Abstract
Chloride salinity has been strongly related to enhanced cadmium (Cd) uptake by plants due to increased solubility in the soil solution, even in agricultural soil with very low levels of cadmium. This finding is relevant because the cadmium content of food crops is an important concern for human health. Therefore, the aim of this study was to predict and discuss the chlorine-enhanced uptake of cadmium by two common crops: maize and tobacco under “non-saline” (1 mM) and “very strongly saline” (200 mM) scenarios using a modified ‘biotic ligand model’ and datasets from a set of soil and hydroponic experiments. Results indicated that predicted cadmium uptake rates (expressed as cadmium in plant μmol m−2 root) by maize and tobacco plants were consistently higher (54 and 15%, respectively) assuming conditions of ‘very strong salinity’ soil compared to the simulated ‘non-saline’ soil. In the light of the results of the present research, valuable information is given on modeled cadmium phytoavailability as an indication of the potential risk due to increased cadmium uptake by crops under saline conditions, especially as the enhancement of cadmium uptake in the presence of Cl− salinity may be a general trend that occurs in many edible crops. The biotic ligand model parameterization applied in the present study attempted to simulate conditions commonly found in natural cadmium and salt-affected soils. However, caution is needed to extrapolate results obtained from these models to real soil conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphate (P2O5) fertilizers and biosolids have been recognized as major sources of cadmium (Cd) in agricultural soils (McBride 2003). Typically, concentrations from 2 to 54 mg Cd kg−1 (P2O5 fertilizer) (Iretskaya et al. 1998) and up to 30 mg Cd kg−1 (biosolids) (Clark 2001) are reported. This is relevant as the Cd content of food crops, which is the main source for human intake of Cd, has become an issue of concern for human health (McBride 2003). Food is the main source of Cd intake for non-smoking individuals who are not occupationally exposed (de Vries et al. 2007). The FAO/World Health Organization (FAO 2007) assessed the mean intake of Cd at 51 μg day−1, about 70% of which was contributed by vegetable products. Smoking may also contribute to Cd intake by humans as tobacco plants have been shown to accumulate Cd in leaves (Macek et al. 2005). Kazi et al. (2009) reports that one cigarette contains approximately 1–3 μg of Cd. Considering that 10–20% of Cd in cigarettes may be inhaled (WHO 1992), smoking 20 cigarettes day−1 may result in an additional daily exposure of 2–6 μg.
In general, Cd in the free ion Cd2+ form has been widely reported to be the determinant of phytoavailability by plants in soils. However, during the last few years, other factors such as salinity have been getting more consideration (Khoshgoftar et al. 2004; Weggler et al. 2004; López-Chuken and Young 2005; Ghallab and Usman 2007; López-Chuken et al. 2010a), even in agricultural soil with very low levels of Cd (McLaughlin et al. 1997). Typical salinity values for irrigation water for agriculture often reach up to 180 mM, especially under arid and semi-arid regions (Khoshgoftar et al. 2004), therefore, salinity in agricultural soils is an extensive and important problem affecting about 45 million ha of the world’s 260 million ha of irrigated land (Qadir and Oster 2004; FAO 2006). Chloride salinity has been strongly related to enhanced Cd uptake by plants due to increased solubility in the soil solution (Norvell et al. 2000). Simple stability calculations by Hahne and Kroontje (1973) indicate that formation of Cd2+–Cl−-complexes should become significant when Cl− concentrations rise above approximately 10 mM, a range easily reached in the soil solution of salt-affected soils (Rengasamy 2006). Effects of Cl−-salinity in increasing the solubility of Cd are due to the formation of Cl−-complexes of Cd, predominantly the 1:1 and 1:2 complexes (CdCl+ and CdCl 02 , respectively). These complexes are less strongly sorbed to soil than free Cd2+ ion, and hence formation of complexes with Cl− tends to shift Cd from the solid to the solution phase, thereby enhancing solubility and mobility (Bäckström et al. 2004).
For the above reasons, the objectives of this work were: (1) to obtain data on saline water-enhanced Cd solubility and its speciation in a long-term biosolids amended soil, (2) to fit a modified biotic ligand model to Cd uptake by maize and tobacco from nutrient solution with variation in Cd2+ activity both with and without the presence of Cd–chloride complexes to enable the independent fitting of ion reaction constants, and (3) to predict and discuss the Cl−-enhanced uptake of Cd by two common crops: maize and tobacco under “normal” and “very strongly saline” scenarios using a modified BLM using datasets from both soil and hydroponic trials.
Materials and methods
Cadmium solubility trial in soil
Topsoil (pH = 5.9) from 0 to 30 cm horizon was collected from a non-vegetated flat field site in a sewage disposal farm run by a large water company in the UK. The farm grows maize for ensiling, which serve as fodder for dairy cattle. Soil samples were sieved to <4 mm and total metal contents were determined by Flame-AAS (Varian SpectrAA 200FS, Australia) following aqua regia digestion. Polystyrene cups were filled with 500 g of air-dried soil (<4 mm) and NaCl solution was then added at rates which were sufficient to produce nearly 50, 100, 150, 200, and 300 mM of NaCl in the soil pore water at water holding capacity (WHC = 250 mL) using deionized water. Porous Rhizon soil moisture samplers (Rhizosphere Research Products. Wageningen, The Netherlands) were inserted at an angle of 45° in the soil and soil solution was extracted for 48 h by applying negative pressure (0.07 MPa), following a stabilization period of 24 h to allow any cation sorption sites on the Rhizon samplers to equilibrate with the soil pore water. A NaCl-free control treatment (soil + deionized water at WHC) was included for the experiment.
Modeling Cd speciation in soil solution
All data obtained from the chemical analysis of the soil pore water were used to speciate Cd using the WHAM-VI speciation model (Tipping et al. 2002). The model was implemented to include Fe(III) competition for binding sites on fulvic acid (FA), by assuming that Fe(OH)3 controls the activity of Fe3+ ions (Tipping et al. 2002). The value of (Fe3+) was computed from the solubility product (K so) of Fe(OH)3 and its standard reaction enthalpy (Eqs. 1, 2)
at 298 K, log10 K so° = 2.7 and the standard reaction enthalpy ΔH° = −25,000 cal mol−1 (S. Lofts, pers. comm.). In addition, it was assumed that 50% DOC was colloidal FA containing 50% C as suggested by Tipping (pers. comm.), temperature was set to 298 K (25°C) and PCO2 was set to the standard atmospheric pressure 0.0003 (atm). Output from WHAM-VI included the activities of the free Cd2+ ion, as well as Cd–chloro and Cd–FA (i.e., Cd-organic) complexes in soil pore water.
Hydroponic trials
Seeds of Zea mays var. Cameron and Nicotiana tabacum var. K326 were germinated in perlite and peat, respectively, and irrigated with a complete nutrient solution (pH = 4.9) containing: (1) macronutrients KNO3 10 mM, KH2PO4 2 mM, MgSO4·7H2O 4 mM, Ca(NO3)2·4H2O 13 mM and (2) micronutrients H3BO3 92 μM, MnCl2·4H2O 18.3 μM, ZnSO4·7H2O 1.53 μM, CuSO4·5H2O 640nM, (NH4)6Mo7O24·4H2O 30nM, FeSO4·7H2O, and Na2EDTA 40 μM. After 2 and 7 weeks, respectively, maize and tobacco seedlings selected for homogeneity were transplanted and grown for 3 (maize) and 1 (tobacco) extra weeks in a 27.5-L hydroponic tray using aerated complete nutrient solution. The complete nutrient solution was replaced with a macronutrient solution (stock 1, pH = 5.1) 2 weeks before transplanting maize plants to 800 mL treatment cups filled with aerated macronutrient solution. This intermediate phase was necessary to avoid possible confounding effects such as competition from micronutrients present in the treatments (Lombi et al. 2001). Tobacco plants were transplanted to 800 mL filled with 12.5 mM Ca(NO3)2. This basic version of the nutrient solution was used to simplify Cd speciation in the nutrient solution with respect to the maize trial. Cadmium was added as Cd(NO3)2·4H2O and Cl− as NaCl. One plant was transplanted per pot, and treatments were applied at the same time (Table 1). These treatments were chosen to be at concentrations commonly found in salt-affected irrigation water used for the production of semi salt-tolerant crops (Khoshgoftar et al. 2004) and principally, to give a range of Cd2+ activities at each Cl− level as predicted from speciation modeling using the program ‘WHAM-VI’ (Tipping et al. 2002), to provide an accurate basis for determining the uptake of Cd–chloride complex forms. In this way it was hoped to determine unequivocal uptake rates for Cd2+ and CdCl+.
Treatment solutions were replaced after an initial 8 h to allow for Cd depletion caused by a rapid approach to a pseudo-equilibrium state between root surface sorption sites (and onto the experimental pots internal surface) and the treatment solutions (López-Chuken et al. 2010b). Maize and tobacco hydroponic trials did not start simultaneously due to the differences in the growing rates between the two plant species.
Throughout the trial, culture containers were topped up with deionized water on a daily basis. In order to maintain constant Cd concentrations in the treated solutions throughout the exposure time of the plants, samples of 5 mL were taken from the treatment cups at intervals of 4, 8, and 12 days after treatment (DAT) for the maize trial and 4, 6, 11, and 12 DAT for the tobacco trial. Following analysis, Cd concentrations in the nutrient solutions were immediately buffered by adding aliquots from a Cd stock solution to return the treatment cups to the original concentrations. Using this method, the Cd concentrations in solution during the maize and tobacco trials showed only a slight variation of ±3.83 and ±4.23%, respectively, compared to the original Cd concentrations shown in Table 1, still, there was a strong correlation between the time-averaged values and the original concentrations (R = 0.99).
Twelve DAT, plants were harvested. Shoots and intact root systems were washed thoroughly with cold deionized water and excess water removed (blotted) before analyses. The trial was conducted under glasshouse controlled conditions (16 h, 26°C/8 h, 24°C during day/night, respectively).
Solution and plant analyses
After solution sampling, routine pH measurements were determined using a combined glass-AgCl electrode (Jenway Ltd. 3010 pH Meter, UK). Total organic carbon (TOC) and total inorganic carbon (TIC) were determined with a ‘Total Organic Carbon Analyser’ (TOC-V CPH/CPN; Shimadzu Corp. Japan). In soil pore water, cations (Na+, Mg2+, K+, Ca2+, and Zn2+) were determined by F-AAS (Varian SpectrAA 200FS, Australia) and anions (Cl−, SO4 2−, NO3 −, and PO4 3−) were measured by ion chromatography (Dionex, CA, USA). Anion (SO4 2−, Cl−, NO3 −, and PO4 3−) and cation (Mg2+, K+, Ca2+, and Na+) concentrations in the hydroponic solutions were assumed to be constant throughout the trial due to their high initial concentrations. Cadmium concentrations in solution were analyzed by Flame-AAS.
After plants were harvested (12 DAT), intact root systems were washed thoroughly with deionized water. The roots were then kept in a solution of H2O2 (1.5%, 10 mL) with ultra-pure water at <4°C to avoid biological contamination prior to scanning for morphological characteristics [e.g., root surface area (RSA)] using WinRIZHO™ (Regent Instruments Inc, Quebec, Canada), a scanner-based image analysis system. Fresh and dry biomass was determined. Plant material was finely milled prior to digestion in hot concentrated HNO3 and analysis of Cd concentrations by Flame-AAS.
Modeling Cd uptake with a “biotic ligand model (BLM)”
Cadmium uptake by plants as a function of free and Cl−-complexed ions in soil pore water was modeled using a form of free ion activity model sometimes called the ‘biotic ligand model’ (BLM). The present research used a model parameterization based on the one described in detail by López-Chuken et al. (2010a, b). This parameterization of the model assumed initial sorption of free metal ions (M2+), or defined metal complex species (e.g., MCl+), from solution onto hypothetical plant root sorption sites also considering competition between cations and protons for sorption sites (Datta and Young 2005).
The nutrient solution experiments were specially designed to implement the BLM to separately model Cd uptake by plants as a function of the free ion Cd2+ and the CdCl+ complexes as modeled by WHAM (VI). In this way, the Cd uptake constants for the free ion, Cd2+, (using Cd uptake data from Cl−-free treatments), and subsequently of the CdCl+ complex (using data from the 40 and 80 mM Cl− treatments) could be independently determined. This approach was intended to enable the independent fitting of ion reaction constants. The best-fit formulation used in the present research is shown in Eq. (3)
where \( K_{{{\text{Cd}}^{{ 2 { + }}} }} \) and \( K_{{{\text{CdCl}}^{ + } }} \) are the absorption reactions for the (Cd2+) and (CdCl+), respectively, and ‘K t R t ’ is a proportionality constant which expresses the assumption that metal concentrations in plant shoots reflects the concentration of metal ions adsorbed on root sites integrated over the growing time of the plant.
Statistical analysis and data quality control
All treatments were replicated threefold in a randomized block design. Soil and plant variables were analyzed using ANOVA. Comparisons of mean values were based on their LSD. A standard reference material (1573a tomato leaves; NIST, Gaithersburg, MD, USA), containing certified concentrations of Cd (1.52 mg kg−1 ± 3%) was used to ensure the quality of the data. This quality standard averaged 1.48 ± SE 0.03 (n = 8) mg kg−1 over the whole trial. For all analyses, blanks and known standard samples were analyzed to ensure consistency.
Results and discussion
Soil experiment
Cadmium solubility and speciation in soil pore water
The large values for loss on ignition (LOI = 24%) and Cd total concentration (58 mg kg−1) of the soil used reveals that this is one of the most heavily amended areas on the sewage disposal site. Chloride addition rates increased nearly proportionally to the Cd solubility in soil (p < 0.001) (Table 2). Background concentrations of Na+ and Cl− in soil were within a ‘non-saline’ range (Abrol et al. 1988) (in mM): 1.34 and 1.37, respectively. It has to be noted that, however, these solubility results were taken as an approximate reference and are unlikely to fully represent conditions in plant growth studies. It has been shown that soil pore water representing the rhizosphere should be preferably obtained from pots with plants, since factors such as pH and TOC (Hamon et al. 1998), and hence the concentration of metals in the soil solution, are significantly affected by the presence of root systems. The degree of solubility and phytoavailability of Cd in soils is affected by numerous other factors including: plant species (root exudates) (Kim et al. 2010), clay (Krishnamurti and Naidu 2000), fertilizer practices (Grant and Sheppard 2008), microbial activity (Jézéquel et al. 2005), and temperature (Moreno et al. 2002).
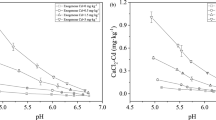
Table 2 shows the distribution of inorganic Cd species present in soil solution. Organic Cd-complexes as affected by the TOC in solution did not show a large variation under the experimental treatment (75.5 ± 13.3 nM, n = 15). Only Cd2+, CdCl+, CdCl 02 , and CdSO 04 species activity were included in the tabulated data as they were the inorganic species that predominated in solution (≈99%) as calculated by WHAM (VI). Cadmium complexation with Cl− ions was enhanced as the respective salt treatment concentration was increased (Table 2). It is noted that the Cd speciation in soil solution shown in Table 2 predicted almost no changes in the activities of the free ion Cd2+ [35.5 nM ± 0.95 (p > 0.05)] despite the wide range of NaCl concentrations in soil solution (50–300 mM in soil). Therefore, a possible cation exchange (Na+ for Cd2+) could not completely explain the differences in Cd content between the Cl− treatments because of the high buffering Cd2+ capacity in soil.
Hydroponic trial
Plant biomass and root morphology
The treatments had no effect (p > 0.05) on the biomass and RSA of both plants, except for maize dry root weight and tobacco fresh root weight between Cl− treatments (Table 3). For both hydroponic experiments, the RSA was included as a means of expressing Cd uptake by plants. It was shown in a previous hydroponic trial that the systematic variation between a dataset is decreased when including this factor (López-Chuken and Young 2010). Thus, Cd uptake in plants was expressed as μmol m−2 RSA.
Plant cadmium concentrations
Table 4 shows that Cd uptake by maize and tobacco plants was not significantly affected by the experimental treatments (p < 0.05), except for the Cd accumulation in roots (expressed as μg of Cd) of both plants. This may indicate that, despite the very low concentrations of Cd selected, the plants were close to the limit of their Cd uptake rate (i.e., the root sorption sites for CdCl+ complexes were saturated). This suggests that after this assumed saturation point, CdCl+ complexes are possibly not being taken up with the same efficiency as the free ion Cd2+ (Boukhars et al. 2000; López-Chuken et al. 2010b).
In general, Cd uptake by both plant species was consistently better explained by the activity of the Cd–Cl−-complexes in solution (R = 0.56 and 0.39, respectively) as compared with the activity of the free ion Cd2+ (R = 0.43 and 0.34, respectively). Several mechanisms have been proposed to explain chloride-enhanced Cd uptake by plants. These mechanisms include: (1) the increase in Cd2+ free ion activity following cation exchange with the salt cation (Bingham et al. 1984), (2) reduced diffusion distances to root surfaces caused by a general increase in Cd concentration in the soil pore water (Smolders et al. 1998), and (3) direct uptake of intact \( {\text{CdCl}}_{n}^{2 - n} \) complexes (Smolders and McLaughlin 1996). It is also possible that more than one mechanism may influence Cd uptake (López-Chuken et al. 2010a). A recent hydroponic trial at constant Cd2+ activity provided strong evidence of uptake of CdCl+ complexes by Brassica juncea [mechanism (3)] in a system in which the possible effect of mechanism (2) was avoided because diffusion was not limiting the uptake of the free ion Cd2+ due to the non-stop circulation of the nutrient solution (López-Chuken et al. 2010b).
The best correlations between Cd species in solution and the Cd uptake by maize plants were achieved by expressing Cd uptake including RSA measurements (i.e., Cd in plant μmol m−2 root) (data not shown). Similarly, Berkelaar and Hale (2000) found that the differences between Cd accumulation in two cultivars of wheat were reduced when expressing Cd uptake as μg cm−2 RSA. Although a previous research showed evidence of the phytoavailability of CdSO 04 complexes using the same maize variety as the present trial (López-Chuken and Young 2010), for the present experiment, due to the small proportion of Cd–sulfate complexes present in solution, it is unlikely that this ion had a substantial effect on Cd uptake by maize plants.
Implications of chloride-enhanced Cd uptake in (saline) agriculture
Saline agriculture
The FAO/World Health Organization (FAO 2007) has set a concentration limit of 0.2 mg Cd kg−1 for vegetables for human consumption. López-Chuken and Young (2005) and López-Chuken et al. (2010a) showed examples of the association between increased Cd content of edible crops (e.g., maize, alfalfa, Indian mustard, and sunflower) and chloride salinity in soils. This effect has been originally reported for potato tubers (McLaughlin et al. 1997), sunflower kernels (Li et al. 1994), and wheat grain (Norvell et al. 2000) in field studies with irrigation containing Cl− (11–34 mM) and with phosphate fertilizers being the major Cd inputs to these soils. The results of those studies suggest that the enhancement of Cd uptake in the presence of Cl− salinity may be a general trend that occurs in many edible crops. However, Cd internal distribution and concentrations in different plant organs is highly species-specific (Ishikawa et al. 2005). Only tolerant crops (hardly any conventional crops) can be successfully produced with waters that exceed about 10 dS m−1 (i.e., 100 mM NaCl) (Abrol et al. 1988).
A preliminary pot trial growing tobacco var. K326 plants during 8 weeks in long-term biosolids amended soil (58 mg kg−1), showed considerable Cd phytoaccumulation in leaves (25 ± 2.4 mg kg−1, dry weight) (López-Chuken et al. unpublished data). Therefore, growing tobacco plants in metal enriched soil may represent an increased risk for smokers’ health since the concentrations found in this trial were considerably greater than the Cd concentrations commonly found in tobacco leaves from agriculture production fields (0.1–6.8 mg kg−1) (Lugon-Moulin et al. 2006).
Predictions on Cd uptake by maize and tobacco plants under simulated saline irrigation
A prime objective of this paper was to test the viability of predicting the uptake of free and complexed Cd species by two common crops grown in simulated Cd-containing soil and saline irrigation using different forms of the BLM. To do this, it was attempted to relate the Cd2+ and CdCl+ uptake constants set by the best-fit BLM parameterisation (Table 5) obtained from the hydroponic experiments to a hypothetical case of extreme salinity in a simulated ‘soil’ (using the Cd speciation dataset from the soil solubility trial). The term salinity refers to the total dissolved concentration of major inorganic ions (i.e., Na+, Ca2+, Mg2+, K+, HCO3, SO4 2−, and Cl−). However, for this hypothetical case, it is assumed that salinity in soil is entirely due to the presence of NaCl.
The BLM suggests that the metal ion activity (M2+) is the best predictor for metal bioavailability. However, this commonly accepted hypothesis has been under scrutiny (López-Chuken et al. 2010a, b). Complications with the BLM arise when M+-ligand complexes are predominant in solution (Berkelaar and Hale 2003; López-Chuken et al. 2010a, b) because mechanisms of possible uptake of M+-complexes by plants are little understood. In the present research, most of the inorganic Cd in soil solution was in the form of Cl− (up to 88.6%) complexes for the Cl− (Table 2). There is growing evidence that the activity of chloro complexes in soil solution must be considered as they have been reported to affect plant Cd uptake from soil solution (Ghallab and Usman 2007; López-Chuken et al. 2010a).
The best-fit BLM predicting the uptake of Cd by maize and tobacco plants was parameterized expressing Cd uptake in the whole plants as μmol m−2 root. Regression coefficients and constants which is used to parameterize the best-fit BLM for maize and tobacco are shown in Table 5, when BLM parameterization assumed two single root sorption sites [without competition between the activities of the divalent (Cd2+) and monovalent Cd ions (CdCl+), respectively] and a single transfer constant from root to shoots (Eq. 3).
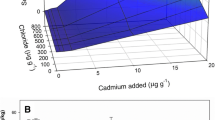
Figures 1 and 2 show the predictions for Cd uptake by tobacco and maize plants under simulated extreme soil salinity conditions. The soil salinity assumed values were: ‘non-saline’ (1 mM) and ‘very strongly saline’ (200 mM), according to the criteria of the Food and Agriculture Organization of the United Nations (Abrol et al. 1988). The free ion Cd2+ activities were selected to include the values shown for the soil solution speciation data [Table 2, (in M) 3.35 × 10−9 (p(Cd2+) = 8.5) to 3.88 × 10−8 (p(Cd2+) = 7.4)] and more widely to be within a range of values (in M) 5.00 × 10−9 (p(Cd2+) = 8.3) to 3.55 × 10−5 (p(Cd2+) = 4.5) commonly found in soils (n = 177) as described by Tye et al. (2003). These simulated conditions did not take into consideration potential physiological stress to plants due to increased salinity and/or Cd concentrations.
The predicted Cd uptake rates by maize plants (Fig. 1) were consistently higher (54%) assuming conditions of ‘very strong salinity’ soil compared to the simulated ‘non-saline’ soil. According to the BLM, this increase should be explained entirely due to the uptake of CdCl+ complexes. In a recent study carried out under real soil conditions at similar Cl− (200 mM) and Cd2+ (p(Cd2+) = 7.0) concentrations using maize plants, López-Chuken et al. (2010a) predicted the Cd uptake rate (μg) for the 200 mM treatment to be 43.6% higher than the zero chloride treatment due to CdCl+ uptake [mechanism (3)]. However, measured Cd uptake (μg) from the same study showed an increase of 123%. Therefore, for that case, the observed difference between modeled and measured Cd uptake could be attributed to mechanism (2) (i.e., CdCl+ acting as a buffer for Cd2+ in soils near the root surface).
On the other hand, it is noted that even when comparing extreme soil salinity conditions, only little increase in the predicted Cd uptake rates by tobacco (≈15%) is observed (Fig. 2). This is according to the trend observed in Table 4, where apparently CdCl+ complexes appeared to saturate their root sorption sites starting at low activities and, therefore, CdCl+ activities greater than this saturation level do not cause any increase in Cd content by tobacco plants. Furthermore, this assumption is supported by the little variation on Cd accumulation rates by tobacco and the drastic decreases in the Cd transfer factor (Table 4) despite the variable total Cd concentration and Cd–Cl− complexes activities present in nutrient solution as the Cl− treatments increased (Table 1).
Bearing in mind the previous information and the anthropogenic Cd inputs to agricultural soils, there is clearly some potential for risk due to increased Cd uptake by crops under saline conditions. The BLM parameterization applied in the present study attempted to simulate conditions commonly found in natural Cd and salt-affected soils. However, caution is needed to extrapolate results obtained from these models to real soil conditions.
Conclusion
-
1.
Cadmium uptake by maize and tobacco plants was generally unaffected by the experimental treatments. This suggests that after an assumed saturation point, Cd–Cl−-complexes are possibly not being taken up with the same efficiency as the free ion Cd2+ and, therefore, CdCl+ activities greater than this saturation level do not cause any increase in Cd content by plants.
-
2.
According to the BLM parameterization used in the present study, the predicted Cd uptake rates (Cd in plant μmol m−2 root) by maize and tobacco plants were consistently higher (54 and 15%, respectively) assuming conditions of ‘very strong salinity’ soil (200 mM) as compared to the simulated ‘non-saline’ soil (1 mM). RSA was found to be an important source of variation of Cd accumulation by the experimental plants used.
-
3.
In view of the results of the present research, there is evidently some potential for risk due to increased Cd uptake by crops under saline agriculture, especially as the enhancement of Cd uptake in the presence of Cl- salinity may be a general trend that occurs in numerous edible crops. While the present study attempted to simulate conditions commonly found in natural Cd and salt-affected soils, caution is needed to extrapolate results obtained from these models to real soil conditions.
References
Abrol IP, Yadav JSP, Massoud FI (1988) Salt-affected soils and their management, FAO Soil Bull. 39. Food and Agriculture Organization of the United Nations, Rome
Bäckström M, Karlsson S, Bäckman L, Folkeson L, Lind B (2004) Mobilisation of heavy metals by deicing salts in a roadside environment. Water Res 38:720–732
Berkelaar E, Hale B (2000) The relationship between root morphology and cadmium accumulation in seedlings of two durum wheat cultivars. Can J Bot 78:381–387
Berkelaar E, Hale B (2003) Cadmium accumulation by durum wheat roots in ligand buffered hydroponic culture: uptake of Cd ligand complexes or enhanced diffusion? Can J Bot 81:755–763
Bingham FT, Sposito G, Strong JE (1984) The effect of chloride on the availability of cadmium. J Environ Qual 13:71–74
Boukhars L, Rada A, Yatribi A (2000) Removal of cadmium coming from urban sludges by peas cultivated in hydroponics with increasing concentrations of chloride. Agrochimica 44:211–220
Clark RB (2001) Marine pollution, 5th edn. Oxford University Press, Oxford, UK, pp 109–112
Datta SP, Young SD (2005) Predicting metal uptake and risk to the human food chain from leaf vegetables grown on soils amended by long-term application of sewage sludge. Water Air Soil Pollut 163:119–136
de Vries W, Römkens PFAM, Schütze G (2007) Critical soil concentrations of cadmium, lead, and mercury in view of health effects on humans and animals. Rev Environ Contam Toxicol 191:91–130
FAO (2006) Land and Plant Nutrition Management Service. FAO, Rome. http://www.fao.org/nr/land/en/. Accessed 20 Nov 2011
FAO/WHO Food and Agriculture Organization and World Health Organization Food Standards (2007) Codex Alimentarius Commission. FAO, Rome. http://www.codexalimentarius.net/download/report/671/al30_13e.pdf. Accessed 20 Nov 2011
Ghallab A, Usman ARA (2007) Effect of sodium chloride-induced salinity on phyto-availability and speciation of Cd in soil solution. Water Air Soil Pollut 185:43–51
Grant CA, Sheppard SC (2008) Fertilizer impacts on cadmium availability in agricultural soils and crops. Hum Ecol Risk Assess 14:210–228
Hahne HC, Kroontje W (1973) Significance of pH and chloride concentration on behavior of heavy metal pollutants: mercury(II), cadmium(II), zinc(II), and lead(II). J Environ Qual 2:444–450
Hamon RE, McLaughlin MJ, Naidu R, Correll R (1998) Long-term changes in cadmium bioavailability in soil. Environ Sci Technol 32:3699–3703
Iretskaya SN, Chien SH, Menon RG (1998) Effect of acidulation of high cadmium containing phosphate rocks on cadmium uptake by upland rice. Plant Soil 201:183–188
Ishikawa S, Ae N, Sugiyama M, Murakami M, Arao T (2005) Genotypic variation in shoot cadmium concentration in rice and soybean in soils with different levels of cadmium contamination. Soil Sci Plant Nutr 51:101–108
Jézéquel K, Perrin J, Lebeau T (2005) Bioaugmentation with a Bacillus sp. to reduce the phytoavailable Cd of an agricultural soil: comparison of free and immobilized microbial inocula. Chemosphere 59:1323–1331
Kazi TG, Jalbani N, Arain MB, Jamali MK, Afridi HI, Shah AQ (2009) Determination of toxic elements in different brands of cigarette by atomic absorption spectrometry using ultrasonic assisted acid digestion. Environ Monit Assess 154:155–167
Khoshgoftar AH, Shariatmadari H, Karimian N, Kalbasi M, van der Zee SEATM, Parker DR (2004) Salinity and zinc application effects on phytoavailability of cadmium and zinc. Soil Sci Soc Am J 68:1885–1889
Kim S, Lim H, Lee I (2010) Enhanced heavy metal phytoextraction by Echinochloa crus-galli using root exudates. J Biosci Bioeng 109:47–50
Krishnamurti GSR, Naidu R (2000) Speciation and phytoavailability of cadmium in selected surface soils of South Australia. Austr J Soil Res 38:991–1004
Li Y-M, Chaney L, Schneiter AA (1994) Effect of soil chloride level on cadmium concentration in sunflower kernels. Plant Soil 167:275–280
Lombi E, Zhao FJ, McGrath SP, Young SD, Sacchi GA (2001) Physiological evidence for a high-affinity cadmium transporter highly expressed in a Thlaspi caerulescens ecotype. New Phytol 149:53–60
López-Chuken UJ, Young SD (2005) Plant screening of halophyte species for cadmium phytoremediation. Z Naturforsch 60:236–243
López-Chuken UJ, Young SD (2010) Modelling sulphate-enhanced cadmium uptake by Zea mays from nutrient solution under conditions of constant free Cd2+ ion activity. J Environ Sci 22:1080–1085
López-Chuken UJ, Young SD, Sánchez-González MN (2010a) The use of chloro-complexation to enhance Cd uptake by Zea mays and Brassica juncea: evaluating a ‘free ion activity model’ and implications for phytoremediation. Int J Phytoremediation 12:680–696
López-Chuken UJ, Young SD, Guzmán-Mar JL (2010b) Evaluating a ‘biotic ligand model’ applied to chloride-enhanced Cd uptake by Brassica juncea from nutrient solution at constant Cd2+ activity. Environ Technol 31:307–318
Lugon-Moulin N, Martin F, Krauss MR, Ramey PB, Rossi L (2006) Cadmium concentration in tobacco (Nicotiana tabacum L.) from different countries and its relationship with other elements. Chemosphere 63:1074–1086
Macek T, Surá M, Pavliková D, Francová K, Scouten WJ, Szekeres M, Sylvestre M, Macková M (2005) Can tobacco have a potentially beneficial effect to our health? Z Naturforsch 60:292–299
McBride MB (2003) Toxic metals in sewage sludge-amended soils: has promotion of beneficial use discounted the risks? Adv Environ Res 8:5–19
McLaughlin MJ, Tiller KG, Smart MK (1997) Speciation of cadmium in soil solutions of saline/sodic soils and relationships with cadmium concentrations in potato tubers (Solanum tuberosum L.). Aust J Soil Res 35:183–198
Moreno DA, Villora G, Hernández J, Castilla N, Romero L (2002) Accumulation of Zn, Cd, Cu, and Pb in Chinese cabbage as influenced by climatic conditions under protected cultivation. J Agric Food Chem 50:1964–1969
Norvell WA, Wu J, Hopkins DG, Welch RM (2000) Association of cadmium in durum wheat grain with soil chloride and chelate-extractable soil cadmium. Soil Sci Soc Am J 64:2162–2168
Qadir M, Oster JD (2004) Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci Total Environ 323:1–19
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57:1017–1023
Smolders E, McLaughlin MJ (1996) Chloride increases Cd uptake in Swiss chard in a resin-buffered nutrient solution. Soil Sci Soc Am J 60:1443–1447
Smolders E, Lambregts RM, McLaughlin MJ, Tiller KG (1998) Effect of soil solution chloride on cadmium availability to Swiss chard. J Environ Qual 27:426–431
Tipping E, Rey-Castro C, Bryan SE, Hamilton-Taylor J (2002) Al(III) and Fe(III) binding by humic substances in freshwaters, and implications for trace metal speciation. Geochim Cosmochim Acta 66:3211–3224
Tye AM, Young SD, Crout NMJ, Zhang H, Preston S, Barbosa-Jefferson VL, Davison W, McGrath SP, Paton GI, Kilham K, Resende L (2003) Predicting the activity of Cd2+ and Zn2+ in soil pore water from the radio-labile metal fraction. Geochim Cosmochim Acta 67:375–385
Weggler K, McLaughlin MJ, Graham RD (2004) Effect of chloride in soil solution on the plant availability of biosolid-borne cadmium. J Environ Qual 33:496–504
WHO World Health Organization, IPCS (International Programme on Chemical Safety) (1992) Cadmium. Environmental Health Criteria 134, Geneva. http://www.inchem.org/documents/ehc/ehc/ehc134.htm
Acknowledgments
U. J. López-Chuken gratefully acknowledges financial support from CONACYT, Mexico (No 137972).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Chuken, U.J., López-Domínguez, U., Parra-Saldivar, R. et al. Implications of chloride-enhanced cadmium uptake in saline agriculture: modeling cadmium uptake by maize and tobacco. Int. J. Environ. Sci. Technol. 9, 69–77 (2012). https://doi.org/10.1007/s13762-011-0018-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-011-0018-2