Abstract
Cadmium (Cd) contamination and soil salinity are the main environmental issues reducing crop productivity. This study aimed to examine the combined effects of salinity (NaCl) and Cd on the physiological and biochemical attributes of quinoa (Chenopodium quinoa Willd.). For this purpose, 30-day-old plants of quinoa genotype “Puno” were transplanted in Hoagland's nutrient solution containing diverse concentrations of Cd: 0, 50, 100, 200 µM Cd, and salinity: 0, 150, and 300 mM NaCl. Results demonstrated that plant growth, stomatal conductance, and pigment contents were significantly lower at all Cd concentrations than the control plants. Quinoa plants exhibited improved growth and tolerance against Cd when grown at a lower level of salinity (150 mM NaCl) combined with Cd. In contrast, the elevated concentration of salinity (300 mM NaCl) combined with Cd reduced shoot and root growth of experimental plants more than 50%. Combined application of salinity and Cd increased Na (25-fold), while lessened the Cd (twofold) and K (1.5-fold) uptake. A blend of high concentrations of Na and Cd caused overproduction of H2O2 (eightfold higher than control) contents and triggered lipid peroxidation. The activities of antioxidant enzymes: ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) were 13, 12, 7 and ninefold higher than control to mitigate the oxidative stress. Due to restricted root to shoot translocation, and greater tolerance potential against Cd, the quinoa genotype, Puno, is suitable for phytostabilization of Cd in saline soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) contamination in soil has become a challenging issue and is primarily contributed by anthropogenic activities, including agricultural practices, mining activities, industrial effluents, and wastewater irrigation (Amjad et al., 2021; Anwar et al., 2021). Since the Cd is not involved in any known biological functions in plants, it is recognized as a non-essential element (Rehman et al., 2019). The concentration of this non-essential heavy metal in the soil varies considerably, and it has been ranked fourth in the list of highly toxic elements for plant growth (Qayyum et al., 2017).
Due to the toxic nature of Cd and being a non-essential element, its uptake, even in traces, causes a wide range of impairments to plant biochemical, physiological, and morphological functions (Zhang et al., 2020). Harmful effects of Cd may compromise plant growth and biomass, damage to chlorophyll, rupturing of cellular membranes, and reduction in nutrient uptake capacity (Amjad et al., 2021; Shanying et al., 2017). Likewise, Cd toxicity causes overproduction of reactive oxygen species (ROS), which are incredibly detrimental to plant tissues. Cd-induced toxicity and oxidative stress triggered by ROS in plants are well documented (Rehman et al., 2019). Oxidative stress is caused by various ROS including hydrogen peroxide (H2O2), superoxide (O2·−), hydroxyl (HO·) radicles, and singlet oxygen (½O2), imparting severe damages to carbohydrates, lipids, proteins and nucleic acids (Anwar et al., 2021; Rehman et al., 2019). The implications of these ROS are mitigated by boosting the activities of antioxidant enzymes such as ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) (Amjad et al., 2021; Souri et al., 2018).
Soil salinity is another serious menace for crop production worldwide, which has affected about 6% of the global land including 20% of areas that have become salinized due to irrigation with salt contaminated groundwater (Abbas et al., 2021; Qadir et al., 2014). Salinity prompts various adverse effects on plants, including osmotic stress, reduced gas exchange, ionic imbalance, chlorophyll concentration, and relative water contents in leaf (Flowers et al., 2015; Hu et al., 2017). These impairments consequently cause a reduction in biomass and grain yield of crops (Abbas et al., 2018). However, the response of plants to salt stress varies with the type of salt-affected soils and plant genotypes (Abbas et al., 2021).
The efficient use of salt-affected soils and waters has become vital for feeding the country's rapidly growing population. Compared to glycophytic crops such as wheat and rice, halophytic grain crops suitable for saline soils need to be incorporated into our cropping system (Shabala et al., 2013). Quinoa (Chenopodium quinoa Willd.) cultivation is regarded as the most appropriate way for sustainable utilization of salt-affected soils (Abbas et al., 2021; Jacobsen et al., 2009). Quinoa is a potential food security crop because of its high nutritional quality grains, and it can be successfully grown on compromised soils (Abbas et al., 2021; Riaz et al., 2020). It is highly suitable for cultivating on metal-contaminated saline soils due to its potential to stabilize the metals within the soil (Amjad et al., 2021; Iftikhar et al., 2021; Parvez et al., 2020). However, to the best of our knowledge, the growth and physiological responses of quinoa genotypes under combined effects of Cd and salinity have not been explored so far, perhaps due to complicated interactions between Na and Cd. There are few contradictory reports regarding this complex interaction. For instance, Rehman et al. (2019) observed increased Cd uptake, while others (Cheng et al., 2018; Mariem et al., 2014) noticed reduced Cd uptake by plants when Cd and salinity were applied together. Hence, the present study was designed with trifold objectives: firstly, to analyze the growth and physiological responses of quinoa genotype “Puno” exposed to the combined application of Cd and salinity; secondly, to evaluate the effects of salinity on the Cd tolerance and phytoremediation potential of quinoa; lastly, to explore the tolerance mechanisms of quinoa against the combined stress of Cd and salinity.
Materials and methods
Experimental description
An experiment based on solution culture was carried out at COMSATS University Islamabad, Vehari Campus (30. 231°N, 72. 211°E) during 2020–2021. The temperature during the study ranged from 10–26 °C. Quinoa genotype “Puno” was used in this experiment because of its better adaptation to salinity and heavy metals (Iftikhar et al., 2021). Thirty-day-old quinoa plants were transplanted to half-strength Hoagland’s nutrient solution (Hoagland & Arnon, 1950) containing macro-nutrients in mM (Ca 2.5, Mg 1.0, S 1.0, K 3.0, N 3.75, P 0.5) and micro-nutrients in µM (Fe 26.0, Cu 0.16, Mn 4.55, Zn 0.38, B 23.15, Mo 0.25, Cl 9.0). Plant samples were allowed to acclimatize for one week in the solution before applying Cd and salinity treatments. The salts of cadmium chloride (CdCl2) and sodium chloride (NaCl) were used in the solution culture to obtain the final concentration of 0, 50, 100, 200 µM Cd, and 0, 150 300 mM of salinity, respectively. Salinity treatments were applied in two splits, whereby the first half was used at the time of the Cd application, while the remaining half was applied two days thereafter. All the treatments were applied alone as well as in combinations. The nutrient solution was replaced every week and with new solution, Cd and salinity treatments were applied as a single dose. The pH was maintained at 6.5 ± 0.2 using 0.1 N HCl or NaOH. Each treatment contained four replicates, with one plant for each replication.
Harvesting and data collection
Four weeks after transplanting in the solution, plants were harvested where the roots were separated from the shoots and washed in 0.01 M HCl solution, followed by rinsing in distilled water to remove the adsorbed Cd as detailed by Iftikhar et al. (2021). Lengths of root/shoot were recorded. Afterward, plant materials were air-dried, followed by oven drying at 70 °C for 48 h, and dry weights were noted.
Digestion of plant material and analyses
The roots and shoots of the experimental plants were ground separately using a porcelain grinder. Both the roots and shoots were separately digested using a blend of HClO4 and HNO3 at 1:2 ratio, respectively. Thereafter, each sample was diluted with the distilled water up to 50 mL volume. Sodium (Na) and potassium (K) ions' concentrations were estimated on a flame photometer (BWB-XP5). However, the Cd concentration was measured on an atomic absorption spectrometer (PerkinElmer Model: Pin AAcle 900F, Inc. USA) using internal standards (CPAchem, Bulgaria) while standardizing the equipment with certified reference material (SRM 1547, peach leaves).
Bioconcentration factor, translocation factor, and tolerance index
Bioconcentration (BCF) and translocation factor (TF) were calculated as described by Rehman et al. (2019). The concentration ratio of Cd in plants and solution was expressed as BCF, whereas the concentration ratio of Cd between root and shoot was denoted as TF. The tolerance index (TI) was calculated by dividing the dry weights of the Cd stressed plant by the dry weights of control plants.
Leaf pigments and stomatal conductance
All types of chlorophyll, chlorophyll a, chlorophyll b, and total chlorophyll values, were assessed by following the procedure outlined by Lichtenthaler (1987). For this analysis, leaf samples (1.0 g) were dipped for few seconds in liquid nitrogen to cease the metabolic processes and ground in 80% hydro-acetone solution. After grinding, the samples were centrifuged at 3000 × g for 10 min and the supernatant was collected. The absorbance of collected supernatant was recorded by UV–Vis spectrophotometer (Lambda 25, PerkinElmer, Inc. USA) at 663.2 and 646.8 nm wavelengths. Stomatal conductance of fully grown second leaf from the top was measured with a portable leaf porometer (Decagon Devices, Pullman, WA, USA).
Membrane stability index (MSI)
The membrane stability index (MSI) of plant leaves was assessed by evaluating the electrical conductance (EC) of the leaf leachate produced in distilled water as detailed by Rehman et al. (2019). The EC was measured at two different time intervals (10 and 30 min.), and two different temperature regimes (40 °C for EC1 and 100 °C for EC2), respectively. The MSI values were calculated as per Eq. 1 given below:
Hydrogen peroxide and lipid peroxidation
Hydrogen peroxide (H2O2) and lipid peroxidation (ROS) determinations were carried out by using the method of Islam et al. (2008). Briefly, 0.5 g leaf samples were ground/homogenized in 0.1% trichloroacetic acid and centrifuged at 12,000 × g for 20 min. One mL (1 mL) of supernatant was taken, and a buffer solution containing 1 mL each of potassium iodide (2 M) and potassium phosphate (10 mM) was added into it while maintaining the pH at 7.0. The absorbance of the reaction mixture was noted at 390 nm wavelength using UV–Vis spectrophotometer, and the concentration of H2O2 in the sample was calculated. Assays for lipid peroxidation were carried out by determining the thiobarbituric acid reactive substances (TBARS) in the sample according to the method devised by Hodges et al. (1999).
Enzymatic activities
The most newly appeared leaves of quinoa plants were used for assessing the activities of antioxidant enzymes; APX, CAT, POD, and SOD. For these assays, 0.5 g leaf samples were ground in 0.1 M phosphate buffer (pH 7.0) under liquid nitrogen. The ground samples were centrifuged at 15,000 × g for 30 min at 4 °C. The enzymatic activity of SOD was estimated based on a 50% reduction of nitro blue tetrazolium (NBT) according to the methods devised by Dhindsa et al. (1981). At the same time, the activity of APX was measured by following the procedures of Nakano and Asada (1981) and considered as l M ascorbate min−1 mg−1 protein. The enzymatic activity of CAT in the leaf sample was assessed as explained by Aebi (1984) and considered l M of H2O2 degraded min−1 mg−1 protein. The enzymatic activity of POD was determined as l M of guaiacol that oxidized min−1 mg−1 protein (Hemeda and Klein 1990).
Statistical analyses
A completely randomized design (CRD) was used in the present study. The data were statistically analyzed using one-way analysis of variance using “Statistix 8.1”. The least significant difference test at a 5% probability level was used for treatment comparison (Steel et al., 1997).
Results
Plant growth
Results demonstrated that root and shoot growth of quinoa genotype “Puno” decreased with the increasing concentration of Cd in the growth medium (Table 1). However, co-application of salinity (150 mM) with various concentrations of Cd enhanced plant growth compared to Cd stressed plants. High doses of salinity and Cd negatively impacted plant growth. The plants exposed to the highest salinity level (300 mM) and Cd concentration showed a remarkable reduction in plant growth. Markedly, the root and shoot lengths were decreased by 55 and 58%, respectively, when plants were grown at the sole treatment of Cd (200 μM). Contrary to these observations, respective reductions in root and shoot were noted as 46 and 47% when plants were supplied with a mild salinity level (150 mM), at the same Cd concentration of 200 μM. Co-application of the highest concentration of Cd (200 μM) and salinity (300 mM) exerted detrimental effects on plant growth with a significant reduction of 64 and 57% in the root and shoot length of quinoa plants, respectively. The dry weights of roots and shoots exhibited a similar trend at higher doses of Cd and salinity. Mild application of salinity at a lower concentration of Cd had a soothing effect on plant growth. It caused a lesser reduction of 43 and 45% in the dry weights of root and shoot, respectively, as compared to the control treatment. In comparison, decreases in dry weights of these plant parts were noted as 57 and 56%, respectively, when plants were grown at higher doses of Cd (200 μM) and salinity (300 mM).
Chlorophyll contents and stomatal conductance
As evident from the data in Table 2, chlorophyll contents of quinoa leaves were negatively influenced at higher doses of Cd and salinity. Except for none, all types of green pigment (chlorophyll a, chlorophyll b, total chlorophyll) and stomatal conductance of quinoa decreased at greater salinity and Cd concentration in the nutrient solution. Opposing these results, plants exposed to lower salinity levels and Cd had a higher concentration of chlorophyll contents and improved stomatal conductance. Increasing Cd level to 200 μM caused respective reductions of 30, 50, 38, and 51% in chlorophyll a, chlorophyll b, total chlorophyll, and stomatal conductance. When a higher concentration of Cd was augmented with salinity, the corresponding reduction in these traits of quinoa plants was 27, 40, 32, and 43%, respectively, compared to control plants. Reductions in the physiological attributes of experimental plants were more pronounced at elevated levels of Cd and salinity (Table 2).
Ionic contents in quinoa
Increasing levels of NaCl in the nutrient solution caused increased accumulation of Na ions both in the below- and aboveground parts of quinoa plants and vice versa (Fig. 1a, b). The combined application of salinity and Cd increased Na ions concentration in roots and shoots of the experimental plants as compared to plants exposed to salinity treatment only. Although mild salinity treatment did not influence the K concentration in quinoa plants (Fig. 1c, d), combined salinity and Cd concentration reduced K ions in the root and shoot of quinoa compared to control treatment.
Effect of salinity (mM) and Cd (µM) on the concentration of Na in roots (a), shoots (b), and K contents in roots (c) and shoot (d) of quinoa plants exposed to different levels of salinity and Cd under hydroponics. The data represent the mean values of four replicates ± SE. The values with different letter(s) are significantly different at 5% significance level
Metal accumulation and tolerance
Accumulation of Cd in the plant parts was positively correlated with metal application in the growth medium. The increasing concentration of Cd in the nutrient solution caused a more significant accumulation of Cd in the roots and shoots of quinoa plants (Table 3). The combined application of salinity and Cd treatments decreased Cd contents in the roots and shoots of quinoa. Still, overall, a greater concentration of metal was reported in roots as compared to shoots. The BCF was higher in plants that received Cd treatments than those treated only with Cd and salinity. Application of salinity along with Cd reduced the BCF value; however, the value of TF was always < 1 regardless of the sole application of Cd or combined with salinity (Table 3). The TI was lessened with increasing levels of salinity and Cd in the growth medium and vice versa. The joint application of mild level of salinity with Cd increased TI of quinoa against Cd stress (Table 3).
Oxidative stress
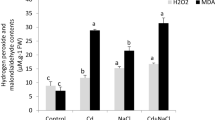
Increasing concentration of Cd imparted oxidative stress in the experimental plants by raising the H2O2 and TBARS levels, as shown in Figs. 2a, b. The combined application of higher doses of salinity and Cd caused additional oxidative damage. Numerically, the higher Cd concentration in the nutrient solution resulted in a sixfold increase to H2O2 and TBARS concentrations than control treatments. In contrast, respective increases in H2O2 and TBARS were noted as 6.7- and 6.8-fold when mild salinity treatment was applied at the same concentration of Cd. However, increases in these stress attributes were reported to be > 8 folds at the highest levels of salinity and Cd treatments compared to control. Membrane stability index (MSI) decreased as the salinity and Cd levels increased in the growth medium (Fig. 2c). Compared with control plants, individual applications of salinity and Cd, respectively, reduced the MSI to 1.1- and 1.8-fold. Still, the effect was multiplied (2.2 folds) when salinity and Cd were applied together to experimental plants.
Effect of salinity (mM) and Cd (µM) on; H2O2 (a), TBARS (b), and MSI (c) of quinoa plants provided with different concentrations of salinity and Cd under hydroponics. The data presented are the means of four replicates ± SE. Values with different letter(s) are significantly different from each other at 5% significance level
Activities of antioxidant enzymes
Activities of antioxidant enzymes; APX, CAT, POD and SOD revealed variable responses to salinity and Cd concentration in the growth medium (Fig. 3a–d). All these enzymes were overexpressed in quinoa plants, exposed to the combined application of higher doses of salinity and Cd. For instance, in plants treated with 200 μM Cd treatment, the respective increase in the aforementioned enzymes was 10.5, 7.2, 11, and 5.5 folds when compared to the control treatment. However, at lower salinity level, the respective activities of APX, CAT, POD, and SOD were only 3.3, 4, 2.5, and 3.6 folds higher with respect to the control plants. The combined treatment of higher quantities of salinity and Cd increased the activities of all these antioxidant enzymes by 12.5-, 9.2-, 13.3-, and 7.5-fold concerning control.
Effect of salinity (mM) and Cd (µM) on activities of enzymes; SOD (a), CAT (b), APX (c), and POD (d) of quinoa plants exposed to different concentrations of salinity and Cd under hydroponics. The data are means of four replicates ± SE. The values with different letter(s) are significantly different from each other at 5% significance level
Discussion
The literature regarding the interaction of salinity and heavy metals is not conclusive due to contradictory outcomes. Few studies have reported that the uptake of heavy metals increases in the presence of salinity (Rehman et al., 2019; Shabir et al., 2018), while others have opposite view point (Cheng et al., 2018; Parvez et al., 2020). The results of the present study were quite variable depending on the levels of salinity and Cd concentration in the growth medium. For instance, the growth of quinoa plants was not greatly hampered up to the salinity level of 150 mM NaCl in the culture, highlighting the salt tolerance potential of this superfood plant (Iftikhar et al., 2021). Quinoa plants exposed to a NaCl concentration of 300 mM showed significantly stunted growth, corroborating the previous research where elevated salinity levels (300 mM) impaired the growth and yield of quinoa plants (Iftikhar et al., 2021; Riaz et al., 2020). The stunted growth of quinoa could be ascribed to the salt-induced osmotic stress, ion toxicity, oxidative stress, and chlorophyll degradation (Abbas et al., 2021). Although redundant plant growth was noticed even at the lowest concentration of Cd (50 μM Cd) in the medium, the higher level of Cd (200 μM) caused a remarkable decline in the biomass of quinoa plants. Previous research (e.g., Amjad et al. (2021) and the references therein) noted such harmful effects of Cd on the growth and yield of quinoa. This is not surprising, considering the non-essentiality of Cd in any physiological or metabolic processes of plants (Rehman et al., 2019). Instead, Cd accumulation within the plant body interferes with nutrients uptake and increases the toxicity of up taken ions, impairs photosynthetic apparatus, slows down the enzymatic activities, and halts the plant–water relations (Hodges et al., 1999; Shabir et al., 2018). These Cd-induced impairments might have caused the decline in the growth and yield of quinoa plants in the current study. The stressing effects of Cd on the experimental plants were lessened during co-application of salinity and Cd, perhaps because salinity improved the Cd tolerance index (TI) of quinoa plants, which being halophytic grows successfully under saline conditions. Another reason for enhanced growth under co-application of Cd and salinity could be the limited uptake of Cd by quinoa under moderate salt stress levels. However, co-application of greater amounts of Cd and salinity did not improve plant growth and TI. Our observations are in line with others, where Iftikhar et al. (2021) found that salinity application eased up the quinoa plants to survive under Pb stress, which would otherwise die under the elevated concentration of non-essential heavy metal. Similarly, Cheng et al. (2018) observed an improved growth of the salt-tolerant plant, Carpobrotus rossii, when jointly treated with Cd and salinity; however, at higher levels of Cd and NaCl, plant growth was decreased. From the current study, it would not be unwise to conclude that a low level of salinity in the growth medium may prove beneficial for some halophytic plant species under heavy metal stress (Cheng et al., 2018; Iftikhar et al., 2021; Manousaki et al., 2009; Zhang et al., 2020).
Chlorophyll contents and stomatal conductance were also declined at higher concentrations of salinity and Cd. Our findings corroborate with other reports (Abbas et al., 2021; Chen et al., 2020; Rehman et al., 2019), where Cd and salinity applications hindered the leaf pigments and stomatal homeostasis. These anomalies can be attributed to the damage to chloroplast membrane and disorder in thylakoids arrangement in photosynthetic apparatus, which are the known consequences of Cd accumulation in plants (Chen et al., 2020; Zhang et al., 2020). Moreover, chlorophyll molecules are damaged due to the toxicity induced by Cd and Na to the macronutrients (Riaz et al., 2020; Zhang et al., 2020). Reduction in stomatal conductance of leaves under Cd stress is ascribed to the non-stomatal limitation of photosynthesis, as noted by (Gallego et al., 2012). Mild application of salinity in combination with Cd improved the stomatal conductance and pigments (Zhang et al., 2020). As salinity positively influences the photosynthetic electron transport chain and maintains the ultrastructure integrity of chloroplasts, hence improving the chlorophyll contents and stomatal functioning (Wali et al., 2016). Moreover, a lower salinity level is well documented to enhance CO2 absorption per unit leaf area, leading to increased stomatal conductance (Iftikhar et al., 2021; Nawaz et al., 2016).
The accumulation of Na by quinoa was boosted with an increasing salt concentration in the nutrient solution, corroborating with the findings of Riaz et al. (2020) and (Iftikhar et al., 2021). Moreover, the growth and yield of the quinoa genotype are prone to high Na contents (Abbas et al., 2021). However, in the combined application of salinity and Cd, Na contents were higher both in the roots and aboveground foliage of plants compared to salinity alone treatments (Zhang et al., 2020). Halophytes, including quinoa, use different strategies to adapt to the medium containing an excessive amount of Na. For instance, some genotypes use an exclusion strategy, while others sequester Na in leaf vacuoles (Abbas et al., 2021; Shabala et al., 2013). In our study, quinoa genotype “Puno” accumulated more Na in shoot than root, indicating that vacuolar sequestration was the prominent mechanism of Na tolerance in this genotype (Iftikhar et al., 2021). A lower degree of salinity did not influence K uptake by the experimental plants either way. Still, increasing salinity levels and a combination of salinity and Cd lessened the K concentration in plant tissues. Similar observations were reported by Iftikhar et al. (2021) while studying Pb and salinity interactions. Literature reveals that K, being an essential macronutrient, has many vital roles in plant metabolism, including osmotic adjustments, enzyme activation, cell enlargement, chlorophyll formation, cytoplasmic pH, and membrane stability (Munns & Tester, 2008). It is well documented that higher K contents in plants exposed to salinity stress usually have better salinity tolerance capacity (Abbas et al., 2021; Riaz et al., 2020). Both the Na and K ions exhibit similar hydration energy as well as ionic radii. Therefore, under salt stress, K channels are occupied by Na for entry into the cell (Marschner, 1995), and additional uptake of Na ions limits the movement of K ions leading to the deficiency of this essential nutrient (Abbas et al., 2021; Parvez et al., 2020). Likewise, the aggregation of Cd in plants also causes limited uptake of different elements, including K (Gallego et al., 2012; Rehman et al., 2019). This controlled uptake of K in the presence of Cd might be due to competition for the same cations’ channels (Amjad et al., 2021; Gallego et al., 2012). It has been reported that Cd interferes with the uptake of K at the root level and its transportation within the cell (Rehman et al., 2019). The limited uptake of K results in malfunctioning and damage of cellular organelles (Shabir et al., 2018).
In our study, Cd uptake increased in plant parts with increasing concentration in the soil while decreased when salinity was applied along with Cd. This is natural tendency in halophytes as is evidenced in previous investigations (Cheng et al., 2018; Lefèvre et al., 2009). These researchers noted that salinity application inhibited the Cd uptake by halophyte plant species: Atriplex halimus L. and Carpobrotus rossii. The activity of Cd2+ is decreased due to the arrangement of the CdCl2 network in the nutrient solution. This reduced activity of Cd2+ is one reason for limited Cd uptake by plants under NaCl stress (López-Chuken et al., 2012; Mariem et al., 2014). Few contradictory reports highlight an increase in the uptake of Cd due to the presence of the salinity (Rehman et al., 2019; Shabir et al., 2018), while others reported that interaction of salinity and Cd was concentration-dependent (Chai et al., 2013; Zhang et al., 2014). Still, the literature on the interaction of salinity and Cd under hydroponics is pretty variable and highlights that in addition to Cd2+ activity in solution, other mechanisms are also involved in the uptake of Cd under saline conditions (Cheng et al., 2018; Mariem et al., 2014; Wali et al., 2015). Another possible reason for the limited uptake of Cd might be the decreased Cd2+ activity on the plasma membrane in the presence of NaCl. For example, an increase in the concentration of cations such as Na+ resulted in reduction of negative potential at the plasma membrane, leading to reduced activity of metal ions, including Cd2+ (Wang et al., 2019). In comparison with bulk solution, the movement of Cd on the outer surface of the plasma membrane was more important for metal uptake by plants (Cheng et al., 2018). However, competition for the exchange of ions through different channels cannot be ruled out for limited uptake of Cd as noted by Mei et al. (2014) in Amaranthus mangostanus plants with reduced uptake of Cd in the presence of NaCl due to competition between Cd2+ and Na+ for Ca2+ channels. In our study, quinoa roots accumulated more Cd than shoots, which can be attributed to root-mediated production of the phytochelatins (PCs) and, ultimately, the formation of Cd-PCs networks. These Cd-PCs complexes are sequestered within root vacuoles, limiting the transfer of Cd from root to shoot (Cheng et al., 2018; Rehman et al., 2019; Zhang et al., 2020).
The bioconcentration factor (BCF) for Cd was > 1 at all Cd concentrations, but the addition of NaCl decreased the BCF. Higher BCF values indicate that experimental plants had the potential to accumulate Cd. Contrary to this, in a soil culture experiment, Amjad et al. (2021) found BCF < 1, possibly because of less availability and uptake of Cd from the soil than hydroponics. The translocation of Cd from root to shoot (TF) was < 1 for all the treatments, either alone or in combination with salinity. Restricted translocation of Cd and some other heavy metals due to the presence of salinity has already been observed in quinoa (Amjad et al., 2021; Iftikhar et al., 2021; Parvez et al., 2020). Lower TF values in quinoa indicate its phytostabilization potential for Cd under saline and non-saline conditions.
The overproduction of ROS (H2O2 and TBARS) was initiated at lower levels of salinity and Cd, which increased at elevated levels of Cd (100 and 200 μM). The increased oxidative stress was demonstrated in decreased MSI due to lipid peroxidation of cell membranes. In line with our results, overproduction of H2O2 and TBARS and resultant membrane damage under salinity and Cd have been observed in different genotypes of quinoa (Amjad et al., 2021; Iftikhar et al., 2021; Parvez et al., 2020). Interestingly, at lower levels of salinity and Cd, oxidative stress and membrane damage were not aggravated. This might be due to the positive effect of salinity, which restricted the uptake of toxic Cd ions by plants. However, the joint application of higher doses of salinity and Cd caused more oxidative damage than their sole applications, perhaps due to excessive uptake of Na ions by plants at improved salinity levels in the growth medium (Iftikhar et al., 2021). Hydrogen peroxide (H2O2) is considered the most damaging among various ROS because of its conversion into noxious hydroxyl anions (Anwar et al., 2021). Therefore, proper mitigation of H2O2 should be accomplished to prevent cellular damage. The detoxification of ROS is achieved through the activation of the antioxidant enzyme system within the cells (Abbas et al., 2021; Shahid et al., 2017a). Superoxide dismutase (SOD) serves as the primary defense system against the damage incurred by ROS because of its involvement in the detoxification of the superoxide radicals (Iftikhar et al., 2021; Natasha et al., 2020). These radicals are converted to H2O2 and oxygen in the presence of SOD (Rehman et al., 2019; Shahid et al., 2017b). The activity of the SOD enzyme was considerably increased due to Cd stress which may lead to the overproduction of H2O2− radicles and improved interaction of SOD and Cd (Natasha et al., 2021; Shahid, 2021). The detoxification of H2O2 is accomplished through its conversion into water and oxygen in the presence of CAT, APX, and POD (Iftikhar et al., 2021). The enhanced enzymatic activities in quinoa under salinity and Cd stress indicate their role in mitigating the effects of H2O2 in quinoa (Amjad et al., 2021; Rehman et al., 2019).
Conclusions
The physiological attributes and growth of quinoa plants were reduced both under sole treatment of Cd or its joint application with salinity (300 mM NaCl). A moderate level of salinity (150 mM NaCl) relieved the quinoa plants from Cd-induced toxicity by inhibiting the aggregation of Cd and activation of the antioxidant enzyme system of plants. Increased tolerance and less uptake of Cd due to moderate salinity level showed that the quinoa genotype named ‘‘Puno’’ was suitable for the phytostabilization and could be successfully cultivated in Cd-contaminated saline and non-saline soils.
Availability of data and material
Data will be available as demanded.
References
Abbas, G., Amjad, M., Saqib, M., Murtaza, B., Asif Naeem, M., Shabbir, A., & Murtaza, G. (2021). Soil sodicity is more detrimental than salinity for quinoa (Chenopodium quinoa Willd.): A multivariate comparison of physiological, biochemical and nutritional quality attributes. Journal of Agronomy and Crop Science, 207, 59–73.
Abbas, G., Chen, Y., Khan, F. Y., Feng, Y., Palta, J. A., & Siddique, K. H. (2018). Salinity and low phosphorus differentially affect shoot and root traits in two wheat cultivars with contrasting tolerance to salt. Agronomy, 8(8), 155.
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Amjad, M., Iqbal, M. M., Abbas, G., Farooq, A. B. U., Naeem, M. A., Imran, M., Murtaza, B., Nadeem, M., & Jacobsen, S.-E. (2021). Assessment of cadmium and lead tolerance potential of quinoa (Chenopodium quinoa Willd) and its implications for phytoremediation and human health. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00826-0(0123456789
Anwar, H., Shahid, M., Natasha, Niazi, N. K., Khalid, S., Tariq, T. Z., Ahmad, S., Nadeem, M., & Abbas, G. (2021). Risk assessment of potentially toxic metal (loid) s in Vigna radiata L. under wastewater and freshwater irrigation. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.129124
Chai, M., Shi, F., Li, R., Liu, L., Liu, Y., & Liu, F. (2013). Interactive effects of cadmium and carbon nanotubes on the growth and metal accumulation in a halophyte Spartina alterniflora (Poaceae). Plant Growth Regulation, 71, 171–179.
Chen, H.-C., Zhang, S.-L., Wu, K.-J., Li, R., He, X.-R., He, D.-N., Huang, C., & Wei, H. (2020). The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. Under Cd stress. Ecotoxicology and Environmental Safety. https://doi.org/10.1016/j.ecoenv.2019.109790.
Cheng, M., Wang, A., Liu, Z., Gendall, A. R., Rochfort, S., & Tang, C. (2018). Sodium chloride decreases cadmium accumulation and changes the response of metabolites to cadmium stress in the halophyte Carpobrotus rossii. Annals of Botany, 122, 373–385.
Dhindsa, R. S., Plumb-Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32, 93–101.
Gallego, S. M., Pena, L. B., Barcia, R. A., Azpilicueta, C. E., Iannone, M. F., Rosales, E. P., Zawoznik, M. S., Groppa, M. D., & Benavides, M. P. (2012). Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environmental and Experimental Botany, 83, 33–46.
Hemeda, H. M., & Klein, B. (1990). Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. Journal of Food Science, 55, 184–185.
Hoagland, D. R., & Arnon, D. I. (1950). The water-culture method for growing plants without soil. Circular, California Agricultural Experiment Station, 347.
Hodges, D. M., DeLong, J. M., Forney, C. F., & Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207, 604–611.
Hu, Y., Hackl, H., & Schmidhalter, U. (2017). Comparative performance of spectral and thermographic properties of plants and physiological traits for phenotyping salinity tolerance of wheat cultivars under simulated field conditions. Functional Plant Biology, 44, 134–142.
Iftikhar, A., Abbas, G., Saqib, M., Shabbir, A., Amjad, M., Shahid, M., Ahmad, I., Iqbal, S., & Qaisrani, S. A. (2021). Salinity modulates lead (Pb) tolerance and phytoremediation potential of quinoa: A multivariate comparison of physiological and biochemical attributes. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00937-8
Islam, E., Liu, D., Li, T., Yang, X., Jin, X., Mahmood, Q., Tian, S., & Li, J. (2008). Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. Journal Hazardous Materials, 154, 914–926.
Jacobsen, S. E., Liu, F., & Jensen, C. R. (2009). Does rootsourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd.). Scientia Horticulture, 122, 281–287.
Lefèvre, I., Marchal, G., Meerts, P., Corréal, E., & Lutts, S. (2009). Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environmental and Experimental Botany, 65, 142–152.
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymology, 148, 350–382.
López-Chuken, U., López-Domínguez, U., Parra-Saldivar, R., Moreno-Jiménez, E., Hinojosa-Reyes, L., Guzmán-Mar, J., & Olivares-Sáenz, E. (2012). Implications of chloride-enhanced cadmium uptake in saline agriculture: Modeling cadmium uptake by maize and tobacco. International Journal of Environmental Science and Technology, 9, 69–77.
Manousaki, E., Kokkali, F., & Kalogerakis, N. (2009). Influence of salinity on lead and cadmium accumulation by the salt cedar (Tamarix smyrnensis Bunge). Journal of Chemical Technology & Biotechnology, 84, 877–883.
Mariem, W., Kilani, B. R., Benet, G., Abdelbasset, L., Stanley, L., Charlotte, P., Chedly, A., & Tahar, G. (2014). How does NaCl improve tolerance to cadmium in the halophyte Sesuvium portulacastrum? Chemosphere, 117, 243–250.
Marschner, H. (1995). Mineral nutrition of higher plants (2nd edn, 889 pp). Academic Press London.
Mei, X., Li, S., Li, Q., Yang, Y., Luo, X., He, B., Li, H., & Xu, Z. (2014). Sodium chloride salinity reduces Cd uptake by edible amaranth (Amaranthus mangostanus L.) via competition for Ca channels. Ecotoxicology and Environmental Safety, 105, 59–64.
Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681.
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880.
Natasha, S. M., Farooq, A. B. U., Rabbani, F., Khalid, S., & Dumat, C. (2020). Risk assessment and biophysiochemical responses of spinach to foliar application of lead oxide nanoparticles: A multivariate analysis. Chemosphere, 125605. https://doi.org/10.1016/j.chemosphere.2019.125605.
Natasha, S. M., Sardar, A., Anwar, H., Khalid, S., Shah, S. H., Shah, A. H., & Bilal, M. (2021). Effect of co-application of wastewater and freshwater on the physiological properties and trace element content in Raphanus sativus: soil contamination and human health. Environmental Geochemistry and Health, 43(6), 2393–2406.
Nawaz, M. F., Gul, S., Tanvir, M. A., Akhtar, J., Chaudary, S., & Ahmad, I. (2016). Influence of NaCl-salinity on Pb-uptake behavior and growth of River Red gum tree (Eucalyptus camaldulensis Dehnh.). Turkish Journal of Agriculture and Forestry, 40, 425–432.
Parvez, S., Abbas, G., Shahid, M., Amjad, M., Hussain, M., Asad, S. A., Imran, M., & Naeem, M. A. (2020). Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicology and Environmental Safety, 187. https://doi.org/10.1016/j.ecoenv.2019.109814.
Qadir, M., Quillérou, E., Nangia, V., Murtaza, G., Singh, M., Thomas, R. J., Drechsel, P., & Noble, A. D. (2014). Economics of salt-induced land degradation and restoration. Natural Resources Forum, 38, 282–295.
Qayyum, M. F., ur Rehman, M. Z., Ali, S., Rizwan, M., Naeem, A., Maqsood, M. A., Khalid, H., Rinklebe, J., & Ok, Y. S. (2017). Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere, 174, 515–523.
Rehman, S., Abbas, G., Shahid, M., Saqib, M., Farooq, A. B. U., Hussain, M., Murtaza, B., Amjad, M., Naeem, M. A., & Farooq, A. (2019). Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicology and Environmental Safety, 171, 146–153.
Riaz, F., Abbas, G., Saqib, M., Amjad, M., Farooq, A., Ahmad, S., Naeem, M. A., Umer, M., Khalid, M. S., & Ahmed, K. (2020). Comparative effect of salinity on growth, ionic and physiological attributes of two quinoa genotypes. Pakistan Journal of Agricultural Sciences, 57, 115–122.
Shabala, S., Hariadi, Y., & Jacobsen, S.-E. (2013). Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. Journal of Plant Physiology, 170, 906–914.
Shabir, R., Abbas, G., Saqib, M., Shahid, M., Shah, G. M., Akram, M., Niazi, N. K., Naeem, M. A., Hussain, M., & Ashraf, F. (2018). Cadmium tolerance and phytoremediation potential of acacia (Acacia nilotica L.) under salinity stress. International Journal of Phytoremediation, 20, 739–746.
Shahid, M. (2021). Effect of soil amendments on trace element-mediated oxidative stress in plants: Meta-analysis and mechanistic interpretations. Journal of Hazardous Materials. https://doi.org/10.1016/j.jhazmat.2020.124881
Shahid, M., Dumat, C., Khalid, S., Schreck, E., Xiong, T., & Niazi, N. K. (2017a). Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. Journal of Hazardous Materials, 325, 36–58.
Shahid, M., Rafiq, M., Niazi, N. K., Dumat, C., Shamshad, S., Khalid, S., & Bibi, I. (2017b). Arsenic accumulation and physiological attributes of spinach in the presence of amendments: An implication to reduce health risk. Environmental Science and Pollution Research, 24, 16097–16106.
Shanying, H., Xiaoe, Y., Zhenli, H., & Baligar, V. C. (2017). Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere, 27, 421–438.
Souri, Z., Karimi, N., & de Oliveira, L. M. (2018). Antioxidant enzymes responses in shoots of arsenic hyperaccumulator, Isatis cappadocica Desv., under interaction of arsenate and phosphate. Environmental Technology, 39, 1316–1327.
Steel, R., Torrie, J., & Dickey, D. (1997). Principles and procedures of statistics: A biometrical approach (3rd ed.). New York.
Wali, M., Fourati, E., Hmaeid, N., Ghabriche, R., Poschenrieder, C., Abdelly, C., & Ghnaya, T. (2015). NaCl alleviates Cd toxicity by changing its chemical forms of accumulation in the halophyte Sesuvium portulacastrum. Environmental Science and Pollution Research, 22, 10769–10777.
Wali, M., Gunsè, B., Llugany, M., Corrales, I., Abdelly, C., Poschenrieder, C., & Ghnaya, T. (2016). High salinity helps the halophyte Sesuvium portulacastrum in defense against Cd toxicity by maintaining redox balance and photosynthesis. Planta, 244, 333–346.
Wang, M., Chen, S., Chen, L., & Wang, D. (2019). Saline stress modifies the effect of cadmium toxicity on soil archaeal communities. Ecotoxicology and Environmental Safety. https://doi.org/10.1016/j.ecoenv.2019.109431
Zhang, S., Ni, X., Arif, M., Zheng, J., Stubbs, A., & Li, C. (2020). NaCl improved Cd tolerance of the euhalophyte Suaeda glauca but not the recretohalophyte Limonium aureum. Plant and Soil, 449, 303–318.
Zhang, X., Gao, B., & Xia, H. (2014). Effect of cadmium on growth, photosynthesis, mineral nutrition and metal accumulation of bana grass and vetiver grass. Ecotoxicology and Environmental Safety, 106, 102–108.
Acknowledgements
The authors are grateful to the Researchers Supporting Project number (RSP-2021/407), King Saud University, Riyadh, Saudi Arabia for the support. The authors are equally indebted to COMSATS University Islamabad, Vehari Campus, for providing research facilities during the research work.
Funding
The authors are grateful to the Researchers Supporting Project number (RSP-2021/407), King Saud University, Riyadh, Saudi Arabia, for the support.
Author information
Authors and Affiliations
Contributions
GA, SA and GMS conceived the research idea. GA and SAA analyzed the data and wrote the manuscript. GA and NA accomplished the experimentation and plant analyses. AAG, MR, SA and MS reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdal, N., Abbas, G., Asad, S.A. et al. Salinity mitigates cadmium-induced phytotoxicity in quinoa (Chenopodium quinoa Willd.) by limiting the Cd uptake and improved responses to oxidative stress: implications for phytoremediation. Environ Geochem Health 45, 171–185 (2023). https://doi.org/10.1007/s10653-021-01082-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-01082-y






