (mean 211 ± 167 µg/kg; n = 28) (Supplementary Table S1). It is important to discuss that the tolerable concentration of arsenic in rice grain for consumption is 200 µg/kg (European Commission, 2015). The same value has also been suggested as the maximum inorganic arsenic concentration in polished white rice, which covers 79% of the international market by Codex Alimentarius, coordinated by the United Nations Food and Agriculture Organization (FAO) and the World Health Organization (WHO) (Codex Alimentarius Commission, 2014). Das et al. (2021a) have thoroughly shown the effect of arsenic-contaminated groundwater on the locally cultivated rice in Raninagar II block, Murshidabad. Approximately, 0.18% of arsenic is accumulated every year in rice grain from deposition in soil which explains the severity of arsenic exposure in agricultural lands.
Observed daily dietary intake of arsenic in three differently aged populations
The present study observed varied contributions of different arsenic-contaminated sources in the daily diet of three different-aged populations (Supplementary Fig. S2). The study observed that majority of people in the study area rely on drinking water withdrawn through tube wells than alternative drinking water sources. The study results revealed that the daily intake rate of arsenic from drinking water through tube well is highest: 26.4 (range 0.31–89.2), 22.6 (range 0.47–56.5) and 15.5 (range 0.18–67.8) µg/kg bw/day for children, teenagers and adults, respectively. The average arsenic ingestion rates from the treated water of Sajaldhara water treatment plants were found to be 7.67 (range 0.43–43.6), 12.3 (range 0.48–27.6) and 8.03 (range 0.23–33.1) µg/kg bw/day, respectively, which are much higher than the provisional tolerable daily dietary intake rate of arsenic (3.0 µg/kg bw/day), recommended by WHO (2011) (Supplementary Fig. S2a). The average daily intake rate of arsenic from pipeline supplied water was found lower than the PTDI value: 2.7 µg/kg bw/day (range 0.99–4.41) in children, 1.71 µg/kg bw/day (range 0.63–2.79) in teenagers and 2.33 µg/kg bw/day (range 0.58–3.35) in adults, respectively. Moreover, arsenic-contaminated rice grains also imposed a significant amount of burden on the daily diets of the populations. Arsenic intake rate from rice grains was found to be higher in children (average 2.75, range 1.10–5.48 µg/kg bw/day) than teenagers (average 1.77, range 1.11–2.04 µg/kg bw/day) and adults (average 1.77, range 0.32–5.34 µg/kg bw/day). The percent contribution of arsenic in daily diet is seemed to be almost highest in children from all the ingestion sources. Dietary intake of arsenic contributed 41, 27, and 40% in children; 35, 44 and 25% in teenagers; and 24, 29 and 35% in adults from tube well water, Sajaldhara plant treated water and pipeline supplied water, respectively (Supplementary Fig. S2b). Contribution from arsenic-contaminated rice grain in daily dietary intake was found to be 44, 28 and 28% for children, teenager and adults, respectively (Supplementary Fig. S2b). The varied range of data in different age groups helps to understand that the daily dietary intake rate depends on both the factors like arsenic concentration in the consumption source and participants’ body weight. It also evinces that both drinking water and rice grain contribute to arsenic toxicity in the population concurrently.
Probability distribution of arsenic in drinking water and biomarkers
The distribution of arsenic in drinking water and biomarkers (urine, hair and nail) samples are highly skewed, which is demonstrated through respective probability distribution plots (Fig. 2). The Q–Q (Quantile–Quantile) plot is a graphical method of ‘goodness-of-fit’ model where the datasets are verified whether they are normally distributed or not in comparison with a theoretical normal distribution. Arsenic concentration in urine (n = 118), hair (n = 118) and nail (n = 118) samples is positively skewed with skewness values of 1.66, 1.91 and 1.29, respectively (Fig. 2b–d). Similar observations were observed by Hinwood et al. (2003) where biological arsenic concentration were all skewed to the right. A standard normal distribution kurtosis value is 3 and is called mesokurtic. The distribution of hair arsenic concentration is leptokurtic with a kurtosis value of 3.78, which signifies that arsenic distribution is heavily tailed with an abundance of outliers, while urine and nail arsenic concentrations are platyurtic (less peaked distribution) with respective kurtosis values of 2.22, and 0.78. Arsenic concentration in drinking water (n = 50) is rightly skewed with a value of 1.29 and platykurtic with a small kurtosis value of 0.55 (Fig. 2a), which signifies that drinking water arsenic distribution is light tailed with a lack of outliers and flat in nature. It can be thus stated that hair arsenic concentration is least symmetric and highly peaked in nature.
Arsenic concentration in biomarkers
The extent of arsenic toxicity is generally assessed through the estimation of arsenic concentration in the biological markers of the human body, i.e. urine, hair and nail. About 70% of inorganic arsenic gets excreted from the body system through urine with a half-life of approximately 4 days (Hughes, 2006; NRC, 1999). Thus, for evaluation of recent or acute exposure, urinary arsenic is used as the major and primary body marker (Buchet et al., 1981; Vahter et al., 1994). The observed mean urinary arsenic concentration in children, teenagers and adults was (11.9 ± 11.3) µg/l (range < 3–42.1 µg/l, n = 21), (11.1 ± 11.9) µg/l (range 3–56.2 µg/l, n = 35) and (11.3 ± 14 µg/l) (range < 3–80 µg/l, n = 176), respectively (Fig. 3). Urinary arsenic concentration range in the male and female studied population was observed as (< 3–48.3 µg/l, n = 69) and (< 3–80 µg/l, n = 66), respectively (Supplementary Table S1). For understanding long-term arsenic exposure, evaluation of arsenic accumulation in hair and nail is the best likely way followed by dermatological manifestations (Shankar et al. 2014; Wade et al., 2015). The impact of arsenic toxicity on human health occurs after a time duration of 2–5 months for hair and 12–18 months for nails (Nowak & Kozlowski, 1998; Pororinskaya & Karpenko, 2009). In the present study, hair arsenic concentrations were found to be 0.84 ± 0.76 mg/kg (range 0.38–2.7 mg/kg, n = 10) in children, 3.07 ± 1.73 mg/kg (range 0.64–5.73 mg/kg, n = 15) in teenagers and 4.41 ± 4.31 mg/kg (range 0.03–24 mg/kg, n = 91) in adults, while nail arsenic concentrations were 2.38 ± 1.62 mg/kg (range 0.5–5 mg/kg, n = 10), 6.18 ± 4.73 mg/kg (range 0.55–14.8 mg/kg, n = 15) and 9.07 ± 6.48 mg/kg (range 1.14–25.3 mg/kg, n = 91) in children, teenagers and adults, respectively (Fig. 3). It is clearly understood that accumulation of arsenic in hair and nail increases with age; from children to teenagers it is increased by 3.65 and 2.6 times and from teenagers to adults it is escalated by 1.44 and 1.47 times. Nail arsenic concentrations seem to be higher than hair arsenic concentrations by 2.83, 2.01 and 2.05 times in children, teenagers and adults, respectively. Contrastingly, the mean arsenic concentration in nail samples of the male and female population (8.41 ± 7.11 and 7.69 ± 7.84 mg/kg) was visibly higher than that of hair samples (4.14 ± 3.74 and 4.33 ± 5.37 mg/kg) (Supplementary Table S1). This justifies the report by NRC (1999) that the rate of deposition of arsenic is faster in nails than in hair. Also, arsenic concentration in the hair and nail of this studied population is seemingly higher than the respective recommended limits for humans (Supplementary Table S1). Higher mean nail arsenic concentration than hair was also observed in populations of other countries like north-eastern Thailand (Wongsasuluk et al., 2018); Vietnam (Nguyen et al., 2019); Cambodia (Gault et al., 2008), etc.
Arsenical skin manifestations
The arsenical body burden scenario discloses that a majority of the villagers are sub-clinically affected without any direct skin lesion, although some of the individuals of the critically exposed population are diagnosed with arsenic-induced symptoms. Arsenical skin manifestations of a few affected individuals from the study area are shown in Supplementary Fig. S3. Different types of skin lesions are observed among the people like raindrop pigmentation in the dorsal part of the hand, feet or whole body, spotted and diffused keratosis, melanosis, even bowens. Leucomelanosis is found too in a few severely affected people who have stopped drinking arsenic-contaminated water; however, we had spotted melanosis earlier. Detailed information about the chronic patients is also described in Supplementary Table S2. In a previous study in the Murshidabad district, researchers found 19% of people with distinct arsenical skin manifestations among the total surveyed villagers (n = 25,274), where the most predominant symptom observed was spotted and diffused melanosis on the trunk (Mukherjee et al., 2005).
Exposure of arsenic with respect to arsenic intake
A Spearman’s correlation has been performed at 95% confidence interval to estimate the relation among arsenic in drinking water and rice grain and arsenic concentration in biomarkers (Fig. 4a). It is observed that the drinking water arsenic is most strongly correlated with nail arsenic (r = 0.60) followed by hair and urine arsenic (r = 0.57 and 0.38), whereas the effect of rice grain arsenic is not significant as it is poorly correlated with urine, hair and nail arsenic (r = 0.18, 0.13 and 0.14). The correlation (r) values (given in Supplementary Table S3a) signify that arsenic from drinking water deposits in the keratin tissues slowly, however strongly. Thus, arsenic accumulation in chronic biomarkers is an easier way to estimate toxicity level than that of acute biomarkers (Brima et al., 2006; Samanta et al., 2004). The effect of arsenic from drinking water is always more than other foodstuffs as it is the direct way of intake inorganic arsenic through contaminated groundwater (Rakhunde et al., 2012; Tokunaga et al., 2005). A considerable decrease in arsenic concentration was observed in urine, hair and nail samples of some children of North 24 Parganas district, West Bengal, when they were given completely arsenic-safe drinking water, i.e. treated surface water (Joardar et al., 2021a). Arsenic-contaminated drinking water is also a proven exposure route in scalp hair reported in other arsenic endemic regions like Thailand (Rujiralai et al., 2018) and Cambodia (Sthiannopkao et al., 2010). In some arsenic-exposed populations from Nadia district, West Bengal, significant dependence was observed between ‘daily arsenic intake through drinking water per body weight’ and ‘arsenic concentration in hair’ (Das et al., 2021c); however, no considerable dependence was observed between ‘arsenic intake through diet’ and ‘arsenic concentration in hair’ using copula-based bivariate mixed regression model (Das et al., 2018). Water arsenic has been the main path of exposure in toenail arsenic concentration as reported earlier (Signes-Pastor et al., 2021). Hair and nail grow slowly and are mostly similar in keratin composition; as a result, the association between toenail and hair arsenic concentration is proved to be quite strong (r = 0.76) (Fig. 4a), which is corroborated in other studies also (Signes-Pastor et al., 2021). Secondly, a principal component analysis (PCA) has been executed to understand the nature of association between ‘dietary intake of arsenic through drinking water or DI and arsenic concentration in the respective biological matrices (Fig. 4b). PCA is usually used to lessen the dimensionality of a data set by finding correlation among the variables in the form of a small number of principal components (Chowdhury et al., 2018; De et al., 2022; Wenning & Erickson, 1994). It clearly shows that the vectors of DI, hair arsenic and nail arsenic are close to each other, explaining that they are directly related to other (quadrant 1). On the other hand, DI and urinary arsenic fall under two opposite quadrant indicating no direct relationship between them. The two principal components explained 47.5% and 33.9% variance, respectively, and the eigenvalues of the four components analysed along with their variance are given in Supplementary Table S3b. Therefore, it can be said that arsenic through the daily dietary intake is directly manifested through the chronic biomarkers like hair and nail compared to urine (Fig. 4b). Hair and nail tissues are made of keratin which is nothing but a scleroprotein, rich in cysteine residues containing sulfhydryl groups where arsenic gets easily bound with Gault et al., 2008. Thus, the most favourable biomarkers of metal exposure for human systems are keratin proteins (Byrne et al., 2010; Kumar et al., 2021; Samanta et al., 2004).
The relation between arsenic intake and excretion through the two chronic biomarkers has been validated through a Kruskal–Wallis test followed by a Dunn’s post hoc test to find out the most fitted biomarker for arsenic toxicity. A Kruskal–Wallis test is used to decide whether there exists a statistically significant difference between the medians of the selected groups (Table 1). The study considered ‘DI’ as an independent variable and ‘arsenic concentrations in biomarkers’ as dependent variables. At 95% confidence interval, a significant difference is found between sample medians as p value is found to be < 0.05 (2.69 × 10–5) and the chi2 value is found to be 21.05 (N = 354). Therefore, a Dunn’s post hoc test is conducted to determine which specific group creates the significant difference. It is observed that the null hypothesis is rejected for hair arsenic because it shows an insignificant p value upon daily intake of arsenic (0.69), while nail arsenic is significant (p = 1.72 × 10–4). The p values along with the hypothesis assumed for the tests are mentioned in Table 1. Thus, on a cumulative approach, it can be concluded that the most imperative biomarker for estimating arsenic toxicity is the nail sample. Toenails are globally known to have more stable and long-term metal accumulations than other biomarkers of recent exposures like urine (Gutiérrez-González et al., 2019; Signes-Pastor et al., 2021).
Exposure of arsenic with respect to age
Accumulation of arsenic in the biomarkers of the studied population is seemed to be age dependent, which is explained by a ‘two-sample paired t test’ (Table 2). For this, a hypothesis is tested to confirm whether any significant difference exists between age and arsenic concentration in different biomarkers. The tstat values (12.83, 20.2 and 17.4) are found to be much higher than tcrit value, 1.98; df = 126, 115 and 115 for urine, hair and nail, respectively. In every case, the null hypothesis (Ho) is rejected, i.e. there exists a significant difference between age and arsenic concentration in the biomarkers. Also, a negative and mild-to-moderate positive correlation is found between age and arsenic concentration in urine (r = − 0.00027), hair (r = 0.111) and nail (r = 0.322) (Table 2). The present study is significant in terms of the arsenic deposition trend in the biomarkers according to the age of the participants. Deposition of arsenic varies with age, which means accumulation of metals occurs with frequency and duration of exposure that too depends on work pattern and occupation (Nath et al., 2008). In the present study, with increase in age, the mean nail arsenic concentration visibly rises (Fig. 5a); however, the percentage of increase gradually drops (Fig. 5b). Mean nail arsenic concentration is highest (14.8 mg/kg) in the old people aged between 60 to 80 years and lowest (2.38 mg/kg) in children aged below 10 years. Hair and nail arsenic concentration increases maximum from children to teenagers by about 265 and 160%, while it decreases towards adults. At the same time, the deposition of arsenic in urine does not follow any exact trend. Urinary arsenic deposition slowly drops in the three consequent age groups from 30 to 60 years by 18.3, 6.4, and 6.7% and suddenly increases in the old people by nearly 142% (Fig. 5b). The mean urinary and hair arsenic concentration is observed to be highest in participants of > 60–80 years (21.9 µg/l and 14.8 mg/kg), whereas their mean hair arsenic concentration (1.95 mg/kg) is comparatively lower than that of other adult groups (Fig. 5a). Around 50–70% of absorbed arsenic is eliminated in kidneys through methylation and the rest of it is stored in hair, nail and other tissues (Nielsen, 2001). Nail arsenic concentration is higher than that of hair in every age gap by almost 3.13 times on an average (range 1.78–7.59), which might be due to having higher arsenic binding proteins in nail than in hair along with a slower growth rate (Maity et al., 2012; Mandal & Suzuki, 2002; Tobin, 2005). Therefore, nail arsenic concentration can be termed as the most effective biomarker for arsenic toxicity irrespective of an individual’s age. Accumulation of arsenic in body tissues takes place according to age and the strength of metabolism (Mitra et al., 2004). In another worst arsenic-affected district of West Bengal, North 24 Parganas, researchers observed that arsenic concentration in the biological matrices of some population increases with age and decreases after 57 years of age (Maity et al., 2012). Some researchers from Pakistan showed that the distribution of arsenic is highest in adults followed by children and old people and it is found to be maximum in nail and blood samples followed by urine and hair (Bibi et al., 2015). The population from Huainan city, China, observed an increasing trend in hair arsenic concentration with age (Fang et al., 2019). However, some studies also observed no significant effect of age on arsenic concentration in chronic biomarkers (Rasheed et al., 2019).
Exposure of arsenic with respect to sex
Gender or sex was found to be a predominant factor in the physical and socioeconomic health of some arsenic-exposed populations in the Murshidabad district (Das, 2013). The observed mean urinary and hair arsenic concentration in the female population (11.6 ± 13.5 µg/l and 4.33 ± 5.37 mg/kg) is approximately 1.15 and 1.05 times higher than that of the male population (10.1 ± 9.51 µg/l and 4.14 ± 3.74 mg/kg) (Supplementary Table S1). However, the observed mean nail arsenic concentration is nearly 1.09 times higher in male population (8.41 ± 7.11 mg/kg, range 0.3–28 mg/kg, n = 58) compared to female ones (7.69 ± 7.84 mg/kg, range 0.35–29 mg/kg, n = 60) (Supplementary Table S1). Exposure of arsenic with respect to sex is tested through arsenic deposition in biomarkers of the studied population. A Chi-square test is conducted considering a null hypothesis (Ho): ‘As concentration in biomarkers is independent of gender’ where arsenic concentration in urine, hair and nail of the male and the female population is categorized according to their respective acceptable range (Table 3). It is observed that approximately 7.25% male population (n = 69) have higher urinary arsenic concentration than the normal range while that of the female population is 10.5% (n = 67), whereas, in another study, higher toxic metals in urine were observed in women than men, which might be a result of their increased gastrointestinal absorption at micronutrient deficiencies like iron and zinc (Berglund et al., 2011). Remarkably, the magnitude of chronic exposure in both the male and female populations is very high than acute exposure. The present study observed 96.6% male (n = 58) and 91.7% female (n = 60) hair samples having arsenic concentrations above the allowable range, whereas nail arsenic is observed to be higher than the normal range in 91.4% of male and 91.7% of female population (n = 58 and 60), respectively. Another study from this district found 95% of the nail, 75% of the hair and 94% of the urine samples with arsenic above the normal levels among total of 3800 biological (nail, urine and hair) samples of the affected villagers (Rahman et al., 2005a). Also, 85% of biological samples were found to contain arsenic above normal range of toxicity, out of the 850 samples from the affected villages of Sagarpara gram panchayats, Murshidabad (Rahman et al., 2005b). In the present study, in each case, the calculated chi2 value (0.13, 0.54 and 0.075 for urine, hair and nail) is observed to be lower than the critical value (3.84), which results in acceptance of the Ho, i.e. deposition of arsenic is not controlled by the sex of the mass (Table 3). Das et al. (2018) found similar observations which corroborated with the present study that gender is neither a significant predictor of arsenic concentration in hair nor of skin lesions. However, some studies proved that gender plays an important role in the metabolism of arsenic or its deposition in body markers. Rahman et al. (2006) showed that Bangladeshi males are more prone to obtain arsenic-induced skin lesions than females when exposed to arsenic-contaminated water from tube wells. Some researchers suggested that adult women have a better methylation capacity of arsenic than men, which is explained by the stimulating effect of estrogens on the synthesis of choline, involved in the re-methylation of homocysteine to methionine (Tseng, 2009). Lindberg et al (2008) showed that pregnant women had higher arsenic methylation efficiency than men because of sex hormones.
Health risk assessment
A health risk assessment has been performed for the studied age groups to evaluate the extent of future threats due to prolonged exposure to arsenic. The results are shown in Fig. 6 with a detailed explanation placed in Supplementary Table S4. Results show that the alternate drinking water sources also provide sufficient health risk; however, the children who consumed drinking water through tube well suffer from higher cancer risk (average 4.53 × 10–3, range 1.5 × 10–5–2 × 10–2) than those who consume alternative drinking water (Fig. 6). Sajaldhara plant treated water and pipeline supplied water, both being the alternative drinking water sources in the arsenic endemic zone, pose higher cancer risk in children (1.49 × 10–3, range 5 × 10–5–9*10–3 and 4.43 × 10–4, range 7.10 × 10–5–8.14 × 10–4, respectively) (Supplementary Table S4) than the allowable value suggested by USEPA; 1 × 10–6 (USEPA, 2005). The average cancer risk value from tube well water is observed as 8.1 × 10–3 and 1.53*10–2 for teenagers and adults with a range of (1.6 × 10–4–1.79 × 10–2) and (1.3 × 10–4–8 × 10–2), respectively (Supplementary Table S4). Similarly, the respective average cancer risk value from Sajaldhara treated water is measured as 4.04 × 10–3 (range 1.7 × 10–4–1.02 × 10–2) and 6.7 × 10–3 (range 1.7 × 10–4–2.47 × 10–2) for teenager and adults, respectively (Supplementary Table S4). Rice grain arsenic too is a potential agent for causing cancer risk as the calculated average values are found to be 4.95 × 10–4 (range 1.8 × 10–4–1.27 × 10–3), 6.19 × 10–4 (range 1.9 × 10–4–1.78*10–3) and 1.67 × 10–3 (range 1.85 × 10–4–5.6 × 10–3) for the three consequent groups of populations. The estimated values being higher than the threshold limit (1 × 10–6) reveal that the studied population faces a significant risk that may cause cancerous diseases in the long future. It has been observed that all the studied age groups are suffering from a considerable amount of non-cancer risk too through every source of drinking water as well as rice grain compared to the threshold value of non-cancer risk provided by the USEPA. The calculated average non-cancer risk is higher in adults followed by teenager and children; HQ (tube well water) are 34, 18 and 10; HQ (Sajaldhara plant treated water) are 14.9, 8.97, and 3.32; and HQ (pipeline supplied water) are 4.65, 1.33, and 0.90 for adults, teenagers and children, respectively (Fig. 6). The rice grain shows the lowest amount of quotient value, which is sufficient to cause non-cancer risk as the calculated average values are 1.10, 1.38 and 3.72 for children, teenager and adults, respectively (Fig. 6). The estimated ranges of HQ values for the three studied group of populations from drinking water and rice grain are presented in Supplementary Table S4. Cumulatively it can be stated that the health risk order is: tube well water > Sajaldhara treated water > rice grain > pipeline supplied water for children and teenagers, while it is tube well water > Sajaldhara treated water > pipeline supplied water > rice grain for adults. The results suggest that health risk of a population depends on various factors such as concentration of the metal in the source, exposure duration of the individuals (age in the present study) and body weight. Also, poor nutritional status in rural Bengal is one more possible reason for suffering from arsenic toxicity (Roychowdhury, 2010; Roychowdhury et al., 2003). Das et al. (2021a) reported that the dug wells are apparently the safest source of drinking water from the same study area. They observed low concentration of arsenic in the studied dug well water samples (mean 3.78 µg/l, range < 3 to 5.5 µg/l). Thus, the use of dug wells should be increased for both drinking and other domestic purposes in these arsenic endemic zones.
Comparative significance of the variables used in health risk assessment
Monte Carlo simulation and sensitivity analysis
High uncertainty is observed during health risk assessment when single point values are used to estimate the risk of a selected population. Therefore, Monte Carlo (MC) technique has been used to quantify the uncertainties of arsenic exposure through drinking water and rice grain. In MC simulation, instead of using a single point value of a variable, a range of the variable is used and repeated several times to achieve a degree of assurance. The values obtained as different input variables were used to reduce the uncertainties caused by distribution factors. The relative significance of the variables associated with the health risk of the populations is then evaluated through sensitivity analysis (Fig. 7), which shows the percent input of the variables (exposure duration or ED, intake rate or IR, arsenic concentration in drinking water/rice grain or C and body weight or BW) upon estimated health risk. A sensitivity analysis study is performed with HQ and CR values, which reveal that concentration of arsenic (C) is apparently the principal variable operational towards health risk among all other factors in all the three age groups. The influence of arsenic concentration in drinking water towards the calculated health risk is found to be highest in teenagers (61.2%), followed by adults (49%) and children (27.6%), whereas, for rice grain it is highest in adults (47.8%), followed by teenagers (45.5%) and children (21%). In the case of children, the second and third most influential factor towards health risk is BW and ED; 47.5% and 8.97% through rice grain and 27.4% and 17.8% through drinking water, respectively (Fig. 7). In adults, both drinking water and rice grain, contribution of ED (17.2% and 20.1%) is higher than BW (9.26% and 12.1%), whereas in teenagers, the contribution of ED (4.62% and 5.81%) is less than BW (13.7% and 17.3%). For drinking water caused health risk, IR is the fourth significant parameter; contribution percentages are 1.36, 9.49 and 9.23%, respectively, in children, teenagers and adults, while the input of IR through contaminated rice grain is 5.63, 20 and 7.34% in the three age groups (Fig. 7). Therefore, it can be concluded that to lessen the health risk, it is required to reduce the contamination at source, i.e. to find the safe drinking water source. As consumption of arsenic-contaminated rice grain also poses a sufficient amount of health risk, cultivation with higher arsenic-contaminated irrigational water should be prohibited. These findings can be supported by the work done by Giri et al. (2020) in the mining areas of Singhbhum, India, where they showed that concentration of the metals in groundwater and exposure duration were the two most significant input variables in assessing the non-cancer risk of populations. Influence of metal concentration and ED was found to be 52.4–53.3% and 40.7–41.4%, respectively, in three age groups. Similarly, Pirsaheb et al. (2021) and Sharafi et al. (2019) showed that the most imperative aspects affecting the HQ value of some Iranian populations were metal concentration and body weight.
Cluster analysis
A dendrogram plot visually groups the variables into different small or large clusters according to their similar nature or relationship (Das et al., 2020). The cluster analysis in the present study also shows that the concentration of the metal (C) is the primary factor, which is directly associated with cancer risk (CR) and non-cancer risk (HQ) (Fig. 8). Being similar in nature, CR and HQ are so closely arranged that their cluster is almost merged. The distinct cluster heights between ‘C’ and ‘CR’ or ‘HQ’ reveal that the concentration of arsenic in drinking water (Fig. 8a) is more intensely linked with health risk than rice grain arsenic concentration (Fig. 8b). The smallest cluster made with intake rate (IR) and body weight (BW) suggests that these two variables are directly dependent on each other, which results into an association with exposure duration (ED).
Conclusion
The population of Raninagar II block, Murshidabad, is significantly suffering from arsenic toxicity through contaminated drinking water and rice grain. Both the conventional drinking water and the alternate drinking water are arsenic-contaminated, which show negligence as well as the lack of cognizance of the authority. Approximately, 60 and 45.5% of drinking water samples from pipeline supplied water and Sajaldhara plant treated water are arsenic-contaminated above the permissible limit in drinking water. The present study concludes that the deposition of arsenic in biomarkers is extremely related to age of the individuals, while it is independent of sex. Nail arsenic concentration is higher than hair arsenic by an average of 3.13 times in each age-group. This study statistically proves that nail arsenic concentration is the most competent biomarker to determine arsenic toxicity in the body system than urine or hair arsenic. Adults have a higher health risk than two other studied age groups and drinking water through tube well imposes maximum health risk compared to Sajaldhara plant treated water or pipeline supplied water. Calculated mean cancer risk from the alternate drinking water sources is undoubtedly higher than the threshold limit. Sensitivity analysis study suggests that the most important factor influencing health risk of the studied population is ‘concentration of arsenic’ in drinking water and rice grain followed by ‘exposure duration’ and ‘body weight’. Therefore, regular and sufficient supply of arsenic-safe drinking water should be addressed by the local governments and policy makers to these arsenic-endemic zones. The arsenic removal plants must be monitored consistently and the pipeline supplied water should be evaluated properly before reaching to the population. Increased usage of dug wells, treated surface water and rainwater harvesting is immediately advised in this sternly arsenic-affected zone for drinking as well as cultivation purposes. Evaluating the hypotheses of the present work against a selected population from an arsenic-unexposed zone comes under the future scope of this study.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request and it can be shared as per the requirement.
References
Abdul, K. S. M., Jayasinghe, S. S., Chandana, E. P., Jayasumana, C., & De Silva, P. M. C. (2015). Arsenic and human health effects: A review. Environmental Toxicology and Pharmacology, 40(3), 828–846. https://doi.org/10.1016/j.etap.2015.09.016
Alexakis, D., & Gamvroula, D. (2014). Arsenic, chromium, and other potentially toxic elements in the rocks and sediments of Oropos-Kalamos basin, Attica, Greece. Applied and Environmental Soil Science, 2014, 718534. https://doi.org/10.1155/2014/718534
Alexakis, D. E., Bathrellos, G. D., Skilodimou, H. D., & Gamvroula, D. E. (2021). Spatial distribution and evaluation of arsenic and zinc content in the soil of a karst landscape. Sustainability, 13(12), 6976. https://doi.org/10.3390/su13126976
Arnold, H. L., Odam, R. B., & James, W. D. (1990). Disease of the skin. Clinical dermatology (pp. 121–122). W.B. Saunders.
Berglund, M., Lindberg, A. L., Rahman, M., Yunus, M., Grandér, M., Lönnerdal, B., & Vahter, M. (2011). Gender and age differences in mixed metal exposure and urinary excretion. Environmental Research, 111(8), 1271–1279. https://doi.org/10.1016/j.envres.2011.09.002
Bhattacharya, P., Samal, A. C., Majumdar, J., & Santra, S. C. (2010). Arsenic contamination in rice, wheat, pulses, and vegetables: A study in an arsenic affected area of West Bengal, India. Water, Air, and Soil Pollution, 213(1), 3–13. https://doi.org/10.1007/s11270-010-0361-9
Bibi, M., Hashmi, M. Z., & Malik, R. N. (2015). Human exposure to arsenic in groundwater from Lahore district, Pakistan. Environmental Toxicology and Pharmacology, 39(1), 42–52. https://doi.org/10.1016/j.etap.2014.10.020
Biswas, B., Chakraborty, A., Chatterjee, D., Pramanik, S., Ganguli, B., Majumdar, K. K., Nriagu, J., Kulkarni, K. Y., Bansiwal, A., Labhasetwar, P., & Bhowmick, S. (2021). Arsenic exposure from drinking water and staple food (rice): A field scale study in rural Bengal for assessment of human health risk. Ecotoxicology and Environmental Safety, 228, 113012. https://doi.org/10.1016/j.ecoenv.2021.113012
Biswas, A., Swain, S., Chowdhury, N. R., Joardar, M., Das, A., Mukherje, M., & Roychowdhury, T. (2019). Arsenic contamination in Kolkata metropolitan city: Perspective of transportation of agricultural products from arsenic-endemic areas. Environmental Science and Pollution Research, 26, 22929–22944. https://doi.org/10.1007/s11356-019-05595-z
Bozack, A. K., Cardenas, A., Quamruzzaman, Q., Rahman, M., Mostofa, G., Christiani, D. C., & Kile, M. L. (2018). DNA methylation in cord blood as mediator of the association between prenatal arsenic exposure and gestational age. Epigenetics, 13(9), 923–940. https://doi.org/10.1080/15592294.2018.1516453
Brima, E. I., Haris, P. I., Jenkins, R. O., Polya, D. A., Gault, A. G., & Harrington, C. F. (2006). Understanding arsenic metabolism through a comparative study of arsenic levels in the urine, hair and fingernails of healthy volunteers from three unexposed ethnic groups in the United Kingdom. Toxicology and Applied Pharmacology, 216(1), 122–130. https://doi.org/10.1016/j.taap.2006.04.004
Buchet, J. P., Lauwerys, R., & Roels, H. (1981). Comparison of the urinary excretion of arsenic metabolites after a single oral dose of sodium arsenite, monomethylarsonate, or dimethylarsinate in man. International Archives of Occupational and Environmental Health, 48(1), 71–79. https://doi.org/10.1007/BF00405933
Byrne, S., Amarasiriwardena, D., Bandak, B., Bartkus, L., Kane, J., Jones, J., Yanez, J., Arriaza, B., & Cornejo, L. (2010). Were Chinchorros exposed to arsenic? Arsenic determination in Chinchorro mummies’ hair by laser ablation inductively coupled plasma-mass spectrometry (LA-ICP-MS). Microchemical Journal, 94(1), 28–35. https://doi.org/10.1016/j.microc.2009.08.006
Casentini, B., Hug, S. J., & Nikolaidis, N. P. (2011). Arsenic accumulation in irrigated agricultural soils in Northern Greece. Science of the Total Environment, 409(22), 4802–4810. https://doi.org/10.1016/j.scitotenv.2011.07.064
Census of India (2011). District Census Handbook, Murshidabad, West Bengal. https://doi.org/10.1016/j.etap.2015.09.016B_MURSHIDABAD.pdf
Chakraborti, D., Das, B., Rahman, M. M., Chowdhury, U. K., Biswas, B., Goswami, A. B., Nayak, B., Pal, A., Sengupta, M. K., Ahamed, S., & Hossain, A. (2009). Status of groundwater arsenic contamination in the state of West Bengal, India: A 20-year study report. Molecular Nutrition and Food Research, 53(5), 542–551. https://doi.org/10.1002/mnfr.200700517
Chakraborti, D., Rahman, M. M., Das, B., Chatterjee, A., Das, D., Nayak, B., Pal, A., Chowdhury, U. K., Ahmed, S., Biswas, B. K., Sengupta, M. K., & Kumar, M. (2017). Groundwater arsenic contamination and its health effects in India. Hydrogeology Journal, 25(4), 1165–1181. https://doi.org/10.1007/s10040-017-1556-6
Chakraborti, D., Rahman, M. M., Das, B., Nayak, B., Pal, A., Sengupta, M. K., Hossain, M. A., Ahamed, S., Sahu, M., Saha, K. C., & Mukherjee, S. C. (2013). Groundwater arsenic contamination in Ganga–Meghna–Brahmaputra plain, its health effects and an approach for mitigation. Environment and Earth Science, 70(5), 1993–2008. https://doi.org/10.1007/s12665-013-2699-y
Chakraborti, D., Sengupta, M. K., Rahman, M. M., Ahamed, S., Chowdhury, U. K., Hossain, A., Mukherjee, S. C., Pati, S., Saha, K. C., Dutta, R. N., & Quamruzzaman, Q. (2004). Groundwater arsenic contamination and its health effects in the Ganga-Meghna-Brahmaputra plain. Journal of Environmental Monitoring, 6(6), 74N-83N. https://doi.org/10.1039/b406573p
Chakraborti, D., Singh, S. K., Rahman, M. M., Dutta, R. N., Mukherjee, S. C., Pati, S., & Kar, P. B. (2018). Groundwater arsenic contamination in the Ganga River Basin: A future health danger. International Journal of Environmental Research and Public Health, 15(2), 180. https://doi.org/10.3390/ijerph15020180
Chakraborty, M., Mukherjee, A., & Ahmed, K. M. (2015). A review of groundwater arsenic in the Bengal Basin, Bangladesh and India: From source to sink. Current Pollution Reports, 1(4), 220–247. https://doi.org/10.1007/s40726-015-0022-0
Chowdhury, N. R., Ghosh, S., Joardar, M., Kar, D., & Roychowdhury, T. (2018). Impact of arsenic contaminated groundwater used during domestic scale post harvesting of paddy crop in West Bengal: Arsenic partitioning in raw and parboiled whole grain. Chemosphere, 211, 173–184. https://doi.org/10.1016/j.chemosphere.2018.07.128
Chowdhury, U. K., Biswas, B. K., Chowdhury, T. R. G., Mandal, B. K., Basu, G. C., Chanda, C. R., Lodh, D., Saha, K. C., Mukherjee, S. K., Roy, S., Kabir, S., Quamruzzaman, Q., & Chakraborti, D. (2000). Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environmental Health Perspectives, 108(5), 393–397. https://doi.org/10.1289/ehp.00108393
Codex Alimentarius Commission (2014). Report of the Eighth Session of the Codex Committee on Contaminants in Food; the Hague, 31 March–4 April 2014. Rep14/CF. Geneva, Switzerland: World Health Organization, 2014, The Netherlands. Food and Agriculture Organization of the United Nations, Rome, Italy. http://bit.ly/1vprMSO
European Commission (2015). Commission Regulation 2015/1006 of 25 June 2015 Amending Regulation (EC) No 1881/2006 As Regards Maximum Levels of Inorganic Arsenic in Foodstuffs.
Das, A. (2013). Socio-economic and gender aspects of arsenicosis—A case study in rural West Bengal (India). IOSR Journal of Human Social Science, 13(1), 74–83.
Das, A., Biswas, A., & Mazumder, D. N. G. (2018). Association between skin lesion and arsenic concentration in hair by mixed bivariate model in chronic arsenic exposure. Environmental Geochemistry and Health, 40(6), 2359–2369. https://doi.org/10.1007/s10653-018-0102-3
Das, A., Das, S. S., Chowdhury, N. R., Joardar, M., Ghosh, B., & Roychowdhury, T. (2020). Quality and health risk evaluation for groundwater in Nadia district, West Bengal: An approach on its suitability for drinking and domestic purpose. Groundwater for Sustainable Development, 10, 100351. https://doi.org/10.1016/j.gsd.2020.100351
Das, A., Joardar, M., Chowdhur, N. R., & Roychowdhury, T. (2021c). Evaluation of health quality in two studied groups of school children from an arsenic-exposed area of West Bengal, India. In J. Dutta, S. Goswami, & A. Mitra (Eds.), Multidimensional approaches to impacts of changing environment on human health (1st ed., pp. 93–112). CRC Press.
Das, A., Joardar, M., Chowdhury, N. R., De, A., Mridha, D., & Roychowdhury, T. (2021b). Arsenic toxicity in livestock growing in arsenic endemic and control sites of West Bengal: Risk for human and environment. Environmental Geochemistry and Health, 43, 3005–3025. https://doi.org/10.1007/s10653-021-00808-2
Das, A., Joardar, M., De, A., Mridha, D., Chowdhury, N. R., Khan, M. T. B. K., Chakrabartty, P., & Roychowdhury, T. (2021a). Pollution index and health risk assessment of arsenic through different groundwater sources and its load on soil-paddy-rice system in a part of Murshidabad district of West Bengal, India. Groundwater for Sustainable Development, 15, 100652. https://doi.org/10.1016/j.gsd.2021.100652
De, A., Mridha, D., Joardar, M., Das, A., Chowdhury, N. R., & Roychowdhury, T. (2022). Distribution, prevalence and health risk assessment of fluoride and arsenic in groundwater from lower Gangetic plain in West Bengal, India. Groundwater for Sustainable Development, 16, 100722.
Fang, F., Wang, Y., Zhu, Z., Yao, Y., Lin, Y., & Wang, J. (2019). Distribution characteristics and influencing factors of heavy metals in scalp hair of Huainan urban residents. Environmental Monitoring and Assessment, 191(7), 1–10. https://doi.org/10.1007/s10661-019-7592-z
Farmer, J. G., & Johnson, L. R. (1990). Assessment of occupational exposure to inorganic arsenic based on urinary concentrations and speciation of arsenic. British Journal of Industrial Medicine, 47, 342–348.
Gault, A. G., Rowland, H. A., Charnock, J. M., Wogelius, R. A., Gomez-Morilla, I., Vong, S., Leng, M., Samreth, S., Sampson, M. L., & Polya, D. A. (2008). Arsenic in hair and nails of individuals exposed to arsenic-rich groundwaters in Kandal province, Cambodia. Science of the Total Environment, 393(1), 168–176. https://doi.org/10.1016/j.scitotenv.2007.12.028
Giri, S., Singh, A. K., & Mahato, M. K. (2020). Monte Carlo simulation-based probabilistic health risk assessment of metals in groundwater via ingestion pathway in the mining areas of Singhbhum copper belt, India. International Journal of Environmental Health Research, 30(4), 447–460. https://doi.org/10.1080/09603123.2019.1599101
Golfinopoulos, S. K., Varnavas, S. P., & Alexakis, D. E. (2021). The status of arsenic pollution in the greek and Cyprus environment: An overview. Water, 13(2), 224. https://doi.org/10.3390/w13020224
Gutiérrez-González, E., García-Esquinas, E., de Larrea-Baz, N. F., Salcedo-Bellido, I., Navas-Acien, A., Lope, V., Gómez-Ariza, J. L., Pastor, R., Pollán, M., & Pérez-Gómez, B. (2019). Toenails as biomarker of exposure to essential trace metals: A review. Environmental Research, 179, 108787. https://doi.org/10.1016/j.envres.2019.108787
Hadi, A., & Parveen, R. (2004). Arsenicosis in Bangladesh: Prevalence and socio-economic correlates. Public Health, 118(8), 559–564. https://doi.org/10.1016/j.puhe.2003.11.002
Halder, S. (2019). Groundwater arsenic contamination in Murshidabad, West Bengal: Current scenario, effects and probable ways of mitigation with special reference to Majhyampur water treatment plant Murshidabad. IOSR Journal of Environmental Science, Toxicology and Food Technology, 13(6), 1–11.
Harvey, C. F., Swartz, C. H., Badruzzaman, A. B. M., Keon-Blute, N., Yu, W., Ali, M. A., Jay, J., Beckie, R., Niedan, V., Brabander, D., Oates, P. M., Ashfaque, K. N., Islam, S., Hemond, H. F., & Ahmed, M. F. (2002). Arsenic mobility and groundwater extraction in Bangladesh. Science, 298(5598), 1602–1606. https://doi.org/10.1126/science.1076978
Hata, A., Endo, Y., Nakajima, Y., Ikebe, M., Ogawa, M., Fujitani, N., & Endo, G. (2007). HPLC-ICP-MS speciation analysis of arsenic in urine of Japanese subjects without occupational exposure. Journal of Occupational Health, 49(3), 217–223. https://doi.org/10.1539/joh.49.217
Hinwood, A. L., Sim, M. R., Jolley, D., de Klerk, N., Bastone, E. B., Gerostamoulos, J., & Drummer, O. H. (2003). Hair and toenail arsenic concentrations of residents living in areas with high environmental arsenic concentrations. Environmental Health Perspectives, 111(2), 187–193. https://doi.org/10.1289/ehp.5455
Hughes, M. F. (2006). Biomarkers of exposure: A case study with inorganic arsenic. Environmental Health Perspectives, 114(11), 1790–1796. https://doi.org/10.1289/ehp.9058
Ioanid, N., Bors, G., & Popa, I. (1961). Beiträgezur Kenntnis des normalen Arsengehaltesron Nägeln und des Gehaltes in den Fällen von Arsenpolyneuritis. International Journal of Legal Medicine, 52(1), 90–94.
Joardar, M., Chowdhury, N. R., Das, A., & Roychowdhury, T. (2021c). Evaluation of health effects and risk assessment of arsenic on an unexposed population from an arsenic-exposed zone of West Bengal, India. In J. Dutta, S. Goswami, & A. Mitra (Eds.), Multidimensional approaches to impacts of changing environment on human health (1st ed., pp. 113–134). CRC Press.
Joardar, M., Das, A., Chowdhury, N. R., Mridha, D., De, A., Majumdar, K. K., & Roychowdhury, T. (2021a). Health effect and risk assessment of the populations exposed to different arsenic levels in drinking water and foodstuffs from four villages in arsenic endemic Gaighata block, West Bengal, India. Environmental Geochemistry and Health, 43, 3027–3053. https://doi.org/10.1007/s10653-021-00823-3
Joardar, M., Das, A., Mridha, D., De, A., Chowdhury, N. R., & Roychowdhury, T. (2021b). Evaluation of acute and chronic arsenic exposure on school children from exposed and apparently control areas of West Bengal India. Expo Health, 13, 33–50. https://doi.org/10.1007/s12403-020-00360-x
Kumar, A., Ali, M., Kumar, R., Kumar, M., Sagar, P., Pandey, R. K., Akhouri, V., Kumar, V., Anand, G., Niraj, P. K., Rani, R., Kumar, S., Kumar, D., Bishwapriya, A., & Ghosh, A. K. (2021). Arsenic exposure in Indo Gangetic plains of Bihar causing increased cancer risk. Science and Reports, 11(1), 1–16. https://doi.org/10.1038/s41598-021-81579-9
Lindberg, A. L., Ekström, E. C., Nermell, B., Rahman, M., Lönnerdal, B., Persson, L. Å., & Vahter, M. (2008). Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environmental Research, 106(1), 110–120. https://doi.org/10.1016/j.envres.2007.08.011
Maity, J. P., Nath, B., Kar, S., Chen, C. Y., Banerjee, S., Jean, J. S., Liu, M., Centeno, J. A., Bhattacharya, P., Chang, C. L., & Santra, S. C. (2012). Arsenic-induced health crisis in peri-urban Moyna and Ardebok villages, West Bengal, India: An exposure assessment study. Environmental Geochemistry and Health, 34(5), 563–574. https://doi.org/10.1007/s10653-012-9458-y
Mandal, D., Biswas, S., Seal, S., Mandal, R., Das, S., & Basu, A. (2022). Arsenic toxicity and its clinical manifestations in Murshidabad district with some potential remedial measures. In Microbes and microbial biotechnology for green remediation (pp. 701–715). Elsevier. https://doi.org/10.1016/B978-0-323-90452-0.00011-6
Mandal, B. K., & Suzuki, K. T. (2002). Arsenic round the world: A review. Talanta, 58(1), 201–235. https://doi.org/10.1016/S0039-9140(02)00268-0
Mitra, S. R., Mazumder, D. G., Basu, A., Block, G., Haque, R., Samanta, S., Ghosh, N., Hira Smith, M. M., Ehrenstein, O. S., & Smith, A. H. (2004). Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India. Environmental Health Perspectives, 112(10), 1104–1109. https://doi.org/10.1289/ehp.6841
Mukherjee, S. C., Saha, K. C., Pati, S., Dutta, R. N., Rahman, M. M., Sengupta, M. K., Ahamed, S., Lodh, D., Das, B., Hossain, M. A., Nayak, B., & Asad, K. A. (2005). Murshidabad-one of the nine groundwater arsenic-affected districts of West Bengal, India. Part II: Dermatological, neurological, and obstetric findings. Clinical Toxicology, 43(7), 835–848.
Mukhopadhyay, B. P., Barua, S., Bera, A., & Mitra, A. K. (2020). Study on the quality of groundwater and its impact on human health: A case study from Murshidabad district, West Bengal. Journal of the Geological Society of India, 96(6), 597–602. https://doi.org/10.1007/s12594-020-1608-8
Nath, B., Sahu, S. J., Jana, J., Mukherjee-Goswami, A., Roy, S., Sarkar, M. J., & Chatterjee, D. (2008). Hydrochemistry of arsenic-enriched aquifer from rural West Bengal, India: A study of the arsenic exposure and mitigation option. Water, Air and Soil Pollution, 190(1), 95–113. https://doi.org/10.1007/s11270-007-9583-x
Nguyen, T. P. M., Nguyen, T. P. T., Bui, T. H., & Nguyen, T. H. (2019). Concentration of arsenic in groundwater, vegetables, human hair and nails in mining site in the Northern Thai Nguyen province, Vietnam: human exposure and risks assessment. Human and Ecological Risk Assessment: an International Journal, 25(3), 602–613. https://doi.org/10.1080/10807039.2018.1483189
Nielsen, F. H. (2001). Trace minerals. Nutrition in health and sickness (9th ed., pp. 328–331). McGraw-Hill.
Nowak, B., & Kozłowski, H. (1998). Heavy metals in human hair and teeth. Biological Trace Element Research, 62(3), 213–228.
NRC. (1999). Arsenic in drinking water. National Academy Press.
Pirsaheb, M., Hadei, M., & Sharafi, K. (2021). Human health risk assessment by Monte Carlo simulation method for heavy metals of commonly consumed cereals in Iran-Uncertainty and sensitivity analysis. Journal of Food Composition and Analysis, 96, 103697. https://doi.org/10.1016/j.jfca.2020.103697
Povorinskaya, O. A., & Karpenko, O. M. (2009). Macro-and trace element status of patients of the elder age groups. Bulletin of Experimental Biology and Medicine, 147(4), 473. https://doi.org/10.1007/s10517-009-0556-5
Rahman, M. M., Ng, J. C., & Naidu, R. (2009). Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environmental Geochemistry and Health, 31(1), 189–200. https://doi.org/10.1007/s10653-008-9235-0
Rahman, M. A., Rahman, A., Khan, M. Z. K., & Renzaho, A. M. (2018). Human health risks and socio-economic perspectives of arsenic exposure in Bangladesh: A scoping review. Ecotoxicology and Environmental Safety, 150, 335–343. https://doi.org/10.1016/j.ecoenv.2017.12.032
Rahman, M. M., Sengupta, K. M., Ahamed, S., Lodh, D., Das, B., Hossain, A., Nayak, M., & Pati, S. (2005a). Murshidabad-one of the nine groundwater arsenic-affected districts of West Bengal India. Part I: Magnitude of contamination and population at risk. Clinical Toxicology, 43(7), 823–834. https://doi.org/10.1080/15563650500357461
Rahman, M. M., Sengupta, M. K., Ahamed, S., Chowdhury, U. K., Lodh, D., Hossain, M. A., Das, B., Chakraborti, D., Saha, K. C., Kaies, I., & Barua, A. K. (2005b). Status of groundwater arsenic contamination and human suffering in a Gram Panchayet (cluster of villages) in Murshidabad, one of the nine arsenic affected districts in West Bengal, India. Journal of Water Health, 3(3), 283–296. https://doi.org/10.2166/wh.2005.038
Rahman, M., Vahter, M., Sohel, N., Yunus, M., Wahed, M. A., Streatfield, P. K., Ekstrom, E., & Persson, L. Å. (2006). Arsenic exposure and age-and sex-specific risk for skin lesions: A population-based case–referent study in Bangladesh. Environmental Health Perspectives, 114(12), 1847–1852. https://doi.org/10.1289/ehp.9207
Rakhunde, R., Jasudkar, D., Deshpande, L., Juneja, H. D., & Labhasetwar, P. (2012). Health effects and significance of arsenic speciation in water. International Journal of Environmental Science Research, 1(4), 92–96.
Rasheed, H., Kay, P., Slack, R., & Gong, Y. Y. (2019). Assessment of arsenic species in human hair, toenail and urine and their association with water and staple food. Journal of Exposure Science and Environmental Epidemiology, 29(5), 624–632. https://doi.org/10.1038/s41370-018-0056-7
Ratnaike, R. N. (2003). Acute and chronic arsenic toxicity. Postgraduate Medical Journal, 79(933), 391–396.
Ravenscroft, P., Brammer, H., & Richards, K. S. (2011). Arsenic pollution: A global synthesis (Vol. 94). Wiley.
RGI (2003). Registrar General of India, SRS analytical studies, Report No. 3, New Delhi.
Roychowdhury, T. (2010). Groundwater arsenic contamination in one of the 107 arsenic-affected blocks in West Bengal, India: Status, distribution, health effects and factors responsible for arsenic poisoning. International Journal of Hygiene and Environmental Health, 213(6), 414–427. https://doi.org/10.1016/j.ijheh.2010.09.003
Roychowdhury, T., Tokunaga, H., & Ando, M. (2003). Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Science of the Total Environment, 308, 15–35. https://doi.org/10.1016/S0048-9697(02)00612-5
Roychowdhury, T., Tokunaga, H., Uchino, T., & Ando, M. (2005). Effect of arsenic-contaminated irrigation water on agricultural land soil and plants in West Bengal, India. Chemosphere, 58(6), 799–810. https://doi.org/10.1016/j.chemosphere.2004.08.098
Rujiralai, T., Juansai, N., & Cheewasedtham, W. (2018). Arsenic determination in soils and hair from schools in past mining activity areas in Ron Phibun district, Nakhon Si Thammarat province, Thailand and relationship between soil and hair arsenic. Chemical Papers, 72(2), 381–391. https://doi.org/10.1007/s11696-017-0287-4
Samal, A. C., Bhattacharya, P., Biswas, P., Maity, J. P., Bundschuh, J., & Santra, S. C. (2021). Variety-specific arsenic accumulation in 44 different rice cultivars (O. sativa L.) and human health risks due to co-exposure of arsenic-contaminated rice and drinking water. Journal of Hazardous Materials, 407, 124804. https://doi.org/10.1016/j.jhazmat.2020.124804
Samanta, G., Sharma, R., Roychowdhury, T., & Chakraborti, D. (2004). Arsenic and other elements in hair, nails, and skin-scales of arsenic victims in West Bengal, India. Science of the Total Environment, 326(1–3), 33–47. https://doi.org/10.1016/j.scitotenv.2003.12.006
Santra, S. C., Samal, A. C., Bhattachary, P., Banerjee, S., Biswas, A., & Majumdar, J. (2013). Arsenic in food chain and community health risk: A study in Gangetic West Bengal. Procedia Environmental Sciences, 18, 2–13. https://doi.org/10.1016/j.proenv.2013.04.002
Shaji, E., Santosh, M., Sarath, K. V., Prakash, P., Deepchand, V., & Divya, B. V. (2021). Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geoscience Frontiers, 12(3), 101079. https://doi.org/10.1016/j.gsf.2020.08.015
Shankar, S., Shanker, U., & Shikha (2014). Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. The Scientific World Journal, 2014, 304524. https://doi.org/10.1155/2014/304524
Sharafi, K., Nodehi, R. N., Yunesian, M., Mahvi, A. H., Pirsaheb, M., & Nazmara, S. (2019). Human health risk assessment for some toxic metals in widely consumed rice brands (domestic and imported) in Tehran, Iran: Uncertainty and sensitivity analysis. Food Chemistry, 277, 145–155. https://doi.org/10.1016/j.foodchem.2018.10.090
Signes-Pastor, A. J., Gutiérrez-González, E., García-Villarino, M., Rodríguez-Cabrera, F. D., López-Moreno, J. J., Varea-Jiménez, E., Pastor-Barriuso, R., & Karagas, M. R. (2021). Toenails as a biomarker of exposure to arsenic: A review. Environmental Research, 195, 110286. https://doi.org/10.1016/j.envres.2020.110286
Sthiannopkao, S., Kim, K. W., Cho, K. H., Wantala, K., Sotham, S., Sokuntheara, C., & Kim, J. H. (2010). Arsenic levels in human hair, Kandal Province, Cambodia: The influences of groundwater arsenic, consumption period, age and gender. Applied Geochemistry, 25(1), 81–90. https://doi.org/10.1016/j.apgeochem.2009.10.003
Tobin, D. J. (2005). The biogenesis and growth of hair. Hair in toxicology. In D. J. Tobin (Ed.), An important bio monitor. The Royal Society of Chemistry.
Tokunaga, H., Roychowdhury, T., Uchino, T., & Ando, M. (2005). Urinary arsenic species in an arsenic-affected area of West Bengal, India (part III). Applied Organometallic Chemistry, 19(2), 246–253. https://doi.org/10.1002/aoc.791
Torres-Sánchez, L., López-Carrillo, L., Rosado, J. L., Rodriguez, V. M., Vera-Aguilar, E., Kordas, K., García-Vargas, G. G., & Cebrian, M. E. (2016). Sex differences in the reduction of arsenic methylation capacity as a function of urinary total and inorganic arsenic in Mexican children. Environmental Research, 151, 38–43. https://doi.org/10.1016/j.envres.2016.07.020
Tseng, C. H. (2009). A review on environmental factors regulating arsenic methylation in humans. Toxicology and Applied Pharmacology, 235(3), 338–350. https://doi.org/10.1016/j.taap.2008.12.016
USEPA (2005). Guidelines for Carcinogen Risk Assessment. Risk Assessment Forum. United States Environmental Protection Agency, Washington, DC. EPA/630/P-03/ 001F
Vahter, M. (1994). Species differences in the metabolism of arsenic compounds. Applied Organometallic Chemistry, 8(3), 175–182.
Vahter, M. (1999). Methylation of inorganic arsenic in different mammalian species and population groups. Science Progress, 82(1), 69–88.
Wade, T. J., Xia, Y., Mumford, J., Wu, K., Le, X. C., Sams, E., & Sanders, W. E. (2015). Cardiovascular disease and arsenic exposure in Inner Mongolia, China: A case control study. Environmental Health, 14(1), 1–10. https://doi.org/10.1186/s12940-015-0022-y
Wenning, R. J., & Erickson, G. A. (1994). Interpretation and analysis of complex environmentaldata using chemometric methods. TrAC, Trends in Analytical Chemistry, 13(10), 446–457.
WHO. (2011). Guidelines for drinking-water quality. World Health Org., 216, 303–304.
Wongsasuluk, P., Chotpantarat, S., Siriwong, W., & Robson, M. (2018). Using hair and fingernails in binary logistic regression for bio-monitoring of heavy metals/metalloid in groundwater in intensively agricultural areas, Thailand. Environmental Research, 162, 106–118. https://doi.org/10.1016/j.envres.2017.11.024
Wu, B., & Chen, T. (2010). Changes in hair arsenic concentration in a population exposed to heavy pollution: Follow-up investigation in Chenzhou City, Hunan Province, Southern China. Journal of Environmental Science, 22(2), 283–289. https://doi.org/10.1016/S1001-0742(09)60106-6
Acknowledgements
We acknowledge the help of ‘field workers’, especially the team members of ‘Bilchatra Anneshan Janaakalyan Samity, a Non-Government Organization (NGO) located in Raninagar for collection of required samples in the field. The authors further acknowledge the studied population for showing their willingness to be involved into the work and providing the samples and related information to carry forward the study. The local Raninagar II gram panchayat office and Block Development Office are well acknowledged. Financial supports from ‘Department of Science & Technology’, Government of West Bengal (Research Project Grant Memo No. 262 (Sanc.)/ST/P/S&T/1G-64/2017, dated 25/3/2018) and Inter University Research Project, RUSA (R-11/1092/19, dated 06/08/2019).
Funding
‘Department of Science & Technology’, Government of West Bengal (Research Project Grant Memo No. 262 (Sanc.)/ST/P/S&T/1G-64/2017, dated 25/3/2018) and Inter University Research Project, RUSA (R-11/1092/19, dated 06/08/2019).
Author information
Authors and Affiliations
Contributions
AD did research planning, analytical work, statistical presentation and initial draft preparation. MJ done analysis, initial draft preparation and checking. NRC contributed to analysis and draft checking. DM and AD were involved in field survey, collection of samples and information from fields. SM did draft checking and data evaluation. JD performed statistical presentation. KKM did identification of dermatological skin manifestations and health check-up of the studied populations. TR did overall supervising the entire research study, monitoring the research project and work plan, manuscript revision and correction.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
The health guideline for human experiment has been followed, and all procedures performed in this study involving human participants were in accordance with the ethical standards of the institute and international ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All the authors are agreed to publish this work in this well renowned journal and no part of this manuscript is either published earlier, or under consideration anywhere. The authors affirm that human research participants provided informed consent for publication of the images in Supplementary Fig. S3.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, A., Joardar, M., Chowdhury, N.R. et al. Significance of the prime factors regulating arsenic toxicity and associated health risk: a hypothesis-based investigation in a critically exposed population of West Bengal, India. Environ Geochem Health 45, 3423–3446 (2023). https://doi.org/10.1007/s10653-022-01422-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01422-6