Abstract
Pot cultivation experiments were conducted to assess the phytoremediation potential of two local agronomic plants, namely Avena sativa and Vicia sativa. Several soils with long-standing contamination and different levels of Polychlorinated biphenyl (PCB) contamination were used for this study. The soil samples came from different regions of Algeria and had different physico-chemical parameters. We studied the influence of these parameters on remediation potential of the two tested plants. The removal rate of the seven PCBs (PCB 28, 52, 101, 138, 153, 156 and 180) was examined after 40 and 90 days. The results showed that the presence of the plants reduced significantly the overall PCB content, ranging initially from 1.33–127.9 mg kg1. After 90 days, the forage plant Vicia sativa allowed us to reach an excess dissipation rate of 56.7% compared to the unplanted control for the most polluted soil. An average dissipation rate of 50% was obtained in the moderately polluted soil. The less contaminated soil had an excess dissipation rate of about 24% for both plants and a predominant dissipation of the low chlorinated PCBs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs), classified as priority persistent organic pollutants (POPs), are a class of synthetic chemicals (Rein et al., 2007; Passatore et al., 2014) whose toxicity to human health and ecosystems has been reported as early as 1964 (Bergman et al., 2013; Erickson & Kaley, 2011; Ross, 2004). These compounds appeared in the industrial world at the beginning of the nineteenth century (Green et al., 2001; Stratton & Sosebee, 1976). Banned by the European community since 1976, PCBs were still used and they remain present in some parts of the world (Eckley & Selin, 2004). In Algeria, PCBs were banned only from 1987 by application of decree 87–182 of 18 August 1987. They had been widely used as a dielectric liquid in electrical transformers (Erickson & Kaley, 2011; Kidd et al., 1998; Ross, 2004) and capacitors in the form of Askarel oil. Askarel oil contains Aroclor with a high content of highly chlorinated PCBs. These PCBs spread from industrial areas to urban and agricultural areas (Fouial-Djebbar et al., 2010; Halfadji et al., 2017). The current contamination is the result of leaks from PCB–containing capacitors and electrical transformers but also of the incineration of this type of equipment (Glüge et al., 2017; Melnyk et al., 2015; Reddy et al., 2019). This contamination appears harmful to the environment and is a concern for human health as it can bioaccumulate in the food chain (Bergman et al., 2013; Tu et al., 2011; Wilkins, 1957). Due to their chemical stability, hydrophobicity and low biodegradability, this contamination is still present in Algeria, at different levels in soils, depending on the sources and activities in the contaminated area.
The elimination of this type of pollutant is therefore a major challenge. Most traditional techniques for remediating PCB—polluted soils often require costly operations (Gan et al., 2009). In addition, the risk of introducing toxic additives or transforming PCBs into potentially more polluting compounds is not excluded. Therefore, "greener" processes were considered. Among them, phytoremediation appears to be an attractive alternative for the in situ treatment of polluted soils by using plants for a possible immobilization, absorption and/or degradation of these pollutants (Abhilash et al., 2009; Macek et al., 2000). Several studies have investigated the efficiency of phytoremediation of PCBs by different plants including alfalfa, tall fescue, switch grass, and others (Chekol, et al., 2004; Li et al., 2013; Meggo et al., 2013; Tu et al., 2011; Wilkins, 1957). However, in situ phytoremediation with climate-adapted plants appears to be more effective. Very few studies address the possible phytoremediation of contaminated soils in North Africa. Among the plants adapted to the Algerian climate, we focused our attention on Vicia sativa that is a common vetch and, on the oat Avena sativa. These two Mediterranean agronomic plants are used as fodder crops, green manures, pastures, hay and cereals for livestock feed (Tuna & Orak, 2007), and they are capable of colonizing poor or degraded soils (Pajuelo et al., 2008). Although very few works were related to their capacity in terms of phytoremediation, it should be noted that Avena sativa has shown interesting potentialities to eliminate a high concentration of phenol (Youn et al., 2012). So, the purpose of the present study was to investigate for the first time the efficiency of Avena sativa and Vicia sativa plants for enhancing remediation of PCBs from weathered Algerian soils. Three different soils, with different levels of contamination, were tested in microcosms to follow the growth and the adaptation of the plants to the contamination level and to estimate their ability to improve the dissipation of a mixture of PCBs into the soil medium.
Materials and Methods
Chemicals
The mixture of 7 PCB congeners (PCB 28, 52, 101, 138, 153, 180, 209) at 100 mg L1, and PCB 156 were purchased from Supelco (Sigma-Aldrich, Saint-Quentin Fallavier, France). In Europe, PCBs 28, 52, 101, 118, 138, 153 and 180 have been defined as indicators for priority screening of PCBs in organic matrices. These indicators were chosen because of their persistence, their abundance in environment and their toxicological properties (Dargnat and Fisson 2010). We have replaced the dioxin-like PCB 118 by PCB 156 for health reasons. Physico–chemical properties of these PCBs are shown in Table S1 (Supplementary materials). Five commercial mixtures of PCBs (Aroclor 1242, 1248, 1254, 1260, 1262) were purchased from Sigma-Aldrich. The four-digit number assigned to these mixtures give information about their structure. The two first digits, i.e., 12, indicate the number of carbons in the biphenyl ring while the two last digits indicate the weight percent of chlorine. Phenanthrene–D10 used as an internal standard and 7–methylbenzopyrene used as a surrogate were obtained from Supelco. Dichloromethane, n–hexane and acetone were of HPLC–grade (purity 99.8%) and were provided by Fisher Scientific (Illkirch, France).
Soil collection and preparation
Soil samples from Algeria were collected from different sites in northern Algeria, as shown in mapping (Fig. 1): soil A was collected near an old PCB–containing transformers and capacitors storage area in an abandoned waste site of Tiaret, soil B originated from a capacitors and electronic storage site of Bouira and soil C was collected from an electronic wastes site of Chelf. In the three selected sites, the initial pollution took place many years ago. The surface soils were collected using a stainless-steel shovel to a depth of 0–25 cm. Altogether, for each selected site, 20 surface soil samples were sampled in different directions, at a distance of 5 m, and mixed to form a composite and representative sample of about 10 kg each. The soil samples were sieved at 2 mm and were packed in ziplock bags, and stored at 20 °C. These three soils Were chosen in relation to their expected pollution level according to their location. The three soils showed the same particle size distribution with a very fine fraction (< 20 μm) representing about 50% of the total dry mass. These soils are fine-textured silty–clay soils (Table 1). Soil B has the highest organic matter (SOM) and organic carbon (OC) content and the lowest pH. These characteristics differ from those corresponding to the two other soils (Table 1). Soil C is distinguished by its high limestone content.
Experimental setup
A three months pot-based laboratory growth trial harvested at 40 days (T1) and 90 days (T2) was conducted to evaluate the phytoremediation capacity of two agronomic plants (P1–Avena sativa and P2–Vicia sativa). Avena sativa (oats) is a cereal suitable for human consumption, but it is commonly used in livestock feed. Vicia sativa (common vetch) is generally considered as a weed, but it can also be grown as a fodder crop. Because of its ability to fix nitrogen, Vicia sativa is often used in combination with a cereal (rye, oats, triticale). It is a particularly water- and input–saving plant (Table S2). The seeds of these two plants came from the local region of Chelf (Algeria) where their culture is adapted to the climate and soils. The seeds were rehydrated during 24 h, and then germinated in cotton soaked in water for 10 days to obtain seedlings ready for sowing. We considered the beginning of our experiment at the planting time, noted T0. For the three soils (A, B, and C), six seedlings of each of the two plants were planted in six different pots (3 pots per time per plants) containing the equivalent of 600 g dry soil (Fig. 2). Three pots per soil, not planted, were also put under the same conditions and served as a reference to assess the dissipation capacity of PCBs by the soil microorganisms. Pots with or without plants were maintained at an average temperature of 21 ± 1 °C with a photoperiod of 10 h (night/light: 14 h/10 h). Soil moisture was maintained continuously by an addition of 3–5 mL of tap water depending of the water retention capacity of each soil sample (Table 1).
PCBs analysis in soils
PCBs were extracted from soil particles using microwave-assisted extraction (MAE) (Portet-Koltalo et al., 2008). Approximately 30 g of wet soil from each site were dried overnight at 35 °C and crushed. Briefly, three sub–samples (5 g) were weighed and transferred to PTFE–lined extraction vessels, to which 1.5°µL of the surrogate at 100 mg L1 and 40 mL of the 50/50 (v/v) n-hexane/acetone solvent mixture were added. The extraction was performed using MAE (MARS-X, CEM Corporation, Matthews, USA) (30 min at 130 °C, 1200 W). The extracts were filtered on a PTFE membrane (0.2 µm) purchased from Phenomenex (Le Pecq, France). After addition of 60 µL of octanol used as a solvent keeper, these extracts were reduced to a volume of about 1.5 mL by rotary evaporation at 45 °C (P = 550 mbar for acetone evaporation and 335 mbar for hexane), under a N2 gas stream. 10µL of per-deuterated phenanthrene Phe–D10 at 100 mg L−1 were added to 990µL of each liquid extract, and then 1µL of the resulting mixture was analyzed by gas chromatography (6850 series, Agilent, Santa Clara, USA) coupled with a mass detector (5975C series, Agilent). The GC–MS system was equipped with a DB5-MS capillary column (5% phenyl: 95% dimethylpolysiloxane, 60 m length × 0.25 mm i.d. × 0.25 µm film thickness, J&W Scientific, USA). Injection in splitless mode was carried out at 285 °C. Separation was performed using He as the carrier gas (1.4 mL min1) according to a temperature gradient: 60 °C for 1.2 min, then up to 190 °C (40 °C min1), then up to 300 °C (4 °C min1), and maintained at 300 °C for 10 min. Target compounds were identified using the Selective ion monitoring (SIM) mode and quantified by internal calibration (Table S3). Extraction recoveries tested in our laboratory, range from 84–107% for the seven PCBs with a relative standard derivation values (n = 5) below 10%. For PCBs, LODs ranged from 0.01–0.02 mg kg1 and LOQs ranged from 0.02 to 0.06 mg kg−1. The decrease in the concentration of the selected congeners was evaluated by calculating a total dissipation percentage TD (%) defined by the following equation:
CT0 is the concentration of PCBs found in the soil at the beginning of the study (T0). CTiPj is the concentration measured at a defined time Ti for a selected plant Pj (where i could be 1 or 2 associated to T, and j could be 0, 1 or 2 associated to P: P0 means unplanted soil, P1 corresponds to soil planted with Avena sativa and P2 to Vicia sativa). Similarly, it appears important to evaluate the actual effect of plant, i.e., without taking into account the contribution of soil microorganism from the unplanted soil P0. For this purpose, we calculated an excess dissipation ED (%) by means of the following relation:
where i = 1 or 2 and j = 1 or 2.
Statistical Methods
Statistical analyses were carried out using the Microsoft excel (Microsoft Inc. USA) software packages. Principal component analysis (PCA) and ANOVA were performed using R Core Team (2019). Factoshiny 2.1 package was used to carry out PCA. (R: A language and environment for statistical computing, foundation for statistical computing, Vienna, Austria. https;//www.R-project.org/).
Results and Discussion
Evaluation of soil pollution before planting
In this work, pollution was mainly due to PCBs. The concentration of the sum of the seven target PCBs (∑7PCBs) (PCB 28, 52, 101, 138, 153, 156, 180) varied according to the soil with respective levels of 127.9 ± 4.2 mg kg−1 (soil A), 15.2 ± 1.0 mg kg−1 (soil B) and 1.33 ± 0.01 mg kg−1 (soil C) (Table 2). As a function of the considered soil, PCB concentrations may vary by a factor ten. By comparing the contents of the three soils with each other, we referred to a low, moderate and high level of contamination for soils C, B and A, respectively. In addition, the comparison with studies of PCB soil contamination levels worldwide shows the consistency of the grades obtained according to the use of the sampling site (Table 3). Then, we compared the PCB profiles obtained in soils with Aroclor formulations by a principal component analysis (Cachada et al., 2009; Cetin, 2016; Jiang et al., 2011; Rachdawong & Christensen, 1997; Wang et al., 2011). Figure 3 shows the graph of variables. The two first dimensions explained 68.3% and 20.2% of the total variation. The correlation of soils B and C in this graph shows that their PCB profiles are similar and related to the Aroclor 1260 formulation (correlation coefficient r > 0.94 – p-value < 0.05), consistent with a high content of hexa and hepta-chlorinated biphenyls. The most contaminated soil A is more likely linked with Aroclor 1254 formulation. These results confirm the pollution from Askarel oils used in transformers and capacitors in Algeria.
Dissipation of the overall PCBs
The study took place from mid–March to early July. The plants developed well whatever the soil. Only a few leaves of plant 1, in the most polluted soil A, show yellowing after ninety days (Fig. 2). So, Avena sativa (P1) and Vicia sativa (P2) were particularly resistant to high levels of PCBs in soils that did not markedly impact on their aerial growth, even if, in the long term, Avena sativa could be more impacted by the toxic effect of the soil A. The concentration values of the individual PCBs and those corresponding to the sum of the 7 congeners are listed according to each soil, plants and time in Table 2. The mean concentrations were calculated, for each planted soil, from the 3 different pots per soil and per plant, for which two extractions were performed (n = 6). For unplanted soils, only one extract per pot was made (n = 3). Whatever the soils and the plants, we could note a greater variability in the measured concentrations for PCB 28 and PCB 52 containing 3 and 4 Cl, respectively. PCBs 28 and 52 are the most volatile compounds and they possess the lowest values of octanol–air partition coefficients (log Koa < 5) (Table S1). Consequently, this could explain their low initial concentrations and bias in measurements (Radonić et al., 2011). Furthermore, the dispersion of the results increased with time and with the presence of plants. This dispersion is more pronounced for the soils planted with the plant P2. The soil system must be considered as a relatively heterogeneous dynamic system involving a combination of multiple factors (Strek & Weber, 1982). The presence of plants complicates this system. In addition, plant 2 developed a network of small roots remaining on the surface. So, the distribution of PCBs in the planted pot containing this plant shows the highest heterogeneity. In addition, Zhou et al. pointed out that the measurement uncertainty is dominated by the sampling procedure (Zhou et al., 2014). Sampling becomes difficult when heterogeneity of the system increases, particularly when plant roots must be separated from the soil. Given the variability of our systems, the results were processed under ANOVA in order to clarify their difference. This treatment was carried out on the total concentrations of the 7 PCBs (Fig. 4). If the observed differences were significant, the dissipation percentages of the 7 PCBs were calculated in relation to their initial mean concentrations or in relation to the mean concentration at the same time in the unplanted soils (Fig. 5).
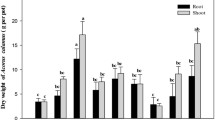
Box plot with error bars of the distribution of the total concentration of the 7 PCBS tested in each time and plant conditions. The bold line in the box indicates the median and the box refers to the 2nd and 3rd quartile. The 1st and 4th quartiles are indicated by the lines below and above each. The lines connect the conditions whose differences are significant and the degree of significance is indicated by the stars (p–value < 0.001(***) –p-value < 0.01(**) and p-value < 0.05 (*)). Circles indicate potential outliers. (a, b and c) refer to soils A, B, C, respectively
Dissipation percentages of the sum of the 7 PCBs at different times (T1, T2) for planted and unplanted soils (P0, P1and P2); calculated in relation to the mean initial concentrations (Dissipation percent) (a) or in relation to the mean concentration at the same time in unplanted soils (Excess Dissipation percent) for the three soils (b)
The total concentration in the unplanted soil A did not change significantly (Fig. 4a). In contrast, the plant effect was significant, with a decrease in PCB concentration when a plant was added to the soil A (p–value < 0.05). The effect of plant 2 was significantly greater (p–value < 0.05) than that of plant 1. The evolution over time did not seem significant in the planted soils and the average excess dissipation was (17.4 ± 22.5) % for P1 and (48.8 ± 23.4) % for P2. So, the vetch Vicia sativa, contributed more efficiently than the oat Avena sativa to the dissipation of PCBs in the highly polluted soil A. For soils with moderate or low contamination levels (soils B and C, respectively), the dissipation mechanism by plants went through a stage of "de–sequestration" of the PCBs and/or transformation of the PCBs which led, after 40 days, to a low increase in the overall concentration of the monitored PCBs compared to the unplanted soil. The transformation of PCBs or the increase of their bioavailability by the root system of plants is to be considered as well as the influence of root exudates on the soil itself, which could likely reactivate the soil microorganisms. Mechanical aeration of the soil by sampling, sieving and potting could also partly contribute to the reactivation of endogenous soil micro–organisms and could explain the dissipation of unplanted soil B (Ma et al., 2015; Pino et al., 2019). These trends can be seen in the soil B (Fig. 4b). In this soil, dissipation by plant 1 was low while the time effect was probably the main factor involved in the degradation process. Soil C exhibited an intermediate behavior between soil A and soil B due to its similarity with the physico-chemical parameters of soil A (Table 1), and its low contaminant content as soil B. Also, there was a tendency for the total concentration of the monitored PCBs to increase over time, for the unplanted soil and for plant 1 at T1 (Fig. 4c). After 90 days, whatever the considered plant, the dissipation was significant (p < 0.05) and an excess dissipation of 30% in average was observed. It could be noted that dissipation by plant 2 began in the first 40 days unlike plant 1 (Fig. 5a and b).
According to their characteristics, the dissipation of organic compounds in soils can follow different processes:
-
(i)
Volatilization in the atmosphere.
-
(ii)
Aqueous leaching.
-
(iii)
Biodegradation by soil microorganisms.
-
(iv)
Absorption and accumulation in plant tissues.
-
(v)
Transformation by plant enzymes (Leroy et al., 2015; Reddy et al., 2019).
The dissipation processes of PCBs are influenced by the age of the pollution, the physico–chemical properties of PCBs and the physico–chemical and microbiological characteristics of the soil. In the case of long-standing pollution, and in soils with very high amounts of fine particles associated with consistent amounts of Soil organic matter (SOM) or organic carbon (OC), it is generally expected that the strong sorption of hydrophobic molecules such as PCBs make them resistant to biodegradation and to aqueous leaching from soils (Passatore et al., 2014; Terzaghi et al., 2018). Considering aqueous leaching as a minor dissipation factor in our silty–clayey soils, the two main dissipation factors were, firstly, biodegradation by soil microorganisms, and secondly, phytoremediation by plants. It has been shown by many authors (Li et al., 2013; Pino et al., 2019) that phytoextraction was relatively weak in the case of PCBs and that rhizodegradation was predominant. The rhizosphere effect is generally characterized by an increase in quality and quantity of the bacterial community. The recent work of Terzaghi (2019) suggests that this effect should be independent of the plant species but rather related to their stage of development. In this study, we evidenced the contribution of plants to the dissipation of PCB in soils A and C. The soil–plant interaction through root exudates may act as an inducer of the biphenyl dioxygenase pathway (Toussaint et al., 2012) or as a biostimulator (Passatore et al., 2014). Then, it results in a higher number of bacteria and an enrichment of PCB degraders. The vetch Vicia sativa, contributed more efficiently than the oat Avena sativa to the dissipation of PCBs in the highly polluted soil. However, the observed plant effect could be due to a slower growth of the plant 1, given the short duration of the experiment (3 months). For soil B, the soil–plant interaction probably increased hydrolytic activity, leading to an increased production of dissolved organic carbon that may increase the bioavailability of PCBs (Fig. 5) (Terzaghi et al., 2019). Indeed, this soil has the highest SOM and OC contents, and the higher amount of nitrogen and the less alkaline pH (Table 1). The density and the diversity of the microorganism population depend on soil pH and on the amount and nature of organic matter (Crampon et al., 2014, 2018). So, the role of OC needs to be clarified for assessing microbial degradation in soils A, B and C. Tremolada et al. (2012) showed that it is very important to know the organic matter composition and the humin content (SOM–insoluble fraction) for a better assessment of POPs accumulation or dissipation in soils. Besides Wang et al. (2019) showed no significant relationship between PCB degradation and organic carbon or clay contents in soils. It can be hypothesized that, unlike soils A and C, the characteristics of soil B, namely a high SOM and OC content and a clay texture, did not allow the enrichment of degradative populations within the rhizosphere bacterial community in the presence of the tested plants.
Dissipation of single PCBs
Similarly, we examined each PCB individually and their dissipation followed the same trends as the sum of the 7 PCBs, except for PCB 28 and to a lesser extent for PCB 52, 138 and 180 (Fig. S1). The mechanisms of PCBs degradation depend strongly on (i) the soil environment, (ii) the nature and amount of soil microorganisms and on (iii) the nature of PCBs, namely on their chlorine number but also on their spatial orientation. More researches on degradation toward coplanar PCBs, with dioxin-like toxicity, were described (Kaya et al., 2018; Meggo et al., 2013; Rosińska & Karwowska, 2017). The degradation of PCBs generally depends on their progressive reduction through dechlorination, with multiple reaction intermediates. This degradation is generally easier when the aromatic rings are not highly substituted by chlorines and when the chlorines are in para/meta position (non-coplanar orientation) (Mackova et al., 2010; Mousa et al., 1998). In this study, we observe a better dissipation for low chlorinated PCBs in planted soils (Fig. S1) probably due to a better aeration of the soil through the root system of the plant. Considering the monitored hexachlorobiphenyl homologs (PCB 138, PCB 153, PCB 156) PCB 138 appeared to be more resistant to dissipation than the two other 6–chlorinated PCBs (Fig. S1). PCB 138 could be a preferential intermediate in the dechlorination of more chlorinated PCBs as suggested by Noma et al., 2007. However, the concentration of less chlorinated congeners remained almost the same so the hypothesis of degradation by dehalogenation of highly chlorinated PCBs seems unlikely (Terzaghi et al., 2019). Although it appears difficult to state from this study, degradation might result from a transformation into different hydroxylated or methoxylated metabolites as observed by Sun et al. (2018).
To better understand the dissipation mechanisms, we correlated, by using principal component analysis (PCA, Fig. 6), the percentages of excess dissipation of each PCB, their physico–chemical characteristics (Table S1) and the characteristics of soils (Table 1) according to soils (Fig. 6a, b) and according to plants (Fig. 6c, d). In Fig. 6-a, the PCA is divided in two main axes that explain 82% of the total variation. The soil parameters are on a same line distributed between these two axes. The line describing the parameters of the PCBs is perpendicular. This latter line opposes, not surprisingly, parameters as the number of chlorines, the octanol/air partition coefficient, the adsorption coefficient into soils to the solubility and the volatilization of the different PCBs. Solubility and volatilization were also correlated to the excess dissipation by plant1 after 90 days. In contrast, the excess dissipation by both plants after 40 days of planting was correlated to the percentage of clay and soil pH, and anticorrelated to the organic carbon and nitrogen levels. The results of the PCA suggest that the physico-chemical parameters of the soils mainly contributed to PCB dissipation at the beginning of the planting. When the growth time increased, soil parameters and PCB characteristics contributed to the excess dissipation by the plant 2. For plant 1, only the intrinsic parameters of these pollutants were predominant. In addition, the graph of individuals shows two distinct clusters. Soils A and C were close together while soil B differ from them (Fig. 6b). In Fig. 6c, the first axis which represents 62% of the total variation mainly considered the PCB parameters. On this dimension, the excess dissipation of PCBs in soil C at T2 and in soil B at T1 were strongly correlated with PCB volatilization. In the weakly polluted soils, dissipation improvement by plants is negatively correlated to the number of chlorines. This result would tend to prove that plants promote the dissipation of low chlorinated PCBs by volatilization; this process appearing to be more pronounced for the least polluted soils. The PCA results suggest also a different dissipation kinetics between these two soils. This result could be explained by the observations of Terzaghi et al. (2019) describing that a biostimulation time was required in order to reach an optimal microbial activity. In their study, this time varies according to the plant species. In our case, it is possible that the soil physical and chemical parameters have an impact on this time. For the soil B, the correlation with these properties decreased over time and another factor predominated, represented by axis 2. For the soil C, relatively close to soil A in term of composition, the correlation with the properties of PCBs appear later. Initially, the excess dissipation was correlated to the second axis of the PCA. This axis is represented by excess dissipation in soil A. The significant dissipation for unplanted soil B and the reverse effects between soils A and B suggest that this second axis could be related to the interaction plant–soil, i.e., the ability of the plant to promote the soil bacterial community. In this PCA, the graph of the individuals (Fig. 6d) distributed the PCBs according to their degree of chlorination on the first dimension and opposed the two plants on the second one. These results suggest that their behavior is different with regard to dissipation.
Conclusion
During this study, remediation of three Algerian soils polluted by Askarel oil was carried out using two plants adapted to Mediterranean climate. The PCB pollution levels ranged from 1.3–127.9 mg kg1. The two studied plants were particularly resistant to high levels of PCBs in soils although Avena sativa could be more sensitive to toxic effects of PCBs in the long term. The “wild” plant Vicia sativa contributed more efficiently to the long–term dissipation of PCBs in the highly polluted soil A. The cultivable plant Avena sativa showed an interesting capacity for PCB dissipation improvement in the moderately polluted soil B. The two plants had the same beneficial effect on the lowest contaminated soil C. In the case of the moderately or highly contaminated soils, the PCB dissipation due to plants was low or not dependent on PCBs properties. The quality of the planted soil had a preponderant effect in the early stages of planting and the intrinsic parameters of the PCBs took over the dissipation mechanisms, with, a priori, a shift depending on the plants. The degradation of PCBs also seems to be governed by the soil–plant interaction and the ability of the latter to shape the bacterial community structure and activity. This shift may simply be temporal, a longer experimental time may be needed to confirm the influence of PCB characteristics on plant dissipation. From the results obtained for soil B, it seems that the high organic matter content may affect not only the sorption of PCBs but also the evolving capacity of the bacteria to degrade PCBs. These findings have yet to be confirmed and the phytoextraction aspect has to be evaluated. However, in view of these results, the use of these two plants, often cultivated in synergy in Algeria, could be effective for the remediation of PCBs, even in highly contaminated soils.
References
Abhilash, P., Jamil, S., & Singh, N. (2009). Transgenic plants for enhanced biodegradation and phytoremediation of organic xenobiotics. Biotechnology Advances, 27(4), 474–488. https://doi.org/10.1016/j.biotechadv.2009.04.002
Backe, C., Cousins, I. T., & Larsson, P. (2004). PCB in soils and estimated air-soil exchange fluxes of selected PCB congeners in the south of Sweden. Environmental Pollution, 128, 59–72. https://doi.org/10.1016/j.envpoprl.2003.08.038
Bergman, Å., Heindel, J. J., Jobling, S., Kidd, K., Zoeller, T. R., World Health Organization. (2013). State of the science of endocrine disrupting chemicals 2012. World Health Organization.
Cachada, A., Lopes, L., Hursthouse, A., Biasioli, M., Grčman, H., Otabbong, E., Davidson, C., & Duarte, A. (2009). The variability of polychlorinated biphenyls levels in urban soils from five European cities. Environmental Pollution, 157(2), 511–518. https://doi.org/10.1016/j.envpol.2008.09.002
Cetin, B. (2016). Investigation of PAHs, PCBs and PCNs in soils around a Heavily Industrialized Area in Kocaeli, Turkey: concentrations, distributions, sources and toxicological effects. Science of the Total Environment, 560, 160–169. https://doi.org/10.1016/j.scitotenv.2016.04.037
Chekol, T., Vough, L. R., & Chaney, R. L. (2004). Phytoremediation of polychlorinated biphenyl-contaminated soils: the rhizosphere effect. Environment International, 30(6), 799–804. https://doi.org/10.1016/j.envint.2004.01.008
Chen, S.-J., Tian, M., Zheng, J., Zhu, Z.-C., Luo, Y., & Mai, B.-X. (2014). Elevated levels of Polychlorinated Biphenyls in plants, air, and soils at an e-waste site in southern China and enantioselective biotransformation of chiral PCBs in plants. Environmental Science and Technology, 48, 3847–3855. https://doi.org/10.1021/es405632v
Crampon, M., Bodilis, J., & Portet-Koltalo, F. (2018). Linking initial soil bacterial diversity and polycyclic aromatic hydrocarbons (PAHs) degradation potential. Journal of Hazardous Materials, 359, 500–509. https://doi.org/10.1016/j.jhazmat.2018.07.088
Crampon, M., Bureau, F., Akpa-Vinceslas, M., Bodilis, J., Machour, N., Le Derf, F., & Portet-Koltalo, F. (2014). Correlations between PAH bioavailability, degrading bacteria, and soil characteristics during PAH biodegradation in five diffusely contaminated dissimilar soils. Environmental Science and Pollution Research, 21(13), 8133–8145. https://doi.org/10.1007/s11356-014-2799-6
Eckley, N., & Selin, H. (2004). All talk, little action: precaution and European chemicals regulation. Journal of European Public Policy, 11(1), 78–105.
Erickson, M. D., & Kaley, R. G. (2011). Applications of polychlorinated biphenyls. Environmental Science and Pollution Research, 18(2), 135–151.
Fan, G., Cang, L., Fang, G., & Zhou, D. (2014). Surfactant and oxidant enhanced electrokinetic remediation of a PCBs polluted soil. Separation and Purification Technology, 123, 106–113.
Fouial-Djebbar, D., Ahmed, A.B.-H., & Budzinski, H. (2010). Determination of organochlorine compounds in coastal marine sediments from the southern west of the Mediterranean Sea. International Journal of Environmental Science & Technology, 7(2), 271–280.
Gan, S., Lau, E., & Ng, H. (2009). Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). Journal of Hazardous Materials, 172(2–3), 532–549. https://doi.org/10.1016/j.jhazmat.2009.07.118
Glüge, J., Steinlin, C., Schalles, S., Wegmann, L., Tremp, J., Breivik, K., Hungerbühler, K. and Bogdal, C. (2017). Import, use, and emissions of PCBs in Switzerland from 1930 to 2100. PloS one 12(10): e0183768.
Green, N. J., Jones, J. L., & Jones, K. C. (2001). PCDD/F deposition time trend to Esthwaite Water, UK, and its relevance to sources. Environmental Science & Technology, 35(14), 2882–2888.
Halfadji, A., Touabet, A., Portet-Koltalo, F., Le Derf, F., & Merlet-Machour, N. (2017). Concentrations and source identification of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in agricultural, urban/residential, and industrial soils, east of Oran (Northwest Algeria). Polycyclic Aromatic Compounds, 39(4), 299–310.
Jiang, Y., Wang, X., Zhu, K., Wu, M., Sheng, G., & Fu, J. (2011). Polychlorinated biphenyls contamination in urban soil of Shanghai: level, compositional profiles and source identification. Chemosphere, 83(6), 767–773. https://doi.org/10.1016/j.chemosphere.2011.02.077
Kaya, D., Imamoglu, I., Sanin, F. D., & Sowers, K. R. (2018). A comparative evaluation of anaerobic dechlorination of PCB-118 and Aroclor 1254 in sediment microcosms from three PCB-impacted environments. Journal of Hazardous Materials, 341, 328–335. https://doi.org/10.1016/j.jhazmat.2017.08.005
Kidd, K. A., Schindler, D. W., Hesslein, R. H., & Muir, D. C. (1998). Effects of trophic position and lipid on organochlorine concentrations in fishes from subarctic lakes in Yukon territory. Canadian Journal of Fisheries and Aquatic Sciences, 55(4), 869–881.
Kobasic, H. V., Calic, V., Tanik, T., Picer, M., Sangulin, J., & Franetovic, M. (2005). Research on the transport of PCBs with leachate water from contaminated soil. Organohalogen Compounds, 1026–4892(67), 1932–1936.
Leroy, M.-C., Legras, M., Marcotte, S., Moncond’Huy, V., Machour, N., Le Derf, F., & Portet-Koltalo, F. (2015). Assessment of PAH dissipation processes in large-scale outdoor mesocosms simulating vegetated road-side swales. Science of the Total Environment, 520, 146–153. https://doi.org/10.1016/j.scitotenv.2015.03.020
Li, Y., Liang, F., Zhu, Y., & Wang, F. (2013). Phytoremediation of a PCB-contaminated soil by alfalfa and tall fescue single and mixed plants cultivation. Journal of Soils and Sediments, 13(5), 925–931. https://doi.org/10.1007/s11368-012-0618-6
Ma, Y., Du, X., Shi, Y., Xu, Z., Fang, J., Li, Z., & Li, F. (2015). Low concentration tailing and subsequent quick line enhanced remediation of volatile chlorinated hydrocarbon contaminated soil by mechanical soil aeration. Chemosphere, 131, 117–123.
Macek, T., Mackova, M., & Káš, J. (2000). Exploitation of plants for the removal of organics in environmental remediation. Biotechnology Advances, 18(1), 23–34.
Mackova, M., Uhlik, O., Lovecka, P., Viktorova, J., Novakova, M., Demnerova, K., Sylvestre, M. and Macek, T. (2010). Bacterial degradation of polychlorinated biphenyls. Geomicrobiology: Molecular and Environmental Perspective, Springer: 347–366.
Martinez, A., Erdman, N. R., Rodenburg, Z. L., Eastling, P. M., & Hornbuckle, K. C. (2012). Spatial distribution of chlordanes and PCB congeners in soil in Cedar Rapids, Iowa, USA. Environmental Pollution, 161, 222–228. https://doi.org/10.1016/j.envpol.2011.10.028
Meggo, R. E., Schnoor, J. L., & Hu, D. (2013). Dechlorination of PCBs in the rhizosphere of switchgrass and poplar. Environmental Pollution, 178, 312–321. https://doi.org/10.1016/j.envpol.2013.02.035
Melnyk, A. H., Wong, A., & Kassen, R. (2015). The fitness costs of antibiotic resistance mutations. Evolutionary Applications, 8(3), 273–283. https://doi.org/10.1016/j.scitotenv.2015.05.092
Mousa, M. A., Ganey, P. E., Quense, J., Madhukar, B. V., Chou, K., Giesy, J. P., Fischer, L. J., & Boyd, S. A. (1998). Altered biologic activities of commercial polychlorinated biphenyl mixtures after microbial reductive dechlorination. Environmental Health Perspectives, 106(suppl 6), 1409–1418.
Nadal, M., Schumacher, M., & Domingo, J. L. (2007). Levels of metals, PCBS, PCNs and PAHs in soils of a highly industrialized chemical/petrochemical area: Temporal trend. Chemosphere, 66, 267–276. https://doi.org/10.1016/j.chemosphere.2006.05.020
Noma, Y., Mitsuhara, Y., Matsuyama, K., & Sakai, S.-I. (2007). Pathways and products of the degradation of PCBS by the sodium dispersion method. Chemosphere, 68, 871–879.
Odabasi, M., Bayram, A., Elbir, T., Seyfioglu, R., Dumanoglu, Y., & Ornektekin, S. (2010). Investigation of soil concentrations of persistent organic pollutants, trace elements, and anions due to iron-steel plant emissions in an industrial region in Turkey. Water, Air, and Soil Pollution, 213, 375–388. https://doi.org/10.1007/s11270-010-0392-2
Pajuelo, E., Rodríguez-Llorente, I. D., Dary, M., & Palomares, A. J. (2008). Toxic effects of arsenic on Sinorhizobium-Medicago sativa symbiotic interaction. Environmental Pollution, 154(2), 203–211.
Passatore, L., Rossetti, S., Juwarkar, A. A., & Massacci, A. (2014). Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): state of knowledge and research perspectives. Journal of Hazardous Materials, 278, 189–202. https://doi.org/10.1016/j.jhazmat.2014.05.051
Perez-Maldonado, I., Costilla-Salazar, R., Ilizaliturri-Hernandez, C. A., Espinosa-Reyes, G., Perez-Vazquez, F. J., & Fernandez-Macias, J. C. (2014). Assessment of the polychlorinated biphenyls (PCBs) levels in soil samples near an electric capacitor manufacturing industry in Morelos, Mexico. Journal of Environmental Science and Health, Part A, 49(11), 1244–1250.
Pino, N. J., Munera, L. M., & Penuela, G. A. (2019). Phytoremediation of soil contaminated with PCBs using different plants and their associated microbial communities. International Journal of Phytoremediation, 21(4), 316–324. https://doi.org/10.1080/15226514.2018.1524832
Portet-Koltalo, F., Oukebdane, K., Dionnet, F., & Desbène, P. L. (2008). Optimisation of the extraction of polycyclic aromatic hydrocarbons and their nitrated derivatives from diesel particulate matter using microwave-assisted extraction. Analytical and Bioanalytical Chemistry, 390(1), 389–398. https://doi.org/10.1007/s00216-007-1684-2
Qi, Z., Chen, T., Bai, S., Yan, M., Lu, S., Buekens, A., Yan, J., Bulmau, C., & Li, X. (2014). Effect of the temperature and particle size on the thermal desorption of PCBs from contaminated soil. Environmental Science and Pollution Research, 21, 4697–4704. https://doi.org/10.1007/s11356-013-2392-4
Rachdawong, P., & Christensen, E. R. (1997). Determination of PCB sources by a principal component method with nonnegative constraints. Environmental Science & Technology, 31(9), 2686–2691.
Radonić, J., Miloradov, M. V., Sekulić, M. T., Kiurski, J., Djogo, M., & Milovanovic, . (2011). The octanol–air partition coefficient, KOA, as a predictor of gas–particle partitioning of polycyclic aromatic hydrocarbons and polychlorinated biphenyls at industrial and urban sites. Journal of the Serbian Chemical Society, 76(3), 447–458.
Reddy, A. V. B., Moniruzzaman, M., & Aminabhavi, T. M. (2019). Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chemical Engineering Journal, 358, 1186–1207. https://doi.org/10.1016/j.cej.2018.09.205
Rein, A., Fernqvist, M. M., Mayer, P., Trapp, S., Bittens, M., & Karlson, U. G. (2007). Degradation of PCB congener by bacterial strains. Applied Microbiology and Biotechnology. https://doi.org/10.1007/s00253-007-1175-6
Rosińska, A., & Karwowska, B. (2017). Dynamics of changes in coplanar and indicator PCB in sewage sludge during mesophilic methane digestion. Journal of Hazardous Materials, 323, 341–349. https://doi.org/10.1016/j.jhazmat.2016.04.016
Ross, G. (2004). The public health implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicology and Environmental Safety, 59(3), 275–291. https://doi.org/10.1016/j.ecoenv.2004.06.003
Ruzickova, P., Klanova, J., Cupr, P., Lammel, G., & Holoubek, I. (2008). An assessment of air-soil exchange of polychlorinated biphenyls and organochlorine pesticides across Central and Southern Europe. Environmental Science and Technology, 42, 179–185. https://doi.org/10.1021/es071406fCCC
Stella, T., Covino, S., Burianova, E., Filipova, A., Kresinova, Z., Voriskova, J., Vetrovsky, T., Baldrian, P., & Cajthaml, T. (2015). Chemical and microbiological characterization of an aged PCB-contaminated soil. Science of Total Environment, 533, 177–186. https://doi.org/10.1016/j.scitotenv.2015.06.019
Stratton, C. L., & Sosebee, J. B. (1976). PCB and PCT contamination of the environment near sites of manufacture and use. Environmental Science & Technology, 10(13), 1229–1233.
Strek, H.J. & Weber, J.B. (1982). Behaviour of polychlorinated biphenyls (PCBs) in soils and plants. Environmental Pollution 28(A): 291–312.
Sun, H., Qi, Y., Zhang, D., Li, Q. X., & Wang, J. (2016). Concentrations, distribution, sources and risk assessment of organohalogenated contaminants in soils from Kenya, Eastern Africa. Environmental Pollution, 209, 177–185. https://doi.org/10.1016/j.envpol.2015.11.040
Sun, J., Pan, L., & Zhu, L. (2018). Formation of hydroxylated and methoxylated polychlorinated biphenyls by Bacillus subtilis: New insights into microbial metabolism. Science of Total Environment, 613–614, 54–61. https://doi.org/10.1016/j.scitotenv.2017.09.063
Terzaghi, E., Vergani, L., Mapelli, F., Borin, S., Raspa, G., Zanardini, E., Morosini, C., Anelli, S., Nastasio, P., Sale, V. M., Armiraglio, S., & Di Guardo, A. (2019). Rhizomediation of weathered PCBs in heavily contaminated agricultural soil: Results of a biostimulation trial in semi field conditions. Science of Total Environment, 686, 484–496. https://doi.org/10.1016/j.scitoenv.2019.05.458
Terzaghi, E., Zanardini, E., Morosini, C., Raspa, G., Borin, S., Mapelli, F., Vergani, L., & Di Guardo, A. (2018). Rhizoremediation half-lives of PCBs: Role of congener composition, organic carbon forms, bioavailability, microbial activity, plant species and soil conditions, on the prediction of fate and persistence in soil. Science of the Total Environment, 612, 544–560. https://doi.org/10.1016/j.scitotenv.2017.08.189
Toussaint, J.-P., Pham, T. T. M., Barriaut, D., & Sylvestre, M. (2012). Plant exudates promote PCB degradation by a rhodococcal rhizobacteria. Applied Microbiology and Biotechnology, 95, 1589–1603. https://doi.org/10.1007/s00253-011-3824-z
Tremolada, P., Guazzoni, N., Smillovitch, L., Moia, F., & Comolli, R. (2012). The effect of the Organic Matter Composition on POP Accumulation in soil. Water Air Soil Pollution, 223, 4539–4556. https://doi.org/10.1007/s11270-012-1216-3
Tu, C., Teng, Y., Luo, Y., Sun, X., Deng, S., Li, Z., Liu, W., & Xu, Z. (2011). PCB removal, soil enzyme activities, and microbial community structures during the phytoremediation by alfalfa in field soils. Journal of Soils and Sediments, 11(4), 649–656. https://doi.org/10.1007/s11368-011-0344-5
Tuna, C., and Orak, A. (2007). The role of intercropping on yield potential of common vetch (Vicia sativa L.)/oat (Avena sativa L.) cultivated in pure stand and mixtures. Journal of Agricultural and Biological Science 2(2): 14–19.
Wang, M., Safe, S., Hearon, S. E., & Phillips, T. D. (2019). Strong adsorption of polychlorinated biphenyls by processed montmorillonite clays: Potential applications as toxin enterosorbents during disasters and floods. Environmental Pollution, 255, 113210. https://doi.org/10.1016/j.envpol.2019.113210
Wang, Y., Luo, C.-L., Li, J., Yin, H., Li, X.-D., & Zhang, G. (2011). Characterization and risk assessment of polychlorinated biphenyls in soils and vegetations near an electronic waste recycling site. SoUth China. Chemosphere, 85(3), 344–350.
Wilkins, D. (1957). A technique for the measurement of lead tolerance in plants. Nature, 180(4575), 37.
Youn, L. S., Kim, B.-N., Choi, Y.-W., Yoo, K.-S., Kim, Y.-H., & Min, J. (2012). Growth response of Avena sativa in amino-acids-rich soils converted from phenol-contaminated soils by Corynebacterium glutamicum. Journal of Microbiology and Biotechnology, 22(4), 541–546. https://doi.org/10.4014/jmb.1108.08089
Zhou, J. L., Siddiqui, E., Ngo, H. H., & Guo, W. (2014). Estimation of uncertainty in the sampling and analysis of polychlorinated biphenyls and polycyclic aromatic hydrocarbons from contaminated soil in Brighton, UK. Science of the Total Environment, 497–498, 163–171.
Acknowledgements
We are thankful to the Algerian Ministry of Higher Education and Scientific Research for providing fruitful funding to complete this research work at the Laboratory of Organic and Bioorganic Chemistry, Reactivity and Analysis, UMR CNRS 6014, IUT Evreux, University of Rouen-Normandy, France. This work was also supported by the European Regional Development Fund (ERDF) N° HN0001343, the European Union’s Horizon 2020 Research Infrastructures program (Grant Agreement 731077), the Région Normandie, and the Laboratoire d’Excellence (LabEx) SynOrg (ANR-11-LABX-0029).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Halfadji, A., Portet-Koltalo, F., Touabet, A. et al. Phytoremediation of PCB: contaminated Algerian soils using native agronomics plants. Environ Geochem Health 44, 117–132 (2022). https://doi.org/10.1007/s10653-021-01049-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-01049-z