Abstract
Salinity and lead (Pb) contamination of soil are important environmental issues. A hydroponics experiment was performed to unravel the effects of salinity on modulation of Pb tolerance and phytoremediation potential of quinoa. Four-week-old plants of quinoa genotype “Puno” were treated with different concentrations of NaCl (0, 150 and 300 mM), Pb (0, 250 and 500 μM) and their combinations. It was noticed that plant biomass, chlorophyll contents and stomatal conductance of quinoa were slightly affected at 150 mM NaCl or 250 μM Pb. However, the higher concentrations of NaCl (300 mM) and Pb (500 μM) caused significant decline in these attributes. The accumulation of Na in quinoa increased under the combined application of salt with highest level of Pb. The uptake of K was not affected at the lower levels of either salinity or Pb, but decreased significantly at their highest levels. The combination of salinity and Pb increased H2O2 contents and caused lipid peroxidation that was mitigated by the activation of antioxidant enzymes (superoxide dismutase, catalase, peroxidase, ascorbate peroxidase). The activities of these enzymes increased by 4-, 3.75-, 5.4- and 2-fold, respectively, in the combined application of 500 μM Pb and 300 mM NaCl with respect to control. A multivariate analysis indicated that Pb tolerance potential of quinoa under combined application of NaCl and Pb was higher at 150 than 300 mM NaCl. The bioconcentration factor and translocation factor for Pb remained less than one either in the absence or presence of salinity. Lead accumulation and tolerance potential indicated that quinoa genotype "Puno" is suitable for phytostabilization of Pb under saline conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing trend of industrialization, urbanization, agriculture and mining has led to an increase in soil contamination with heavy metals (Kushwaha et al., 2018; Pourrut et al., 2011). Among different heavy metals, lead (Pb) is regarded as one of the dangerous contaminants and its continued use has put environment as well as human health at risk (Kushwaha et al., 2018). Lead is naturally present in the earth crust in relatively low concentration (< 50 mg kg−1 soil); however, the recent increase in anthropogenic activities has considerably increased the concentration and forms of Pb present in soil (Arias et al., 2010).
Lead has been reported to cause interferences with plant photosynthetic processes, uptake of nutrients, growth of seedlings, permeability of membranes, activities of various enzymes and water balance either directly or indirectly (Ahmad et al., 2018; Kumar et al., 2012; Shahid et al., 2011). When present in elevated concentration, Pb causes disturbance in normal functioning of chloroplasts by inhibiting the enzymes that take part in the biosynthesis of chlorophyll, fixation of carbon dioxide and the complexation of proteins with pigments in photosystems (Sharma & Dubey, 2005). It also damages the donor and acceptor sites, electron-transfer reactions and oxygen evolving complex (Pourrut et al., 2011) and triggers the generation of reactive oxygen species (ROS) which cause cellular toxicity leading to peroxidation of lipids and leakage of membranes (Malecka et al., 2001; Natasha, 2020). Plants respond to these elevated levels of ROS by the activation of various enzymatic antioxidants such as catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX) and superoxide dismutase (SOD) and non-enzymatic antioxidants (carotenoids, tocopherols and ascorbic acid) (Gratão et al., 2005; Mishra et al., 2009; Parvez et al., 2020).
Agricultural soils are not only being contaminated with heavy metals, but also facing the issue of soil salinization which has already affected above 6% of total land and above 20% of irrigated agricultural land all over the globe (Qadir et al., 2014). Soil salinization severely affects the growth and productivity of crops by inducing osmotic stress and ionic imbalance (Munns & Tester, 2008; Negrão et al., 2017). Additionally, it also causes oxidative stress, reduction in stomatal conductance and pigment contents of leaves (Abbas et al., 2017; Hu et al., 2017). The abovementioned negative effects eventually lead to diminish productivity of plants grown on saline soils (Abbas et al., 2017; Qadir et al., 2014). The extent of damage caused by salinity is dependent on the kind of salt affected soils and the type of plants grown on these soils (Shabala et al., 2013).
Food security is being threatened globally due to an exponential increase in the world population; hence, the productive use of saline soils and waters is imperative. Cultivation of halophytes is considered as the most promising and suitable approach to deal with the menace of salinization (Jacobsen et al., 2009). Quinoa (Chenopodium quinoa Willd.) a halophyte has emerged as an exemplary food crop (Shabala et al., 2013) due to its remarkable tolerance to salinity (Riaz et al., 2020; Ruiz-Carrasco et al., 2011) and drought stress (Razzaghi et al., 2015). It can produce highly nutritious seeds even if cultivated under extremely contaminated environment (Abbas et al., 2021; Iqbal et al., 2019). In our previous study, we found the antagonistic effect between Na+ and As uptake i.e. increase in Na+ uptake decreased As contents in quinoa tissues. Moreover, quinoa has the potential for phytostabilization of toxic metals in the presence of salinity (Amjad et al., 2021; Parvez et al., 2020).
The findings on interaction of salinity with Pb are not conclusive. Salinity may increase or decrease Pb tolerance of plants (Li et al., 2019; Manousaki & Kalogerakis, 2009). As for quinoa, its growth and physiological responses under the combined stresses of salinity and Pb have not been studied yet. Therefore, the present study was conducted to explore (a) the physiological and biochemical attributes of quinoa under the co-contamination of salinity and Pb (b) the effects of salinity on Pb tolerance and phytoremediation potential of quinoa.
Materials and method
Experimental description
The current hydroponics experiment was carried out at the Department of Environmental Sciences, COMSATS University Islamabad, Vehari Campus during 2019–2020. Four-week-old, healthy plants of quinoa genotype “Puno” (originated from Denmark) were transplanted in polystyrene sheets, and these sheets were made afloat on half strength Hoagland’s nutrient solution (Hoagland & Arnon, 1950). The solution contained macro-nutrients in mM (S 1.0, Mg 1.0, Ca 2.5, K 3.0, P 0.5, N 3.75) and micro-nutrients in μM (Cu 0.16, Mo 0.25, Zn 0.38, Cl 9.0, Mn 4.55, Fe 26.0, B 23.15). All the chemicals utilized in the current experiment were of high purity and were procured from Sigma-Aldrich. After acclimatization of one week, salinity treatments (0, 150, 300 mM NaCl) were applied in two splits in two successive days. After one-week gap, treatments of Pb (0, 250 and 500 μM Pb) were applied alone and in combination with NaCl using lead nitrate salt [Pb (NO3)2]. Each treatment had four replications with one plant per replicate. Solution pH was regularly monitored and maintained at 6.5 ± 0.2 by either NaOH (1 N) or HCl (1 N). Nutrient solution was changed on weekly basis throughout the experiment. The average minimum and maximum temperature during the study was 10 and 26 °C, respectively.
Harvesting of plants and growth measurements
Harvesting of plants was done after four weeks of treatments exposure, and plants were divided into shoots and roots. The roots were carefully washed in 0.01 M HCl and subsequently washed with distilled water for removing surface adsorbed Pb (Parvez et al., 2020). Root and shoot lengths were measured. Afterwards, roots and shoots were subjected to air drying. Dry weight of samples was measured after oven drying at 70 °C till constant weight.
Ionic analysis
For analyzing the ionic contents, shoots and roots were finely ground in a porcelain grinder and subjected to digestion in 1:2 mixture of HClO4 and HNO3. Following digestion, samples were filtered and concentrations of Na and K were analyzed by flame photometer (BWB-XP5). The concentration of Pb in digested samples was determined by atomic absorption spectrophotometer (PerkinElmer Model: PinAAcle 900F, Inc. USA), using certified reference material (NIST SRM 1547, peach leaves) internal standards (CPAchem, Bulgaria) and reagent blanks.
Bioconcentration factor, translocation factor and tolerance index
The calculations of bioconcentration factor (BCF) and translocation factor (TF) were done according to Shabbir et al. (2020) as given below.
-
BCF = Concentration of Pb in plant/concentration of Pb in solution.
-
TF = Concentration of Pb in shoot/concentration of Pb in root.
Tolerance index (TI) was calculated according to given equation as described by Parvez et al. (2020).
Stomatal conductance, leaf pigments and membrane stability index
Stomatal conductance of fully expanded second leaf from the top was estimated before harvesting using a portable leaf porometer (Decagon Devices, Pullman, WA, USA). The pigment contents [chlorophyll a (Chl a), chlorophyll b (Chl b) and total chlorophyll (Total Chl)] were determined by following Lichtenthaler (1987). The leaf samples (1.0 g) were put into liquid nitrogen for few seconds in order to protect metabolites by stopping the metabolic processes. Afterwards, the samples were ground in a hydro-acetone solution (80% acetone) to obtain leaf extract. After grinding, the mixture was subjected to centrifugation for 10 min at 3000×g. The supernatant of the centrifuged samples was collected, and a UV–Vis spectrophotometer (Lambda 25, PerkinElmer, Inc. USA) was used for recording the absorbance of samples at specified wavelengths (663.2 and 646.8 nm). The method described by Sairam et al. (2002) was adopted for measuring the membrane stability index (MSI) of leaves.
Hydrogen peroxide (H 2 O 2 )
The H2O2 contents of leaves were determined by following the method described by Islam et al. (2008). Briefly, 0.5 g leaf sample was homogenized in trichloroacetic acid (0.1%) under liquid nitrogen. After homogenization, the sample was subjected to centrifugation at 12,000×g for 20 min. Following centrifugation, the reaction mixture (pH-7.0) was prepared by mixing supernatant (1 mL) with potassium phosphate buffer (1 mL, 10 mM) and potassium iodide (1 mL, 2 M). Immediately, the absorbance was recorded at 390 nm wavelength by UV–Vis spectrophotometer. The concentration of H2O2 was estimated through a standard curve of H2O2.
Lipid peroxidation
Thiobarbituric acid reactive substances (TBARS) were measured according to Hodges et al. (1999) in order to determine the lipid peroxidation. The leaf samples were weighed (0.5 g) and homogenized at 4 °C using a hydro-acetone solution (80% acetone) under liquid nitrogen. The homogenized samples were mixed with butyl hydroxytoluene (BHT) and thiobarbituric acid (TBA) and incubated at 95 °C. Thereafter, the samples were subjected to centrifugation at 12,000×g for 10 min, and their absorbance was recorded at 532 nm wavelength by UV–Vis spectrophotometer. The TBARS contents were presented in nmol g−1 on fresh weight basis.
Activities of antioxidant enzymes
To measure the activities of antioxidant enzyme [SOD (EC 1.15.1.1), CAT (EC 1.11.1.6), POD (1.11.1.7) and APX (EC 1.11.1.11], the leaf samples (youngest fully expanded leaves) were weighed (0.25 g) and ground under liquid nitrogen using a 0.1 M phosphate buffer of pH 7.0. After grinding, the leaf extract was subjected to 30 min centrifugation at 15,000×g and 4 °C temperature. For determining the activity of SOD, the method given by Dhindsa et al. (1981) was followed. The activity of SOD was related to 50% reduction of nitroblue tetrazolium (NBT). The method of Nakano and Asada (1981) was followed for measuring the activity of APX which was presented as μM ascorbate min−1 mg−1 protein. The contents of POD were measured by following Hemeda and Klein (1990) and were expressed as μM of guaiacol oxidized min−1 mg−1 protein. In order to determine the activity of CAT, the method provided by Aebi (1984) was used. The contents of CAT were presented as μM of H2O2 degraded min−1 mg−1 protein.
Statistical analysis
The current study was carried out in a completely randomized design (CRD). All the data were analyzed by statistical software “Statistix 8.1.” The analysis of the data was done by one-way analysis of variance (ANOVA). Treatments means were compared by least significant difference (LSD) test at 5% probability level (Steel et al., 1997). Principal component analysis (PCA) and Pearson correlation matrices were drawn using “XLSTAT 2014.”
Results
Plant growth
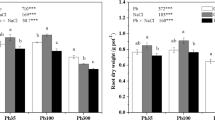
Growth of quinoa plants did not affect significantly at the lower levels of both salinity (150 mM NaCl) and Pb (250 μM Pb) treatments (Table 1). However, the highest levels of salinity (300 mM NaCl) and Pb (500 μM) caused significant reduction in plant growth as compared to control. Interestingly, when lower level of salinity was combined with both levels of Pb, plant growth was improved. However, the combination of higher level of salinity and Pb caused the highest reduction in plant growth attributes. Shoot length decreased by 34% at the highest level of Pb treatment (500 μM). When lower (150 mM) and higher (300 mM) levels of NaCl were combined with the higher level of Pb, the respective reduction in shoot length was 24% and 47%, while in root length it was 32% and 58%, compared to control treatment. Dry weights of shoot and root decreased by 30% and 34% at the higher level of Pb alone treatment. The combination of the lower and higher levels of salinity with Pb caused 18% and 43% decrease in shoot dry weight, while 24% and 56% in root dry weight, respectively, compared to control treatment.
Leaf chlorophyll contents and stomatal conductance
Chlorophyll contents (chl a, chl b and total chl) and stomatal conductance of quinoa leaves decreased as the levels of salinity and Pb were increased in the nutrient solution (Table 2). When the lower level of salinity was combined with Pb treatments, chlorophyll contents and stomatal conductance were improved. The higher level of Pb (500 μM) caused 17%, 35%, 21% and 40% decrease in chl a, chl b and total chl and stomatal conductance, respectively. For the lower level of salinity applied in combination with higher level of Pb, the respective reduction in abovementioned attributes was 13%, 28%, 16% and 31% as compared to control. The combination of the higher level of salinity with higher level of Pb resulted in 48%, 57%, 50% and 69% respective decrease in chl a, chl b, total chl and stomatal conductance in comparison with control treatment.
Ionic contents
The contents of Na in root and shoot increased with the corresponding increase in NaCl concentration in the growth medium (Fig. 1a, b). The application of salinity along with the lower level of Pb did not increase the contents of Na, but the combination of salinity with the higher level of Pb significantly increased Na contents with respect to salinity alone treatments. The contents of K in root and shoot neither decreased at the lower level of salinity nor at Pb, but decreased at the higher levels of both stresses in comparison with control treatment (Fig. 1c, d). The combination of salinity and Pb further decreased the contents of K in quinoa plants with respect to control treatment.
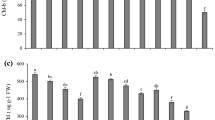
Lead accumulation and tolerance
The combination of the lower level of salinity with Pb treatments did not increase Pb contents, but the combination of the higher level of salinity with Pb increased Pb contents both in root and shoot of quinoa (Table 3). Root accumulated more Pb than shoot for all the applied levels of Pb in the nutrient solution. The BCF was less than one for all the Pb treatments (Table 3). The combination of salinity with each level of Pb increased the value of BCF. Root to shoot translocation of Pb was expressed as TF, which was also less than one for all the Pb treatments. Addition of salt to each Pb treatment did not significantly increase the TF in quinoa. There was a decline in tolerance index (TI) with an increase in the levels of Pb and salinity in the nutrient solution (Table 3). On the other hand, the combination of the lower level of salinity with the lower and higher level of Pb increased the TI from 85–95% and 70–82%, respectively. However, in the combined treatments of the higher level of salinity with lower and higher level of Pb, TI was decreased to 69% and 57%, respectively.
Oxidative stress attributes
The contents of H2O2 and TBARS increased more under salinity stress as compared to Pb stress (Fig. 2a, b). The combination of salinity and Pb caused more oxidative damage than the either stress alone. The higher level of salinity alone caused 2.0- and 2.5-fold increase, respectively in the contents of H2O2 and TBARS in comparison with control plants. The higher level of Pb stress, respectively, caused 2.0- and 1.9-fold enhancement in H2O2 and TBARS contents as compared to control treatment. The combined treatment of the higher level of salinity and Pb resulted in fourfold increase in the contents of H2O2 and TBARS in comparison with control treatment.
Effect of different levels of salinity and Pb on H2O2 contents (a), TBARS (b) contents and membrane stability index (c) of quinoa genotype “Puno” grown in hydroponics. Data are presented as mean of four replications ± SE. The values sharing different letters indicate significant difference at 5% probability level
Membrane stability index (MSI) declined with an increase in the levels of Pb and salinity (Fig. 2c). The lower levels of either salinity or Pb alone did not cause any damage to membranes. However, the higher levels of Pb and salinity stress, either alone or in combination, significantly reduced MSI. The highest reduction in MSI (47%) was noticed in the combined treatment of the higher levels of Pb (500 μM) and salinity (300 mM NaCl).
Antioxidant enzymes activities
Antioxidant enzymes (SOD, CAT, POD and APX) showed varied response against salinity and Pb stress. The activities of all the antioxidant enzymes were higher in the combined treatments as compared to individual treatments of Pb and salinity (Fig. 3a–d). Under the higher level of salinity, the activities of SOD, POD, CAT and APX were 3.3-, 2.0-, 3.0- and 1.5-fold higher than control. In the joint application of 250 μM Pb and 300 mM NaCl, the respective enhancement in the activities of above enzymes was 4.6-, 3.5-, 5.3- and twofold higher than control. The activities of these enzymes increased by 4-, 3.75-, 5.4- and twofold in the combined application of 500 μM Pb and 300 mM NaCl with respect to control.
Multivariate analyses
Principal component analysis (PCA) and Pearson correlation matrix were performed to determine the correlations between different observations and response variables of quinoa under salinity and Pb stress (Fig. 4a, b; Tables S1, S2 and S3). The PCA divided all the variables in to eighteen factors (F1 to F18), but only five factors had the main contribution. These five factors contributed 71%, 10%, 8%, 5% and 2% variability, respectively. All the variables were clustered in two groups. Oxidative stress attributes (H2O2, TBARS, SOD, CAT, POD), Na and Pb contents were grouped together, whereas the rest of the parameters were grouped together. Pearson correlation showed that oxidative stress attributes, Na and Pb contents had negative correlations with the rest of the parameters (Table S1); therefore, they were grouped on the opposite side of the axis (Fig. 4b). The response of different treatments was also illustrated by PCA (Fig. 4a). The lower level of salinity alone or in combination with Pb had similar effect; therefore, these treatments were scatted around positive x-axis. The higher level of salinity alone or in combination with Pb had similar effect; therefore, these treatments were clustered around negative x-axis. This opposite clustering of lower and higher levels of salinity and Pb was also depicted from our results. We observed severe reduction in growth and physiological attributes of quinoa under the elevated levels of salinity, Pb and their combination.
Discussion
The current study was carried out to unravel the effects of salinity on Pb tolerance and phytoremediation potential of quinoa by exploring different physiological and biochemical attributes. We found that plant growth and biomass were not decreased to great extent at 150 mM NaCl concentration which confirmed the halophyte nature of quinoa (Parvez et al., 2020; Riaz et al., 2020). However, at 300 mM NaCl, plant growth and biomass production were severely declined due to salt stress confirming our previous study on quinoa (Parvez et al., 2020). Decline in plant biomass at elevated levels of salinity may be due to osmotic stress, toxicity and imbalance of ions (Abbas et al., 2017; Munns & Tester, 2008), oxidative stress (Parvez et al., 2020) and reduced gas exchange and chlorophyll contents (Hu et al., 2017; Parvez et al., 2020).
In case of Pb, we found that plant growth was not much affected at the lower level of Pb (250 μM), but decreased considerably at the elevated level (500 μM). In our recent study on quinoa, we found that the higher levels of Pb were detrimental for growth and yield of quinoa (Amjad et al., 2021). The results regarding the interaction of salinity and Pb were quite interesting and unexpected. When the lower level of salinity was combined with Pb, plant growth and TI were increased, whereas, under the combination of the higher level of salinity and Pb, plant growth and TI of quinoa were decreased. Similarly, growth enhancement at the lower level and growth reduction at the higher level of salinity and Pb combination was noticed by Manousaki and Kalogerakis (2009) in a halophyte plant (Atriplex halimus L.). It has been reported that the lower levels of salinity (Parvez et al., 2020; Riaz et al., 2020) and Pb (Liu et al., 2000; Manousaki & Kalogerakis, 2009) are beneficial for some plants. Shahid et al. (2019) recently proposed that some trace elements including Pb can induce phytohormesis in which plant growth increases at low levels, while decreases at high levels. The reduction in plant biomass to greater extent in the combined application of elevated level of salinity and Pb may be attributed to negative effects on nutrient uptake, chlorophyll formation, transpiration rates, enzyme activities and oxidative stress (Amjad et al., 2021; Nawaz et al., 2016; Parvez et al., 2020).
Chlorophyll contents of leaves (Chl a, Chl b, total Chl) and stomatal conductance were considerably reduced under the higher levels of Pb and salinity stress. Similar reduction in these physiological attributes had been noticed under the elevated levels of salt (Koyro & Eisa, 2008; Parvez et al., 2020) and Pb stress (Ahmad et al., 2018; Lamhamdi et al., 2013; Nawaz et al., 2016). According to Pourrut et al. (2011), the higher level of Pb stress results in many physiological disorders in plants including disruption of chlorophyll molecules. The combination of the lower level of salinity with both levels of Pb had positive effects on these attributes (Manousaki & Kalogerakis, 2009; Nawaz et al., 2016). The improvement in stomatal conductance under moderate level of salinity and Pb may be due to more absorption of CO2 per unit leaf area (Nawaz et al., 2016; Rawat & Banerjee, 1998). Liu et al. (2000) found that small quantity of Pb in the growth medium had positive effects on pigment contents of some plants. In our study, these attributes were decreased to greater extent under the combination of the higher level of salinity with both levels of Pb. So, under these conditions it can be assumed that chlorophyll structure might had been degraded due to the damaging effects of various ROS (Pourrut et al., 2011), as we found increased H2O2 and TBARS contents in quinoa leaves under the combined stress of salinity and Pb. Moreover, chlorophyll molecule is destroyed due to disordering of grana and thylakoid membranes and replacement of main nutrients by Na and Pb (Akinci et al., 2010; Haseeb et al., 2018; Riaz et al., 2020).
We found that the lower levels of either salinity or Pb did not cause oxidative stress (H2O2 and TBARS contents) in quinoa. However, the higher levels of both salinity and Pb caused oxidative stress, which is conferred by over production of H2O2 and TBARS in this study (Fig. 2a, b). As a result of this oxidative stress, cell membranes suffered from lipid peroxidation which decreased the stability of membranes (Fig. 2c). These results are corroborated the previous findings that either salinity (Abbas et al., 2017; Parvez et al., 2020) or Pb (Amjad et al., 2021; Murtaza et al., 2019) induced greater production of H2O2 and TBARS and caused membrane damage in host plants. Being a very strong oxidant, H2O2 causes severe toxicity to plants particularly when it is transformed to hydroxyl anions. Hence to avoid toxicity in plants, efficient and quick detoxification of H2O2 is inevitable. Various antioxidant enzymes accomplish the mitigation of H2O2 in different components of the cell (Natasha et al., 2020; Parvez et al., 2020). Different enzymes work in a conjugated way during the detoxification of ROS (Pourrut et al., 2011). Among different antioxidant enzymes, SOD plays the most crucial role in the mitigation of superoxide radicals (Parvez et al., 2020). We found that the activity of SOD was increased in quinoa under the elevated levels of Pb and salinity stress. Superoxide radicals are converted into H2O2 and oxygen due to the activation of SOD (Pourrut et al., 2011; Shahid et al., 2014). Increase in SOD activity under Pb stress may be due to elevation in O2·– content or direct interaction between of Pb and SOD (Pourrut et al., 2011). We noticed a slight decline in SOD activity in the combined higher levels of salinity and Pb, which may be due to the binding of metal ions to the active sites of enzyme or interruption in enzyme biosynthesis due to the raised level of ROS (Gupta et al., 2009; Pourrut et al., 2011). The activities of other measured enzymes (POD, CAT, APX) also enhanced as the stress levels were increased. These enzymes accomplish the detoxification of H2O2 by its conversion into molecular oxygen and water (Pourrut et al., 2011; Shahid et al., 2014). The activities of these enzymes were the highest in the combined treatments of salinity and Pb, indicating more oxidative stress in the combined application of both stresses. Activation of these antioxidants under salinity and Pb stress is well documented in the past (Murtaza et al., 2019; Natasha et al., 2020).
In line with many previous reports (Parvez et al., 2020; Riaz et al., 2020), the accumulation of Na in quinoa increased with the corresponding increase in salt level of the medium. In a recent field experiment, we concluded that quinoa yield and grain quality was more declined on salt affected soil with more Na contents as compared to saline soil with less Na concentration. (Abbas et al., 2021). The combination of salinity with Pb was quite interesting. We found that the lower level of Pb had no effect, whereas the higher level of Pb further increased Na accumulation in quinoa. Such variation in Na uptake in the presence of Pb had been noticed in few studies (Lakra et al., 2006; Li et al., 2019; Singh et al., 2003). It has been reported that the mechanism of salt tolerance among quinoa genotypes is variable. Some genotypes exclude toxic ions at root level and some undergo vacuolar sequestration in shoot tissues (Parvez et al., 2020; Shabala et al., 2013). In our case, more amount of Na was accumulated in shoot than root, which demonstrated that salt tolerance mechanism adopted by genotype “Puno” was vacuolar sequestration.
The contents of K neither decreased in the lower levels of salinity and Pb nor in their combination. That might be one of the reasons of less decrease in plant biomass in the combined treatment of the lower level of salinity and Pb. Being an essential element, K has many important physiological functions in plants such as enzyme activation, osmotic adjustment, chlorophyll formation, cell enlargement, homeostasis of membrane potential and regulation of cytoplasmatic pH (Munns & Tester, 2008; Shabala, 2003). Therefore, the plants which accumulate more K under salt stress conditions usually are more salt tolerant and they produce more biomass (Amjad et al., 2015; Riaz et al., 2020). Contrarily, at the higher level of salinity and its combination with Pb, the uptake of K was drastically decreased in quinoa. It has been investigated that the hydration energy and ionic radii of both Na and K are very similar. Hence, at the elevated levels of salt stress, Na cross cell membranes by using K channels (Marschner, 1995). When excessive amount of Na enters the cell, the uptake of K is decreased significantly resulting in the lower salt tolerance potential of plants (Abbas et al., 2017; Adolf et al., 2012; Parvez et al., 2020). Similarly, Pb uptake in plants also results in the limited uptake of many essential ions including K, leading to growth inhibition (Lakra et al., 2006; Li et al., 2019). The strong negative interaction between K and Pb might be due to their similar ionic radii (Sharma & Dubey, 2005); therefore, both ions compete for the same K channels for their entry into the plant cells (Amjad et al., 2021; Pourrut et al., 2011).
We found that Pb contents increased in quinoa as the amount of Pb was increased in the nutrient solution. The lower level of salinity did not increase Pb contents, but the higher level of salinity combined with Pb further increased Pb contents in quinoa. The literature about the interaction between salinity and Pb is not conclusive. In line with our results, Pb uptake by Eucalyptus camaldulensis (Nawaz et al., 2016) and Cucumis sativus (Taghipour & Jalali, 2019) was increased in the presence of salinity under hydroponic and soil conditions, respectively. On the other hand, Manousaki and Kalogerakis (2009) found that Pb uptake by Atriplex halimus was decreased under salinity stress mainly due to less solubility and mobility of Pb in soil in usual range of soil pH (Abbaspour et al., 2008). In our study, salinity-induced higher accumulation of Pb may be due to an increase in the permeability of roots under the elevated levels of both stresses (Wang et al., 2006) or due to the enhanced mobility of Pb under hydroponic growth conditions (Acosta et al., 2011; Nawaz et al., 2016).
The BCF for Pb was less than one for all the Pb treatments indicating less potential of quinoa plant to accumulate Pb in shoot and root. Although BCF increased due to salinity, BCF value remained less than one as observed by Amjad et al. (2021). Similar results regarding BCF had been noticed in various plants under salinity and Pb combination (Nawaz et al., 2016; Taghipour & Jalali, 2019). Root-to-shoot translocation of Pb (TF) was also less than one for Pb alone and the combined treatments of salinity and Pb. More accumulation of Pb in roots and less translocation to shoot even in the presence of salinity had been observed in many studies (Li et al., 2019; Manousaki & Kalogerakis, 2009; Nawaz et al., 2016; Taghipour & Jalali, 2019). The limited accumulation and translocation of Pb might be due to the binding of Pb at cell walls and root surfaces (Butcher, 2009). According to Pourrut et al. (2011), the reasons of less translocation of Pb from roots to aerial parts are precipitation of Pb in intercellular spaces, Pb immobilization by negatively charged pectins within the cell wall, sequestration in the vacuoles of rhizodermal and cortical cells and accumulation in plasma membranes.
If the aerial parts of plants such as leaves and seeds accumulate higher amounts of heavy metals (Pb in our case), food quality and human health may be deteriorated. The reported threshold concentration of Pb is 0.2–0.5 mg kg−1 (Kabata-Pendias, 2010). If Pb contents are higher than this range, it may cause non-carcinogenic and carcinogenic effects to humans. The limited uptake and translocation of Pb from root to shoot are an indication that quinoa can be grown on Pb-contaminated soils as a food crop without health hazards (Amjad et al., 2021). Moreover, the lower level of salinity did not increase the uptake of Pb from soil. The lower translocation potential of quinoa for Pb indicated its phytostabilization character for Pb-contaminated saline and non-saline soils which is quite beneficial for limiting the entry of Pb into food chain through consumption of quinoa.
The response of different treatments and variables was also explained by multivariate analyses. This data analysis technique is regarded as very ideal to trace covariance and correlation among different treatments and variables (Murtaza et al., 2019; Natasha et al., 2020). In our study, Pearson correlation and PCA showed strong negative correlation of Na and Pb contents with plant biomass, chlorophyll contents, stomatal conductance and K contents. Oxidative stress attributes had positive correlation with Na and Pb contents. It was also demonstrated that the lower and higher levels of salinity in combination with Pb had different effects. Increase in Pb tolerance potential of quinoa under the lower level of salinity was also verified by the close association of these treatments in PCA. Our results were in accordance with the opposite clustering of lower and higher levels of salinity, Pb and their combinations.
Conclusion
We concluded that growth, chlorophyll and stomatal conductance of quinoa were not affected at the lower levels of salinity (150 mM NaCl) or Pb (250 μM). However, the higher levels of salinity (300 mM NaCl) and Pb (500 μM) alone or in combination caused pronounced decline in these attributes. Under the combination of lower level of salinity with Pb, plants growth and physiological attributes were improved. The contents of Na increased in the combined treatments of salinity with the higher level of Pb. The combination of salinity and Pb induced oxidative stress that was mitigated by the activation of antioxidant enzymes. Lead accumulation in quinoa increased under higher level of salinity stress, but its root to shoot translocation remained unchanged. Lead uptake and tolerance potential of quinoa genotype "Puno" indicated its suitability for phytostabilization of Pb under normal and saline conditions.
Availability of data and materials
Data will be available as demanded.
References
Abbas, G., Amjad, M., Saqib, M., Murtaza, B., Asif Naeem, M., Shabbir, A., et al. (2021). Soil sodicity is more detrimental than salinity for quinoa (Chenopodium quinoa Willd.): A multivariate comparison of physiological, biochemical and nutritional quality attributes. Journal of Agronomy and Crop Science, 207, 59–73
Abbas, G., Saqib, M., Akhtar, J., & Murtaza, G. (2017). Physiological and biochemical characterization of Acacia stenophylla and Acacia albida exposed to salinity under hydroponic conditions. Canadian Journal of Forest Research, 47, 1293–1301
Abbaspour, A., Kalbasi, M., Hajrasuliha, S., & Fotovat, A. (2008). Effect of organic matter and salinity on ethylenediaminetetraacetic acid–extractable and solution species of cadmium and lead in three agricultural soils. Communications in Soil Science and Plant Analysis, 39, 983–1005
Acosta, J. A., Jansen, B., Kalbitz, K., Faz, A., & Martínez-Martínez, S. (2011). Salinity increases mobility of heavy metals in soils. Chemosphere, 85, 1318–1324
Adolf, V. I., Shabala, S., Andersen, M. N., Razzaghi, F., & Jacobsen, S. E. (2012). Varietal differences of quinoa’s tolerance to saline conditions. Plant and Soil, 357, 117–129
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126
Ahmad, I., Akhtar, M. J., Mehmood, S., Akhter, K., Tahir, M., Saeed, M. F., et al. (2018). Combined application of compost and Bacillus sp. CIK-512 ameliorated the lead toxicity in radish by regulating the homeostasis of antioxidants and lead. Ecotoxicology and Environmental Safety, 148, 805–812
Akinci, I. E., Akinci, S., & Yilmaz, K. (2010). Response of tomato (Solanum lycopersicum L.) to lead toxicity: Growth, element uptake, chlorophyll and water content. African Journal of Agricultural Research, 5, 416–423
Amjad, M., Akhtar, S. S., Yang, A., Akhtar, J., & Jacobsen, S. E. (2015). Antioxidative response of quinoa exposed to iso-osmotic, ionic and non-ionic salt stress. Journal of Agronomy and Crop Science, 201, 452–460
Amjad, M., Iqbal, M. M., Abbas, G., et al. (2021). Assessment of cadmium and lead tolerance potential of quinoa (Chenopodium quinoa Willd) and its implications for phytoremediation and human health. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00826-0
Arias, J. A., Peralta-Videa, J. R., Ellzey, J. T., Ren, M., Viveros, M. N., & Gardea-Torresdey, J. L. (2010). Effects of Glomus deserticola inoculation on Prosopis: Enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environmental and Experimental Botany, 68, 139–148
Butcher, D. J. (2009). Phytoremediation of lead in soil: Recent applications and future prospects. Applied Spectroscopy Reviews, 44, 123–139
Dhindsa, R. S., Plumb-Dhindsa, P., & Thorpe, T. A. (1981). Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. Journal of Experimental Botany, 32, 93–101
Gratão, P. L., Polle, A., Lea, P. J., & Azevedo, R. A. (2005). Making the life of heavy metal-stressed plants a little easier. Functional Plant Biology, 32, 481–494
Gupta, D. K., Nicoloso, F. T., Schetinger, M. R. C., Rossato, L. V., Pereira, L. B., Castro, G. Y., et al. (2009). Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. Journal of Hazardous Materials, 172, 479–484
Haseeb, M., Basra, S. M., Afzal, I., & Wahid, A. (2018). Quinoa response to lead: Growth and lead partitioning. International Journal of Agriculture and Biology, 20, 338–344
Hemeda, H. M., & Klein, B. (1990). Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. Journal of Food Science, 55, 184–185
Hoagland, D. R., & Arnon, D. I. (1950). The water-culture method for growing plants without soil. (2nd ed.). Circular California Agricultural Experiment Station.
Hodges, D. M., DeLong, J. M., Forney, C. F., & Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207, 604–611
Hu, Y., Hackl, H., & Schmidhalter, U. (2017). Comparative performance of spectral and thermographic properties of plants and physiological traits for phenotyping salinity tolerance of wheat cultivars under simulated field conditions. Functional Plant Biology, 44, 134–142
Iqbal, S., Basra, S. M. A., Afzal, I., Wahid, A., Saddiq, M. S., Hafeez, M. B., et al. (2019). Yield potential and salt tolerance of quinoa on salt-degraded soils of Pakistan. Journal of Agronomy and Crop Science, 205, 13–21
Islam, E., Liu, D., Li, T., Yang, X., Jin, X., Mahmood, Q., et al. (2008). Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. Journal of Hazardous Materials, 154, 914–926
Jacobsen, S. E., Liu, F., & Jensen, C. R. (2009). Does root-sourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd.). Scientia Horticulture, 122, 281–287
Kabata-Pendias, A. (2010). Trace elements in soils and plants. CRC Press.
Koyro, H. W., & Eisa, S. (2008). Effect of salinity on composition, viability and germination of seeds of Chenopodium quinoa Willd. Plant and Soil, 302, 79–90
Kumar, A., Prasad, M. N., & Sytar, O. (2012). Lead toxicity, defense strategies and associated indicative biomarkers in Talinum triangul grown hydroponically. Chemosphere, 89, 1056–1065
Kushwaha, A., Hans, N., Kumar, S., & Rani, R. (2018). A critical review on speciation, mobilization and toxicity of lead in soil–microbe–plant system and bioremediation strategies. Ecotoxicology and Environmental Safety, 147, 1035–1045
Lakra, N., Mishra, S. N., Singh, D. B., & Tomar, P. C. (2006). Exogenous putrescine effect on cation concentration in leaf of Brassica juncea seedlings subjected to Cd and Pb along with salinity stress. Journal of Environmental Biology, 27, 263–269
Lamhamdi, M., El-Galiou, O., Bakrim, A., Nóvoa-Muñoz, J. C., Arias-Estévez, M., Aarab, A., et al. (2013). Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi Journal of Biological Sciences, 20, 29–36
Li, X., Zhang, X., Wang, X., Yang, X., & Cui, Z. (2019). Bioaugmentation-assisted phytoremediation of lead and salinity co-contaminated soil by Suaeda salsa and Trichoderma asperellum. Chemosphere, 224, 716–725
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382
Liu, D., Jiang, W., Liu, C., Xin, C., & Hou, W. (2000). Uptake and accumulation of lead by roots, hypocotyls and shoots of Indian mustard [Brassica juncea (L.)]. Bioresource Technology, 71, 273–277
Malecka, A., Jarmuszkiewicz, W., & Tomaszewska, B. (2001). Antioxidative defense to lead stress in subcellular compartments of pea root cells. Acta Biochimica Polonica, 48, 687–698
Manousaki, E., & Kalogerakis, N. (2009). Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): Metal uptake in relation to salinity. Environmental Science and Pollution Research, 16, 844–854
Marschner, H. (1995). Mineral nutrition of higher plants. Academic Press.
Mishra, M., Mishra, P. K., Kumar, U., & Prakash, V. (2009). NaCl phytotoxicity induces oxidative stress and response of antioxidant systems in Cicer arietinum L. cv. Abrodhi. Botany Research International, 2, 74–82
Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681
Murtaza, B., Naeem, F., Shahid, M., Abbas, G., Shah, N. S., Amjad, M., et al. (2019). A multivariate analysis of physiological and antioxidant responses and health hazards of wheat under cadmium and lead stress. Environmental Science and Pollution Research, 26, 362–370
Nakano, Y., & Asada, K. (1981). Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867–880
Natasha, S. M., Farooq, A. B. U., Rabbani, F., Khalid, S., & Dumat, C. (2020). Risk assessment and biophysiochemical responses of spinach to foliar application of lead oxide nanoparticles: A multivariate analysis. Chemosphere, 245, 125605
Nawaz, M. F., Gul, S., Tanvir, M. A., Akhtar, J., Chaudary, S., & Ahmad, I. (2016). Influence of NaCl-salinity on Pb-uptake behavior and growth of River Red gum tree (Eucalyptus camaldulensis Dehnh.). Turkish Journal of Agriculture and Forest, 40, 425–432
Negrão, S., Schmöckel, S. M., & Tester, M. (2017). Evaluating physiological responses of plants to salinity stress. Annals of Botany, 119, 1–11
Parvez, S., Abbas, G., Shahid, M., Amjad, M., Hussain, M., et al. (2020). Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicology and Environmental Safety, 187, 109814
Pourrut, B., Shahid, M., Dumat, C., Winterton, P., & Pinelli, E. (2011). Lead uptake, toxicity, and detoxification in plants. Reviews of Environmental Contamination and Toxicology, 213, 113–136
Qadir, M., Quillérou, E., Nangia, V., Murtaza, G., Singh, M., Thomas, R. J., et al. (2014). Economics of salt-induced land degradation and restoration. Natural Resources Forum, 38, 282–295
Rawat, J. S., & Banerjee, S. P. (1998). The influence of salinity on growth, biomass production and photosynthesis of Eucalyptus camaldulensis Dehnh. and Dalbergia sissoo Roxb. seedlings. Plant and Soil, 205, 163–169
Razzaghi, F., Jacobsen, S. E., Jensen, C. R., & Andersen, M. N. (2015). Ionic and photosynthetic homeostasis in quinoa challenged by salinity and drought—mechanisms of tolerance. Functional Plant Biology, 42, 136–148
Riaz, F., Abbas, G., Saqib, M., Amjad, M., Farooq, A., Ahmad, S., et al. (2020). Comparative effect of salinity on growth, ionic and physiological attributes of two quinoa genotypes. Pakistan Journal of Agricultural Science, 57, 115–122
Ruiz-Carrasco, K., Antognoni, F., Coulibaly, A. K., Lizardi, S., Covarrubias, A., Martínez, E. A., et al. (2011). Variation in salinity tolerance of four lowland genotypes of quinoa (Chenopodium quinoa Willd.) as assessed by growth, physiological traits, and sodium transporter gene expression. Plant Physiology and Biochemistry, 49, 1333–1341
Sairam, R. K., Rao, K. V., & Srivastava, G. (2002). Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Science, 163, 1037–1046
Shabala, S. (2003). Regulation of potassium transport in leaves: From molecular to tissue level. Annals of Botany, 92, 627–634
Shabala, S., Hariadi, Y., & Jacobsen, S. E. (2013). Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. Journal of Plant Physiology, 170, 906–914
Shabbir, A., Abbas, G., Asad, S. A., Razzaq, H., Anwar-ul-Haq, M., & Amjad, M. (2020). Effects of arsenite on physiological, biochemical and grain yield attributes of quinoa (Chenopodium quinoa Willd): Implications for phytoremediation and health risk assessment. International Journal of Phytoremediation. https://doi.org/10.1080/15226514.2020.1865266
Shahid, M., Niazi, N. K., Rinklebe, J., Bundschuh, J., Dumat, C., & Pinelli, E. (2019). Trace elements-induced phytohormesis: A critical review and mechanistic interpretation. Critical Reviews in Environmental Science and Technology, 50, 1984–2015
Shahid, M., Pinelli, E., Pourrut, B., & Dumat, C. (2014). Effect of organic ligands on lead-induced oxidative damage and enhanced antioxidant defense in the leaves of Vicia faba plants. Journal of Geochemical Exploration, 144, 282–289
Shahid, M., Pinelli, E., Pourrut, B., Silvestre, J., & Dumat, C. (2011). Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicology and Environmental Safety, 74, 78–84
Sharma, P., & Dubey, R. S. (2005). Lead toxicity in plants. Brazilian Journal of Plant Physiology, 17, 35–52
Singh, R. P., Tripathi, R. D., Dabas, S., Rizvi, S. M. H., Ali, M. B., Sinha, S. K., et al. (2003). Effect of lead on growth and nitrate assimilation of Vigna radiata (L.) Wilczek seedlings in a salt affected environment. Chemosphere, 52, 1245–1250
Steel, R., Torrie, J., & Dickey, D. (1997). Principles and procedures of statistics: A biometrical approach. (3rd ed.). McGraw-Hill.
Taghipour, M., & Jalali, M. (2019). Impact of some industrial solid wastes on the growth and heavy metal uptake of cucumber (Cucumis sativus L.) under salinity stress. Ecotoxicology and Environmental Safety, 182, 109347
Wang, G., Su, M. Y., Chen, Y. H., Lin, F. F., Luo, D., & Gao, S. F. (2006). Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environmental Pollution, 144, 127–135
Acknowledgements
The authors are highly thankful to the Higher Education Commission of Pakistan for providing financial support.
Funding
The authors are highly thankful to the Higher Education Commission of Pakistan for providing financial support.
Author information
Authors and Affiliations
Contributions
G.A. and M.S. conceived the research idea. G.A. and M.A. analyzed the data and wrote the manuscript. A.I. and A.S. accomplished the experimentation and plant analyses. M.S., I.A., S.I. and S.A.Q. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iftikhar, A., Abbas, G., Saqib, M. et al. Salinity modulates lead (Pb) tolerance and phytoremediation potential of quinoa: a multivariate comparison of physiological and biochemical attributes. Environ Geochem Health 44, 257–272 (2022). https://doi.org/10.1007/s10653-021-00937-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00937-8