Abstract
Environmental arsenic exposure is associated with increased risk of non-cancerous chronic diseases and a variety of cancers in humans. The aims of this study were to carry out for the first time a health risk assessment for two common arsenic exposure routes (drinking water and soil ingestion) in children living in the most important agricultural areas in the Yaqui and Mayo valleys in Sonora, Mexico. Drinking water sampling was conducted in the wells of 57 towns. A cross-sectional study was done in 306 children from 13 villages in the valleys. First morning void urine samples were analyzed for inorganic arsenic (InAs) and monomethyl and dimethyl arsenic (MMA and DMA) by HPLC/ICP-MS. The results showed a wide range of arsenic levels in drinking water between 2.7 and 98.7 μg As/L. Arsenic levels in agricultural and backyard soils were in the range of < 10–27 mg As/kg. The hazard index (HI) = ∑hazard quotient (HQ) for drinking water, agricultural soil, and backyard soil showed values > 1 in 100% of the study towns, and the carcinogenic risk (CR) was greater than 1E−04 in 85%. The average of arsenic excreted in urine was 31.7 μg As/L, and DMA had the highest proportion in urine, with averages of 77.8%, followed by InAs and MMA with 11.4 and 10.9%, respectively, percentages similar to those reported in the literature. Additionally, positive correlations between urinary arsenic levels and HI values were found (r = 0.59, P = 0.000). These results indicated that this population is at high risk of developing chronic diseases including cancer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human exposure to environmental arsenic occurs primarily through ingestion of drinking water, food, soil ingestion, and inhalation [1, 2]. Arsenic exposure affects millions of people in the world, and is associated with skin lesions; lung, kidney, skin, and liver cancers; and neurologic and cardiovascular pathologies [2, 3].
Recent studies show that the most important environmental media containing arsenic are foods and drinking water [4, 5], which highlights the importance of including a “multi source approach” in the risk assessment in order to provide a better understanding of human exposure.
Drinking water has been considered the predominant pathway of human exposure to arsenic in agricultural areas of arid regions, and arsenic in drinking is a priority research topic and global health problem [6]. Drinking water may be obtained from a number of sources (surface water, rainwater, groundwater) depending on local availability. Climate change is expected to severely impact both the quality and availability of water in arid zones [7]; thus, deep aquifers could become the main source of drinking water. Except for localized sources of anthropogenic contamination, the highest aqueous arsenic concentrations tend to be found in groundwater from deep aquifers in Latin America [8] because of natural water-rock interaction processes and the high solid/solution ratios found in aquifers [7].
Residential soils may represent a significant exposure path because arsenic can be accumulated in the human body via direct inhalation, ingestion, and dermal contact absorption. Resuspension of fine soil particles is a dominating process in arid environments for dust generation [9]. Increasing superficial temperature linked to climate change promotes the loss of moisture in the soil, which causes a decrease in the cohesion of the fine particles, favoring their suspension. Agricultural and mining activities include processes of removal of the soil layer, which increases the emission of particulate matter into the atmosphere representing an exposure pathway of inhalation and ingestion [10].
The state of Sonora is located in northwestern Mexico, neighboring Arizona in the USA. The main economic activities of Sonora include mining and agriculture. The most important agricultural areas are located in the central and southern parts of the state, just at the end of the Yaqui river basin, primarily close to the coast, and two of the most productive areas are the Yaqui and Mayo valleys.
Because of the scarce availability of surface water from dams in the state, the main source of drinking water of the population is from deep wells. Previous studies reported that drinking water is one of the most important paths of arsenic exposure in some towns of Sonora [11, 12], but to our knowledge, and despite the high population living in such areas, there is no published research regarding health risk assessment for simultaneous arsenic exposure (drinking water and soil ingestion) in the residential areas from the Yaqui and Mayo agricultural valleys.
Information about arsenic exposure by the major environmental routes in these agricultural communities is needed for risk assessment and decision-making policies [13]. Urinary arsenic is a reliable biomarker to evaluate recent exposure to inorganic arsenic, and its determination in biological samples supports risk assessment studies [3, 14, 15]. In addition, the use of this biomarker has increased the power of recent studies because exposure misclassification is minimized [11, 13˗15].
Human health risk assessment is the process used to estimate the nature and probability of adverse health effects in humans by chemicals in contaminated environmental media such as drinking water and soils. There are a number of important studies related to these pathways [16, 17]; however, studies are scarce in communities with arsenic agrarian legacy including agricultural areas such as the Yaqui and Mayo areas, where there have been reports of heavy application of pesticides containing lead arsenate and sodium arsenate since the 1950s [18].
Therefore, the aims of this study were (i) to carry out for the first time in this study area the health risk assessment by two common exposure routes (drinking water and soils) in children living in the most important agricultural areas in the Yaqui and Mayo valleys in Sonora, (ii) to show the most complete picture of the arsenic levels and spatial location of the major drinking water wells in these agricultural areas, and (iii) to present for the first time data showing the arsenic levels in soils from these agricultural and residential zones.
Materials and Methods
Study Area
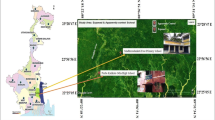
The Yaqui and Mayo valleys are two of the most important agricultural areas in Mexico, located in southwest Sonora (Fig. 1). Water from the Yaqui and Mayo rivers are the most important source of drinking water for the Mayo and the Yaqui Valley respectively; this latter valley is known as the “cradle of the Green Revolution for agriculture” [18]. Both valleys have a modern irrigation system, with a total of approximately 396,187 ha under irrigation. Of these, 86% of the water used in the Yaqui valley comes from the “Alvaro Obregon” dam and 14% is produced by 600 wells, while in the Mayo valley, 88% of the irrigation water comes from the “Adolfo Ruiz Cortines” dam (Fig. 1) and 12% is produced by approximately 180 wells [19].
The Yaqui and Mayo valleys produce approximately 2,662,711 t/year of crops and vegetables for local consumption and export to the USA. The historic use of large amounts of pesticides is common in the area, and there are reports of heavy application of lead and sodium arsenate since the early 1950s together with organochlorine mixtures [18].
In both valleys, there are approximately 29 communities with populations greater than 2500 residents [19] where the only source of drinking water comes from deep wells. Previous studies of some wells in these Yaqui Valley communities showed high arsenic concentrations [20]. So far, there are no reported data for arsenic in the groundwater from the Mayo valley communities.
Sampling and Analytical Methods
Environmental Sampling
An exploratory study of arsenic groundwater was conducted in 57 communities from southern Sonora, Mexico (Fig. 2). In addition, agricultural and backyard soils were sampled in 25 communities located in the Yaqui and Mayo valleys (Fig. 1), to identify the towns with the highest arsenic levels in their wells and soils, and to obtain arsenic biomarker samples from children residing in these communities. Based on these criteria, 13 communities were chosen from the Yaqui and Mayo valleys to conduct a cross-sectional study (Fig. 1): nine in the Yaqui valley—Cocorit, Bacum, Campo 5, SJ. Bacum, Campo 47, Tobarito, Cd. Obregon, Vicam, and Potam—and four in the Mayo valley—B. Juarez, Etchojoa, Navojoa, and Buaysiacobe (Fig. 1).
Well Drinking Water
Drinking water samples (n = 300) were collected directly from each well according to the Mexican Norm [21] because it represents the main source of human consumption for each study community. Duplicate samples and field blanks were taken for quality control. Samples were stored in polypropylene bottles and packaged on ice and transported in sealed coolers to the laboratory in the subsequent 24 h. The samples were preserved with concentrated nitric acid (pH < 2) and were stored at − 20 °C prior to analysis of total arsenic. All plastic bottles were soaked using detergent, deionized water, and 20% (v/v) nitric acid for 3 days, and finally with deionized water once again.
Soils
Two hundred fifty soils were sampled from the Yaqui and Mayo valleys respectively (n = 500), taken from residential backyards and from the surrounding agricultural fields [18]. These samples were collected to a depth of 5–10 cm and stored at room temperature in plastic bags.
Biological Sampling
Ethical Issues
The protocol of urine collection was approved by the Human Subject Committee of the Technological Institute of Sonora (ITSON). A signed consent for each participant and a signed parental consent for each child were obtained.
Recruitment
Children between 5 and 16 years (n = 304) were recruited through meetings with medical personnel from the Health Sector in each of the 13 towns selected. They had similar socioeconomic status, nutritional habits, and health services. The participation rate was higher than 95%. The inclusion criteria required consumption of water from the local well and residence time of at least 2 years in the local town.
Urine Collection
First morning void urine samples were obtained in 100-mL polypropylene bottles and kept on ice and transported to the Technological Institute of Sonora, where they were kept frozen at − 20 °C. Frozen samples were shipped to the University of Arizona and stored at − 80°C until the analysis was performed.
Analytical Methods
Analysis of Arsenic in Drinking Water
Water samples were analyzed for total arsenic according to USEPA method 3015 [22], except that the sample volume was modified to 3.0 mL and the nitric acid concentration was changed to 25% v/v. For quality control purposes, the Standard Reference Water, SMR 1640 (NIST, Gaithersburg, MD), was used. The recovery was between 90 and 106%, and the percentage CV was below 3.0%. Total arsenic was determined using inductively coupled plasma/mass spectrometry (ICP/MS) with an Agilent 7500a ICP-MS equipped with an ASX-500 autosampler (Agilent Technologies, Inc., Santa Clara, CA). The detection limit was 0.1 μg/L.
Analysis of Arsenic in Soils
Soil samples were analyzed for arsenic content using an Innov-XT400 portable X-ray fluorescence (XRF) analyzer with a miniature, rugged, X-ray tube excitation source. The XT400 XRF analyzer utilizes a Hewlett-Packard (HP) iPAQ personal data assistant for data storage. The certified standard NIST SRM-2702 (inorganics in marine sediments) was also analyzed by the XT400 with recoveries ranging from 90 to 110%. The detection limit for arsenic is 10 mg/kg. In order to verify the XRF analysis, 10% of the samples were analyzed using acid digestion coupled with plasma atomic emission spectrometry (ICP-AES), in accordance with USEPA Method 3050B/6010B. ICP-AES analyses were performed using a Perkin-Elmer 4200 DV coupled with a hydride generation device at the University of Sonora. When compared, the XRF results were within 10% agreement with the ICP-AES results.
Analysis of Arsenic in Urine
Total urinary arsenic and speciation of arsenic metabolites [As(III), As(V), MMA(V), DMA(V)] were measured at the Arizona Laboratory for Emerging Contaminants (ALEC) at the University of Arizona, in Tucson, using HPLC/ICP-MS (Agilent Technologies, Inc., Santa Clara, CA) as described previously [20, 23]. The detection limits were as follows (μg/L): As III 0.12, As V 0.21, MMA (V) 0.12, and DMA (V) 0.12.
It is important to note that urine samples from Vicam and Buaysiacobe could not be analyzed for arsenic because problems were encountered in sending samples to the USA due to cross-border customs restrictions.
Risk Assessment
Risk assessment for arsenic exposure through contaminated water and soil ingestion was carried out to estimate the non-cancer (chronic) and cancer risk for children living in the studied agriculture valleys. Estimation of risk was calculated based on equations detailed in USEPA [24]. Average daily dose (ADD) was determined by the following equations:
where C is the mean arsenic concentration in soil (mg/kg) or water (mg/L). Conservative estimates of soil ingestion rates (IngR) were chosen for children (200 mg/day), and IR was the ingested water (L/day). In this study, exposure frequency (EF) was 365 days/year, and the averaging time (AT) was ED × 365 days for non-cancer risk and 70 × 365 days for carcinogenic risk. Exposure duration (ED in years), body weight (BW in kilograms), and IR were taken from the survey of each participant. The oral arsenic dose through drinking water was calculated using the daily volume of water consumed by each individual, arsenic concentration in tap water from each study subject’s household, and the weight of each participant.
The potential non-cancer risk, hazard quotient (HQ), and carcinogenic risk (CR) were determined using the following equations:
where HQ is an estimate of the daily arsenic exposure to children that is likely to be without an appreciable risk of deleterious effects during a lifetime. Therefore, HQ ≤ 1 suggests unlikely adverse health effects, whereas HQ > 1 suggests the probability of adverse health effects. An HQ > 10 is considered to be high chronic risk [24]. RfD is the oral reference dose for arsenic (3E−04 mg/kg day), and SF0 (1.5 mg/kg day) is the slope factor for arsenic. The hazard index (HI) was used to assess the overall potential non-carcinogenic health risk posed by more than one pathway. For this study, the HI was calculated as the sum of the HQ for water and soils.
Statistical Analysis
The mean ± standard deviation was calculated for the anthropometric, demographic, arsenic water intake and oral dose variables. Total arsenic and arsenic species were transformed to a log scale to calculate geometric means and confidence intervals. In addition, ADD, HQ, and HI data were log transformed, allowing parametric statistics to be used. The Pearson correlation coefficient was used to evaluate the association between arsenic levels in urine and the ADD, HQ, and HI values. All statistical analyses were computed using the Software Stata 9.0 (College Station, TX, USA, 2005).
Results
Total Arsenic in Drinking Water
Total arsenic levels in the drinking water from the wells or household tap water of the major towns in southern Sonora ranged from 2.7 to 98.7 μg/L (Table 1). According to Mexican legislation [25], a maximum permissible level for arsenic in drinking water is 25 μg As/L. Using this criterion, 12.3% of the samples exceeded this standard value. In addition, 56% of the sampled towns had arsenic levels greater than 10 μg As/L, which is the current US EPA drinking water standard limit, with Potam, Vicam, and Buaysiacobe, reporting the highest arsenic levels with 98.7, 37.7, and 74.5 μg As/L, respectively. Potam and Vicam water sources are related to the Yaqui River, which represents the main water source in the state of Sonora, and it is located within an arsenic province of natural origin (Yaqui River basin). Buaysiacobe is related to the Mayo River (Fig. 2), which belongs to a different hydrologic basin,
Total Arsenic Levels in Soils
The total arsenic levels in soil are shown in Table 2. Average levels of arsenic in agricultural and backyards soils were in the range of < 10–27 and 10.4–21.1 mg As/kg, respectively. The agricultural soils showed slightly higher arsenic values, and 28% of the soil samples exceeded the Mexican legislation limit of 22 mg As/kg [26]. Vicam and Potam, with the highest arsenic levels in drinking water, also had the highest arsenic concentrations in soils, with 27 and 26 mg As/kg, respectively, and Buaysiacobe presented the highest arsenic concentrations in soils from the Mayo valley with an average value of 23.3 mg As/kg (Table 2).
Daily Arsenic Intake of Children
Children from the 13 communities (n = 306) had similar socioeconomic status and nutritional habits. The mean age was 9.1 ± 1.3 years, with a mean residence time of 8.5 ± 0.4 years and mean body weight of 33.9 ± 10.3 kg. The number of male and female participants in our study was equal (Table 3)
The average water consumption was 1.6 L/day, with a wide range between 0.5 and 3.0 L/day. Table 3 presents the arsenic intake values for our study population. Children from Potam and Buaysiacobe had the highest values with 165 ± 54 μg As/day and 138.8 ± 64.7 μg As/day, respectively. The children from these two villages had the highest volume of water consumption and also had the highest arsenic levels in their drinking water wells (Table 1). Overall, the oral dose of arsenic ingestion in our study children had a mean value of 1.7 ± 1.6 μg As/kg day (Table 3).
Non-carcinogenic Risk and Carcinogenic Risk
The calculated hazard quotients (HQs), using body weight, age, water intake for each child, and arsenic concentration in water and soil for each studied community, are presented in Table 4.
The HQ values considering only the drinking water route were highest for Potam and Vicam in the Yaqui valley, with values of HQ = 15.9 and HQ = 5.7, respectively. In the Mayo valley, Buaysiacobe had the highest value with HQ = 16.1 (Table 4). The carcinogenic risk (CR) values ranged from 3E−05 to 9E−04 considering only the arsenic drinking water exposure (Table 4), with the highest values being for Potam, Buaysiacobe, and Vicam. The ranges of HQ values for the soil ingestion route were similar in agricultural and backyard soils for both valleys (range 0.19 to 0.53), and the CR values for the agricultural and backyard soils from both valleys occurred in the same range (1.1E−05 to 3.3 E−05).
The integrated risk (HI) is a useful tool for evaluating the risk of exposure to arsenic by different routes (in our case, drinking water and soil ingestion). HI for the Yaqui communities ranged from 1.48 to 16.43, and the highest values correspond to Potam (16.43) and Vicam (6.63), and for the Mayo Valley, the HI values ranged from 2.56 to 16.98, with the children from Buaysiacobe having the highest value.
Total Arsenic in Urine
There was a good agreement between the values obtained for total arsenic and the sum of arsenic species [As (V), As (III), MMA (V), and DMA (V)], indicating that in this study the latter was not influenced by seafood arsenic consumption (r = 0.99, P < 0.0011).
The average value of sum of arsenic species excreted in urine for the total children (n = 203) was 31.7 μg As/L, and 27% of them excreted levels above the value of WHO concern, i.e., 50 μg As/L [27].
The arsenic species in urine of the children were measured (Table 5). The methylated metabolite, DMA, was the major species of total arsenic in urine with 23.7 μg As/L and a relative proportion of 77.8%, followed by inorganic arsenic (∑As III + As V) with a mean value of 3.5 μg As/L and 11.4%, and MMA presented a low value of 3.3 μg As/L and relative proportion of 10.9% (Table 5).
A positive correlation was also observed between the arsenic levels excreted in urine and the ADDwater (r = 0.5851, P = 0.0000), but this was not significant for AADsoil (r = − 0.09, P = 0.2467). The same behavior was shown for HQwater (r = 0.5758, P = 0.000) and HI (r = 0.5865, P = 0.000), but it was not significant for HQsoil (r = − 0.09, P = 0.2447) (Fig. 3a–e).
Discussion
Using the Official Mexican Norm of arsenic in drinking water [25] with a limit value of 25 μg/L, Potam, Vicam, and Esperanza were 3.9, 1.5, and 1.4 times above the Norm, respectively. In the Mayo Valley, only Buaysiacobe and Bacobampo were above the Mexican Guideline, by 3.0 and 1.1 times, respectively. Considering that the children had a mean residence time of 8.5 years (range 2–17 years) in these towns and that their main consumption of drinking water had been from the local well, they have been chronically exposed to excessive amounts of this metalloid. Our results are similar to those reported in previous studies in some of these communities [20, 28,29,30]. On the other hand, in the north-central part of Mexico, there are a few recent reports of the presence of inorganic arsenic in drinking water, where the major sources are deep wells, but with levels higher than those reported in our study [31,32,33,34]; some of these locations have been identified as areas with “high arsenic levels,” with evidence of clinical symptoms in the exposed population [35], while in our study population the arsenic exposure levels from drinking water are low to moderate (< 100 μg As/L). The presence of arsenic in groundwater is mainly controlled by the hydrogeological environment, but anthropogenic activities such as mining and agriculture can also contribute [8]. Mexican authorities mitigate the arsenic problem for urban areas, but the population living in arid rural areas is commonly exposed to arsenic through drinking water pumped from wells. The studied areas from this work are hosted by two valleys related to different hydrological basins where the Yaqui River and the Mayo River are located. Both areas represent the main source of drinking water to the population in Sonora state as well as irrigation water for the most important agricultural areas in Mexico. However, to our knowledge, there are not previously documented studies that estimate the risk related to arsenic exposure through drinking water and soil ingestion in children living at rural areas in northern Mexico.
The wide range in water consumption by the children in this work was similar to that reported for children from Arizona [3]. This US state has similar environmental conditions as Southern Sonora, and additionally, the children from the Yaqui and Mayo valleys spend more time in outdoor activities than children in Arizona, at extreme temperatures (5–48 °C), which causes high water consumption, especially during the summer season.
The HQs from drinking water in the 13 studied communities were as follows: 15% were below the safe value < 1, and 85% were > 1. In addition, 15% of the communities had values of HQ > 10. Thus, the children in our study are at high risk of developing some non-cancer health effects such as skin lesions and respiratory illness. Arsenic exposure in early life has been related to respiratory diseases during adulthood [3]. Moreover, in the study area several diseases such as acute respiratory infections, diarrhea, parasitoids, caries, and dermatitis have been reported [36]. On the other hand, our HQ results were similar to those reported for the groundwater of Pakistan (HQ = 0.1 to 11) [37], but lower than those reported in Kandal, Cambodia, for water (HQ = 0.67 to 28) [38]. For carcinogen risk, the USEPA (2009) considers a value of cancer risk lower than 1E−06 as negligible, and values between 1E−06 to 1E−04 are considered an acceptable range [39]. The cancer risk values in our study, considering only the arsenic exposure through drinking water, indicate that 69% of the study communities were above of the safe value of 1E−04 and that more than 233 children (approximately 80%) were exposed chronically to arsenic by this route. The highest percentages were for children from Potam, Buaysiacobe, and Vicam, suggesting that intake of arsenic via consumption of drinking water could induce carcinogenic effects over the long term.
Rural populations in the Yaqui and Mayo valleys are a disadvantaged group because they also live in agricultural areas and are exposed to multiple pollutants. Residential soils are commonly impacted by the agricultural activities. Soil and dust ingestion are important pathways because the fine granulometric fraction of polluted soil easily adheres to children’s hands, and also because fine particles are suspended in the air and become breathable. In the Yaqui Valley, the towns of Potam, Vicam, and SJ. Bacum were found to have arsenic levels in agricultural soils that were 1.2, 1.2, and 1.1 times, respectively, above the Official Mexican Norm of arsenic in soils with a limit value of 22 μg As/kg [26]. In the Mayo Valley, agricultural soils from Buaysiacobe and Huatabampo were above the Mexican Guideline by 1.06 and 1.01 times, respectively. However, risk assessment from this work shows that HQ for arsenic exposure through soil ingestion (agricultural and backyard) showed that 100% of the children were below the safe value (Table 4), thus soil ingestion does not represent a particular risk for the exposed children.
When the levels of arsenic in soil samples found in this study were compared with reports from other rural areas located in Mexico and cities worldwide, lower levels were found than those reported in areas near mining sites located in the north-central part of Mexico, with values between 7.0 and 17, 384 mg/kg [40], but higher from those sites recently reported in suburban areas with agricultural activities with values in the range of 1.0 to 10.4 mg/kg [34].
On the other hand, arsenic concentrations in our soil samples were similar than those reported by Moreno-Rodríguez et al. [10] and García-Rico et al. [41] in other arid zones located in the northwestern part of Mexico, with arsenic levels between 2.5 to 20 mg/kg and 12.3 to 28.8 mg/kg respectively, and with agricultural soils in cities from China [42]. For agricultural areas, the use of pesticides such as lead arsenate and arsenic pentoxide is considered the principal source of arsenic.
It is important to note that average levels of arsenic in agricultural and backyards soils were similar. This behavior may indicate a common source of arsenic. Thus, children of these communities have been exposed chronically to this metalloid since children spend important time playing in their backyards and also in the agricultural sites, which are near their households.
When HI was calculated adding the HQ from drinking water, the HI was increased to values that were higher than the safe value (HI ≤ 1). For the pathways examined in our study, drinking water had the greater contribution to HI (39 to 96%), followed by backyard soils (2.7 to 28%) and agricultural soils (3.3 to 34%). Also, the values of the integrated risk in soils (agricultural and backyard soils) of the present study were higher than the previously reported value (HI = 0.6) [17] for other metals in the soils from the same area. These results indicate the importance of including the most important routes of arsenic exposure in the risk assessment studies, to show a more reliable scenario.
Previous studies [30, 43] on the analysis of health risks in arsenic-contaminated towns showed the importance of making an integrated approach. In our study, we have included environmental and biological monitoring (drinking water, soils, and urine). On the other hand, newer studies report that dust inhalation and food consumption are also important sources of arsenic [3,4,5, 44].
In the absence of seafood consumption (previous 48 h), the average arsenic levels excreted in human urine is approximately of 50 μg As/L. Values higher than 200 μg As/L are considered abnormal [45]. For the children in this study, the average urinary arsenic value was 31.7 μg As/L, lower than the national maximum concern level [31], and was within the range reported by other studies in mining and industrial areas in Mexico [31,46, 47], even though a recent study carried out in the Comarca Lagunera, Mexico, showed higher levels of urinary arsenic in children (average of 141 μg As/L) exposed to higher arsenic levels through drinking water (152.13 ± 49.35 μg As/L) [31]. Our study population showed large variability in arsenic excretion among the studied towns (2.2 to 333 μg As/L) (Table 5), with a non-linear association with the levels of arsenic in drinking water, as has been shown in previous studies, which suggests that this variability could be partially explained by genetic factors and individual characteristics such as sex, ethnicity, age, exposure routes, and the duration of exposure [20, 23, 29, 48, 49].
For the children in this study, the average percentages of methylated arsenic metabolites in urine were typically within the “regular range” reported in the literature for children exposed to arsenic in Mexico and other countries (range of 10–30% InAs, 10–20% MMA, and 60–80% DMA) [31, 49,50,51], even though the pattern of excretion in our children was different than that reported for some populations in Argentina [52, 53], Chile [54], and Taiwan [55]; the differences in the arsenic biotransformation could be explained by the presence of genetic polymorphisms in the methylation enzymes of these different ethnic groups and the diet [31, 56].
We found a positive and significant association with moderate correlation (r = 0.59, P = 0.0000) between the variables: urinary arsenic and the individual estimated AADwater, HQwater, and HI values (Table 4), respectively, which confirms that the most important variable for this association is the average daily dose (ADD). This is because the ADD calculation includes two of the most important variables obtained by the questionnaire from each child: volume of drinking water consumption [57] and weight of each participant. In addition, the concentration of arsenic in the drinking water of each study town is a key variable, which has also been included in the ADD calculation [57]. The ADD and HQ values from soil, however, did not present significant relationships with the levels of arsenic excreted in urine (P = 0.2467), this is in agreement with the minor contribution from HQAs to HI in the soil samples. The results from this work highlight the contribution of risk assessment studies in the interpretation of biomarkers of exposure. Even though the contribution to HQAs through soil ingestion is minimal, it is very important to analyze the bioaccessibility of arsenic in soil, in order to calculate the real HQ contribution to the HI value for our population.
Study Limitations
This study did not include the contribution of soil inhalation, and there was a lack of data for arsenic exposure through food ingestion. Further studies are highly encouraged to include this medium. In addition, the sample size should be increased to validate the results in a larger epidemiological study.
Conclusion
For the first time, the health risk assessment for two common arsenic exposure routes (drinking water and soil ingestion) was reported in children living in the most important agricultural areas in Sonora, Mexico. The HI values were above 1, and the carcinogenic risk (CR) was greater than 1E−04, indicating for our children high potential risk of developing chronic diseases including cancer. Positive and significant correlations between urinary arsenic levels and the individual estimated HI values were found in these children, with the higher contribution for ADD from drinking water. Thus, further studies regarding biomarkers and identification of point and non-point sources for arsenic should be conducted in the same study area in the near future. For this scenario, it is urgent to implement remediation strategies that decrease cumulative arsenic exposure in this susceptible population.
References
Liang CP, Wang SW, Kao YH, Chen JS (2016) Health risk assessment of groundwater arsenic pollution in southern Taiwan. Environ Geochem Health 38:1271–1281
Cubadda F, D’Amato M, Mancini FR, Aureli F, Raggi A, Busani L, Mantovani A (2015) Assessing human exposure to inorganic arsenic in high-arsenic areas of Latium: a biomonitoring study integrated with indicators of dietary intake. Ann Ig 27:39–51
Beamer PI, Klimecki WT, Loh M, Van Horne YO, Sugeng AJ, Lothrop N, Billheimer D, Guerra S, Lantz RC, Canales RA, Martinez FD (2016) Association of children’s urinary CC16 levels with arsenic concentrations in multiple environmental media. Int J Environ Res Public Health 13(5):521
Kurzius-Spencer M, Burgess JL, Harris RB, Hartz V, Roberge J, Huang S, Hsu C-H, O’Rourke MK (2014) Contribution of diet to aggregate arsenic exposures—an analysis across populations. J Exp Sci Environ Epidemiol 24(2):156–162
Kurzius-Spencer M, O’Rourke MK, Hsu C-H, Hartz V, Harris RB, Burgess JL (2013) Measured versus modeled dietary arsenic and relation to urinary arsenic excretion and total exposure. J Exp Sci and Environ Epidemiol 23:442–449
Tang J, Bian J, Li Z, Li Y, Yang W, Liang S (2017) Comparative study on the hydrogeochemical environment at the major drinking water based arsenism areas. Appl Geochem 77:62–67
Bondu R, Cloutier V, Rosa E, Benzaazoua M (2016) A review and evaluation of the impacts of climate change on geogenic arsenic in groundwater from fractured bedrock aquifers. Water Air Soil Poll 227(9):296
Bundschuh J, Nath B, Bhattacharya P, Liu CW, Armienta MA, Moreno López MV, Lopez D, Jean JS, Cornejo L, Macedo LFL, Filho AT (2012) Review: arsenic in the human food chain: the Latin American perspective. Sci Total Environ 429:92–106
Sharratt BS, Feng G (2006) Evidence of direct suspension of soil particulates on the Columbia Plateau. International Conference on Aeolian Research
Moreno-Rodríguez V, Del Rio-Salas R, Adams DK, Ochoa-Landin L, Zepeda J, Gómez-Alvarez A, Palafox-Reyes J, Meza-Figueroa D (2015) Historical trends and sources of TSP in a Sonoran desert city: can the North America Monsoon enhance dust emissions? Atmos Environ 110:111–121
Roberge J, O’Rourke MK, Meza-Montenegro MM, Gutiérrez-Millán LE, Burgess JL, Harris RB (2012) Binational arsenic exposure survey: methodology and estimated arsenic intake from drinking water and urinary arsenic concentrations. Int J Environ Res Public Health 9:1051–1067
Burgess JL, Meza MM, Josyula AB, Poplin GS, Kopplin MJ, McClellen H, Sturup S, Lantz RC (2007) Environmental arsenic exposure and urinary 8-OHdG in Arizona and Sonora. Clin Toxicol 45:490–498
Caceres D, Pino P, Montesinos N, Atalah E, Amigo H, Loomis D (2005) Exposure to organic arsenic in drinking water and total urinary arsenic concentration in a Chilean population. Environ Res 98:151–159
Burgess JL, Kurzius-Spencer M, O’Rourke MK, Littau SR, Roberge J, Meza-Montenegro MM, Gutiérrez-Millán LE, Harris RB (2013) Environmental arsenic exposure and serum matrix metalloproteinase-9. Expo Sci Environ Epidemiol 23(2):163–169
Calderon RL, Hudgens EE, Carty C, He B, Le XC, Rogers J, Thomas DJ (2013) Biological and behavioral factors modify biomarkers of arsenic exposure in a U.S. population. Environ Res 126:134–144
Mendoza-Cano O, Sánchez-Piña RA, Barrón-Quintana J, Cuevas-Arellano HB, Escalante-Minakata P, Solano-Barajas R (2017) Riesgos potenciales de salud por consumo de agua con arsénico en Colima, México. Salud Publica Mex 59(1):34–40
Meza-Montenegro MM, Gandolfi AJ, Santana-Alcántar ME, Gomez-Alvarez A, Mendivil-Quijada H, Valencia M, Meza-Figueroa D (2012) Metals in residential soils and cumulative risk assessment in Yaqui and Mayo agricultural valleys, northern Mexico. Sci Total Environ 433:472–481
Meza-Montenegro MM, Valenzuela-Quintanar AI, Balderas-Cortés JJ, Yañez-Estrada L, Gutiérrez-Coronado ML, Cuevas-Robles A, Gandolfi AJ (2013) Exposure assessment of organochlorine pesticides, arsenic, and lead in children from the major agricultural areas in Sonora, Mexico. Arch Environ Contam Toxicol 64:519–527
Instituto Nacional de Estadística y Geografía (INEGI). 2010. Microdatos. http://www.inegi.org.mx/est/contenidos/proyectos/accesomicrodatos/. Accessed 06 Sep 2017
Meza MM, Kopplin MJ, Burgess JL, Gandolfi AJ (2008) Urinary arsenic methylation in children exposed at low-level in the Yaqui Valley, Sonora. J Environ Toxicol Chem 90(5):957–970
NOM-230-SSA1–2002. Norma Oficial Mexicana Salud ambiental. Agua para uso y consumo humano. Requisitos sanitarios que se deben cumplir en los sistemas de abastecimiento públicos y privados durante el manejo del agua. Procedimientos sanitarios para el muestreo. Diario Oficial de la Federación 2005
United States Environmental Protection Agency. 2007. Microwave assisted acid digestion of aqueous samples and extracts, Method 3015A. https://www.epa.gov/hw-sw846/sw-846-test-method-3015a-microwave-assisted-acid-digestion-aqueous-samples-and-extracts. Accessed 07 Sep 2017
Meza MM, Yu L, Rodríguez YY, Guiad M, Thompson D, Gandolfi AJ, Klimecki WT (2005) Developmentally restricted genetic determinants of human arsenic metabolism: association between genetic urinary methylated arsenic and CYT 19 polymorphism in children. Environ Health Persp 113:775–781
United States Environmental Protection Agency. 2009. Highlights of the Child-Specific Exposure Factors Handbook (Final Report). USEPA, Washington, DC, EPA/600/R-08/135, 2009
NOM-127-SSA1–1994. Norma Oficial Mexicana. Agua para uso y consumo humano. Límites permisibles de calidad. Diario Oficial de la Federación 2000
NOM-147-SEMARNAT/SSA1–2004. NORMA Oficial Mexicana, Que establece criterios para determinar las concentraciones de remediación de suelos contaminados por arsénico, bario, berilio, cadmio, cromo hexavalente, mercurio, níquel, plata, plomo, selenio, talio y/o vanadio. Diario Oficial de la Federación 2007
World Health Organization (WHO). 2001. Arsenic and Arsenic Compounds, 2nd edn. Environmental Health Criteria 224. Geneva 2001. (accessed Sep. 6 2017). http://www.inchem.org/documents/ehc/ehc/ehc224.htm
Wyatt CJ, Fimbres C, Romo L, Mendéz RO, Grijalva M (1998) Incidence of heavy metal contamination in water supplies in Northern Mexico. Environ Res 76:114–119
Meza MM, Kopplin MJ, Burgess JL, Gandolfi AJ (2004) Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora México. Environ Res 96:119–126
Maldonado JF, Meza-Figueroa D, Dévora AG, García-Rico L, Burgess JL, Lantz RC, Yáñez-Estrada L, Martínez-Cinco MA, Balderas JJ, Mondaca I, Meza-Montenegro MM (In Press 2018) An integrated health risk assessment of indigenous children exposed to arsenic in Sonora, Mexico Human Ecol Risk Assess doi: https://doi.org/10.1080/10807039.2018.1449098
Recio-Vega R, Gonzalez-Cortes T, Olivas-Calderon E, Lantz RC, Gandolfi AJ, Gonzalez-De Alba C (2015) In utero and early childhood exposure to arsenic decreases lung function in children. J Appl Toxicol 35:358–366
González-Horta C, Ballinas-Casarrubias L, Sánchez-Ramírez B, Ishida MC, Barrera-Hernández A, Gutiérrez-Torres D, Zacarias OL, Saunders RJ, Drobná Z, Mendez MA, García-Vargas G, Loomis D, Stýblo M, Del Razo LM (2015) A concurrent exposure to arsenic and fluoride from drinking water in Chihuahua, Mexico. Int J Environ Res Public Health 12:4587–4601
Cárdenas-González M, Osorio-Yáñez C, Gaspar-Ramírez O, Pavković M, Ochoa-Martínez A, López-Ventura D, Medeiros M, Barbier OC, Pérez-Maldonado IN, Sabbisetti VS, Bonventre JV, Vaidya VS (2016) Environmental exposure to arsenic and chromium in children is associated with kidney injury molecule-1. Environ Res 150:653–662
Pérez-Vázquez J, Flores-Ramírez R, Ochoa-Martínez AC, Carrizales-Yáñez L, Ilizaliturri-Hernández CA, Moctezuma-González J, Pruneda-Álvarez LG, Ruiz-Vera T, Orta-García ST, González-Palomo AK, Pérez-Maldonado IN (2016) Human health risks associated with heavy metals in soil in different areas of San Luis Potosí, México. Hum Ecol Risk Assess 22:323–336
Del Razo LM, Garcia-Vargas G, Hernandez MC, Gómez-Muñoz CME (1999) Profile of urinary arsenic metabolites in children chronically exposed to inorganic arsenic in Mexico. In: Chappel WR, Abernathy CO, Calderon RL (eds) Arsenic exposure and health effects. Elsevier, Oxford, pp 281–287
Secretaria de Salubridad y Asistencia. Prevalence of the major diseases in children between 5–14 years. Report Sanitary Jurisdiccion IV Cajeme 2008 (Prevalencia de las principales enfermedades en niños entre 5–14 años. Jurisdicción Sanitaria IV. Cajeme 2008 SSA, Secretaria de Salubridad y Asistencia)
Waqas H, Shan A, Khan YG, Nawaz R, Rizwan M, Saif-Ur- Rehman M, Shakoor MB, Ahmed W, Jabeen M (2017) Human health risk assessment of arsenic in groundwater aquifers of Lahore, Pakistan. Hum Ecol Risk Assess 23(4):836–850
Phan K, Sthiannopkao S, Kim K-W, Wong MH, Sao V, Hashim JH, Yasin MSM, Aljunid SM (2010) Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Res 44(19):5777–5788
United States Environmental Protection Agency- IRIS. 2014. Toxicological Review of Inorganic Arsenic (Preliminary Assessment Materials). U.S. Environmental Protection Agency, Washington, DC, EPA/630/R-14/101, 2014
Razo I, Carrizales L, Castro J, Díaz-Barriga F, Monroy M (2004) Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water Air Soil Pollut 152(1–4):129–152
García-Rico L, Meza-Figueroa D, Gandolfi AJ, Del Río-Salas R, Romero FM, Meza-Montenegro MM (2016) Dust–metal sources in an urbanized arid zone: implications for health-risk assessments. Arch Environ Contam Toxicol 70:522–533
Wei B, Yang L (2010) A review of heavy contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94:99–107
Jasso-Pineda Y, Espinosa-Reyes G, González-Mille D, Razo-Soto I, Carrizales L, Torres-Dosal A, Mejía-Saavedra J, Monroy M, Ize AI, Yarto M, Díaz-Barriga F (2007) An integrated health risk assessment approach to the study of mining sites contaminated with arsenic and lead. Integr Environ Assess Manag 3:344–350
Vu CT, Lin C, Yeh G, Villanueva MC (2017) Bioaccumulation and potential sources of heavy metal contamination in fish species in Taiwan: assessment and possible human health implications. Environ Sci Pollut Res 24(23):19422–19434
Agency for Toxic Substances and Disease Registry (ATSDR) (2000) Toxicological profile for arsenic. Public Health Services, Atlanta, United States Department of Health and Human Services
Ochoa-Martinez AC, Orta-Garcia ST, Rico-Escobar EM, Carrizales-Yañez L, Martin Del Campo JD, Pruneda-Alvarez LD, Ruiz-Vera T, Gonzalez-Palomo AK, Piña-Lopez IG, Torres-Dosal A, Pérez-Maldonado IN (2016) Exposure assessment to environmental chemicals in children from Ciudad Juarez, Chihuahua, Mexico. Arch Environ Contam Toxicol 70:657–670
Trejo-Acevedo A, Díaz-Barriga F, Carrizales L, Domínguez G, Costilla R, Ize-Lema I, Yarto-Ramírez M, Gavilán-García A, Mejía-Saavedra JJ, Pérez-Maldonado IN (2009) Exposure assessment of persistent organic pollutants and metals in Mexican children. Chemosphere 74:974–980
Concha G, Vogler G, Nermell B (2002) Intra-individual variation in the metabolism of inorganic arsenic. Int Arch Environ Health 75:576–580
Sun G, Xu X, Li Y, Jin B, Li SX (2007) Urinary arsenic metabolites in children and adults exposed to arsenic in drinking water in Inner Mongolia, China. Environ Health Persp 115:648–652
Chowdhury UK, Rahman MM, Segunpta MK, Lodth D, Chanda CR, Roy S, Quamruzzaman Q, Tokunaga H, Ando M, Chakraborti D (2003) Pattern of excretion of arsenic compounds (arsenite, arsenate, MMA V, DMA V) in urine of children compared to adults from arsenic exposed area in Blangadesh. J Environ Sci Health 38:87–113
Kalman DA, Hughes J, Van Belle G, Burbacher T, Bolgiano D, Coble K, Mottet NK, Polissar L (1990) The effect of variable environmental arsenic contamination of urinary concentrations of arsenic species. Environ Health Persp 89:145–151
Vahter M, Concha G, Nermell B, Nilsson R, Dulout F, Natarajan AT (1995) A unique metabolism of inorganic arsenic in native Andean women. Eur J Pharmacol 293:455–462
Concha G, Nermell B, Vahter MV (1998) Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina. Environ Health Perspect 106:355–359
Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE (1996) Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Perspect 104:620–628
Chiou HY, Hsueh YM, Hsieh LL, Hsu LI, Hsu YH, Hsieh FI, Wei ML, Chen HC, Yang HT, Leu LC, Chu TH, Chen-Wu C, Yang MH, Chen CJ (1997) Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat Res 386:197–207
Weinshilboum RM, Otterness DM, Szumlanski CL (1999) Methylation pharmacogenetics: catecol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol 39:19–52
National Research Council (NRC) Subcommittee on Arsenic in Drinking Water. Arsenic in Drinking Water. Washington (DC): National Academies Press (US); 1999. 6, Biomarkers of Arsenic Exposure. Available from: https://www.ncbi.nlm.nih.gov/books/NBK230898/
Acknowledgments
We are grateful to Dr. A. Jay Gandolfi of the University of Arizona for making the arsenic investigation in Sonora, Mexico, a reality, and for his support in consolidating our research group in the arsenic field. In addition, we would like to thank Dr. Paul W. Kilpatrick for helping with the English edition. This research was supported by CONACYT-FONSALUD Grant 000000233976, the NIEHS Superfund Basic Research Program at the University of Arizona (ES 04940), and the PROFAPI_00396 and PROFAPI_539 Grants at ITSON.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol of urine collection was approved by the Human Subject Committee of the Technological Institute of Sonora (ITSON). A signed consent for each participant and a signed parental consent for each child were obtained.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
García-Rico, L., Meza-Figueroa, D., Jay Gandolfi, A. et al. Health Risk Assessment and Urinary Excretion of Children Exposed to Arsenic through Drinking Water and Soils in Sonora, Mexico. Biol Trace Elem Res 187, 9–21 (2019). https://doi.org/10.1007/s12011-018-1347-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1347-5